Ichthyoplankton Assemblages from the Coasts of Hamsilos Nature Park, Sinop, Southern Black Sea: Biodiversity, Abundance, and Relationships with Environmental Variables
Abstract
1. Introduction
2. Materials and Methods
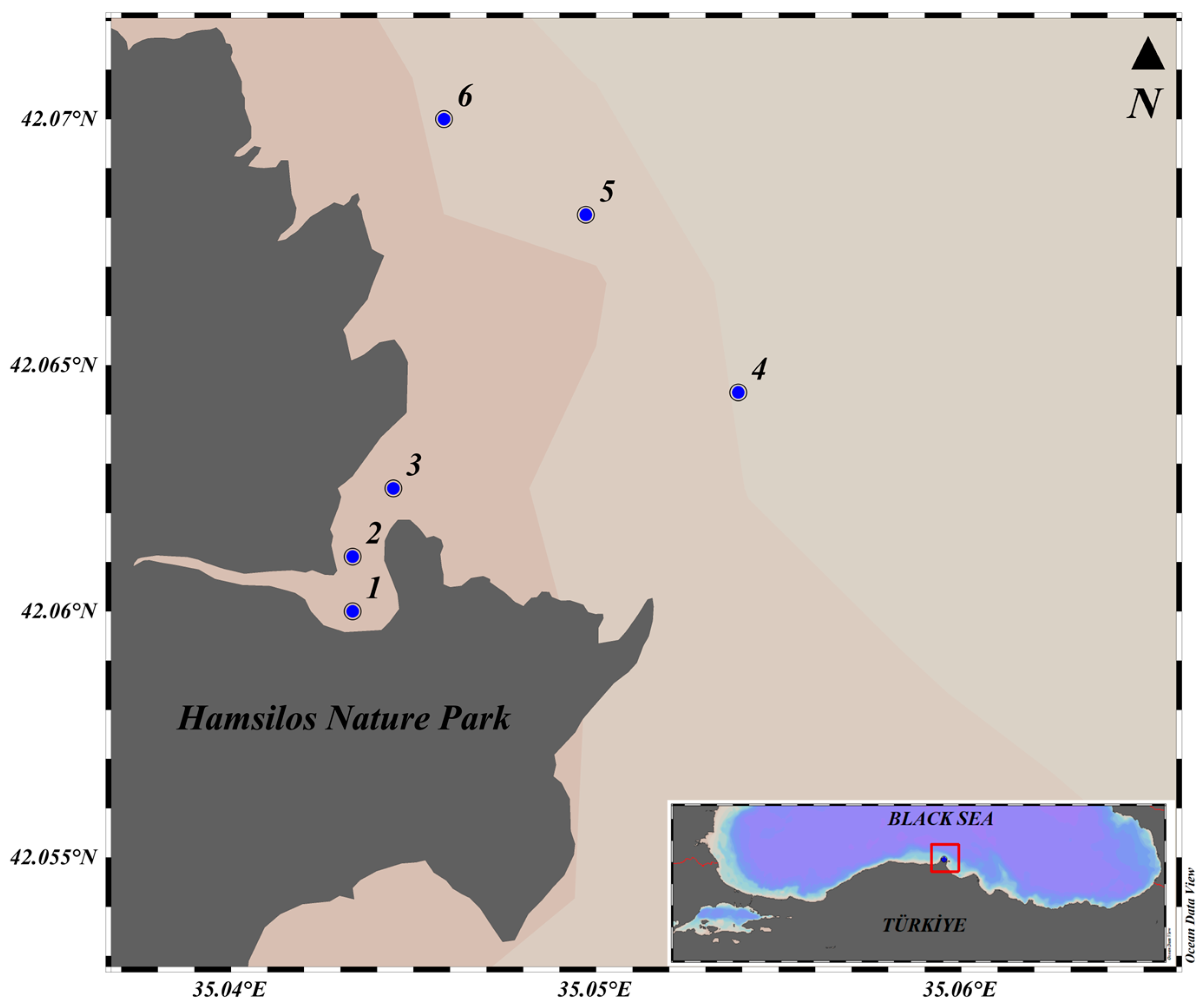
2.1. Study Area and Methodology
2.2. Statistical Analyses of Data
3. Results
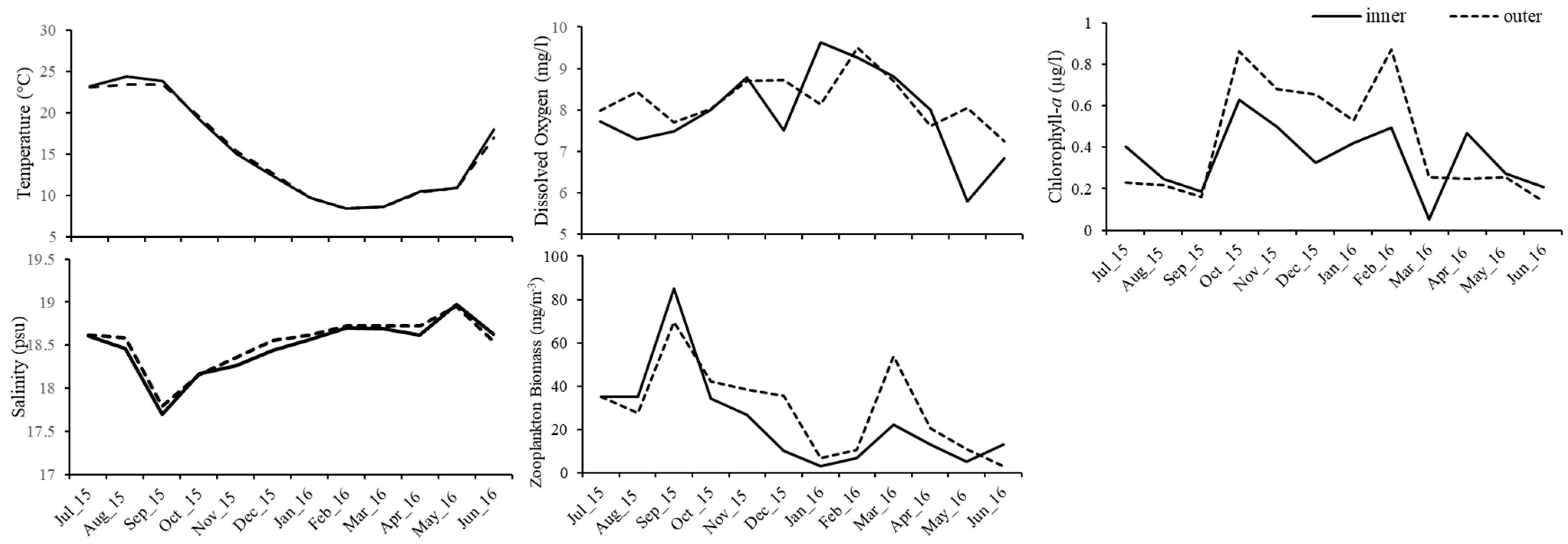
3.1. Abiotic and Biotic Environmental Factors
3.2. Ichthyoplankton Assemblage Structure
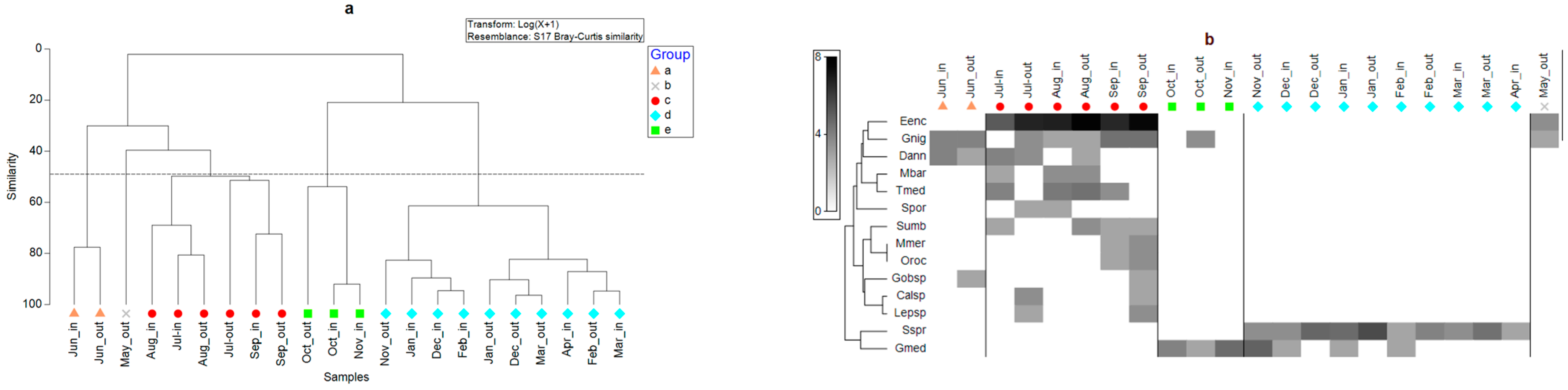
3.2.1. Temporal Variations
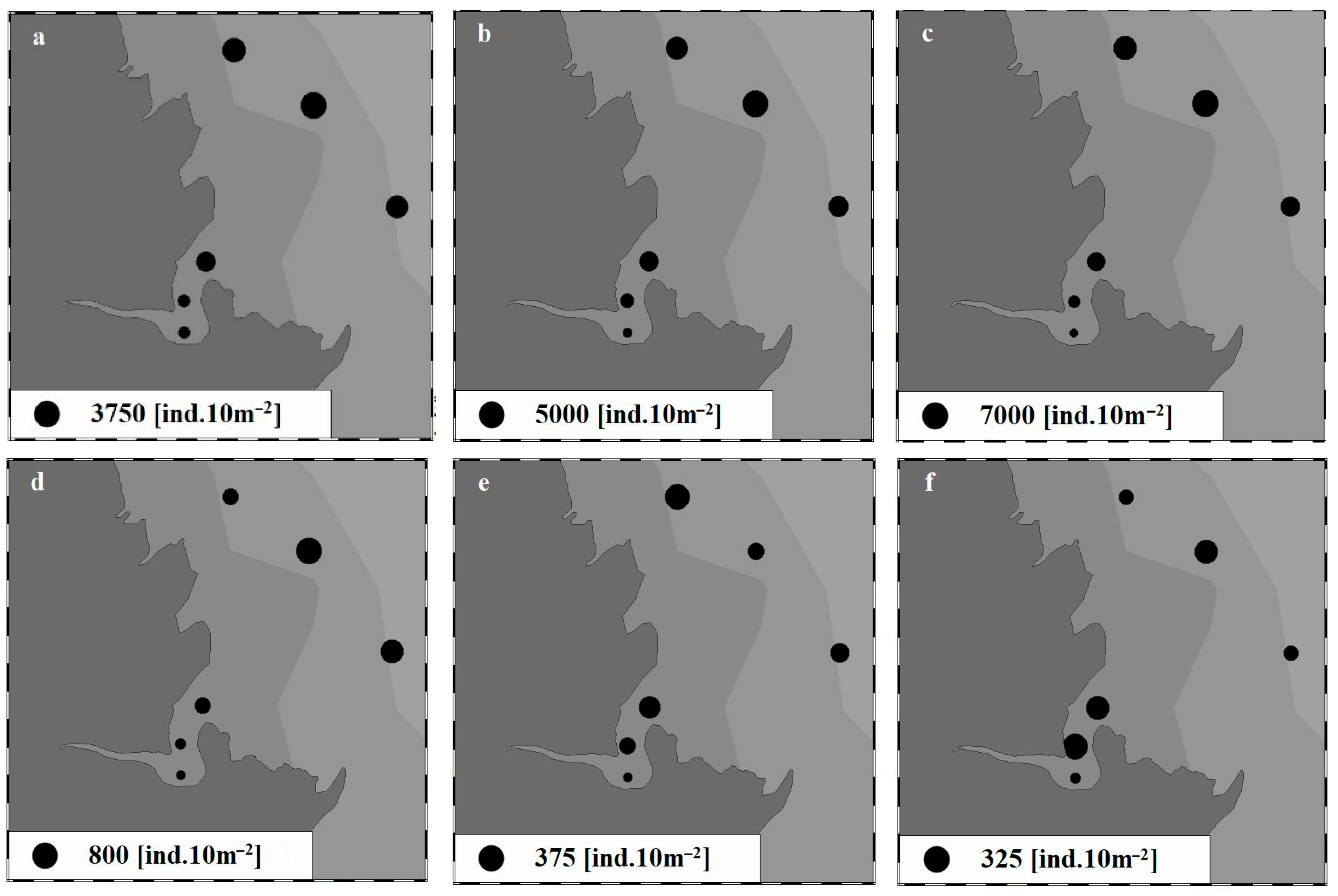
3.2.2. Spatial Variations
3.2.3. Relationships of Ichthyoplankton Assemblages with Environmental Variables
4. Discussion
4.1. Ichthyoplankton Composition
4.2. Environmental Relationship of Ichthyoplankton
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macedo-Soares, L.; Garcia, C.; Freire, A.; Muelbert, J. Large-scale ichthyoplankton and water mass distribution along the south Brazil shelf. PLoS ONE 2014, 9, e91241. [Google Scholar] [CrossRef] [PubMed]
- Duke, E.; Burton, R. Efficacy of metabarcoding for identification of fish eggs evaluated with mock communities. Ecol. Evol. 2020, 10, 3463–3476. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Possingham, H.; Muelbert, J. Incorporating early life stages of fishes into estuarine spatial conservation planning. Aquatic Conserv. Mar. Freshw. Ecosyst. 2015, 26, 1013–1030. [Google Scholar] [CrossRef]
- Nynatten, A.; Gallage, K.; Lujan, N.; Mandrak, N.; Lovejoy, N. Ichthyoplankton metabarcoding: An efficient tool for early detection of invasive species establishment. Mol. Ecol. Resour. 2023, 23, 1319–1333. [Google Scholar] [CrossRef]
