Characterization of Escherichia coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. E. coli Identification and Characterization
2.3. Antibiotic Susceptibility Evaluation
2.4. Data Analysis
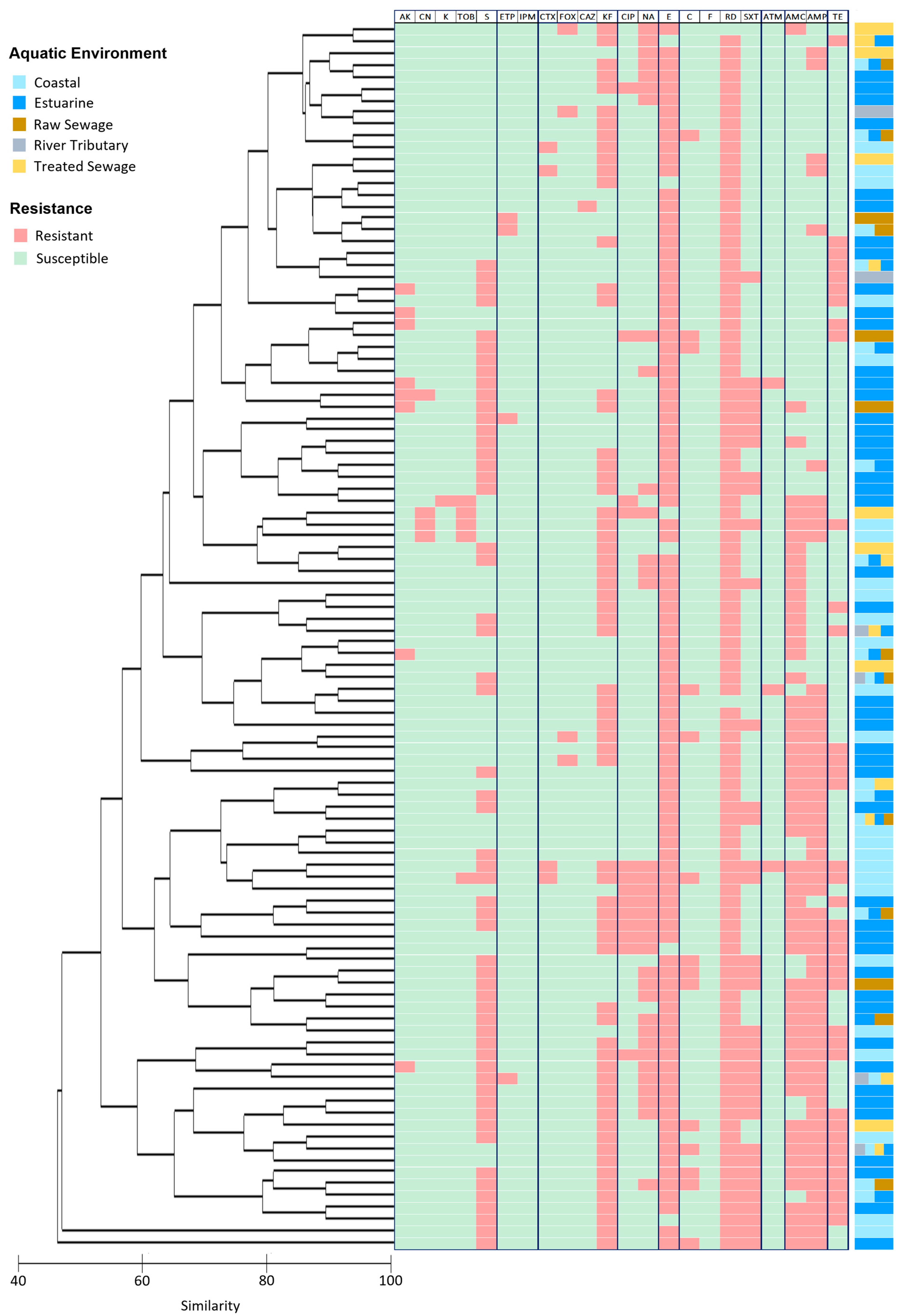
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carreño, A.; Lloret, J. Environmental impacts of increasing leisure boating activity in Mediterranean coastal waters. Ocean Coast. Manag. 2021, 209, 105693. [Google Scholar] [CrossRef]
- Giampaoli, S.; Romano Spica, V. Health and safety in recreational waters. Bull. World Health Organ. 2014, 92, 79. [Google Scholar] [CrossRef] [PubMed]
- DeFlorio-Barker, S.; Wing, C.; Jones, R.M.; Dorevitch, S. Estimate of incidence and cost of recreational waterborne illness on United States surface waters. Environ. Health Glob. Access. Sci. Source. 2018, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, L.; Kay, D. Recreational Water and Infection: A Review of Recent Findings. Curr. Environ. Health Rep. 2015, 2, 85–94. [Google Scholar] [CrossRef]
- Korajkic, A.; McMinn, B.R.; Harwood, V. Relationships between Microbial Indicators and Pathogens in Recreational Water Settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Solimini, A.; Pantanella, F.; Cummins, E. The potential human exposure to antibiotic resistant-Escherichia coli through recreational water. Sci. Total Environ. 2019, 650 Pt 1, 786–795. [Google Scholar] [CrossRef]
- EC, European Commission Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 64, 37–51.
