Biogeochemical Fe-Redox Cycling in Oligotrophic Deep-Sea Sediment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bio-Redox Reactions of Fe in Abyssal Sediment
2.3. Bioinformatic Analysis
2.3.1. DNA Extraction, 16S rRNA Gene Amplification, and High-Throughput Sequencing
2.3.2. Data Processing and Statistical Analysis
2.3.3. Data Submission and Accession Numbers
2.4. Analytical Methods
2.4.1. Measurements of Total Fe(II), Total Fe and Nitrate
2.4.2. X-ray Diffraction Analysis
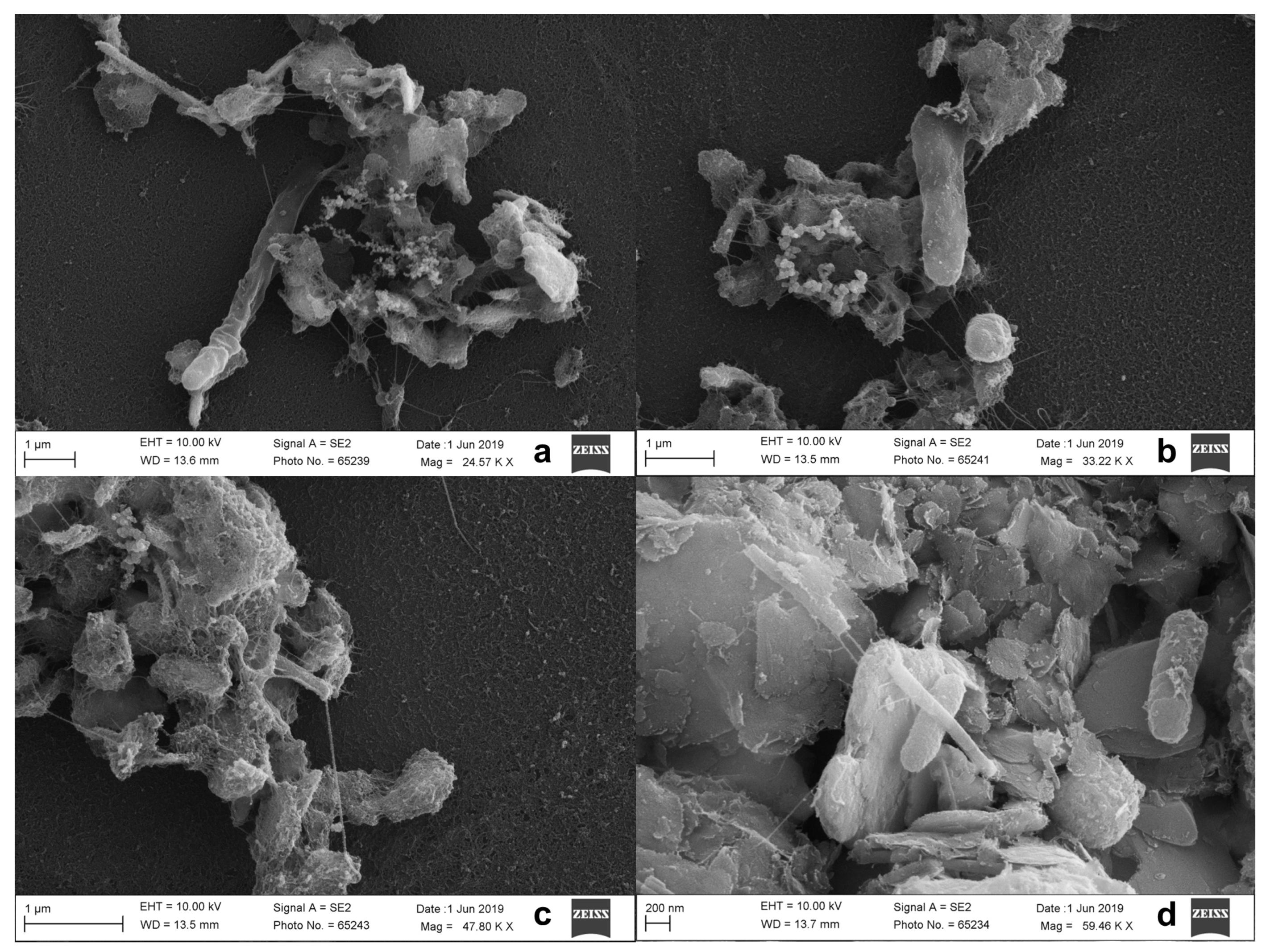
2.4.3. Scanning Electron Microscopy Analysis
2.4.4. Major and Trace Element Analysis
3. Results and Discussion
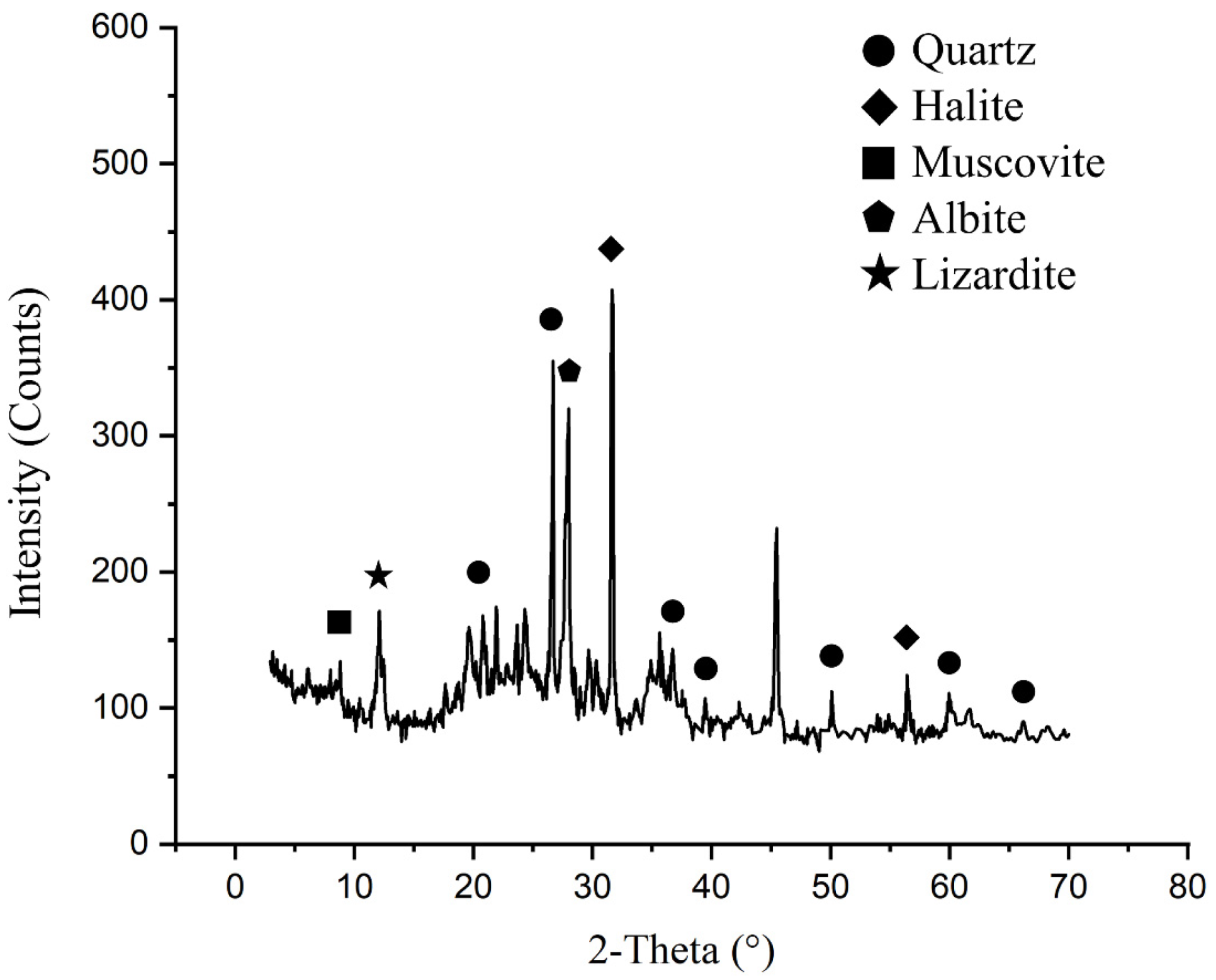
3.1. Characterization of the Abyssal Sediment
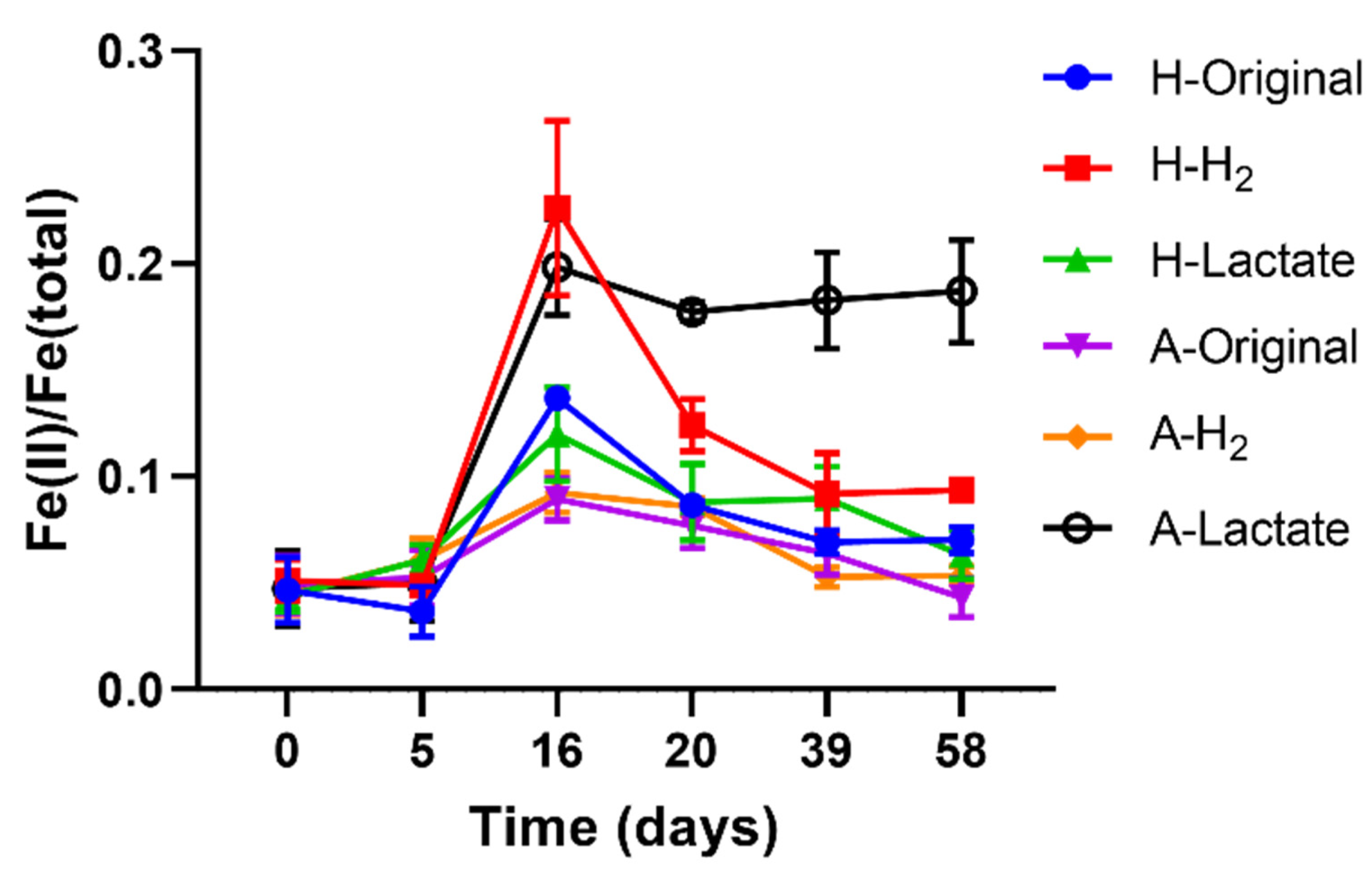
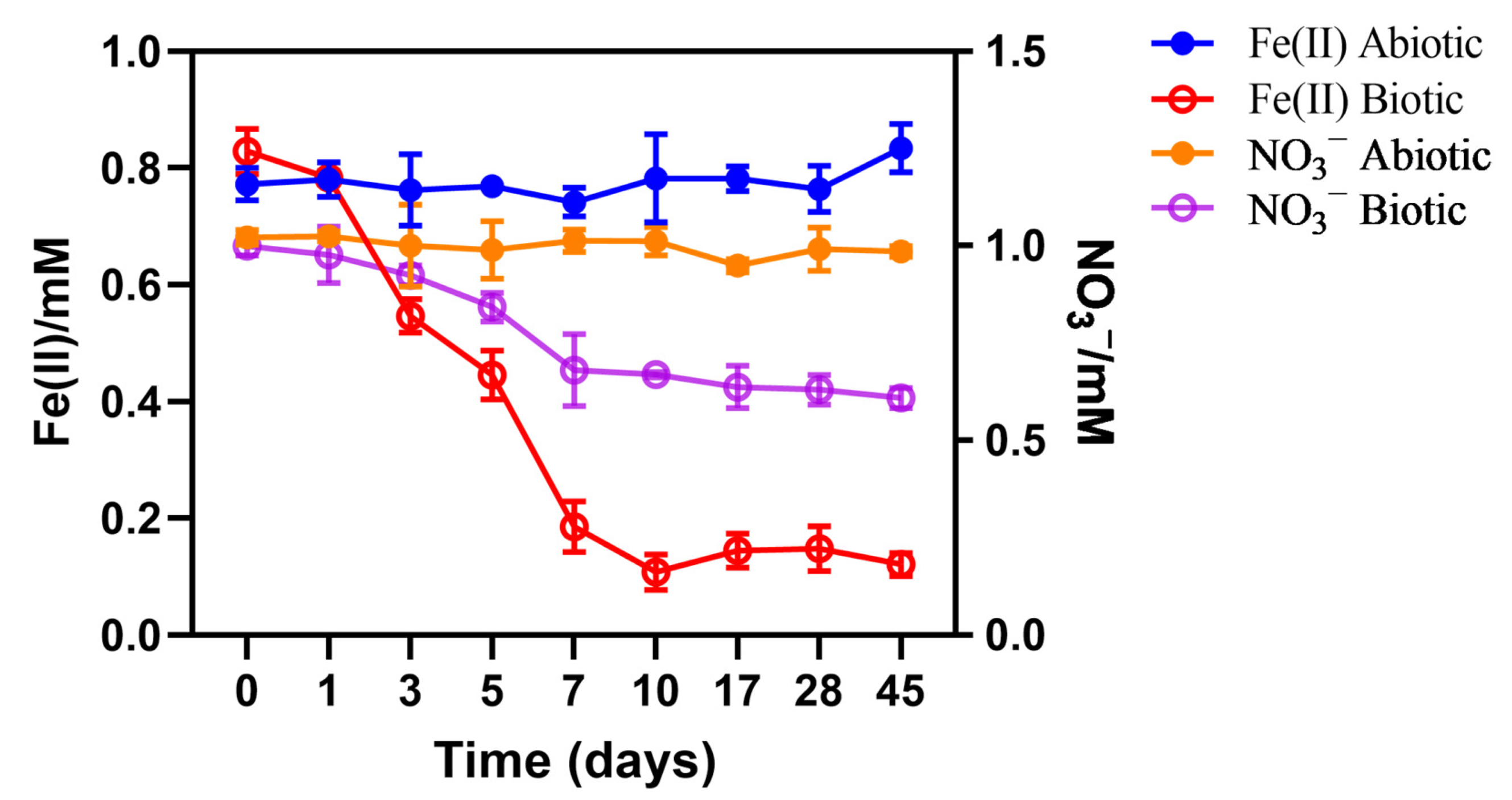
3.2. Fluctuation of Fe-Redox State in the Abyssal Sediment
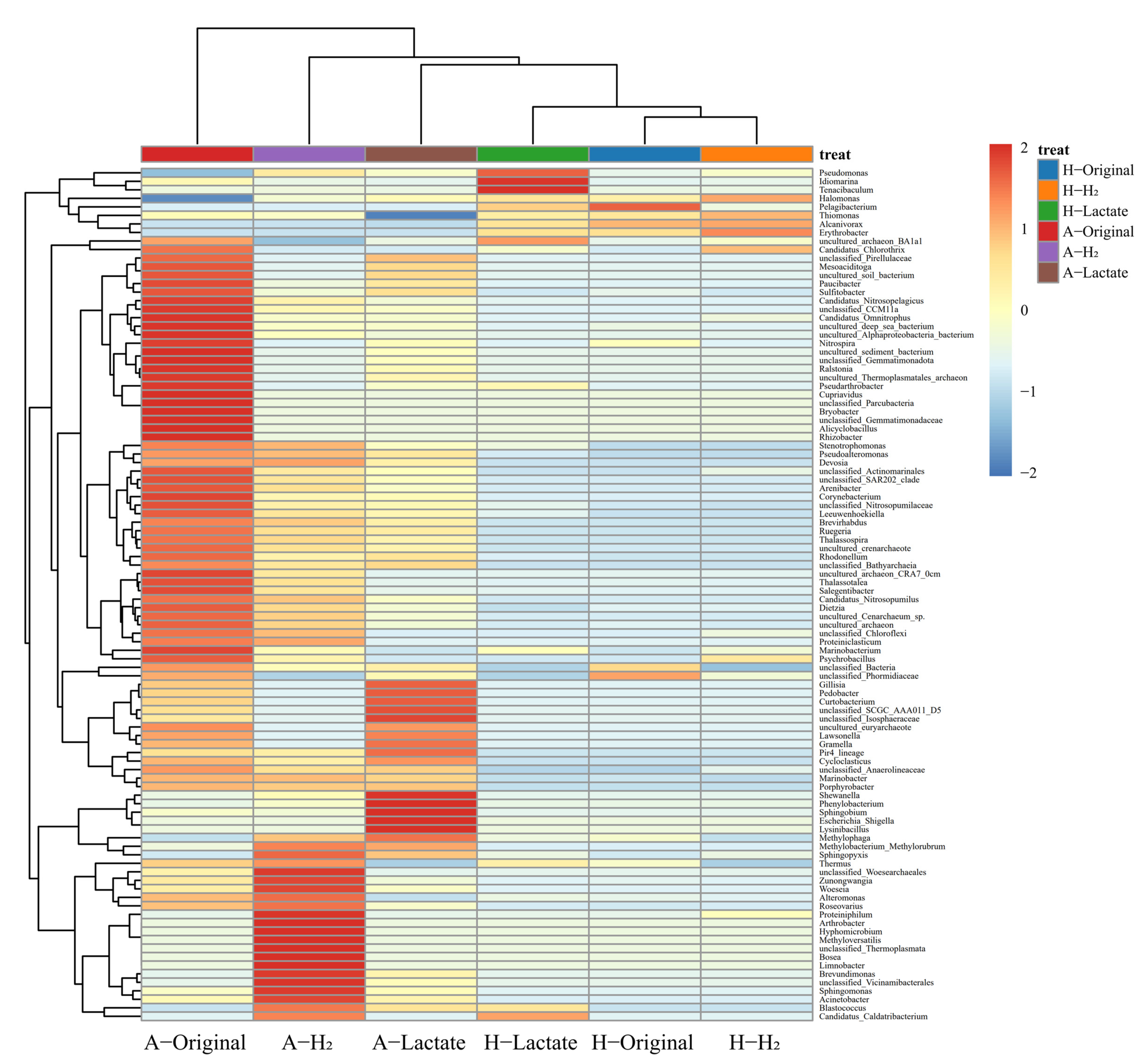
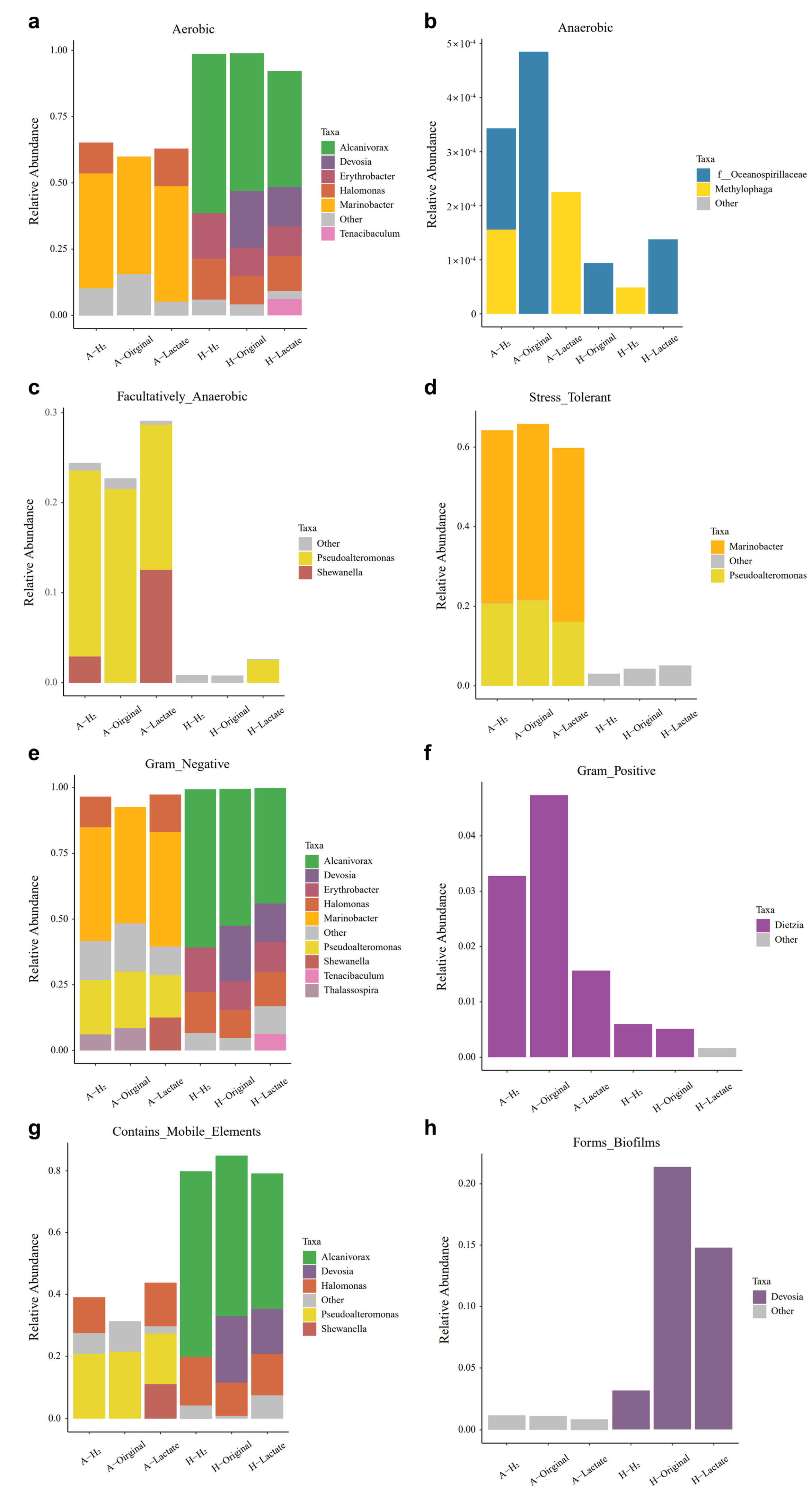
3.3. Pressure Controlling the Microbial Community Composition in Abyssal Sediment
3.4. The Dynamics between Microbial Community and Fe-Redox Fluctuation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyd, P.W.; Ellwood, M.J. The Biogeochemical Cycle of Iron in the Ocean. Nat. Geosci. 2010, 3, 675–682. [Google Scholar] [CrossRef]
- Lu, J.; Yu, P.; Zhang, J.; Guo, Z.; Li, Y.; Wang, S.; Hu, Z. Biotic/Abiotic Transformation Mechanisms of Phenanthrene in Iron-Rich Constructed Wetland under Redox Fluctuation. Water Res. 2024, 261, 122033. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Baars, O.; Guo, D.; Whitham, J.; Srivastava, S.; Dong, H. Mineral-Bound Trace Metals as Cofactors for Anaerobic Biological Nitrogen Fixation. Environ. Sci. Technol. 2023, 57, 7206–7216. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, Q.; Sheng, Y.; Chen, C.; Yu, G.; Kappler, A. Coupled Iron Cycling and Organic Matter Transformation across Redox Interfaces. Nat. Rev. Earth Environ. 2023, 4, 659–673. [Google Scholar] [CrossRef]
- He, H.; Zhang, C.-G.; Xia, J.-L.; Peng, A.-A.; Yang, Y.; Jiang, H.-C.; Zheng, L.; Ma, C.-Y.; Zhao, Y.-D.; Nie, Z.-Y.; et al. Investigation of Elemental Sulfur Speciation Transformation Mediated by Acidithiobacillus Ferrooxidans. Curr. Microbiol. 2009, 58, 300–307. [Google Scholar] [CrossRef]
- Sheng, Y.; Dong, H.; Kukkadapu, R.K.; Ni, S.; Zeng, Q.; Hu, J.; Coffin, E.; Zhao, S.; Sommer, A.J.; McCarrick, R.M. Lignin-Enhanced Reduction of Structural Fe (III) in Nontronite: Dual Roles of Lignin as Electron Shuttle and Donor. Geochim. Cosmochim. Acta 2021, 307, 1–21. [Google Scholar] [CrossRef]
- Danovaro, R.; Snelgrove, P.V.; Tyler, P. Challenging the Paradigms of Deep-Sea Ecology. Trends Ecol. Evol. 2014, 29, 465–475. [Google Scholar] [CrossRef]
