Survival and Swimming Performance of Small-Sized Gobiidae Implanted with Mini Passive Integrated Transponders (PIT-Tags)
Abstract
1. Introduction
2. Materials and Methods
2.1. Tagging
2.2. Fish Holding
2.3. Behavioral Tests
2.4. Statistics
3. Results
3.1. Survival and Tag Retention
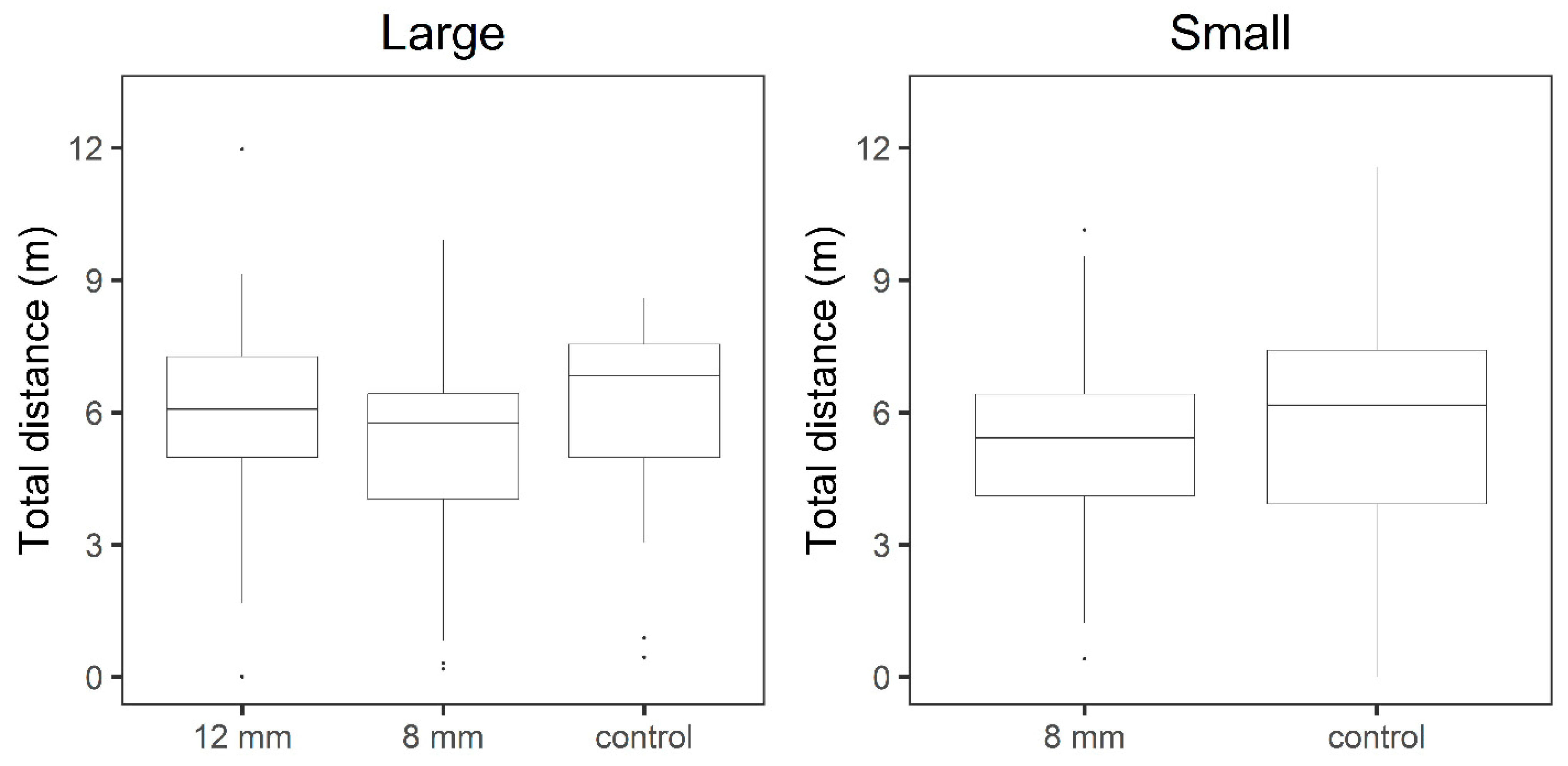
3.2. Behavioral Tests
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A Mechanistic Approach to Ecology. Trends Ecol. Evol. 2004, 19, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Thorstad, E.B.; Rikardsen, A.H.; Alp, A.; Okland, F. The Use of Electronic Tags in Fish Research: An Overview of Fish Telemetry Methods. Turk. J. Fish. Aquat. Sci. 2013, 13, 881–896. [Google Scholar]
- Gibbons, W.J.; Andrews, K.M. PIT Tagging: Simple Technology at Its Best. Bioscience 2004, 54, 447–454. [Google Scholar] [CrossRef]
- Nyqvist, D.; Hedenberg, F.; Calles, O.; Österling, M.; von Proschwitz, T.; Watz, J. Tracking the Movement of PIT-Tagged Terrestrial Slugs (Arion Vulgaris) in Forest and Garden Habitats Using Mobile Antennas. J. Molluscan Stud. 2020, 86, 79–82. [Google Scholar] [CrossRef]
- Keeler, R.A.; Breton, A.; Peterson, D.P.; Cunjak, R.A. Apparent Survival and Detection Estimates for PIT-Tagged Slimy Sculpin in Five Small New Brunswick Streams. Trans. Am. Fish. Soc. 2007, 136, 281–292. [Google Scholar] [CrossRef]
- Watz, J.; Bergman, E.; Piccolo, J.J.; Greenberg, L. Ice Cover Affects the Growth of a Stream-Dwelling Fish. Oecologia 2016, 181, 299–311. [Google Scholar] [CrossRef]
- Quintella, B.R.; Andrade, N.O.; Espanhol, R.; Almeida, P.R. The Use of PIT Telemetry to Study Movements of Ammocoetes and Metamorphosing Sea Lampreys in River Beds. J. Fish Biol. 2005, 66, 97–106. [Google Scholar] [CrossRef]
- Watz, J.; Calles, O.; Carlsson, N.; Collin, T.; Huusko, A.; Johnsson, J.; Nilsson, P.A.; Norrgård, J.; Nyqvist, D. Wood Addition in the Hatchery and River Environments Affects Post-release Performance of Overwintering Brown Trout. Freshw. Biol. 2019, 64, 71–80. [Google Scholar] [CrossRef]
- Breen, M.J.; Ruetz, C.R.; Thompson, K.J.; Kohler, S.L. Movements of Mottled Sculpins (Cottus Bairdii) in a Michigan Stream: How Restricted Are They? Can. J. Fish. Aquat. Sci. 2009, 66, 31–41. [Google Scholar] [CrossRef]
- Brönmark, C.; Skov, C.; Brodersen, J.; Nilsson, P.A.; Hansson, L.-A. Seasonal Migration Determined by a Trade-off between Predator Avoidance and Growth. PLoS ONE 2008, 3, e1957. [Google Scholar] [CrossRef]
- Schwinn, M.; Baktoft, H.; Aarestrup, K.; Koed, A. A Comparison of the Survival and Migration of Wild and F1-Hatchery-Reared Brown Trout (Salmo Trutta) Smolts Traversing an Artificial Lake. Fish. Res. 2017, 196, 47–55. [Google Scholar] [CrossRef]