- World Health Organization. WHO Guidelines on Recreational Water Quality: Volume 1: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021; Available online: https://iris.who.int/handle/10665/342625 (accessed on 15 April 2024).
- USEPA. Ambient Water Quality Criteria for Bacteria; USEPA: Washington, DC, USA, 1986. [Google Scholar]
- Edberg, S.C.; Rice, E.W.; Karlin, R.J.; Allen, M.J. Escherichia coli: The best biological drinking water indicator for public health protection. Symp. Ser. Soc. Appl. Microbiol. 2000, 88, 106S–116S. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Wang, W.; Zhang, J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Hadi, A.Z.; Griffith, J.F.; Ishii, S.; Sadowsky, M.J. Large scale analysis of virulence genes in Escherichia coli strains isolated from Avalon Bay, CA. Water Res. 2010, 44, 5463–5473. [Google Scholar] [CrossRef]
- Luo, C.; Walk, S.T.; Gordon, D.M.; Feldgarden, M.; Tiedje, J.M.; Konstantinidis, K.T. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc. Natl. Acad. Sci. USA 2011, 108, 7200–7205. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Memona, H. Molecular and immunological studies of pathogenic Escherichia coli in meat samples collected from different localities of Lahore. IJCMB 2010, 1, 218–224. [Google Scholar]
- Yu, D.; Banting, G.; Neumann, N.F. A review of the taxonomy, genetics, and biology of the genus Escherichia and the type species Escherichia coli. Can. J. Microbiol. 2021, 67, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Wang, W.; Li, K.; Akbar, A.; Zhang, J. Isolation and identification of infection-causing bacteria in dairy animals and determination of their antibiogram. J. Food Qual. 2021, 2021, 2958304. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Carlos, C.; Pires, M.M.; Stoppe, N.C.; Hachich, E.M.; Sato, M.I.; Gomes, T.A.; Amaral, L.A.; Ottoboni, L.M. Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol. 2010, 10, 161. [Google Scholar] [CrossRef]
- Stange, C.; Sidhu, J.P.S.; Tiehm, A.; Toze, S. Antibiotic resistance and virulence genes in coliform water isolates. Int. J. Hyg. Environ. Health 2016, 679, 823–831. [Google Scholar] [CrossRef]
- Franz, E.; Veenman, C.; van Hoek, A.H.; de Roda Husman, A.; Blaak, H. Pathogenic Escherichia coli producing Extended-Spectrum β-Lactamases isolated from surface water and wastewater. Sci. Rep. 2015, 5, 14372. [Google Scholar] [CrossRef]
- Escobar-Páramo, P.; Le Menac’h, A.; Le Gall, T.; Amorin, C.; Gouriou, S.; Picard, B.; Skurnik, D.; Denamur, E. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 2006, 8, 1975–1984. [Google Scholar] [CrossRef]
- Ndlovu, T.; Le Roux, M.; Khan, W.; Khan, S. Co-detection of virulent Escherichia coli genes in surface water sources. PLoS ONE 2015, 10, e0116808. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, J.; Rashid, R.B.; Sultana, R.; Saima, S.; Jahan Prima, M.; Begum, A.; Mackie Jensen, P.K. Is It Human or Animal? The Origin of Pathogenic E. coli in the Drinking Water of a Low-Income Urban Community in Bangladesh. Trop. Med. Infect. 2021, 6, 181. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- LeStrange, K.; Markland, S.M.; Hoover, D.G.; Sharma, M.; Kniel, K.E. An evaluation of the virulence and adherence properties of avian pathogenic Escherichia coli. One Health 2017, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 2011, 62, 1–10. [Google Scholar] [CrossRef]
- Markland, S.M.; LeStrange, K.J.; Sharma, M.; Kniel, K.E. Old Friends in New Places: Exploring the Role of Extraintestinal E. coli in Intestinal Disease and Foodborne Illness. Zoonoses Public Health 2015, 62, 491–496. [Google Scholar] [CrossRef]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 2018, 9, e00470-18. [Google Scholar] [CrossRef]
- Nappier, S.P.; Liguori, K.; Ichida, A.M.; Stewart, J.R.; Jones, K.R. Antibiotic Resistance in Recreational Waters: State of the Science. Int. J. Environ. Res. Public Health 2020, 17, 8034. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet. Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10.1128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; Available online: https://iris.who.int/handle/10665/311820 (accessed on 10 May 2024).