- Zhang, H.; Xian, W.; Liu, S. Seasonal variations of the ichthyoplankton assemblage in the Yangtze estuary and its relationship with environmental factors. PeerJ 2019, 7, e6482. [Google Scholar] [CrossRef]
- Jiang, Y.; Lin, B.; He, H.; Ding, G.; Yan, L.; Zhang, G.; Liu, M.; Zheng, L. Species composition and assemblages of ichthyoplankton in Sansha Bay, Fujian Province, China. Front. Mar. Sci. 2021, 8, 758089. [Google Scholar] [CrossRef]
- Govoni, J.J. Fisheries oceanography and the ecology of early life histories of fishes: A perspective over fifty years. Sci. Mar. 2005, 69, 125–137. [Google Scholar] [CrossRef]
- Frantine-Silva, W.; Sofia, S.; Órsi, M.; Almeida, F. Dna barcoding of freshwater ichthyoplankton in the neotropics as a tool for ecological monitoring. Mol. Ecol. Resour. 2015, 15, 1226–1237. [Google Scholar] [CrossRef]
- Koslow, J.A.; Wright, M. Ichthyoplankton sampling design to monitor marine fish populations and communities. Mar. Policy 2016, 68, 55–64. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, C.; Chen, Z.; Liu, S.; Zhang, H.; Xian, W. Spring ichthyoplankton assemblage structure in the Yangtze Estuary under environmental factors. Front. Mar. Sci. 2021, 8, 806096. [Google Scholar] [CrossRef]
- Zhang, H.; Xian, W.; Liu, S. Autumn ichthyoplankton assemblage in the Yangtze Estuary shaped by environmental factors. PeerJ 2016, 4, e1922. [Google Scholar] [CrossRef] [PubMed]
- Uygun, O.; Hoşsucu, B. Temporal dynamics of the ichthyoplankton assemblages in the central Aegean Sea during one year and the effects of ecological factors. Reg. Stud. Mar. Sci. 2024, 72, 103439. [Google Scholar] [CrossRef]