- Závorka, L.; Aldvén, D.; Näslund, J.; Höjesjö, J.; Johnsson, J.I. Inactive Trout Come out at Night: Behavioral Variation, Circadian Activity, and Fitness in the Wild. Ecology 2016, 97, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Castro-Santos, T.; Haro, A.; Walk, S. A Passive Integrated Transponder (PIT) Tag System for Monitoring Fishways. Fish. Res. 1996, 28, 253–261. [Google Scholar] [CrossRef]
- Moser, M.L.; Corbett, S.C.; Keefer, M.L.; Frick, K.E.; Lopez-Johnston, S.; Caudill, C.C. Novel Fishway Entrance Modifications for Pacific Lamprey. J. Ecohydraulics 2019, 4, 71–84. [Google Scholar] [CrossRef]
- Ovidio, M.; Dierckx, A.; Benitez, J.-P. Movement behaviour and fishway performance for endemic and exotic species in a large anthropized river. Limnologica 2023, 99, 126061. [Google Scholar] [CrossRef]
- Bartoň, D.; Brabec, M.; Sajdlová, Z.; Souza, A.T.; Duras, J.; Kortan, D.; Blabolil, P.; Vejřík, L.; Kubečka, J.; Šmejkal, M. Hydropeaking Causes Spatial Shifts in a Reproducing Rheophilic Fish. Sci. Total Environ. 2022, 806, 150649. [Google Scholar] [CrossRef]
- Crossin, G.T.; Heupel, M.R.; Holbrook, C.M.; Hussey, N.E.; Lowerre-Barbieri, S.K.; Nguyen, V.M.; Raby, G.D.; Cooke, S.J. Acoustic Telemetry and Fisheries Management. Ecol. Appl. 2017, 27, 1031–1049. [Google Scholar] [CrossRef]
- Brown, R.S.; Eppard, M.B.; Murchie, K.J.; Nielsen, J.L.; Cooke, S.J. An Introduction to the Practical and Ethical Perspectives on the Need to Advance and Standardize the Intracoelomic Surgical Implantation of Electronic Tags in Fish. Rev. Fish Biol. Fish. 2011, 21, 1–9. [Google Scholar] [CrossRef]
- Baras, E.; Westerloppe, L.; Mélard, C.; Philippart, J.-C.; Bénech, V. Evaluation of Implantation Procedures for PIT-Tagging Juvenile Nile Tilapia. North Am. J. Aquac. 1999, 61, 246–251. [Google Scholar] [CrossRef]
- Brown, R.S.; Cooke, S.J.; Anderson, W.G.; McKinley, R.S. Evidence to Challenge the “2% Rule” for Biotelemetry. N. Am. J. Fish. Manag. 1999, 19, 867–871. [Google Scholar] [CrossRef]
- Winter, J. Underwater Biotelemetry. In Fisheries Techniques; American Fisheries Society: Bethesda, MD, USA, 1983; pp. 371–395. [Google Scholar]
- Vollset, K.W.; Lennox, R.J.; Thorstad, E.B.; Auer, S.; Bär, K.; Larsen, M.H.; Mahlum, S.; Näslund, J.; Stryhn, H.; Dohoo, I. Systematic Review and Meta-Analysis of PIT Tagging Effects on Mortality and Growth of Juvenile Salmonids. Rev. Fish Biol. Fish. 2020, 30, 553–568. [Google Scholar] [CrossRef]