- Leng, D.; Shao, S.; Xie, Y.; Wang, H.; Liu, G. A Brief Review of Recent Progress on Deep Sea Mining Vehicle. Ocean Eng. 2021, 228, 108565. [Google Scholar] [CrossRef]
- Zhou, R.; Bai, B.; Cai, G.; Chen, X. Thermo-Hydro-Mechanic-Chemical Coupling Model for Hydrate-Bearing Sediment within a Unified Granular Thermodynamic Theory. Comput. Geotech. 2024, 167, 106057. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.; Yang, K.; Park, H.; Elliott, W.C.; Spivack, A.; Koo, T.; Kim, G.; Morono, Y.; Henkel, S.; et al. Naturally Occurring, Microbially Induced Smectite-to-Illite Reaction. Geology 2019, 47, 535–539. [Google Scholar] [CrossRef]
- Sheng, Y.; Dong, H.; Coffin, E.; Myrold, D.; Kleber, M. The Important Role of Enzyme Adsorbing Capacity of Soil Minerals in Regulating β-Glucosidase Activity. Geophys. Res. Lett. 2022, 49, e2021GL097556. [Google Scholar] [CrossRef]
- Gao, X.; Han, Z.; Zhao, Y.; Zhou, G.; Lyu, X.; Qi, Z.; Liu, F.; Tucker, M.E.; Steiner, M.; Han, C. Interaction of Microorganisms with Carbonates from the Micro to the Macro Scales during Sedimentation: Insights into the Early Stage of Biodegradation. J. Environ. Manag. 2024, 356, 120714. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E. Microbial Iron-Redox Cycling in Subsurface Environments. Biochem. Soc. Trans. 2012, 40, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, Z.; Wang, Z.; Wang, L.; Fang, J.; Liu, R. Community Composition and Functional Characterization of Microorganisms in Surface Sediment of the New Britain Trench. Curr. Microbiol. 2024, 81, 282. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Dissimilatory metal reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]
- Dong, H.; Coffin, E.S.; Sheng, Y.; Duley, M.L.; Khalifa, Y.M. Microbial Reduction of Fe(III) in Nontronite: Role of Biochar as a Redox Mediator. Geochim. Cosmochim. Acta 2023, 345, 102–116. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular Electron Transfer via Microbial Nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Dong, Y.; Shi, L.; Jiang, Y. Enhancing Electrical Outputs of the Fuel Cells with Geobacter Sulferreducens by Overexpressing Nanowire Proteins. Microb. Biotechnol. 2023, 16, 534–545. [Google Scholar] [CrossRef]
- Penas, D.; Pereira, A.S.; Tavares, P. Direct Evidence for Ferrous Ion Oxidation and Incorporation in the Absence of Oxidants by Dps from Marinobacter hydrocarbonoclasticus. Angew. Chem. 2019, 131, 1025–1030. [Google Scholar] [CrossRef]
- Nagata, T.; Tamburini, C.; Arístegui, J.; Baltar, F.; Bochdansky, A.B.; Fonda-Umani, S.; Fukuda, H.; Gogou, A.; Hansell, D.A.; Hansman, R.L. Emerging Concepts on Microbial Processes in the Bathypelagic Ocean–Ecology, Biogeochemistry, and Genomics. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1519–1536. [Google Scholar] [CrossRef]