- Henriques, I.S.; Fonseca, F.; Alves, A.; Saavedra, M.J.; Correia, A. Occurrence and diversity of integrons and beta-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 2006, 157, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Alm, E.W.; Zimbler, D.; Callahan, E.; Plomaritis, E. Patterns and persistence of antibiotic resistance in faecal indicator bacteria from freshwater recreational beaches. J. Appl. Microbiol. 2014, 117, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.S.; Pereira, A.; Araújo, S.M.; Castro, B.B.; Correia, A.C.; Henriques, I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 2014, 5, 426. [Google Scholar] [CrossRef]
- ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and coliform bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. International Organization for Standardization: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/55832.html (accessed on 15 April 2024).
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Current Protocols in Molecular Biology; Green Publishing Associates and Wiley: New York, NY, USA, 2003. [Google Scholar]
- Chern, E.C.; Siefring, S.; Paar, J.; Doolittle, M.; Haugland, R.A. Comparison of quantitative PCR assays for Escherichia coli targeting ribosomal RNA and single copy genes. Lett. Appl. Microbiol. 2011, 52, 298–306. [Google Scholar] [CrossRef]
- Ferdous, J.; Hossain, Z.Z.; Tulsiani, S.; Rashid, R.B.; Jensen, P.K.M.; Begum, A. Optimization and Validation of Real Time PCR Assays for Absolute Quantification of toxigenic Vibrio cholerae and Escherichia coli. Trop. Biomed. 2016, 33, 641–651. Available online: https://www.msptm.org/files/Vol33No4/641-651-Begum-A.pdf (accessed on 15 May 2024).
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Le Van, P.; Le Huy, C.; Gia, K.N.; Weintraub, A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 2005, 43, 755–760. [Google Scholar] [CrossRef] [PubMed]
- CLSI, Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 15 April 2024).
- EUCAST, The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 12.0; European Committee on Antimicrobial Susceptibility Testing: Sweden, 2022; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 15 April 2024).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PR IM ER-E Ltd.: Plymouth, UK, 1994. [Google Scholar]
- Gotkowska-Płachta, A.; Gołaś, I.; Korzeniewska, E.; Koc, J.; Rochwerger, A.; Solarski, K. Evaluation of the distribution of fecal indicator bacteria in a river system depending on different types of land use in the southern watershed of the Baltic Sea. Environ. Sci. Pollut. Res. Int. 2016, 23, 4073–4085. [Google Scholar] [CrossRef]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of antibiotic resistance genes (ARGs) via integrons in Escherichia coli: A risk to human health. Environ. Pollut. 2020, 266 Pt 2, 115260. [Google Scholar] [CrossRef]
- Rebello, R.C.; Regua-Mangia, A.H. Potential enterovirulence and antimicrobial resistance in Escherichia coli isolates from aquatic environments in Rio de Janeiro, Brazil. Sci. Total Environ. 2014, 490, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Regua-Mangia, A.H.; Bezerra, R.M.P.; Esparis, C.M.; Teixeira, L.M. Escherichia coli enteroagregativa (EAEC): Filotipagem e resistência a antimicrobianos em um enteropatógeno emergente. Ver. Patol. Trop. 2009, 38, 27–34. [Google Scholar] [CrossRef][Green Version]
