Dietary Addition of Selenium Attenuates Cadmium-Induced Liver Injury in Grass Carp (Ctenopharyngodon idellus) by Reducing Oxidative Stress and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Compound Feed
2.2. Experimental Fish Management and Experimental Design
2.3. Analysis of Malondialdehyde and Antioxidative Enzyme Activity
2.4. Apoptosis-Related Gene Expression Levels Analysis
2.5. TUNEL Method to Detect Apoptosis in Liver Tissues
2.6. Statistical Analysis
3. Results
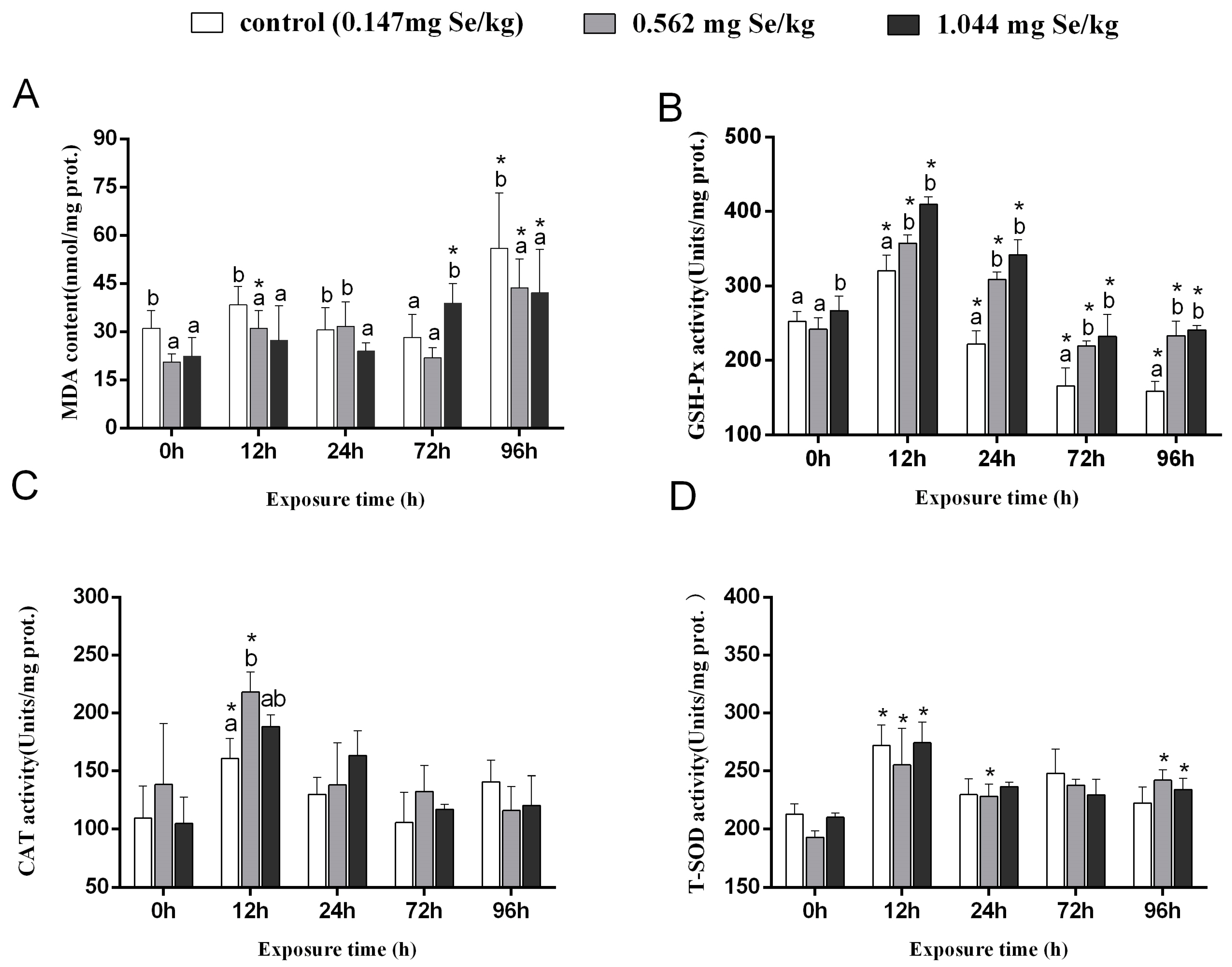
3.1. MDA Content and Antioxidant Enzyme Activity
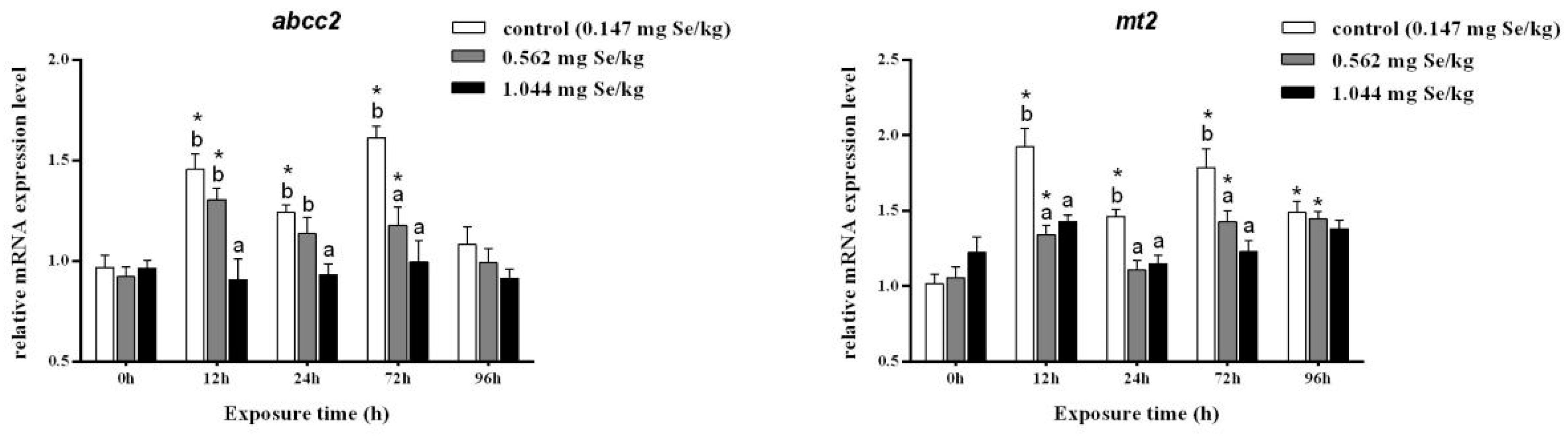
3.2. Expression Levels of Heavy Metal Scavenging Genes
3.3. Expression Levels of Apoptosis Gene
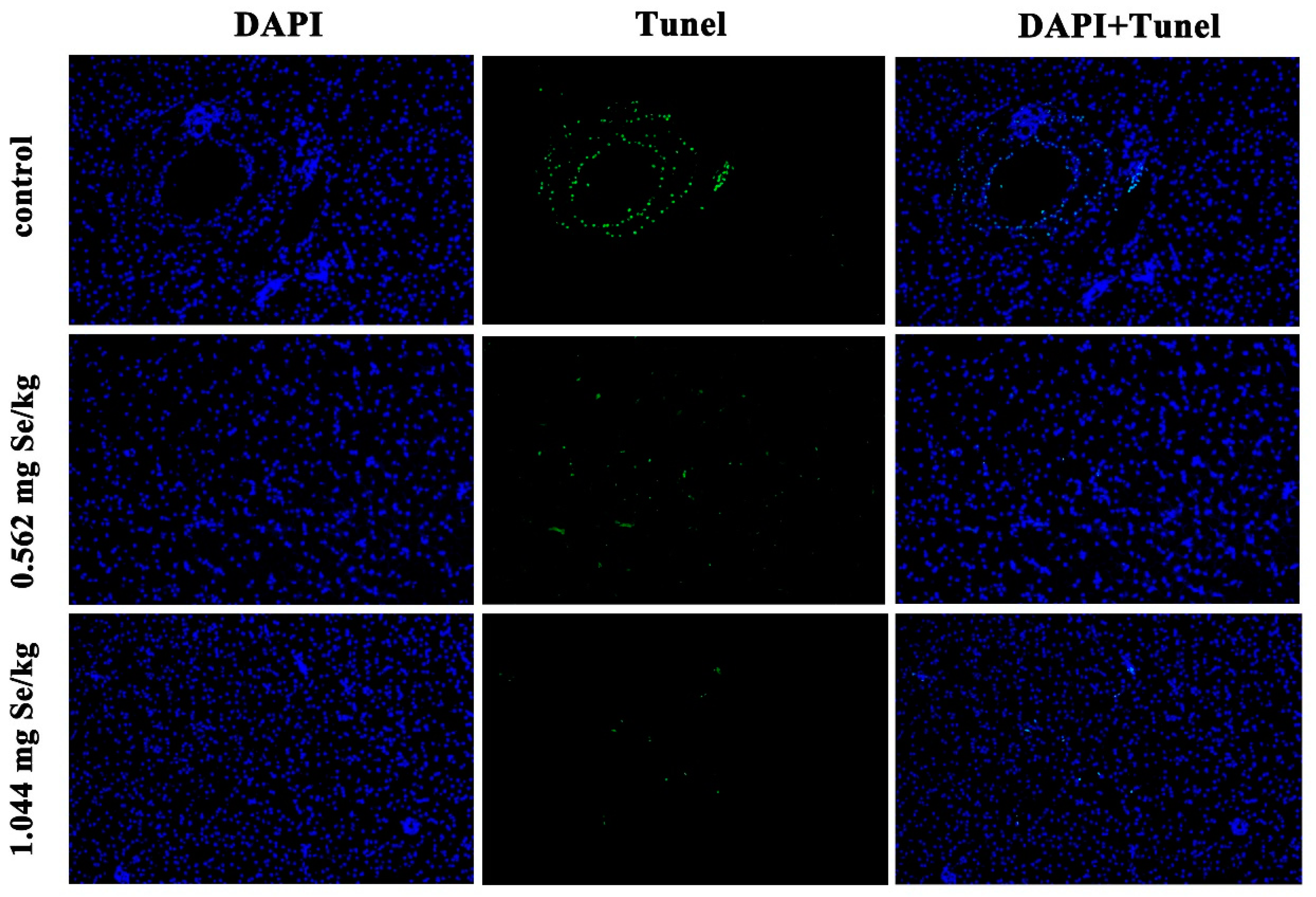
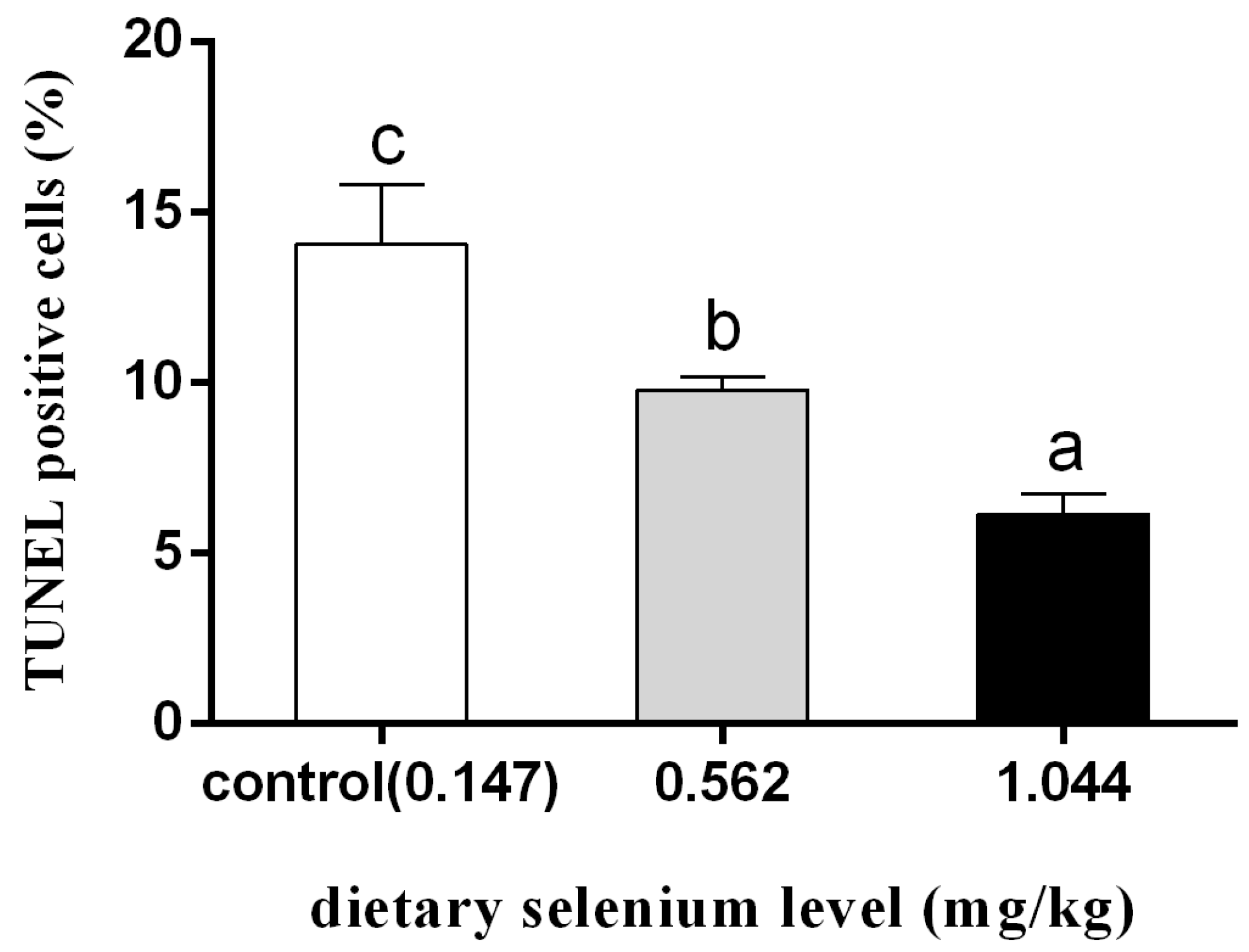
3.4. Effect of Dietary Selenium on Apoptosis of Grass Carp Liver Cells under Cadmium Stress
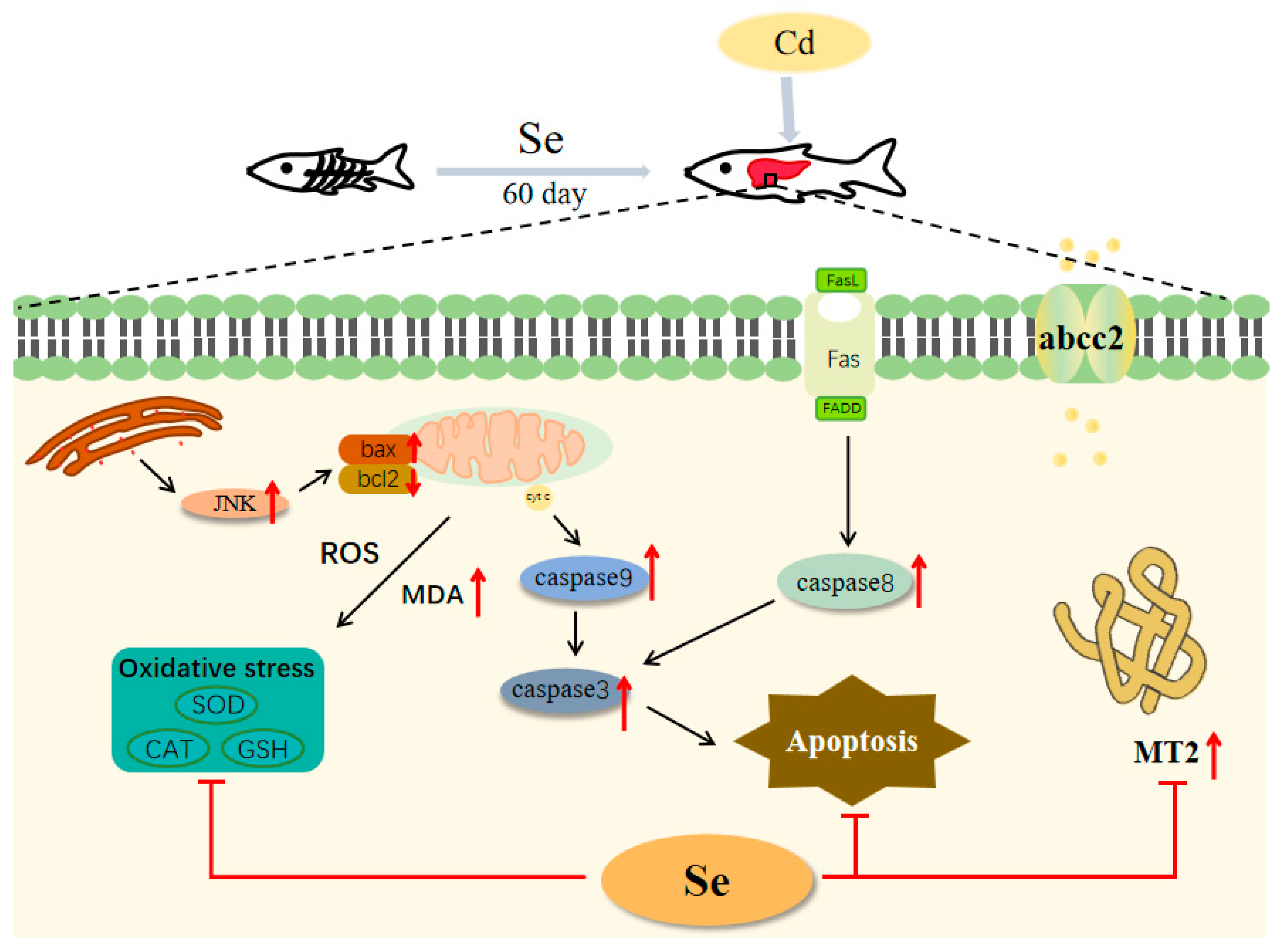
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lucia, M.; Andre, J.M.; Gonzalez, P.; Baudrimont, M.; Gontier, K.; Maury-Brachet, R.; Davail, S. Impact of cadmium on aquatic bird Cairina moschata. Biometals 2009, 22, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Roccheri, M.C.; Agnello, M.; Bonaventura, R.; Matranga, V. Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem. Biophys. Res. Commun. 2004, 321, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, I.J.M.; Gant, C.M.; v Huizen, S.; Maatman, R.; Navis, G.; Bakker, S.J.L.; Laverman, G.D. Lifestyle-Related Exposure to Cadmium and Lead is Associated with Diabetic Kidney Disease. J. Clin. Med. 2020, 9, e2432. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, M.G.; Ma, Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem. Res. Toxicol. 2008, 21, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lo, L.; Chan, W. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish (Danio rerio). Mol. Cell. Endocrinol. 2000, 159, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, Z.; Qiao, P.P.; Liu, S.; Zhang, L.; He, P.H.; Zhang, X.Y.; Wang, Y.N.; Min, W.P. Cadmium induces liver cell apoptosis through caspase-3A activation in purse red common carp (Cyprinus carpio). PLoS ONE 2013, 8, e83423. [Google Scholar] [CrossRef]
- Krumschnabel, G.; Ebner, H.L.; Hess, M.W.; Villunger, A. Apoptosis and necroptosis are induced in rainbow trout cell lines exposed to cadmium. Aquat. Toxicol. 2010, 99, 73–85. [Google Scholar] [CrossRef]
- Chan, P.K.; Cheng, S.H. Cadmium-induced ectopic apoptosis in zebrafish embryos. Arch. Toxicol. 2003, 77, 69–79. [Google Scholar] [CrossRef]
- Bai, H.; Yang, F.; Jiang, W.J.; Hu, A.M.; Chang, H.F.; Zhang, Y.L.; Jiang, L.; Lin, S.X.; Lu, Z.T.; Zhang, C.Y.; et al. Molybdenum and cadmium co-induce mitophagy and mitochondrial dysfunction via ROS-mediated PINK1/Parkin pathway in Hepa1-6 cells. Ecotoxicol. Environ. Saf. 2021, 224, e112618. [Google Scholar] [CrossRef]
- Ouyang, C.; Ke, H.Y.; Zhou, J.; Zhao, J.L.; Ma, X.; Liu, Y.M.; Li, X.H.; Li, W.W. Renal injury induced by cadmium chloride and the protective effect of vitamin C in mice. J. Hyg. Res. 2022, 51, 791–807. [Google Scholar]
- Tan, C.; Gao, Z.Y.; Wang, X.; Zhou, H.H.; Mai, K.S.; He, G. Nutritional effect of yeast selenium and its application in aquaculture. Hebei Fish. 2018, 7, 52–54+60. [Google Scholar]
- Zheng, R.L.; Chen, D.Y.; Su, J.Y.; Lai, J.; Wang, C.Y.; Chen, H.T.; Ning, Z.H.; Liu, X.; Tian, X.G.; Li, Y.H.; et al. Inhibition of HAdV-14 induced apoptosis by selenocystine through ROS-mediated PARP and p53 signaling pathways. J. Trace Elem. Med. Biol. 2023, 79, 127213. [Google Scholar] [CrossRef]