- Blöthe, M.; Roden, E.E. Composition and Activity of an Autotrophic Fe(II)-Oxidizing, Nitrate-Reducing Enrichment Culture. Appl. Environ. Microbiol. 2009, 75, 6937–6940. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Ward, T.; Larson, J.; Meulemans, J.; Hillmann, B.; Lynch, J.; Sidiropoulos, D.; Spear, J.R.; Caporaso, G.; Blekhman, R.; Knight, R. BugBase Predicts Organism-Level Microbiome Phenotypes. BioRxiv 2017, 133462. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, L.; Dong, H.; Li, Z.; Zhang, Y.; Hu, J.; Chen, H.; Chen, Y. Bio-Weathering of a Uranium-Bearing Rhyolitic Rock from Xiangshan Uranium Deposit, Southeast China. Geochim. Cosmochim. Acta 2020, 279, 88–106. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, J.; Dong, H. Effects of Organic Ligands on the Antibacterial Activity of Reduced Iron-Containing Clay Minerals: Higher Extracellular Hydroxyl Radical Production Yet Lower Bactericidal Activity. Environ. Sci. Technol. 2023, 57, 6888–6897. [Google Scholar] [CrossRef]
- Xia, Q.; Jin, Q.; Chen, Y.; Zhang, L.; Li, X.; He, S.; Guo, D.; Liu, J.; Dong, H. Combined Effects of Fe(III)-Bearing Nontronite and Organic Ligands on Biogenic U(IV) Oxidation. Environ. Sci. Technol. 2022, 56, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.K.; González, E.; Burwicz-Galerne, E.; Braack, M.; Wallmann, K. NN-TOC v1: Global Prediction of Total Organic Carbon in Marine Sediments Using Deep Neural Networks. EGUsphere 2024. [Google Scholar] [CrossRef]
- Debbarma, R.; Singh, S.K.; Waikhom, G.; Biswas, P.; Meena, D.K.; Choudhary, B.K. Chapter 12—Biofloc Technology: A Strategic Way to Waste Recycling in Aquaculture. In Organic Farming, 2nd ed.; Sarathchandran, M.U., Thomas, S., Meena, D.K., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2023; pp. 395–419. ISBN 978-0-323-99145-2. [Google Scholar] [CrossRef]
- Joshi, P.; Gorski, C.A. Anisotropic Morphological Changes in Goethite during Fe2+-Catalyzed Recrystallization. Environ. Sci. Technol. 2016, 50, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liang, X.; Ma, L.; Huang, J.; He, H.; Zhu, J. Adsorption of REEs on Kaolinite and Halloysite: A Link to the REE Distribution on Clays in the Weathering Crust of Granite. Chem. Geol. 2019, 525, 210–217. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Li, R.; Liu, D.; Bian, L.; Chen, Y.; Pan, Z.; Boyanov, M.I.; Kemner, K.M.; Wen, J.; et al. Effect of Siderophore DFOB on U(VI) Adsorption to Clay Mineral and Its Subsequent Reduction by an Iron-Reducing Bacterium. Environ. Sci. Technol. 2022, 56, 12702–12712. [Google Scholar] [CrossRef]