- Pereira, A.; Santos, A.; Tacão, M.; Alves, A.; Henriques, I.; Correia, A. Genetic diversity and antimicrobial resistance of Escherichia coli from Tagus estuary (Portugal). Sci. Total Environ. 2013, 461–462, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.M.; Matthews, B.; Stratton, H.M.; Katouli, M. Pathogenic Escherichia coli found in sewage treatment plants and environmental waters. Appl. Environ. Microbiol. 2012, 78, 5536–5541. [Google Scholar] [CrossRef]
- Alfinete, N.W.; Bolukaoto, J.Y.; Heine, L.; Potgieter, N.; Barnard, T.G. Virulence and phylogenetic analysis of enteric pathogenic Escherichia coli isolated from children with diarrhoea in South Africa. Int. J. Infect. Dis. 2022, 114, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Johura, F.T.; Parveen, R.; Islam, A.; Sadique, A.; Rahim, M.N.; Monira, S.; Khan, A.R.; Ahsan, S.; Ohnishi, M.; Watanabe, H.; et al. Occurrence of Hybrid Escherichia coli Strains Carrying Shiga Toxin and Heat-Stable Toxin in Livestock of Bangladesh. Front. Public Health 2017, 4, 287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fegan, N.; Gobius, K.S. Pathogenic Escherichia coli and One Health implications. Curr. Top. Microbiol. Immunol. 2013, 366, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Brando, R.J.; Sacerdoti, F.; Amaral, M.M.; Bernal, A.M.; Da Rocha, M.; Belardo, M.; Palermo, M.S.; Ibarra, C.A. Detection of plasma anti-lipopolysaccharide (LPS) antibodies against enterohemorrhagic Escherichia coli (EHEC) in asymptomatic kindergarten teachers from Buenos Aires province. Rev. Argent. Microbiol. 2024, 56, 25–32. [Google Scholar] [CrossRef]
- Cho, S.; Hiott, L.M.; Barrett, J.B.; McMillan, E.A.; House, S.L.; Humayoun, S.B.; Adams, E.S.; Jackson, C.R.; Frye, J.G. Prevalence and characterization of Escherichia coli isolated from the Upper Oconee Watershed in Northeast Georgia. PLoS ONE 2018, 13, e0197005. [Google Scholar] [CrossRef]
- Lim, M.A.; Kim, J.Y.; Acharya, D.; Bajgain, B.B.; Park, J.H.; Yoo, S.J.; Lee, K. A Diarrhoeagenic Enteropathogenic Escherichia coli (EPEC) Infection Outbreak That Occurred among Elementary School Children in Gyeongsangbuk-Do Province of South Korea Was Associated with Consumption of Water-Contaminated Food Items. Int. J. Environ. Res. Public Health 2020, 17, 3149. [Google Scholar] [CrossRef]
- Bolukaoto, J.Y.; Singh, A.; Alfinete, N.; Barnard, T.G. Occurrence of Hybrid Diarrhoeagenic Escherichia coli Associated with Multidrug Resistance in Environmental Water, Johannesburg, South Africa. Microorganisms 2021, 9, 2163. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, N.; Karambwe, S.; Mudau, L.S.; Barnard, T.; Traore, A. Human Enteric Pathogens in Eight Rivers Used as Rural Household Drinking Water Sources in the Northern Region of South Africa. Int. J. Environ. Res. Public Health 2020, 17, 2079. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, F.A.; Emaneini, M.; Hosseini, H.; Iman-Eini, H.; Aligholi, M.; Jabalameli, F.; Haghi-Ashtiani, M.T.; Taherikalani, M.; Mirsalehian, A. Molecular analysis of typical and atypical enteropathogenic Escherichia coli (EPEC) isolated from children with diarrhoea. J. Med. Microbiol. 2013, 62 Pt 2, 191–195. [Google Scholar] [CrossRef]
- Lan, R.; Alles, M.C.; Donohoe, K.; Martinez, M.B.; Reeves, P.R. Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect. Immun. 2004, 72, 5080–5088. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Garcia, T.; Navarro-Garcia, F. Enteroaggregative Escherichia coli pathotype: A genetically heterogeneous emerging foodborne enteropathogen. FEMS Immunol. Med. Microbiol. 2012, 66, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Santos, A.C.M.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of Hybrid- and Hetero-Pathogenic Escherichia coli and Their Potential Implication in More Severe Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef]