- Öztürk, R.Ç. Genetic identification of ichthyoplankton in the Black Sea and their abundance and community assemblages. Turk. J. Fish. Aquat. Sci. 2023, 23, TRJFAS23514. [Google Scholar] [CrossRef]
- Bat, L.; Şahin, F.; Satılmış, H.H.; Üstün, F.; Özdemir, Z.B.; Kıdeys, A.E.; Shulman, G.E. The changed ecosystem of the Black Sea and its impact on anchovy fisheries. J. Fish. Sci. 2007, 4, 191–227. [Google Scholar] [CrossRef]
- Demirel, N.; Zengin, M.; Ulman, A. First large-scale eastern Mediterranean and Black Sea stock assessment reveals a dramatic decline. Front. Mar. Sci. 2020, 7, 103. [Google Scholar] [CrossRef]
- Turkstat. Turkish Statistical Institute, Fishery Statistics. Available online: http://www.turkstat.gov.tr/ (accessed on 28 June 2024).
- FAO. FishStat, License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/fishery/statistics-query/en/capture/capture_quantity (accessed on 4 July 2024).
- Erdem, Y.; Özdemir, S.; Özsandıkçı, U.; Büyükdeveci, F. Fishery infrastructures of Sinop Province. Turk. J. Marit. Mar. Sci. 2018, 4, 20–32. (In Turkish) [Google Scholar]
- Erdem, Y.; Özdemir, S.; Özsandıkçı, U.; Büyükdeveci, F. Technical features of nets used industrial fisheries in the western Black Sea (Sinop Province). Turk. J. Marit. Mar. Sci. 2019, 5, 74–87. (In Turkish) [Google Scholar]
- Korkmaz, A.Ş.; Çoskun, T. Socioeconomic indicators of industrial fishing vessels:A case study of Sinop Province. J. Aquacult. Eng. Fish. Res. 2016, 2, 208–216. (In Turkish) [Google Scholar] [CrossRef]
