The Isotopic Composition of Selected Phosphate Sources (δ18O-PO4) from the Area of the Vistula and Bug Interfluve (Poland)
Abstract
1. Introduction
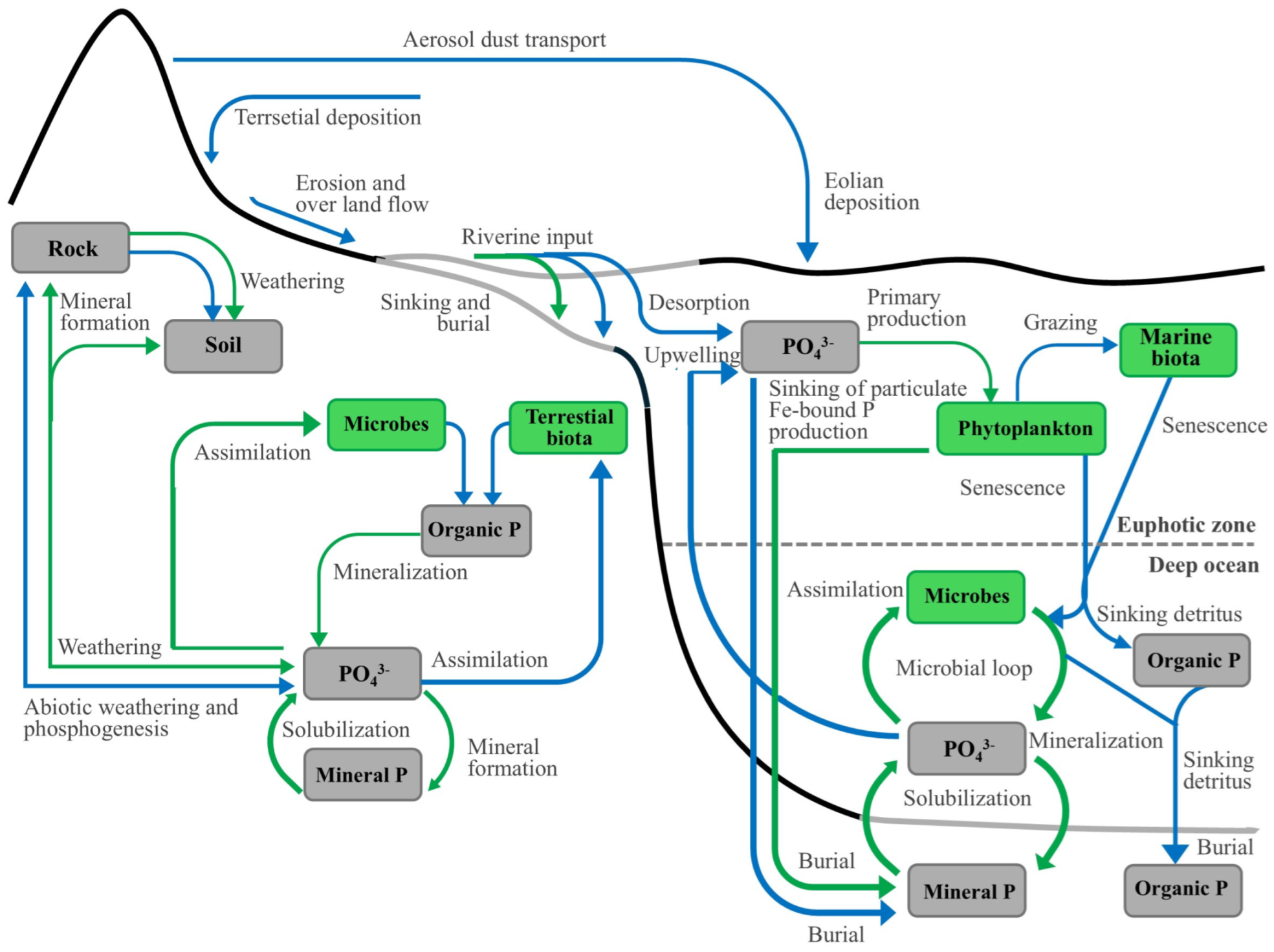
1.1. Global Phosphorus Cycle
1.2. The Human Impact on the Global Phosphorus Cycle

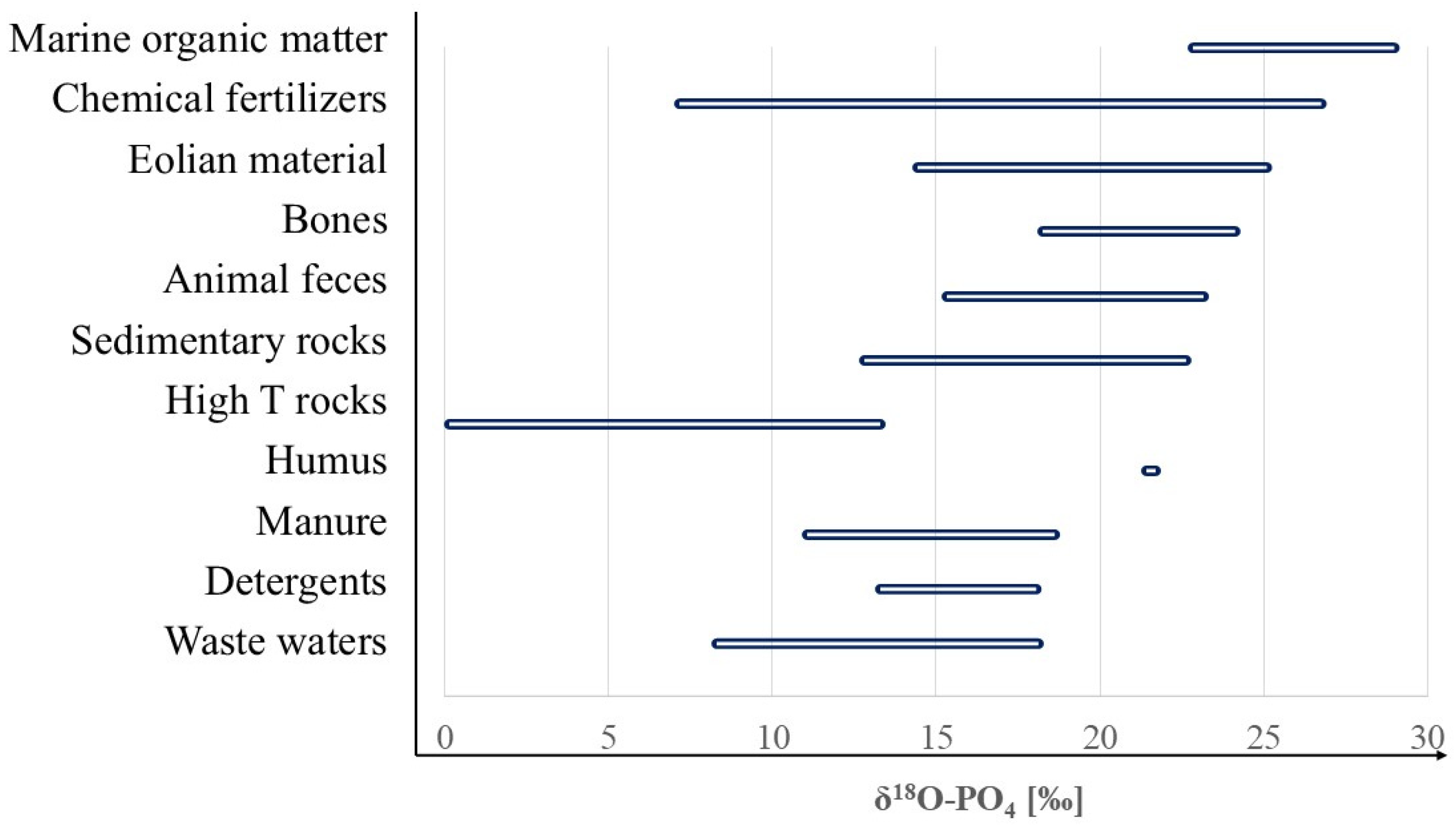
1.3. How to Use δ18O-PO4 as a Tracer for Sources and the Cycling of Phosphates
2. Study Area
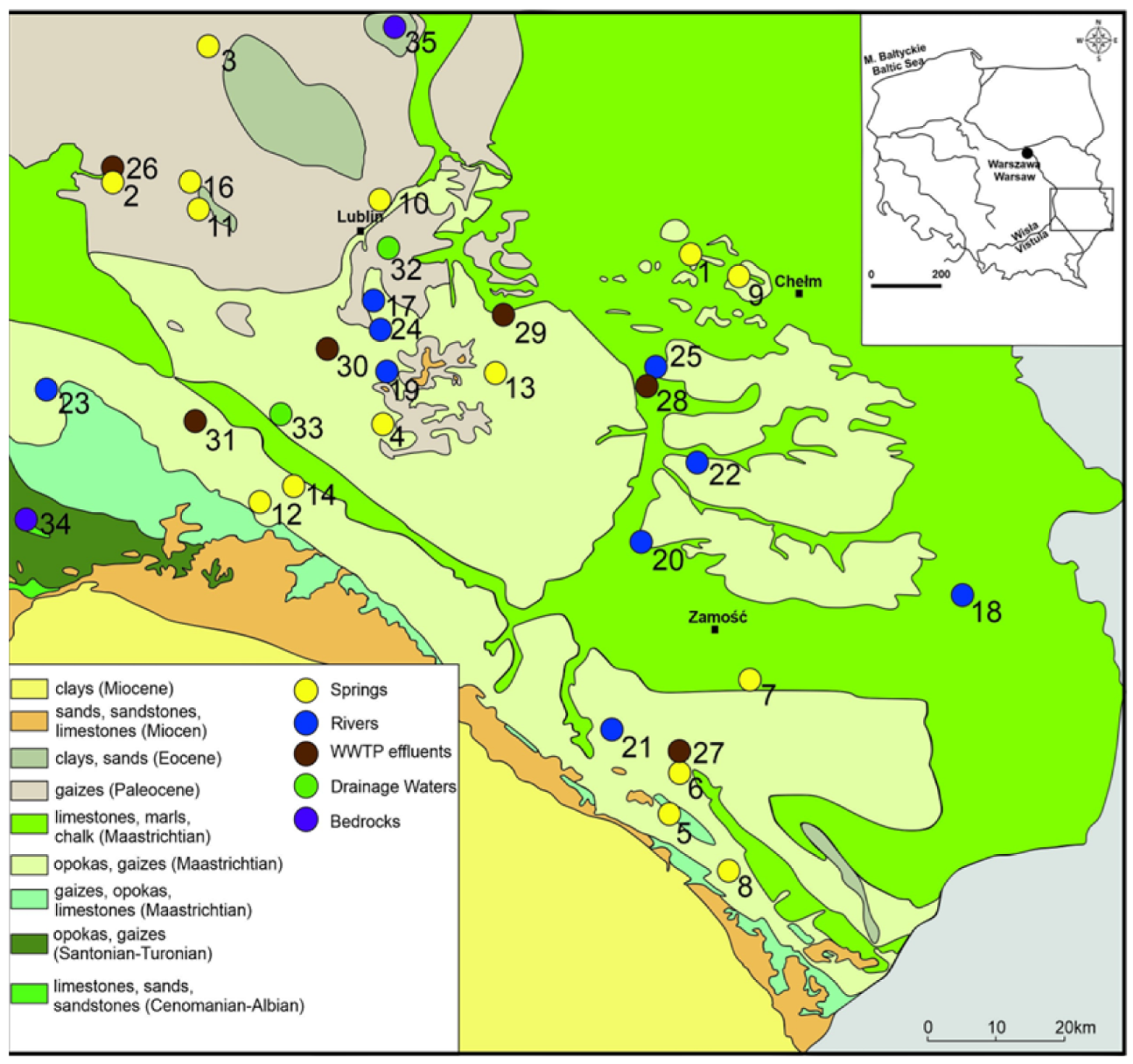
3. Materials and Methods
3.1. Sample Collection
3.2. Methods
Physicochemical Analysis
3.3. Phosphate Extraction from Water and Rock Samples
3.3.1. Water Samples
3.3.2. Bedrock Samples
3.3.3. Isotopic Analysis of δ18O-PO4 in Phosphates