- Clermont, O.; Condamine, B.; Dion, S.; Gordon, D.M.; Denamur, E. The E phylogroup of Escherichia coli is highly diverse and mimics the whole E. coli species population structure. Environ. Microbiol. 2021, 23, 7139–7151. [Google Scholar] [CrossRef]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef]
- Hu, J.; Afayibo, D.J.A.; Zhang, B.; Zhu, H.; Yao, L.; Guo, W.; Wang, X.; Wang, Z.; Wang, D.; Peng, H.; et al. Characteristics, pathogenic mechanism, zoonotic potential, drug resistance, and prevention of avian pathogenic Escherichia coli (APEC). Front. Microbiol. 2022, 13, 1049391. [Google Scholar] [CrossRef]
- Leekitcharoenphon, P.; Johansson, M.H.K.; Munk, P.; Malorny, B.; Skarżyńska, M.; Wadepohl, K.; Moyano, G.; Hesp, A.; Veldman, K.T.; Bossers, A.; et al. Genomic evolution of antimicrobial resistance in Escherichia coli. Sci Rep. 2021, 11, 15108. [Google Scholar] [CrossRef] [PubMed]
- Wight, J.; Byrne, A.S.; Tahlan, K.; Lang, A.S. Anthropogenic contamination sources drive differences in antimicrobial-resistant Escherichia coli in three urban lakes. Appl. Environ. Microbiol. 2024, 90, e0180923. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020.
- Tiberi, S.; Sotgiu, G.; D’Ambrosio, L.; Arbex, M.A.; Arrascue, E.A.; Alffenaar, J.W.; Caminero, J.A.; Gaga, M.; Gualano, G.; Skrahina, A.; et al. Comparison of effectiveness and safety of imipenem/clavulanate-versusmeropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur. Respir. J. 2016, 47, 1758–1766. [Google Scholar] [CrossRef]
- Skurnik, D.; Lacheeb, S.; Bernede, C.; le Menac’h, A.; Elbaz, S.; Mohler, J.; Denamur, E.; Andremont, A.; Ruimy, R. Integrons and antibiotic resistance in phylogenetic group B2 Escherichia coli. Microb. Drug. Resist. 2009, 15, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Barbosa-Vasconcelos, A.; Mendes, A.; Vaz-Pires, P.; Martins da Costa, P. High prevalence of multidrug-resistant Escherichia coli and Enterococcus spp. in river water, upstream and downstream of a wastewater treatment plant. J. Water Health 2014, 12, 426–435. [Google Scholar] [CrossRef]
- Bhowmik, A.; Shah, S.T.; Goswami, S.; Sirajee, A.S.; Ahsan, S. Predominance of Multidrug Resistant Escherichia coli of Environmental Phylotype in Different Environments of Dhaka, Bangladesh. Trop. Med. Infect. Dis. 2023, 8, 226. [Google Scholar] [CrossRef]
- Svenungsson, B.; Lagergren, A.; Ekwall, E.; Evengård, B.; Hedlund, K.O.; Kärnell, A.; Löfdahl, S.; Svensson, L.; Weintraub, A. Enteropathogens in adult patients with diarrhea and healthy control subjects: A 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 2000, 30, 770–778. [Google Scholar] [CrossRef]
- Pollard, D.R.; Johnson, W.M.; Lior, H.; Tyler, S.D.; Rozee, K.R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J. Clin. Microbiol. 1990, 28, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Tornieporth, N.G.; John, J.; Salgado, K.; de Jesus, P.; Latham, E.; Melo, M.C.; Gunzburg, S.T.; Riley, L.W. Differentiation of pathogenic Escherichia coli strains in Brazilian children by PCR. J. Clin. Microbiol. 1995, 33, 1371–1374. [Google Scholar] [CrossRef]