- Clark, S.R. Effects of Passive Integrated Transponder Tags on the Physiology and Swimming Performance of a Small-Bodied Stream Fish. Trans. Am. Fish. Soc. 2016, 145, 1179–1192. [Google Scholar] [CrossRef]
- Watson, J.R.; Goodrich, H.R.; Cramp, R.L.; Gordos, M.A.; Franklin, C.E. Assessment of the Effects of microPIT Tags on the Swimming Performance of Small-Bodied and Juvenile Fish. Fish. Res. 2019, 218, 22–28. [Google Scholar] [CrossRef]
- Bangs, B.L.; Falcy, M.R.; Scheerer, P.D.; Clements, S. Comparison of Three Methods for Marking a Small Floodplain Minnow. Anim. Biotelemetry 2013, 1, 18. [Google Scholar] [CrossRef]
- Swarr, T.R.; Myrick, C.A.; Fitzpatrick, R.M. Tag Retention in and Effects of Passive Integrated Transponder Tagging on Survival and Swimming Performance of a Small-Bodied Darter. J. Fish Biol. 2022, 100, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Nyqvist, D.; Schiavon, A.; Candiotto, A.; Mozzi, G.; Eggers, F.; Comoglio, C. PIT-Tagging Italian Spined Loach (Cobitis Bilineata): Methodology, Survival and Behavioural Effects. J. Fish Biol. 2023, 102, 575–580. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Goerig, E.; He, P.; Lauder, G.V. Applied Aspects of Locomotion and Biomechanics. Fish Physiol. A 2022, 39, 91–140. [Google Scholar]
- Tudorache, C.; De Boeck, G.; Claireaux, G. Forced and Preferred Swimming Speeds of Fish: A Methodological Approach. In Swimming Physiology of Fish; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–108. [Google Scholar]
- Ficke, A.D.; Myrick, C.A.; Kondratieff, M.C. The Effects of PIT Tagging on the Swimming Performance and Survival of Three Nonsalmonid Freshwater Fishes. Ecol. Eng. 2012, 48, 86–91. [Google Scholar] [CrossRef]
- Nyqvist, D.; Schiavon, A.; Candiotto, A.; Tarena, F.; Comoglio, C. Survival and Swimming Performance in Small-Sized South European Cypriniformes Tagged with Passive Integrated Transponders. J. Ecohydraulics 2024, 9, 248–258. [Google Scholar] [CrossRef]
- Newby, N.C.; Binder, T.R.; Stevens, E.D. Passive Integrated Transponder (PIT) Tagging Did Not Negatively Affect the Short-Term Feeding Behavior or Swimming Performance of Juvenile Rainbow Trout. Trans. Am. Fish. Soc. 2007, 136, 341–345. [Google Scholar] [CrossRef]
- Mueller, R.P.; Moursund, R.A.; Bleich, M.D. Tagging Juvenile Pacific Lamprey with Passive Integrated Transponders: Methodology, Short-Term Mortality, and Influence on Swimming Performance. N. Am. J. Fish. Manag. 2006, 26, 361–366. [Google Scholar] [CrossRef]
- Knaepkens, G.; Maerten, E.; Tudorache, C.; De Boeck, G.; Eens, M. Evaluation of Passive Integrated Transponder Tags for Marking the Bullhead (Cottus Gobio), a Small Benthic Freshwater Fish: Effects on Survival, Growth and Swimming Capacity. Ecol. Freshw. Fish 2007, 16, 404–409. [Google Scholar] [CrossRef]