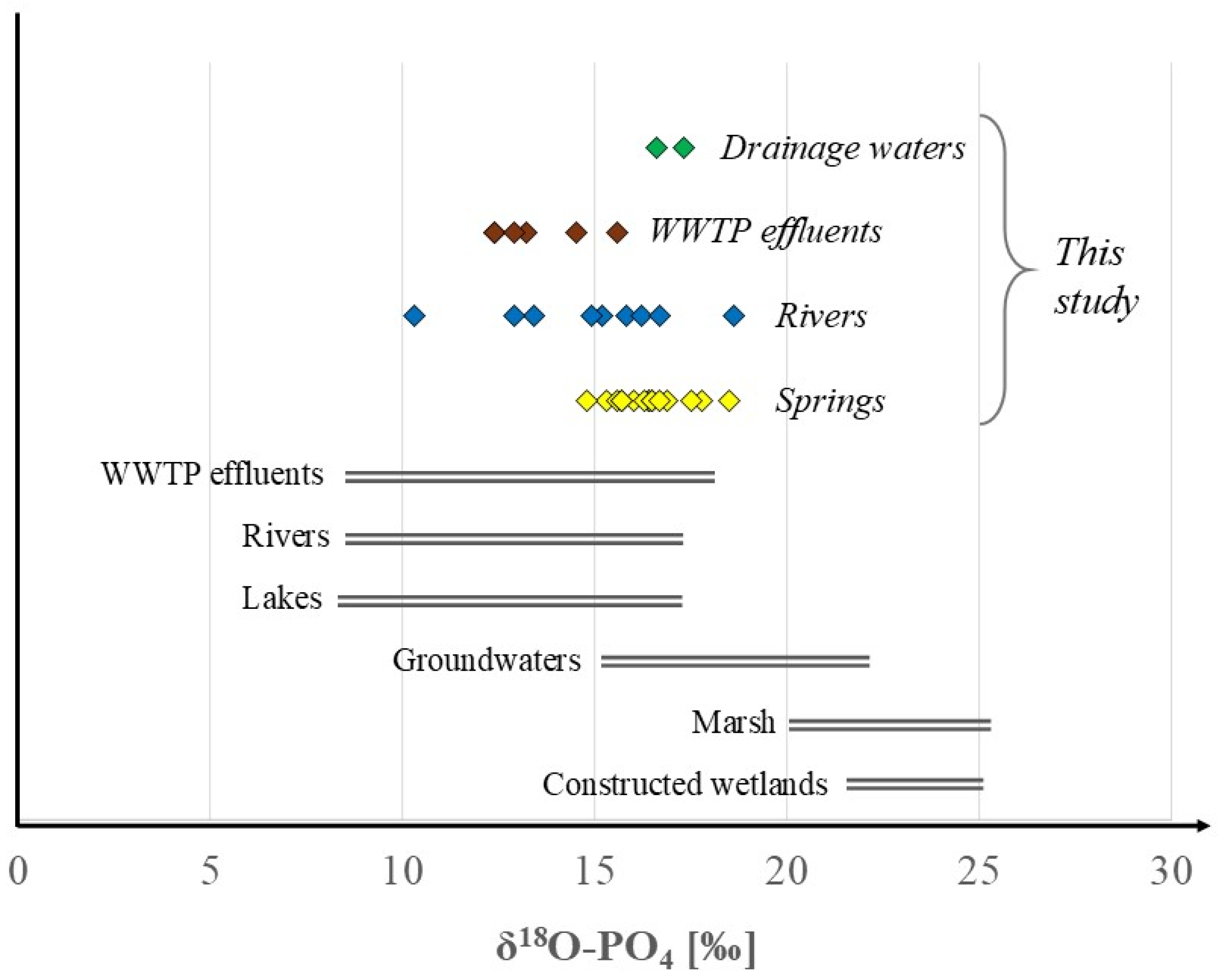
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mackey, K.R.M.; Paytan, A. Phosphorus Cycle. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 322–334. [Google Scholar] [CrossRef]
- Tiessen, H. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions: Plant Ecophysiology; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7. [Google Scholar] [CrossRef]
- O’Neill, P. Environmental Chemistry, 2nd ed.; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Filippelli, G.M. The Global Phosphorus Cycle: Past, Present, and Future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Vollenweider, R.A. The Scientific Basis for Lake and Stream Eutrophication with Particular Reference to Phosphorus and Nitrogen as Eutrophication Factors; Technical Report DAS/DS1/68.2; Organization for Economic Cooperation and Development: Paris, France, 1968. [Google Scholar]
- Regulation on the Classification of Ecological Status, Ecological Potential, Chemical Status and the Method of Classifying the Status of Surface Water Bodies as Well as Environmental Quality Standards for Priority Substances. Available online: https://leap.unep.org/en/countries/pl/national-legislation/regulation-classification-ecological-status-ecological-1 (accessed on 25 September 2024).