- Satılmış, H.H.; Gordina, A.D.; Bat, L.; Bircan, R.; Çulha, M.; Akbulut, M.; Kıdeyş, A.E. Seasonal distribution of fish eggs and larvae off Sinop (the Southern Black Sea) in 1999–2000. Acta Oecol. 2003, 24, 275–280. [Google Scholar] [CrossRef]
- Satılmış, H.H.; Bat, L.; Birinci Özdemir, Z.; Üstün, F.; Şahin, F.; Kıdeyş, A.E.; Erdem, Y. Composition of eggs and larvae fish and macrogelatinious zooplankton in Sinop Region (the Central Black Sea) during 2002. EU J. Fish. Aquat. Sci. 2006, 23, 135–140. (In Turkish) [Google Scholar]
- Satılmış, H.H.; Mavruk, S.; Bat, L.; Avşar, D. Seasonal changes of ichthyoplankton assemblages of Sinop coasts in southern of the Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2014, 14, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ersoy Karaçuha, M. Crustacea (Arthropoda) Fauna of Zostera Facies in Sinop Peninsula Coasts and Their Bioecological Features. Master’s Thesis, Ondokuz Mayıs University, Samsun, Türkiye, 2006. (In Turkish). [Google Scholar]
- Uygun, O. Composition of Fish Larvae in Sinop-Akliman Coast of the Black Sea. Master’s Thesis, Sinop University, Sinop, Türkiye, 2015. (In Turkish). [Google Scholar]
- Üstün, F. Seasonal cycle of zooplankton abundance and biomass in Hamsilos Bay, Sinop, Southern Black Sea. Turkey. J. Nat. Hist. 2019, 53, 365–389. [Google Scholar] [CrossRef]
- Santos, R.V.S.; Severi, W. Dynamics of early life-history stages of fish along an estuarine gradient. Fish. Oceanogr. 2019, 28, 402–418. [Google Scholar] [CrossRef]
- MAF. Ministry of Agriculture and Forestry, Sinop-Hamsilos Natural Park. Available online: https://bolge10.tarimorman.gov.tr/Menu/48/Sinop-Hamsilos-Tabiat-Parki (accessed on 3 March 2023). (In Turkish)