- Schiavon, A.; Comoglio, C.; Candiotto, A.; Hölker, F.; Ashraf, M.U.; Nyqvist, D. Survival and Swimming Performance of a Small-Sized Cypriniformes (Telestes Muticellus) Tagged with Passive Integrated Transponders. J. Limnol. 2023, 82, 1–7. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Letcher, B.H. Implanting 8-mm Passive Integrated Transponder Tags into Small Brook Trout: Effects on Growth and Survival in the Laboratory. N. Am. J. Fish Manag. 2017, 37, 605–611. [Google Scholar] [CrossRef]
- Schumann, D.A.; Graeb, K.N.; Wagner, M.D.; Graeb, B.D.; Prenosil, E.; Hoekwater, J. Suitability of Surgically Implanted 8-mm Passive Integrated Transponder Tags for Small-bodied Fishes. J. Appl. Ichthyol. 2020, 65, 682–692. [Google Scholar] [CrossRef]
- Tiffan, K.F.; Perry, R.W.; Connor, W.P.; Mullins, F.L.; Rabe, C.D.; Nelson, D.D. Survival, Growth, and Tag Retention in Age-0 Chinook Salmon Implanted with 8-, 9-, and 12-mm PIT Tags. N. Am. J. Fish Manag. 2015, 35, 845–852. [Google Scholar] [CrossRef]
- Cary, J.B.; Holbrook, J.L.; Reed, M.E.; Austin, T.B.; Steffensen, M.S.; Kim, S.; Pregler, K.C.; Kanno, Y. Survival of Upper Piedmont Stream Fishes Implanted with 8-mm Passive Integrated Transponder Tags. Trans. Am. Fish. Soc. 2017, 146, 1223–1232. [Google Scholar] [CrossRef]
- Moore, D.M.; Brewer, S.K. Evaluation of Visual Implant Elastomer, PIT, and p-Chip Tagging Methods in a Small-Bodied Minnow Species. N. Am. J. Fish Manag. 2021, 41, 1066–1078. [Google Scholar] [CrossRef]
- Pennock, C.A. Effects of PIT Tags on Red Shiner Cyprinella Lutrensis and Sand Shiner Notropis Stramineus. tkas 2017, 120, 87–93. [Google Scholar] [CrossRef]
- Fernholz, J. Influence of Pit Tags on Growth and Survival of Banded Sculpin (Cottus Carolinae): Implications for Endangered Grotto Sculpin (Cottus Specus). Ph.D. Thesis, Southeast Missouri State University, Cape Girardeau, MO, USA, 2018. [Google Scholar]
- Patzner, R.A.; Van Tassell, J.L.; Kovacic, M.; Kapoor, B.G. The Biology of Gobies; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Cookingham, M.N.; Ruetz Iii, C.R. Evaluating Passive Integrated Transponder Tags for Tracking Movements of Round Gobies. Ecol. Freshw. Fish 2008, 17, 303–311. [Google Scholar] [CrossRef]
- Thorlacius, M.; Hellström, G.; Brodin, T. Behavioral Dependent Dispersal in the Invasive Round Goby Neogobius Melanostomus Depends on Population Age. Curr. Zool. 2015, 61, 529–542. [Google Scholar] [CrossRef]
- Harding, J.M.; Allen, D.M.; Haffey, E.R.; Hoffman, K.M. Site Fidelity of Oyster Reef Blennies and Gobies in Saltmarsh Tidal Creeks. Estuaries Coasts 2020, 43, 409–423. [Google Scholar] [CrossRef]