- Rączka, J.; Skąpski, K.; Tyc, T. Water Resources in Poland—Protection and Utilization; Fundacja Przyjazny Kraj: Warszawa, Russia, 2021; pp. 5–47. (In Polish) [Google Scholar]
- Paytan, A.; McLaughlin, K. The Oceanic Phosphorus Cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.; Young, M.B.; Paytan, A.; Kendall, C. The Oxygen Isotopic Composition of Phosphate: A Tracer for Phosphate Sources and Cycling; IAEA-TECDOC-1695; IAEA: Vienna, Austria, 2013; pp. 93–110. [Google Scholar]
- Tamburini, F.; Pfahler, V.; von Sperber, C.; Frossard, E.; Bernasconi, S.M. Oxygen Isotopes for Unraveling Phosphorus Transformations in the Soil–Plant System: A Review. Soil Sci. Soc. Am. J. 2014, 78, 38–46. [Google Scholar] [CrossRef]
- Blake, R.E.; O’Neil, J.R.; Surkov, A.V. Biogeochemical cycling of phosphorus: Insights from oxygen isotope effects of phosphoenzymes. Am. J. Sci. 2005, 305, 596–620. [Google Scholar] [CrossRef]
- McLaughlin, K.; Kendall, C.; Silva, S.; Stuart-Williams, H.; Paytan, A. A precise method for the analysis of δ18O of dissolved inorganic phosphate in seawater. Limnol. Oceanogr. Methods 2004, 2, 202–212. [Google Scholar] [CrossRef]
- Gruau, G.; Legeas, M.; Riou, C.; Gallacier, E.; Martineau, F.; Hénin, O. The oxygen isotope composition of dissolved anthropogenic phosphates: A new tool for eutrophication research? Water Res. 2005, 39, 232–238. [Google Scholar] [CrossRef]
- Elsbury, K.E.; Paytan, A.; Ostrom, N.E.; Kendall, C.; Young, M.B.; McLaughlin, K.; Rollog, M.E.; Watson, S. Using Oxygen Isotopes of Phosphate to Trace Phosphorus Sources and Cycling in Lake Erie. Environ. Sci. Technol. 2009, 43, 3108–3114. [Google Scholar] [CrossRef]
- Pistocchi, C.; Mészáros, É.; Frossard, E.; Bünemann, E.K.; Tamburini, F. In or Out of Equilibrium? How Microbial Activity Controls the Oxygen Isotopic Composition of Phosphate in Forest Organic Horizons with Low and High Phosphorus Availability. Front. Environ. Sci. 2020, 8, 564778. [Google Scholar] [CrossRef]
- Paytan, A.; Roberts, K.; Watson, S.; Peek, S.; Chuang, P.-C.; Defforey, D.; Kendall, C. Internal loading of phosphate in Lake Erie Central Basin. Sci. Total Environ. 2017, 579, 1356–1365. [Google Scholar] [CrossRef]
- Tcaci, M.; Barbecot, F.; Hélie, J.-F.; Surridge, B.W.J.; Gooddy, D.C. A New Technique to Determine the Phosphate Oxygen Isotope Composition of Freshwater Samples at Low Ambient Phosphate Concentration. Environ. Sci. Technol. 2019, 53, 10288–10294. [Google Scholar] [CrossRef]
- Michalczyk, Z.; Wilgat, T. Waters. In The Natural Environment of the Lublin Region; Uziak, S., Turski, R., Eds.; LTN; EN: Brussels, Belgium, 2008; pp. 113–209. (In Polish) [Google Scholar]
- Mazurek, S.; Roszkowska-Remin, J.; Bienko, T. New geological criteria for domestic phosphorite deposits—A discussion. Gospod. Surowcami Miner.—Miner. Resour. Manag. 2024, 40, 63–83. [Google Scholar] [CrossRef]
- Gebus-Czupyt, B.; Chmiel, S.; Trembaczowski, A.; Wach, B. The studies of nitrates (δ15N, δ18O) and phosphates (δ18O) isotopic composition as a tool for identifying eutrophic water pollution. In Proceedings of the 9th National Conference Petrological and Mineralogical Research in Geology, Warsaw, Poland, 28–29 March 2019. (In Polish). [Google Scholar]
- Mullen, M.D. Phosphorus in Soils—Biological Interactions. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Fillipelli, G.M. Phosphorus and Life on a Water World. Geophys. Res. Lett. 2022, 49, e2021GL097346. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The trace element composition of apatite and its application to detrital provenance studies. Earth-Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Yoder, C.H.; Stepien, K.R.; Dudrick, R.N. The distribution of carbonate in apatite: The environment model. Am. Miner. 2023, 108, 1072–1079. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.-Y.; Zhao, Y.-H. Trace elements in apatite from Gejiu Sn polymetallic district: Implications for petrogenesis, metallogenesis and exploration. Ore Geol. Rev. 2022, 145, 104880. [Google Scholar] [CrossRef]