- Zheng, S.F.; Jin, X.; Chen, M.H.; Shi, Q.X.; Zhang, H.F.; Xu, S.W. Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers. Chemosphere 2019, 214, 25–34. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci. Total Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef]
- Talas, Z.S.; Orun, I.; Ozdemir, I.; Erdogan, K.; Alkan, A.; Yilmaz, I. Antioxidative role of selenium against the toxic effect of heavy metals (Cd+2, Cr+3) on liver of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2008, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cao, J.W.; Xu, T.C.; Liu, T.J.; Zhu, M.R.; Guo, M.R. Selenium deficiency induced inflammation and apoptosis via NF-κB and MAPKs pathways in muscle of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2023, 138, 108847. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Wang, S.S.; Zhang, C.Q.; Hu, X.D.; Zhou, L.; Li, Y.H.; Xu, L.C. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicol. Environ. Saf. 2020, 192, e110266. [Google Scholar] [CrossRef]
- Zheng, L.; Feng, L.; Jiang, W.D.; Wu, P.; Tang, L.; Kuang, S.Y.; Zeng, Y.Y.; Zhou, X.Q.; Liu, Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70. [Google Scholar] [CrossRef]
- Liu, L.; Liang, X.; Li, J.; Fang, J.; Alam, M. Effects of dietary selenium on growth performance and oxidative stress in juvenile grass carp (Ctenopharyngodon idellus). Aquacult. Nutr. 2018, 24, 1296–1303. [Google Scholar] [CrossRef]
- Su, C.F.; Luo, L.; Wen, H.; Chen, X.C.; Sheng, X.S.; Chen, Z. Effects of selenium on growth, nutritional composition and digestive enzyme activity of grass carp (Ctenopharyngodon idella). J. Shanghai Ocean Univ. 2007, 02, 124–129. [Google Scholar]