- Fortini, N. Nuovo Atlante Dei Pesci Delle Acque Interne Italiane: Guida Completa Ai Pesci, Ciclostomi e Crostacei Decapodi Di Acque Dolci e Salmastre; Aracne Editrice: Ariccia, Italy, 2016. [Google Scholar]
- Paradisi, S.; Miotti, E.; Miotti, L. Pesci d’acqua Dolce Del Friuli Venezia Giulia; CO.EL: Udine, Italy, 2021. [Google Scholar]
- Freyhof, J. Padogobius Bonelli (Errata Version Published in 2018). The IUCN Red List of Threatened Species 2011: E.T41541A136572424. 2011. Available online: https://www.iucnredlist.org/species/41541/136572424 (accessed on 5 September 2024).
- Bolland, J.D.; Cowx, I.G.; Lucas, M.C. Evaluation of VIE and PIT Tagging Methods for Juvenile Cyprinid Fishes. J. Appl. Ichthyol. 2009, 25, 381–386. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Nyqvist, D.; Comoglio, C.; Manes, C. The Effect of In-Flume Habituation Time and Fish Behaviour on Estimated Swimming Performance. J. Ecohydraulics 2024, 9, 239–247. [Google Scholar] [CrossRef]
- Miklósi, A.; Topal, J.; Csányi, V. Development of Open-Field and Social Behavior of the Paradise Fish (Macropodus opercularis L.). Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1992, 25, 335–344. [Google Scholar] [CrossRef]
- Watz, J. Structural Complexity in the Hatchery Rearing Environment Affects Activity, Resting Metabolic Rate and Post-release Behaviour in Brown Trout Salmo Trutta. J. Fish Biol. 2019, 95, 638–641. [Google Scholar] [CrossRef]
- Tudorache, C.; Viaene, P.; Blust, R.; Vereecken, H.; De Boeck, G. A Comparison of Swimming Capacity and Energy Use in Seven European Freshwater Fish Species. Ecol. Freshw. Fish 2008, 17, 284–291. [Google Scholar] [CrossRef]
- Walter, T.; Couzin, I.D. TRex, a Fast Multi-Animal Tracking System with Markerless Identification, 2D Body Posture Estimation and Visual Field Reconstruction. eLife 2021, 10, e64000. [Google Scholar] [CrossRef]
- Domenici, P.; Blake, R. The Kinematics and Performance of Fish Fast-Start Swimming. J. Exp. Biol. 1997, 200, 1165–1178. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Chi-Squared Test and Fisher’s Exact Test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef]
- Jepsen, N.; Schreck, C.; Clements, S.; Thorstad, E.B. A Brief Discussion on the 2% Tag/Bodymass Rule of Thumb. In Aquatic Telemetry: Advances and Applications; FAO: Rome, Italy, 2005; pp. 255–259. [Google Scholar]
- Perals, D.; Griffin, A.S.; Bartomeus, I.; Sol, D. Revisiting the Open-Field Test: What Does It Really Tell Us about Animal Personality? Anim. Behav. 2017, 123, 69–79. [Google Scholar] [CrossRef]