- Bruand, E.; Fowler, M.; Storey, C.; Darling, J. Apatite trace element and isotope applications to petrogenesis and provenance. Am. Miner. 2017, 102, 75–84. [Google Scholar] [CrossRef]
- Qiu, K.-F.; Zhou, T.; Chew, D.; Hou, Z.-L.; Müller, A.; Yu, H.-C.; Lee, R.G.; Chen, H.; Deng, J. Apatite trace element composition as an indicator of ore deposit types: A machine learning approach. Am. Miner. 2024, 109, 303–314. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The Global Phosphorus Cycle. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 585–643. [Google Scholar] [CrossRef]
- Liu, Y.; Villalba, G.; Ayres, R.U.; Schroder, H. Global Phosphorus Flows and environmental Impacts from a Consumption Perspective. J. Ind. Ecol. 2008, 12, 229–247. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Energy Environ 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Schlesinger, W.H. The Global Cycles of Nitrogen and Phosphorus: Biogeochemistry: An Analysis of Global Change, 2nd ed.; Academic Press: San Diego, CA, USA, 1997; pp. 383–401. [Google Scholar]
- IFA. The Global “4R” Nutrient Stewardship Framework—Developing Fertilizer Best Management Practices for Delivering Economic, Social and Environmental Benefits; International Fertilizer Industry Association: Paris, France, 2009. [Google Scholar]
- Sapek, A. Phosphorus in the human food chain and the environment in Poland. Inżynieria Ekol. 2009, 21, 62–72. (In Polish) [Google Scholar]
- Turner, B.L.; Raboy, V. Phosphorus cycle. In AccessScience; McGraw Hill: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, S.; Sheng, H.; Liu, X.; Hua, H.; Liu, X.; Zhang, Y. Human Perturbation of the Global Phosphorus Cycle: Changes and Consequences. Environ. Sci. Technol. 2018, 52, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Sardans, J. Human-driven global nutrient imbalances increase risks to health. Eco-Environ. Health 2023, 2, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Von Sperber, C.; Tamburini, F.; Brunner, B.; Bernasconi, S.M.; Frossard, E. The oxygen isotope composition of phosphate released from phytic acid by the activity of wheat and Aspergillus niger phytase. Biogeosciences 2015, 12, 4175–4184. [Google Scholar] [CrossRef]
- Longinelli, A.; Nuti, S. Revised phosphate-water isotopic temperature scale. Earth Planet. Sci. Lett. 1973, 19, 373–376. [Google Scholar] [CrossRef]
- Gebus-Czupyt, B.; Wach, B. Application of δ18O-PO4 analysis to recognize phosphate pollutions in eutrophic water. Ecohydrol. Hydrobiol. 2022, 22, 21–39. [Google Scholar] [CrossRef]
- Michalczyk, Z.; Chmiel, S.; Głowacki, S.; Zielińska, B. Changes of springs’ yield of Lublin Upland and Roztocze Region in 1998–2008. J. Water Land Dev. 2008, 12, 113–125. [Google Scholar] [CrossRef]
- Michalczyk, Z. (Ed.) Źródła Wyżyny Lubelskiej i Roztocza; Wydawnictwo UMCS: Lublin, Poland, 2001; pp. 1–298. (In Polish) [Google Scholar]
- Michalczyk, Z.; Chmiel, S.; Głowacki, S.; Sposób, J.; Zielińska, B. Discharge of the springs of the Lublin Upland and Roztocze. Ecohydrol. Hydrobiol. 2020, 20, 599–609. [Google Scholar] [CrossRef]
- Chełmicki, W.; Jokiel, P.; Michalczyk, Z.; Moniewski, P. Distribution, discharge and regional characteristics of springs in Poland. Episodes 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Harasimiuk, M.; Brzezińska-Wójcik, T. Structural control on the relief in the Lublin Upland and the Roztocze region. Przegląd Geol. 2014, 62, 51–56. (In Polish) [Google Scholar]
- Available online: https://www.pgi.gov.pl/jubileusz/100-naj/wydarzen/11310-odkrycie-fosforytow-w-rejonie-annopola-rachowa.html (accessed on 24 March 2024).
- Machalski, M.; Komorowski, A.; Harasimiuk, M. New perspectives of exploration of Cretaceous marine vertebrates in the abandoned phosphate mine in Annopol on the Vistula. Przegląd Geol. 2009, 57, 638–641. (In Polish) [Google Scholar]
- Bardet, N.; Fischer, V.; Machalski, M. Large predatory marine reptiles from the Albian–Cenomanian of Annopol, Poland. Geol. Mag. 2016, 153, 1–16. [Google Scholar] [CrossRef]