- Barwick, M.; Maher, W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar. Environ. Res. 2003, 56, 471–502. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Y.; Zhang, H.J.; Liu, X.Y. Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp (Cyprinus carpio) var. Color. Fish Physiol. Biochem. 2011, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Xu, Z.; Liu, T.Q.; Min, Y.H.; Li, S. Ameliorative Effects of Selenium on Cadmium-Induced Injury in the Chicken Ovary: Mechanisms of Oxidative Stress and Endoplasmic Reticulum Stress in Cadmium-Induced Apoptosis. Biol. Trace Elem. Res. 2018, 184, 463–473. [Google Scholar] [CrossRef]
- Liu, L.L.; Yang, B.Y.; Cheng, Y.P.; Lin, H.J. Ameliorative Effects of Selenium on Cadmium-Induced Oxidative Stress and Endoplasmic Reticulum Stress in the Chicken Kidney. Biol. Trace Elem. Res. 2015, 167, 308–319. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zheng, S.F.; Wang, S.C.; Liu, Q.Q.; Xu, S.W. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef]
- Li, J.L.; Jiang, C.Y.; Li, S.; Xu, S.W. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol. Environ. Saf. 2013, 96, 103–109. [Google Scholar] [CrossRef]
- Li, K.; Feng, T.J.; Liu, L.Y.; Liu, H.M.; Huang, K.X.; Zhou, J. Hepatic Proteomic Analysis of Selenoprotein T Knockout Mice by TMT: Implications for the Role of Selenoprotein T in Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 227–233. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radical Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.J.; Wang, X.H.; Wang, Z.Y.; Wei, Z.B.; Wang, L.S. Metal accumulation and antioxidant defenses in the freshwater fish (Carassius auratus) in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J. Hazard. Mater. 2014, 275, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Shen, H.; Wang, X.R.; Wu, J.C.; Xue, Y.Q. Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish (Carassius auratus). Chemosphere 2004, 55, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Pillet, M.; Castaldo, G.; De Weggheleire, S.; Bervoets, L.; Blust, R.; De Boeck, G. Limited oxidative stress in common carp (Cyprinus carpio) exposed to a sublethal tertiary (Cu, Cd and Zn) metal mixture. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hermesz, E.; Abrahám, M.; Nemcsók, J. Tissue-specific expression of two metallothionein genes in common carp during cadmium exposure and temperature shock. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 128, 457–465. [Google Scholar] [CrossRef]
- Park, J.D.; Liu, Y.P.; Klaassen, C.D. Protective effect of metallothionein against the toxicity of cadmium and other metals. Toxicology 2001, 163, 93–100. [Google Scholar] [CrossRef]
- Knapen, D.; Reynders, H.; Bervoets, L.; Verheyen, E.; Blust, R. Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat. Toxicol. 2007, 82, 163–172. [Google Scholar] [CrossRef]
- Tan, S.W.; Li, H.; Jin, Y.; Yu, H. In vitro and in vivo effects of sublethal cadmium on the expression of MT2 and ABCC2 genes in grass carp (Ctenopharyngodon idellus). Ecotoxicol. Environ. Saf. 2014, 108, 258–264. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Della, T.C.; Zaja, R.; Loncar, J.; Smital, T.; Focardi, S.; Corsi, I. Interaction of ABC transport proteins with toxic metals at the level of gene and transport activity in the PLHC-1 fish cell line. Chem.-Biol. Interact. 2012, 198, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ahmad, I.; Maria, V.L.; Serafim, A.; Bebianno, M.J.; Pacheco, M.; Santos, M.A. Hepatic metallothionein concentrations in the golden grey mullet (Liza aurata)-Relationship with environmental metal concentrations in a metal-contaminated coastal system in Portugal. Mar. Environ. Res. 2010, 69, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Payen, L.; Sparfel, L.; Courtois, A.; Vernhet, L.; Guillouzo, A.; Fardel, O. The drug efflux pump MRP2: Regulation of expression in physiopathological situations and by endogenous and exogenous compounds. Cell Biol. Toxicol. 