- Sheng, Y.; Hu, J.; Kukkadapu, R.; Guo, D.; Zeng, Q.; Dong, H. Inhibition of Extracellular Enzyme Activity by Reactive Oxygen Species upon Oxygenation of Reduced Iron-Bearing Minerals. Environ. Sci. Technol. 2023, 57, 3425–3433. [Google Scholar] [CrossRef]
- Cui, S.; Wang, R.; Chen, Q.; Pugliese, L.; Wu, S. Geobatteries in Environmental Biogeochemistry: Electron Transfer and Utilization. Environ. Sci. Ecotechnol. 2024, 22, 100446. [Google Scholar] [CrossRef]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F. A Critical Review of Mineral–Microbe Interaction and Co-Evolution: Mechanisms and Applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, C.; Chen, L.; Li, Y.; Hua, T.; Li, Y.; Wang, C.; Jin, S.; Ding, H.; Lu, A. Reduction, Mineralization, and Magnetic Removal of Chromium from Soil by Using a Natural Mineral Composite. Environ. Sci. Ecotechnol. 2022, 11, 100181. [Google Scholar] [CrossRef]
- Wang, H.; Bao, W.; Sarwar, M.T.; Tian, L.; Tang, A.; Yang, H. Mineral-Enhanced Manganese Ferrite with Multiple Enzyme-Mimicking Activities for Visual Detection of Disease Markers. Inorg. Chem. 2023, 62, 8418–8427. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Dong, H.; Wang, H.; Zhao, L.; Huang, L.; Wu, L. Biological Reduction of Structural Fe (III) in Smectites by a Marine Bacterium at 0.1 and 20 MPa. Chem. Geol. 2016, 438, 1–10. [Google Scholar] [CrossRef]
- Nixon, S.L.; Bonsall, E.; Cockell, C.S. Limitations of Microbial Iron Reduction under Extreme Conditions. FEMS Microbiol. Rev. 2022, 46, fuac033. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional Controls on Hydrogen Generation during Serpentinization of Ultramafic Rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- He, Y.; Zeng, X.; Xu, F.; Shao, Z. Diversity of Mixotrophic Neutrophilic Thiosulfate-and Iron-Oxidizing Bacteria from Deep-Sea Hydrothermal Vents. Microorganisms 2022, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Huang, J.; Guo, X.; Zhang, Y.; Liu, D.; Wu, R.; He, H.; Wang, J. Diversity of the Microbial Community and Cultivable Protease-Producing Bacteria in the Sediments of the Bohai Sea, Yellow Sea and South China Sea. PLoS ONE 2019, 14, e0215328. [Google Scholar] [CrossRef]
- Hoshino, T.; Doi, H.; Uramoto, G.-I.; Wörmer, L.; Adhikari, R.R.; Xiao, N.; Morono, Y.; D’Hondt, S.; Hinrichs, K.-U.; Inagaki, F. Global Diversity of Microbial Communities in Marine Sediment. Proc. Natl. Acad. Sci. USA 2020, 117, 27587–27597. [Google Scholar] [CrossRef]
- Sharma, V.; Vashishtha, A.; Jos, A.L.M.; Khosla, A.; Basu, N.; Yadav, R.; Bhatt, A.; Gulani, A.; Singh, P.; Lakhera, S.; et al. Phylogenomics of the Phylum Proteobacteria: Resolving the Complex Relationships. Curr. Microbiol. 2022, 79, 224. [Google Scholar] [CrossRef]