- Cywicka, K. Geological Report on Reconnaissance Research for Clay Raw Material for the Production of Building Ceramics in the Eastern Part of the Lublin Voivodeship, in the Area of the Following Towns: Leszkowice, Brzeziny, Serniki, Czerniejów; Milejów Central Geological Archive of the Polish Geological Institute: Warsaw, Poland, 1980. (In Polish) [Google Scholar]
- Krawczyk, M.; Ryzner, K.; Skurzyński, J.; Jary, Z. Lithological indicators of loess sedimentation of SW Poland. Contemp. Trends Geosci. 2017, 6, 94–111. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kulus, M.; Grzelak, J.; Radzikowska, M.; Oziembłowski, M.; Domagała, Z.; Krajcarz, M.T. Assessing weaning stress—Relations between enamel hypoplasia, δ18O and δ13C values in human teeth obtained from early modern cemeteries in Wroclaw, Poland. Ann. Anat.—Anat. Anz. 2020, 232, 151546. [Google Scholar] [CrossRef]
- Lécuyer, C.; Fourel, F.; Seris, M.; Amiot, R.; Goedert, J.; Simon, L. Synthesis of In-House Produced Calibrated Silver Phosphate with a Large Range of Oxygen Isotope Compositions. Geostand. Geoanalytical Res. 2019, 43, 681–688. [Google Scholar] [CrossRef]
- Davies, C.L.; Surridge, B.W.J.; Gooddy, D.C. Phosphate oxygen isotopes within aquatic ecosystems: Global data synthesis and future research priorities. Sci. Total Environ. 2014, 496, 563–575. [Google Scholar] [CrossRef]
- Smith, A.C.; Pfahler, V.; Tamburini, F.; Blackwell, M.S.A.; Granger, S.J. A review of phosphate oxygen isotope values in global bedrocks: Characterising a critical endmember to the soil phosphorus system. J. Plant Nutr. Soil Sci. 2021, 184, 25–34. [Google Scholar] [CrossRef]
- Gooddy, D.C.; Lapworth, D.J.; Bennett, S.A.; Heaton, T.H.E.; Williams, P.J.; Surridge, B.W.J. A multi-stable isotope framework to understand eutrophication in aquatic ecosystems. Water Res. 2016, 88, 623–633. [Google Scholar] [CrossRef]
- Gooddy, D.C.; Lapworth, D.J.; Ascott, M.J.; Bennett, S.A.; Heaton, T.H.E.; Surridge, B.W.J. Isotopic Fingerprint for Phosphorus in Drinking Water Supplies. Environ. Sci. Technol. 2015, 49, 9020–9028. [Google Scholar] [CrossRef]
- Granger, S.J.; Heaton, T.H.E.; Pfahler, V.; Blackwell, M.S.A.; Yuan, H.; Collins, A.L. The oxygen isotopic composition of phosphate in river water and its potential sources in the Upper River Taw catchment, UK. Sci. Total Environ. 2017, 574, 680–690. [Google Scholar] [CrossRef]
| Sampling Date | Sample No. | Sample Name | Q [dm3/s] | T [°C] | pH | Conductivity [μS/cm] | Dissolved O2 | ORP [mV] | Na+ [mg/dm3] | NH4+ [mg/dm3] | K+ [mg/dm3] | Ca2+ [mg/dm3] | Mg2+ [mg/dm3] | HCO3− [mg/dm3] | F− [mg/dm3] | Cl− [mg/dm3] | NO2− [mg/dm3] | NO3− [mg/dm3] | SO42− [mg/dm3] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/dm3] | [%] | |||||||||||||||||||
| Springs | ||||||||||||||||||||
| 14 October 2021 | 1 | Bezek | 7.5 | 9.6 | 7.5 | 596 | 7.5 | 67.2 | 104.0 | 1.1 | <0.01 | 1.7 | 130.5 | 0.9 | 317.8 | 0.1 | 20.3 | 0.1 | 21.1 | 21.5 |
| 29 September 2021 | 2 | Celejów | 44.7 | 9.2 | 7.5 | 678 | 2.3 | 21.4 | 102.0 | 7.0 | <0.01 | 1.9 | 129.6 | 24.2 | 461.2 | 0.3 | 14.4 | <0.01 | 7.0 | 29.0 |
| 22 November 2021 | 3 | Dębiny | 16.9 | 9.1 | 7.7 | 446 | 6.1 | 55.5 | 131.0 | 1.3 | <0.01 | 1.0 | 104.8 | 2.0 | 271.2 | 0.1 | 6.2 | <0.01 | 10.8 | 33.9 |
| 30 September 2021 | 4 | Gałęzów | 34.0 | 9.1 | 7.6 | 563 | 10.1 | 88.5 | 107.7 | 1.7 | <0.01 | 1.0 | 121.0 | 3.2 | 316.6 | 0.1 | 14.9 | <0.01 | 20.2 | 13.2 |
| 23 November 2021 | 5 | Husiny | 42.0 | 8.8 | 7.5 | 487 | 3.1 | 27.0 | 94.6 | 1.6 | <0.01 | 1.2 | 92.2 | 1.4 | 229.7 | 0.1 | 5.2 | <0.01 | 18.7 | 28.9 |
| 22 November 2021 | 6 | Krasnobród | 39.7 | 8.8 | 7.7 | 431 | 5.9 | 52.6 | 135.0 | 2.2 | <0.01 | 1.3 | 100.1 | 3.1 | 285.0 | 0.1 | 6.5 | <0.01 | 14.5 | 20.4 |
| 22 November 2021 | 7 | Łabunie | 24.3 | 9.5 | 7.1 | 692 | 4.3 | 40.3 | 150.0 | 8.3 | <0.01 | 5.0 | 136.4 | 16.2 | 405.1 | 0.2 | 27.3 | <0.01 | 17.5 | 23.7 |
| 23 November 2021 | 8 | Łosiniec | 46.7 | 8.7 | 7.4 | 495 | 3.2 | 28.5 | 104.4 | 2.9 | <0.01 | 1.6 | 96.5 | 3.1 | 262.3 | 0.1 | 5.4 | <0.01 | 7.7 | 25.9 |