- Nyqvist, D.; Schiavon, A.; Candiotto, A.; Comoglio, C. Interspecific Differences in Swimming Performance, Behavior and Survival between Native Italian Gudgeon (Gobio Benacensis Pollini, 1816) and Non-Native European Gudgeon (Gobio Gobio Linnaeus, 1758). Eur. Zool. J. 2024, 91, 906–914. [Google Scholar] [CrossRef]
- Fraser, D.F.; Gilliam, J.F.; Daley, M.J.; Le, A.N.; Skalski, G.T. Explaining Leptokurtic Movement Distributions: Intrapopulation Variation in Boldness and Exploration. Am. Nat. 2001, 158, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Nyqvist, D.; Tarena, F.; Candiotto, A.; Comoglio, C. Individual Activity Levels and Presence of Conspecifics Affect Fish Passage Rates over an In-Flume Barrier. Ecol. Freshw. Fish 2024, e12787. [Google Scholar] [CrossRef]
- Domenici, P. Escape Responses in Fish: Kinematics, Performance and Behavior. In Fish Locomotion: An Eco-Ethological Perspective; CRC Press: Boca Raton, FL, USA, 2010; pp. 123–170. [Google Scholar]
- Tierney, K.B.; Kasurak, A.V.; Zielinski, B.S.; Higgs, D.M. Swimming Performance and Invasion Potential of the Round Goby. Env. Biol. Fish. 2011, 92, 491–502. [Google Scholar] [CrossRef]
- Katopodis, C. Introduction to Fishway Design; Freshwater Institute, Central and Arctic Region Department of Fisheries and Oceans: Winnipeg, MB, Canada, 1992. [Google Scholar]
- Mesa, M.G.; Copeland, E.S.; Christiansen, H.E.; Gregg, J.L.; Roon, S.R.; Hershberger, P.K. Survival and Growth of Juvenile Pacific Lampreys Tagged with Passive Integrated Transponders (PIT) in Freshwater and Seawater. Trans. Am. Fish Soc. 2012, 141, 1260–1268. [Google Scholar] [CrossRef]
- Wargo Rub, A.M.; Sandford, B.P.; Butzerin, J.M.; Cameron, A.S. Pushing the Envelope: Micro-Transmitter Effects on Small Juvenile Chinook Salmon (Oncorhynchus Tshawytscha). PLoS ONE 2020, 15, e0230100. [Google Scholar] [CrossRef]
- Jepsen, N.; Christoffersen, M.; Munksgaard, T. The Level of Predation Used as an Indicator of Tagging/Handling Effects. Fish. Manag. Ecol. 2008, 15, 365–368. [Google Scholar] [CrossRef]
- Wilson, A.D.; Hayden, T.A.; Vandergoot, C.S.; Kraus, R.T.; Dettmers, J.M.; Cooke, S.J.; Krueger, C.C. Do Intracoelomic Telemetry Transmitters Alter the Post-Release Behaviour of Migratory Fish? Ecol. Freshw. Fish 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Šmejkal, M.; Blabolil, P.; Bartoň, D.; Duras, J.; Vejřík, L.; Sajdlova, Z.; Kočvara, L.; Kubečka, J. Sex-Specific Probability of PIT Tag Retention in a Cyprinid Fish. Fish. Res. 2019, 219, 105325. [Google Scholar] [CrossRef]