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; pp. 101–111. [Google Scholar] [CrossRef]
- D’Ancona, U. Clupeoidei, in Uova, Larve e Stadi Giovanili di Teleostei, Fauna Flora Golfo di Napoli; Pub. Stazione Zoologica Di Napoli: Monographia, Napoli, 1931–1956; p. 1064. [Google Scholar]
- Dekhnik, T.V. Ichthyoplankton of the Black Sea; Cernova Moria Haukova: Kyiv, Ukraine, 1973; p. 234. (In Russian) [Google Scholar]
- Russell, F.S. The Eggs and Planktonic Stages of British Marine Fishes; Academic Press: London, UK, 1976; p. 524. [Google Scholar]
- Mater, S.; Çoker, T. Türkiye denizleri ihtiyoplankton atlası; Ege University Press: İzmir, Türkiye, 2004; p. 210. (In Turkish) [Google Scholar]
- Rodríguez, J.M.; Alemany, F.; Garcia, A. A Guide to the Eggs and Larvae of 100 Common Western Mediterranean Sea Bony Fish Species; FAO: Rome, Italy, 2017; p. 256. [Google Scholar]
- WoRMS Editorial Board, 2024, World Register of Marine Species. Available online: https://www.marinespecies.org/about.php#cite_worms (accessed on 4 May 2024). [CrossRef]
- Smith, P.E.; Richardson, S. Standard techniques for pelagic. FAO Fish. Tech. Pap. 1977, 175, 27–73. [Google Scholar]
- Bellan-Santini, D. Influence des pollutions sur le peuplement des amphipodes dans la biocénose des algues photophiles. Tethys 1981, 10, 185–194. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Legendre, P. Species associations: The Kendall coefficient of concordance revisited. J. Agric. Biol. Environ. Stat. 2005, 10, 226–245. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Wilhm, J.L. Use of biomass units in Shannon’s formula. Ecology 1968, 49, 153–156. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Schlitzer, R. Ocean Data View. 2024. Available online: https://odv.awi.de (accessed on 10 April 2024).