| 14 October 2021 | 9 | Nowosiółki | 8.2 | 9.5 | 7.5 | 606 | 8.0 | 71.4 | 109.0 | 2.1 | <0.01 | 2.6 | 140.0 | 2.8 | 330.0 | 0.1 | 26.9 | <0.01 | 27.6 | 27.3 |
| 29 September 2021 | 10 | Pliszczyn | 52.9 | 9.5 | 7.2 | 635 | 6.1 | 53.6 | 96.0 | 5.9 | <0.01 | 1.4 | 129.8 | 22.1 | 420.9 | 0.0 | 22.5 | <0.01 | 10.0 | 34.2 |
| 29 September 2021 | 11 | Rogalów | 62.3 | 9.6 | 7.5 | 684 | 6.1 | 53.6 | 67.3 | 8.3 | <0.01 | 3.2 | 129.2 | 19.4 | 416.4 | 0.2 | 16.8 | <0.01 | 5.9 | 37.7 |
| 13 October 2021 | 12 | Słodków | 41.6 | 9.5 | 7.2 | 532 | 4.7 | 42.4 | 112.4 | 5.4 | <0.01 | 2.7 | 112.3 | 14.1 | 372.7 | 0.1 | 10.4 | <0.01 | 16.2 | 15.6 |
| 30 September 2021 | 13 | Stryjno Małe | 78.0 | 9.4 | 7.4 | 599 | 7.2 | 63.4 | 100.3 | 4.7 | <0.01 | 1.6 | 130.4 | 12.1 | 390.2 | 0.0 | 19.1 | <0.01 | 23.2 | 24.5 |
| 13 October 2021 | 14 | Sulów | 57.8 | 9.3 | 7.4 | 546 | 4.9 | 43.4 | 105.2 | 3.8 | <0.01 | 2.0 | 112.9 | 12.8 | 342.2 | 0.1 | 10.0 | 0.1 | 14.3 | 18.2 |
| 13 October 2021 | 15 | Święta Otylia (Urzędów) | 17.5 | 10.5 | 7.4 | 659 | 4.7 | 43.5 | 123.0 | 4.5 | <0.01 | 2.5 | 129.7 | 2.4 | 338.5 | 0.1 | 13.5 | <0.01 | 12.6 | 29.2 |
| 29 September 2021 | 16 | Wąwolnica | 93.0 | 9.5 | 7.7 | 692 | 4.0 | 35.6 | 53.7 | 4.5 | <0.01 | 3.2 | 133.4 | 13.4 | 374.9 | 0.2 | 13.9 | <0.01 | 9.2 | 56.3 |
| Rivers | ||||||||||||||||||||
| 15 July 2021 | 1 | Czerniejówka (Mętów) | 309 | 14.1 | 7.6 | 614 | 9.4 | 98.6 | 143.0 | 5.2 | <0.01 | 2.5 | 108.8 | 5.8 | 286.5 | 0.0 | 22.7 | <0.01 | 12.6 | 26.0 |
| 06 December 2021 | 18 | Huczwa (Werbkowice) | 3064 | 10.4 | 8.3 | 747 | 12.1 | 88.9 | 116.5 | 12.2 | 0.3 | 4.7 | 151.2 | 18.1 | 535.7 | 0.2 | 22.5 | <0.01 | 3.3 | 24.4 |
| 15 July 2021 | 19 | Kosarzewka (Bychawka) | 1201 | 14.9 | 7.8 | 560 | 11.1 | 110.0 | 172.0 | 4.6 | <0.01 | 1.9 | 104.6 | 8.1 | 365.5 | 0.1 | 19.6 | <0.01 | 10.7 | 19.9 |
| 23 November 2021 | 20 | Łabuńka (Krzak) | 2175 | 6.3 | 7.9 | 713 | 10.1 | 84.0 | 76.0 | 15.8 | <0.01 | 5.4 | 133.6 | 12.4 | 409.1 | 0.0 | 37.5 | 0.2 | 12.8 | 29.7 |
| 23 November 2021 | 21 | Wieprz (Guciów) | 1030 | 7.0 | 7.9 | 381 | 10.1 | 88.0 | 98.3 | 5.2 | 0.1 | 2.4 | 89.8 | 5.4 | 264.1 | 0.1 | 8.1 | <0.01 | 5.5 | 17.2 |
| 06 December 2021 | 22 | Wolica | 750 | 2.4 | 8.0 | 645 | 12.6 | 95.5 | 94.0 | 9.3 | <0.01 | 3.0 | 146.3 | 22.2 | 525.7 | 0.0 | 11.5 | <0.01 | 3.4 | 9.9 |
| 13 October 2021 | 23 | Wyżnica | 880 | 9.0 | 7.9 | 497 | 11.6 | 101.2 | 114.0 | 8.6 | <0.01 | 3.7 | 105.7 | 6.9 | 296.7 | 0.1 | 18.1 | <0.01 | 8.1 | 21.6 |
| 15 July 2021 | 24 | Tuszów | 153 | 11.4 | 7.5 | 514 | 10.9 | 110.2 | 104.3 | 3.5 | <0.01 | 1.5 | 101.9 | 5.4 | 286.1 | 0.1 | 16.6 | <0.01 | 18.4 | 22.0 |
| 06 December 2021 | 25 | Wieprz (Krasnystaw) | 12,600 | 2.7 | 7.8 | 627 | 12.2 | 92.2 | 117.0 | 8.3 | <0.01 | 3.0 | 117.3 | 12.0 | 387.8 | 0.1 | 16.2 | 0.1 | 7.4 | 18.8 |
| WWTP effluents | ||||||||||||||||||||
| 29 September 2021 | 26 | Celejów | - | 16.1 | 7.5 | 1450 | 4.8 | 49.0 | 13.5 | 133.8 | 25.4 | 42.4 | 193.2 | 28.5 | 698.6 | 0.2 | 174.8 | 6.5 | 52.3 | 58.5 |
| 23 November 2021 | 27 | Krasnobród | - | 11.0 | 7.4 | 1143 | 6.8 | 62.6 | 110.0 | 115.9 | 3.3 | 33.6 | 134.7 | 2.9 | 405.8 | 0.1 | 132.1 | 5.4 | 11.4 | 68.0 |
| 06 December 2021 | 28 | Krasnystaw | - | 11.3 | 7.6 | 1780 | 6.1 | 56.6 | 122.0 | 167.8 | 3.6 | 36.4 | 176.4 | 21.3 | 757.3 | 0.0 | 175.5 | 5.4 | 25.2 | 70.0 |
| 30 September 2021 | 29 | Piaski | - | 12.8 | 7.4 | 1540 | 1.8 | 18.3 | 86.0 | 95.4 | 72.5 | 29.3 | 145.7 | 12.6 | 749.3 | 0.1 | 117.7 | 0.1 | 1.0 | 30.0 |
| 13 October 2021 | 30 | Piotrowice | - | 17.9 | 7.1 | 1380 | 3.3 | 36.3 | 9.0 | 208.3 | <0.01 | 42.7 | 142.6 | 28.3 | 547.9 | 0.3 | 272.5 | 5.2 | 31.1 | 90.0 |
| 13 November 2021 | 31 | Urzędów | - | 15.3 | 7.6 | 1440 | 6.2 | 63.5 | 85.0 | 104.9 | 86.8 | 29.7 | 146.6 | 4.4 | 789.0 | 0.1 | 110.6 | <0.01 | 0.3 | 57.8 |
| Drainage waters | ||||||||||||||||||||
| 31 May 2023 | 32 | Świdnik (near airport) | - | - | - | 1665 | - | - | - | 269.1 | <0.01 | 97.7 | 198.0 | 26.9 | 430.7 | 0.0 | 486.7 | 1.2 | 203.0 | 13.8 |
| 31 May 2023 | 33 | Wilkołaz | - | - | - | 578 | - | - | - | 26.9 | <0.01 | 2.5 | 114.6 | 7.7 | 218.4 | 0.1 | 66.6 | 0.2 | 41.0 | 41.5 |