- Cristóbal, H.A.; Benito, J.; Lovrich, G.A.; Abate, C.M. Phylogenentic and Enzymatic Characterization of Psychrophilic and Psychrotolerant Marine Bacteria Belong to γ-Proteobacteria Group Isolated from the Sub-Antarctic Beagle Channel, Argentina. Folia Microbiol. 2015, 60, 183–198. [Google Scholar] [CrossRef]
- Takai, K.; Inagaki, F.; Nakagawa, S.; Hirayama, H.; Nunoura, T.; Sako, Y.; Nealson, K.H.; Horikoshi, K. Isolation and Phylogenetic Diversity of Members of Previously Uncultivated ε-Proteobacteria in Deep-Sea Hydrothermal Fields. FEMS Microbiol. Lett. 2003, 218, 167–174. [Google Scholar]
- Edwards, K.J.; Rogers, D.R.; Wirsen, C.O.; McCollom, T.M. Isolation and Characterization of Novel Psychrophilic, Neutrophilic, Fe-Oxidizing, Chemolithoautotrophic α- and γ-Proteobacteria from the Deep Sea. Appl. Environ. Microbiol. 2003, 69, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-S.; Kahng, H.-Y.; Lee, D.-H.; Lee, S.B. Tenacibaculum Jejuense Sp. Nov., Isolated from Coastal Seawater. Int. J. Syst. Evol. Microbiol. 2012, 62, 414–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urakami, T.; Komagata, K. Characterization of Species of Marine Methylotrophs of the Genus Methylophaga. Int. J. Syst. Bacteriol. 1987, 37, 402–406. [Google Scholar] [CrossRef]
- Nataro, J.P.; Bopp, C.A.; Fields, P.I.; Kaper, J.B.; Strockbine, N.A. Escherichia, Shigella, and Salmonella. In Manual of Clinical Microbiology; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 603–626. ISBN 978-1-68367-411-5. [Google Scholar]
- Roy, M.; Khara, P.; Basu, S.; Dutta, T.K. Catabolic Versatility of Sphingobium Sp. Strain PNB Capable of Degrading Structurally Diverse Aromatic Compounds. J. Bioremed. Biodeg. 2013, 4, 2. [Google Scholar] [CrossRef]
- Jo, J.H.; Choi, G.-M.; Lee, S.-Y.; Im, W.-T. Phenylobacterium Aquaticum Sp. Nov., Isolated from the Reservoir of a Water Purifier. Int. J. Syst. Evol. Microbiol. 2016, 66, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Hussain, F.; Habib, N.; Wadaan, M.A.M.; Ahmed, I.; Im, W.-T.; Hozzein, W.N.; Zhi, X.-Y.; Li, W.-J. Phenylobacterium Deserti Sp. Nov., Isolated from Desert Soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 4722–4727. [Google Scholar] [CrossRef]
- Green, P.N.; Ardley, J.K. Review of the Genus Methylobacterium and Closely Related Organisms: A Proposal That Some Methylobacterium Species Be Reclassified into a New Genus, Methylorubrum Gen. Nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 2727–2748. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Ghodake, G.S.; Bharagava, R.N.; Kim, D.S.; Nair, S.; Shin, H.S. Efficient Bioconversion of Sugarcane Bagasse into Polyhydroxybutyrate (PHB) by Lysinibacillus Sp. and Its Characterization. Bioresour. Technol. 2021, 324, 124673. [Google Scholar] [CrossRef]