- Schiavon, A.; Comoglio, C.; Candiotto, A.; Spairani, M.; Hölker, F.; Tarena, F.; Watz, J.; Nyqvist, D. Navigating the Drought: Upstream Migration of a Small-Sized Cypriniformes (Telestes Muticellus) in Response to Drying in a Partially Intermittent Mountain Stream. Knowl. Manag. Aquat. Ecosyst. 2024, 6, 9. [Google Scholar] [CrossRef]
- Osugi, T.; Yanagisawa, Y.; Mizuno, N. Feeding of a Benthic Goby in a River Where Nektonic Fishes Are Absent. Environ. Biol. Fishes 1998, 52, 331–343. [Google Scholar] [CrossRef]
- Jepsen, N.; Thorstad, E.B.; Havn, T.; Lucas, M.C. The Use of External Electronic Tags on Fish: An Evaluation of Tag Retention and Tagging Effects. Anim. Biotelemetry 2015, 3, 49. [Google Scholar] [CrossRef]

| Size | Group | n | Length (mm) | Weight (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Min | Max | Median | IQR | Min | Max | |||
| Small | 8 mm | 76 | 45 | 41–47 | 35 | 49 | 0.9 | 0.7–1.1 | 0.3 | 2.2 |
| control | 76 | 44 | 41–46 | 35 | 49 | 0.9 | 0.7–1.0 | 0.4 | 1.4 | |
| Large | 8 mm | 38 | 57 | 53–64 | 50 | 76 | 2.3 | 0.8 | 1.3 | 4.4 |
| 12 mm | 42 | 57 | 54–60 | 50 | 73 | 2.0 | 0.9 | 0.8 | 4.1 | |
| control | 40 | 57 | 53–61 | 50 | 76 | 2.2 | 1.0 | 1.1 | 5.8 | |
| Size | Group | n | Dead (n) | Mortality (%) | Loss (n) | Loss (%) | Failure (%) |
|---|---|---|---|---|---|---|---|
| Small | 8 mm | 76 | 0 | 0 | 3 | 3.9 | 3.9 |
| control | 76 | 0 | 0 | - | - | 0 | |
| Large | 8 mm | 38 | 1 | 2.6 | 0 | 0 | 2.6 |
| 12 mm | 42 | 2 | 4.7 | 4 | 9.5 | 14.3 | |
| control | 40 | 0 | 0 | - | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyqvist, D.; Schiavon, A.; Ashraf, M.U.; Candiotto, A.; Palazzi, A.; Parolini, M.; Comoglio, C. Survival and Swimming Performance of Small-Sized Gobiidae Implanted with Mini Passive Integrated Transponders (PIT-Tags). Water 2024, 16, 2745. https://doi.org/10.3390/w16192745
Nyqvist D, Schiavon A, Ashraf MU, Candiotto A, Palazzi A, Parolini M, Comoglio C. Survival and Swimming Performance of Small-Sized Gobiidae Implanted with Mini Passive Integrated Transponders (PIT-Tags). Water. 2024; 16(19):2745. https://doi.org/10.3390/w16192745
Chicago/Turabian StyleNyqvist, Daniel, Alfredo Schiavon, Muhammad Usama Ashraf, Alessandro Candiotto, Adriano Palazzi, Marco Parolini, and Claudio Comoglio. 2024. "Survival and Swimming Performance of Small-Sized Gobiidae Implanted with Mini Passive Integrated Transponders (PIT-Tags)" Water 16, no. 19: 2745. https://doi.org/10.3390/w16192745
APA StyleNyqvist, D., Schiavon, A., Ashraf, M. U., Candiotto, A., Palazzi, A., Parolini, M., & Comoglio, C. (2024). Survival and Swimming Performance of Small-Sized Gobiidae Implanted with Mini Passive Integrated Transponders (PIT-Tags). Water, 16(19), 2745. https://doi.org/10.3390/w16192745