2002, 18, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Li, Q.; Li, J.; Cui, Z.B. Molecular analysis, developmental function and heavy metal-induced expression of ABCC5 in zebrafish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 158, 46–55. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Fontaínhas-Fernandes, A.A.; Pinto-Carnide, O.; Matos, M.; Coimbra, A.M. Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat. Toxicol. 2013, 128–129, 183–189. [Google Scholar] [CrossRef]
- Pathak, N.; Mitra, S.; Khandelwal, S. Cadmium induces thymocyte apoptosis via caspase-dependent and caspase-independent pathways. J. Biochem. Mol. Toxicol. 2013, 27, 193–203. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Miao, Y.S.; Muhammad, I.; Tian, E.; Hu, W.J.; Wang, J.; Wang, B.; Li, R.; Li, J.C. Colistin-induced autophagy and apoptosis involves the JNK-Bcl2-Bax signaling pathway and JNK-p53-ROS positive feedback loop in PC-12 cells. Chem.-Biol. Interact. 2017, 277, 62–73. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Hockenbery, D.M.; Oltvai, Z.N.; Yin, X.M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993, 75, 241–251. [Google Scholar] [CrossRef]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef]
- Liu, X.M.; Shi, L.; Wang, W.; Jin, M.H.; Du, H.Y.; Liu, Y.; Sun, L.; Sun, Z.W. Mitochondrial damage induced by cadmium chloride in human hepatoma cell line SMMC-7721. Teratog. Carcinog. Mutagen. 2010, 22, 276–278. [Google Scholar]