| Sampling Date | Sample No. | Localization and Kind of Collected Sample | PO43− [mg/dm3] | δ18O-PO4 av [‰] | Bedrock Type |
|---|---|---|---|---|---|
| Springs | |||||
| 14 October 2021 | 1 | Bezek | 0.33 | 15.3 ± 0.3 | Opokas |
| 29 November 2021 | 2 | Celejów | 0.30 | 16.4 ± 0.6 | Gaizes |
| 22 November 2021 | 3 | Dębiny | 0.21 | 15.6 ± 0.5 | Opokas |
| 30 November 2021 | 4 | Gałęzów | 0.21 | 18.5 ± 0.4 | Opokas |
| 23 November 2021 | 5 | Husiny | 1.09 | 14.8 ± 0.4 | Gaizes |
| 22 November 2021 | 6 | Krasnobród | 0.76 | 16.0 ± 0.3 | Opokas |
| 22 November 2021 | 7 | Łabunie | 0.41 | 16.9 ± 0.4 | Marls |
| 23 November 2021 | 8 | Łosiniec | 0.59 | 17.8 ± 0.6 | Opokas |
| 14 October 2021 | 9 | Nowosiółki | 0.34 | 17.8 ± 0.5 | Opokas |
| 29 November 2021 | 10 | Pliszczyn | 0.54 | 16.4 ± 0.4 | Gaizes |
| 29 November 2021 | 11 | Rogalów | 0.21 | 16.3 ± 0.3 | Gaizes |
| 13 October 2021 | 12 | Słodków | 0.34 | 16.5 ± 0.2 | Opokas |
| 30 November 2021 | 13 | Stryjno Małe | 0.58 | 17.5 ± 0.3 | Opokas |
| 13 October 2021 | 14 | Sulów | 0.49 | 15.7 ± 0.3 | Opokas |
| 13 October 2021 | 15 | Święta Otylia (Urzędów) | 0.38 | 15.7 ± 0.5 | Opokas |
| 29 November 2021 | 16 | Wąwolnica | 0.54 | 16.7 ± 0.2 | Gaizes |
| Rivers | |||||
| 15 July 2021 | 17 | Czerniejówka (Mętów) | 0.65 | 15.2 ± 0.5 | Opokas |
| 06 December 2021 | 18 | Huczwa (Werbkowice) | 0.23 | 14.9 ± 0.4 | Marls/chalks |
| 15 July 2021 | 19 | Kosarzewka (Bychawka) | 0.40 | 13.4 ± 0.2 | Opokas |
| 23 November 2021 | 20 | Łabuńka (Krzak) | 0.42 | 15.8 ± 0.5 | Opokas/marls/chalks |
| 23 November 2021 | 21 | Wieprz (Guciów) | 0.64 | 18.6 ± 0.4 | Gaizes/opokas |
| 06 December 2021 | 22 | Wolica (Orłów Drewniany) | 0.30 | 16.2 ± 0.3 | Opokas/chalks |
| 13 October 2021 | 23 | Wyżnica (Bór) | 0.30 | 10.3 ± 0.3 | Opokas |
| 15 July 2021 | 24 | Tuszów (Tuszów) | 0.45 | 12.9 ± 0.3 | Gaizes/opokas |
| 06 December 2021 | 25 | Wieprz (Krasnystaw) | 0.43 | 16.7 ± 0.3 | Opokas/marls/chalks |
| WWTP effluents | |||||
| 29 November 2021 | 26 | Celejów | 26.50 | 12.4 ± 0.3 | - |
| 23 November 2021 | 27 | Krasnobród | 9.90 | 15.6 ± 0.3 | - |
| 06 December 2021 | 28 | Krasnystaw | 3.20 | 13.2 ± 0.3 | - |
| 30 November 2021 | 29 | Piaski | 16.90 | 12.4 ± 0.4 | - |
| 13 October 2021 | 30 | Piotrowice | 7.70 | 14.5 ± 0.1 | - |
| 13 November 2021 | 31 | Urzędów | 3.00 | 12.9 ± 0.2 | - |
| Drainage waters | |||||
| 31 May 2023 | 32 | Świdnik (near airport) | 1.90 | 16.6 ± 0.3 | - |
| 31 May 2023 | 33 | Wilkołaz | 0.16 | 17.3 ± 0.4 | - |
| Bedrocks | |||||
| 20 July 2022 | 34 | Phosphorite concrection near Annopol | - | 21.2 ± 0.3 | Sandstone |
| 19 July 2022 | 35 | Phosphorite from Brzeziny (near Lubartów) | - | 20.5 ± 0.5 | Sands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebus-Czupyt, B.; Chmiel, S.; Kończak, M.; Huber, M.; Stienss, J.; Radzikowska, M.; Stępniewski, K.; Pliżga, M.; Zielińska, B. The Isotopic Composition of Selected Phosphate Sources (δ18O-PO4) from the Area of the Vistula and Bug Interfluve (Poland). Water 2024, 16, 2809. https://doi.org/10.3390/w16192809
Gebus-Czupyt B, Chmiel S, Kończak M, Huber M, Stienss J, Radzikowska M, Stępniewski K, Pliżga M, Zielińska B. The Isotopic Composition of Selected Phosphate Sources (δ18O-PO4) from the Area of the Vistula and Bug Interfluve (Poland). Water. 2024; 16(19):2809. https://doi.org/10.3390/w16192809
Chicago/Turabian StyleGebus-Czupyt, Beata, Stanisław Chmiel, Magdalena Kończak, Miłosz Huber, Jacek Stienss, Magdalena Radzikowska, Krzysztof Stępniewski, Mariusz Pliżga, and Beata Zielińska. 2024. "The Isotopic Composition of Selected Phosphate Sources (δ18O-PO4) from the Area of the Vistula and Bug Interfluve (Poland)" Water 16, no. 19: 2809. https://doi.org/10.3390/w16192809
APA StyleGebus-Czupyt, B., Chmiel, S., Kończak, M., Huber, M., Stienss, J., Radzikowska, M., Stępniewski, K., Pliżga, M., & Zielińska, B. (2024). The Isotopic Composition of Selected Phosphate Sources (δ18O-PO4) from the Area of the Vistula and Bug Interfluve (Poland). Water, 16(19), 2809. https://doi.org/10.3390/w16192809