- Schmidt, H.; Knop, C.; Franke, S.; Aleksic, S.; Heesemann, J.; Karch, H. Development of PCR for screening of enteroaggregative Escherichia coli. J. Clin. Microbiol. 1995, 33, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stapleton, A.E.; Russo, T.A.; Scheutz, F.; Brown, J.J.; Maslow, J.N. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect. Immun. 1997, 65, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Wannemuehler, Y.M.; Nolan, L.K. Evolution of the iss gene in Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2360–2369. [Google Scholar] [CrossRef]
- Morales, C.; Lee, M.D.; Hofacre, C.; Maurer, J.J. Detection of a novel virulence gene and a Salmonella virulence homologue among Escherichia coli isolated from broiler chickens. Foodborne Pathog. Dis. 2004, 1, 160–165. [Google Scholar] [CrossRef]



| Pathogenic Type | Gene Detection | n (%) |
|---|---|---|
| Commensal | - | 176 (65%) |
| DEC | - | 96 (35%) |
| ETEC | eltB | 6 (6%) |
| estA | 27(28%) | |
| EHEC | vt1 | 11 (12%) |
| vt2 | 3 (3%) | |
| vt1 + eaeA | 3 (3%) | |
| vt2 + eaeA | 8 (8%) | |
| aEPEC | eaeA | 12 (13%) |
| EIEC | ipaH | 10 (10.5%) |
| EAEC | pCVD | 6 (6%) |
| Hybrid | - | 10 (10.5%) |
| Pathogenic Type | Gene Detection Combination | n |
|---|---|---|
| EHEC/ETEC | vt1 + eltB | 1 |
| vt1 + estA | 1 | |
| aEPEC/ETEC | eaeA + estA | 1 |
| EAEC/ETEC | pCVD + estA | 1 |
| EAEC/EAEC | pCVD + vt1 | 2 |
| EAEC/aEPEC | pCVD + eaeA | 2 |
| EHEC | vt1 + vt2 + eaeA | 2 |
| Class of Antibiotic | Antibiotic | Code | Disk Content (µg) | E. coli Isolates | |
|---|---|---|---|---|---|
| Resistant | Susceptible | ||||
| Aminoglycosides | Amikacin | AK | 30 | 9 | 263 |
| Gentamicin | CN | 10 | 5 | 267 | |
| Kanamycin | K | 30 | 3 | 269 | |
| Streptomycin | S | 10 | 142 | 130 | |
| Tobramycin | TOB | 10 | 6 | 266 | |
| Carbapenems | Ertapenem | ETP | 10 | 4 | 268 |
| Imipenem | IPM | 10 | 0 | 272 | |
| Cephalosporines | Cefotaxime | CTX | 5 | 4 | 268 |
| Cefoxitin | FOX | 30 | 5 | 267 | |
| Ceftazidime | CAZ | 30 | 1 | 271 | |
| Cephalothin | KF | 30 | 150 | 122 | |
| Fluoroquinolones | Ciprofloxacin | CIP | 5 | 15 | 257 |
| Nalidixic acid | NA | 30 | 52 | 220 | |
| Macrolides | Erythromycin | E | 15 | 266 | 6 |
| Miscellaneous Agents | Chloramphenicol | C | 30 | 15 | 257 |
| Nitrofurantoin | F | 300 | 0 | 272 | |
| Rifampicin | RD | 5 | 270 | 2 | |
| Trimethoprim-sulfamethoxazole | SXT | 25 | 64 | 208 | |
| Monobactams | Aztreonam | ATM | 30 | 3 | 269 |
| Penicillins | Amoxicillin—Clavulanic acid | AMC | 30 | 124 | 148 |
| Ampicillin | AMP | 10 | 92 | 180 | |
| Tetracyclines | Tetracycline | TE | 30 | 46 | 226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.; Bordalo, A.A.; Machado, A. Characterization of Escherichia coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach. Water 2024, 16, 2695. https://doi.org/10.3390/w16182695
Gomes L, Bordalo AA, Machado A. Characterization of Escherichia coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach. Water. 2024; 16(18):2695. https://doi.org/10.3390/w16182695
Chicago/Turabian StyleGomes, Lúcia, Adriano A. Bordalo, and Ana Machado. 2024. "Characterization of Escherichia coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach" Water 16, no. 18: 2695. https://doi.org/10.3390/w16182695
APA StyleGomes, L., Bordalo, A. A., & Machado, A. (2024). Characterization of Escherichia coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach. Water, 16(18), 2695. https://doi.org/10.3390/w16182695