- Duan, Y.J.; Duan, J.; Feng, Y.; Huang, X.L.; Fan, W.; Wang, K.Y.; Ouyang, P.; Deng, Y.Q.; Du, Z.J.; Chen, D.F.; et al. Hepatoprotective Activity of Vitamin E and Metallothionein in Cadmium-Induced Liver Injury in Ctenopharyngodon idellus. Oxid. Med. Cell. Longev. 2018, 2018, 9506543. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Z.; Zhang, Y.; Yang, J.; Liu, Q.; Zhao, R.H.; Hamid, S.; Wang, H.; Xu, S.W.; Zhang, Z.W. Antagonistic effects of selenium against necroptosis injury via adiponectin-necrotic pathway induced by cadmium in heart of chicken. RSC Adv. 2017, 7, 44438–44446. [Google Scholar] [CrossRef]
- Miao, Z.R.; Miao, Z.Y.; Shi, X.; Wu, H.; Yao, Y.J.; Xu, S.W. The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotoxicol. Environ. Saf. 2022, 231, 113176. [Google Scholar] [CrossRef]
- Zhang, T.T.; Sun, S.Y.; Gavrilović, A.; Li, D.P.; Tang, R. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in L8824 cells. Ecotoxicol. Environ. Saf. 2023, 262, 115337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, J.; Ge, J.; Wang, L.L.; Li, N.; Sun, X.T.; Cao, H.B.; Li, J.L. Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis. Toxicol. In Vitro 2017, 44, 349–356. [Google Scholar] [CrossRef]

| Basic Formula | Content (g/kg) | Nutritional Composition | |
|---|---|---|---|
| Casein | 260 | Crude protein (%) | 32.68 |
| Corn Starch | 200 | Crude Ash (%) | 10.26 |
| Wheat starch | 186 | Moisture (%) | 8.71 |
| Soybean isolate protein | 138 | Crude fat (%) | 5.03 |
| Cellulose | 100 | ||
| Fish oil | 31 | ||
| Mineral premix | 20 | ||
| Soybean oil | 18 | ||
| Calcium dihydrogen phosphate | 15 | ||
| Choline chloride (50%) | 10 | ||
| Selenium premix | 10 | ||
| Sodium carboxymethyl cellulose | 10 | ||
| Vitamin premixes | 1.5 | ||
| Ethoxyquin (30%) | 0.5 | ||
| Target Gene | Primer Sequence (5′-3′) | GenBank Number |
|---|---|---|