- Sharma, M.; Khurana, H.; Singh, D.N.; Negi, R.K. The Genus Sphingopyxis: Systematics, Ecology, and Bioremediation Potential-A Review. J. Environ. Manag. 2021, 280, 111744. [Google Scholar] [CrossRef]
- Gan, C.; Wu, R.; Luo, Y.; Song, J.; Luo, D.; Li, B.; Yang, Y.; Xu, M. Visualizing and Isolating Iron-Reducing Microorganisms at the Single-Cell Level. Appl. Environ. Microbiol. 2021, 87, e02192-20. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Xu, J.; Fu, Z.; Wu, S.; Ni, J.; Hu, B. Insight into Efficient Removal of Cr(VI) by Magnetite Immobilized with Lysinibacillus Sp. JLT12: Mechanism and Performance. Chemosphere 2021, 262, 127901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Guo, D.; Dong, H. Differential Degradation of Petroleum Hydrocarbons by Shewanella Putrefaciens under Aerobic and Anaerobic Conditions. Front. Microbiol. 2024, 15, 1389954. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Balkwill, D.L.; Romine, M.F.; Shi, T. Ecology, Physiology, and Phylogeny of Deep Subsurface Sphingomonas sp. J. Ind. Microbiol. Biotechnol. 1999, 23, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kieft, T.L.; Fredrickson, J.K.; Onstott, T.C.; Gorby, Y.A.; Kostandarithes, H.M.; Bailey, T.J.; Kennedy, D.W.; Li, S.W.; Plymale, A.E.; Spadoni, C.M.; et al. Dissimilatory Reduction of Fe(III) and Other Electron Acceptors by a Thermus Isolate. Appl. Environ. Microbiol. 1999, 65, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.P.; Lonergan, D.J. Hydrogen and Formate Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese by Alteromonas Putrefaciens. Appl. Environ. Microbiol. 1989, 55, 700–706. [Google Scholar] [CrossRef]
- Jakus, N.; Blackwell, N.; Straub, D.; Kappler, A.; Kleindienst, S. Presence of Fe(II) and Nitrate Shapes Aquifer-Originating Communities Leading to an Autotrophic Enrichment Dominated by an Fe(II)-Oxidizing Gallionellaceae sp. FEMS Microbiol. Ecol. 2021, 97, fiab145. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Kublanov, I.V.; Tran, H.; Korzhenkov, A.A.; Lünsdorf, H.; Nechitaylo, T.Y.; Gavrilov, S.N.; Toshchakov, S.V.; Golyshin, P.N. Biology of Archaea from a Novel Family Cuniculiplasmataceae (Thermoplasmata) Ubiquitous in Hyperacidic Environments. Sci. Rep. 2016, 6, 39034. [Google Scholar] [CrossRef]