| β-actin | F: CACTGTGCCCATCTACGA R: CCATCTCCTGCTCGAAGTC | DQ211096.1 |
| bax | F: TCTCTGGCTGCAGTATGTGG R: CAAGGTTGTCATGGCTCAGA | KT697992 |
| bcl2 | F: AGATGGCGTCCCAGGTAGAT R: GCTGACCGTACAACTCCACA | JQ713862 |
| mt2 | F: ATGGATCCTTGCGACTGCG R: CATTGACAGCAGCTGGAGCC | KC678701 |
| abcc2 | F: ACTATACTCCTAGCTGATGGATCCA R: CTGAAGGTGTGGTATCAAAGAACAC | KC256782 |
| jnk | F: ATGGCTGAAATGGTCAGAGG R: GAGTCTGCAGGGAAGAGCAC | KT757312 |
| caspas-e3 | F: GGCACTGCATCATCATCAAC R: CATGGGCAACTGCTGTTAAA | JQ793789 |
| caspase-8 | F: TGAACTCACACTGCCTTTCG R: CACTGCTCAACGCAGGATTA | KM016991 |
| caspase-9 | F: AGGCAAACCACAATCGTTTC R: AAGGACAGTTCTGGCCATTG | JQ793787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-L.; Ma, P.; Li, M.; Li, D.-P.; Tang, R. Dietary Addition of Selenium Attenuates Cadmium-Induced Liver Injury in Grass Carp (Ctenopharyngodon idellus) by Reducing Oxidative Stress and Apoptosis. Water 2024, 16, 2691. https://doi.org/10.3390/w16182691
Zhang Y-L, Ma P, Li M, Li D-P, Tang R. Dietary Addition of Selenium Attenuates Cadmium-Induced Liver Injury in Grass Carp (Ctenopharyngodon idellus) by Reducing Oxidative Stress and Apoptosis. Water. 2024; 16(18):2691. https://doi.org/10.3390/w16182691
Chicago/Turabian StyleZhang, Yu-Ling, Pin Ma, Min Li, Da-Peng Li, and Rong Tang. 2024. "Dietary Addition of Selenium Attenuates Cadmium-Induced Liver Injury in Grass Carp (Ctenopharyngodon idellus) by Reducing Oxidative Stress and Apoptosis" Water 16, no. 18: 2691. https://doi.org/10.3390/w16182691
APA StyleZhang, Y.-L., Ma, P., Li, M., Li, D.-P., & Tang, R. (2024). Dietary Addition of Selenium Attenuates Cadmium-Induced Liver Injury in Grass Carp (Ctenopharyngodon idellus) by Reducing Oxidative Stress and Apoptosis. Water, 16(18), 2691. https://doi.org/10.3390/w16182691