- Greening, C.; Cabotaje, P.R.; Alvarado, L.E.V.; Leung, P.M.; Land, H.; Rodrigues-Oliveira, T.; Ponce-Toledo, R.I.; Senger, M.; Klamke, M.A.; Milton, M.; et al. Minimal and Hybrid Hydrogenases Are Active from Archaea. Cell 2024, 187, 3357–3372.e19. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Zhang, D.; Chen, S.; Sun, Y.; Wu, J. Catalysis of Oxygen Reduction Reaction by an Iron-Reducing Bacterium Isolated from Marine Corrosion Product Layers. J. Electroanal. Chem. 2016, 774, 83–87. [Google Scholar] [CrossRef]
- Garber, A.I.; Nealson, K.H.; Okamoto, A.; McAllister, S.M.; Chan, C.S.; Barco, R.A.; Merino, N. FeGenie: A Comprehensive Tool for the Identification of Iron Genes and Iron Gene Neighborhoods in Genome and Metagenome Assemblies. Front. Microbiol. 2020, 11, 499513. [Google Scholar] [CrossRef]
- Ross, D.E.; Ruebush, S.S.; Brantley, S.L.; Hartshorne, R.S.; Clarke, T.A.; Richardson, D.J.; Tien, M. Characterization of Protein-Protein Interactions Involved in Iron Reduction by Shewanella Oneidensis MR-1. Appl. Environ. Microbiol. 2007, 73, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Jian, H.; Zhang, B.; Li, S.; Wang, F.; Zeng, X.; Gao, L.; Bartlett, D.H.; Yu, J.; et al. Environmental Adaptation: Genomic Analysis of the Piezotolerant and Psychrotolerant Deep-Sea Iron Reducing Bacterium Shewanella Piezotolerans WP3. PLoS ONE 2008, 3, e1937. [Google Scholar] [CrossRef]
- Akunna, J.C.; Bizeau, C.; Moletta, R. Nitrate and Nitrite Reductions with Anaerobic Sludge Using Various Carbon Sources: Glucose, Glycerol, Acetic Acid, Lactic Acid and Methanol. Water Res. 1993, 27, 1303–1312. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Various Electron Donors for Biological Nitrate Removal: A Review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Kendall, B.; Anbar, A.D.; Kappler, A.; Konhauser, K.O. The Global Iron Cycle. In Fundamentals of Geobiology; Knoll, A.H., Canfield, D.E., Konhauser, K.O., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 65–92. ISBN 978-1-118-28081-2. [Google Scholar]
- Eloe, E.A.; Lauro, F.M.; Vogel, R.F.; Bartlett, D.H. The Deep-Sea Bacterium Photobacterium Profundum SS9 Utilizes Separate Flagellar Systems for Swimming and Swarming under High-Pressure Conditions. Appl. Environ. Microbiol. 2008, 74, 6298–6305. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically Conductive Bacterial Nanowires Produced by Shewanella oneidensis Strain MR-1 and Other Microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef]


| C, H, N, S (%) | C (%) | H (%) | N (%) | S (%) | C/N | C/H | |||||
| 0.22 | 0.682 | 0.053 | 0.639 | 4.177 | 0.3238 | ||||||
| Major elements (%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | |||||
| 49.11 | 14.05 | 9.78 | 4.69 | 2.91 | 4.49 | ||||||
| K2O | MnO | TiO2 | P2O5 | LOI | FeO | ||||||
| 2.07 | 1.2 | 0.759 | 0.356 | 10.28 | 0.36 | ||||||
| Trace elements (μg/g) | Li | Be | Sc | V | Cr | Co | Ni | Cu | Zn | Ga | Rb |
| 45.2 | 1.52 | 24.2 | 171 | 90.6 | 72.1 | 168 | 331 | 149 | 17.5 | 61.1 | |
| Sr | Y | Mo | Cd | In | Sb | Cs | Ba | La | Ce | Pr | |
| 231 | 77.3 | 11.1 | 0.394 | 0.118 | 1.88 | 5.52 | 735 | 49 | 67 | 12.5 | |
| Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |
| 55.5 | 12.4 | 3.28 | 10.2 | 2.39 | 14 | 2.89 | 7.55 | 1.28 | 7.72 | 1.05 | |
| W | Re | Tl | Pb | Bi | Th | U | Nb | Ta | Zr | Hf | |
| 3.44 | 0.006 | 1.79 | 35.5 | 0.638 | 7.73 | 1.5 | 10.8 | 1.11 | 129 | 3.75 | |
| Genus/Species | H-Original | H-H2 | H-Lactate | A-Original | A-H2 | A-Lactate | |
|---|---|---|---|---|---|---|---|
| Fe-reducing microorganisms | Shewanella | 0.0637 | 0.0553 | 0.023 | 0.998 | 5.407 | 20.679 |
| S. putrefaciens | 0 | 0.0164 | 0 | 0 | 0 | 0.00369 | |
| S. oneidensis | 0 | 0.00614 | 0.00628 | 0 | 0 | 0.00738 | |
| Unclassified S. sp. | 0.0637 | 0.0328 | 0.0167 | 0.998 | 5.407 | 20.668 | |
| Thalassospira | 0.00554 | 0.00615 | 0.00209 | 5.233 | 3.864 | 2.438 | |
| Thermodesulfovibrio | 0.00139 | 0 | 0 | 0 | 0 | 0 | |
| Lysinibacillus | 0 | 0.00205 | 0 | 0.00833 | 0 | 0.0148 | |
| Thermus | 0.00692 | 0.00205 | 0.0126 | 0.00625 | 0.00557 | 0.00123 | |
| Alteromonas | 0.166 | 0.191 | 0.149 | 0.281 | 0.443 | 0.0701 | |
| Sphingomonas | 0.00139 | 0.0123 | 0.00837 | 0.0167 | 0.0445 | 0.0184 | |
| Fe-oxidizing microorganisms | Marinobacter | 4.46 | 2.356 | 2.99 | 34.458 | 29.995 | 28.034 |
| Uncultured M. sp. | 0.370 | 0.154 | 0.234 | 1.013 | 0.665 | 0.873 | |
| M. lacisalsi | 0.032 | 0.047 | 0.015 | 1.215 | 0.846 | 0.216 | |
| M. hydrocarbonc-lasticus | 1.292 | 0.918 | 1.029 | 16.617 | 14.954 | 19.154 | |
| Thiomonas | 0.0263 | 0.0307 | 0.0293 | 0.0125 | 0.0111 | 0.00861 | |
| Sulfolobus | 0 | 0 | 0 | 0 | 0 | 0.00246 | |
| Methyloversatilis | 0 | 0 | 0 | 0.00208 | 0.420 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, D.; Xia, Q.; Li, G.; Li, X.; Li, Y.; Hu, D.; Hu, J.; Zhou, Z.; Sheng, Y. Biogeochemical Fe-Redox Cycling in Oligotrophic Deep-Sea Sediment. Water 2024, 16, 2740. https://doi.org/10.3390/w16192740
Zhan D, Xia Q, Li G, Li X, Li Y, Hu D, Hu J, Zhou Z, Sheng Y. Biogeochemical Fe-Redox Cycling in Oligotrophic Deep-Sea Sediment. Water. 2024; 16(19):2740. https://doi.org/10.3390/w16192740
Chicago/Turabian StyleZhan, Di, Qingyin Xia, Gaoyuan Li, Xinyu Li, Yang Li, Dafu Hu, Jinglong Hu, Ziqi Zhou, and Yizhi Sheng. 2024. "Biogeochemical Fe-Redox Cycling in Oligotrophic Deep-Sea Sediment" Water 16, no. 19: 2740. https://doi.org/10.3390/w16192740
APA StyleZhan, D., Xia, Q., Li, G., Li, X., Li, Y., Hu, D., Hu, J., Zhou, Z., & Sheng, Y. (2024). Biogeochemical Fe-Redox Cycling in Oligotrophic Deep-Sea Sediment. Water, 16(19), 2740. https://doi.org/10.3390/w16192740