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Int.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 15 May 2024).
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Şahin, A.; Düzgüneş, E. Spatial and temporal variation in the distribution and abundance of pelagic fish eggs and larvae off Giresun, south-eastern Black Sea, Turkey. Acta Ichthyol. Piscat. 2019, 49, 159–169. [Google Scholar] [CrossRef]
- Almatar, S.; Lone, K.; Abu-Rezq, T.; Yousef, A. Spawning frequency, fecundity, egg weight and spawning type of silver pomfret, Pampus argenteus (Euphrasen) (Stromateidae), in Kuwait waters. J. Appl. Ichthyol. 2004, 20, 176–188. [Google Scholar] [CrossRef]
- Muhling, B.; Beckley, L. Seasonal variation in horizontal and vertical structure of larval fish assemblages off south-western Australia, with implications for larval transport. J. Plankt. Res. 2007, 29, 967–983. [Google Scholar] [CrossRef]
- Joyeux, J.; Pereira, B.; Almeida, H. The flood-tide ichthyoplanktonic community at the entrance into a Brazilian tropical estuary. J. Plankt. Res. 2004, 26, 1277–1287. [Google Scholar] [CrossRef]
- Klimova, T.; Podrezova, P. Seasonal distribution of the Black Sea ichthyoplankton near the Crimean Peninsula. Reg. Stud. Mar. Sci. 2018, 24, 260–269. [Google Scholar] [CrossRef]
- Gaudy, R.; Champalbert, G. Space and time variations in zooplankton distribution south of Marseilles. Oceanol. Acta 1998, 21, 793–802. [Google Scholar] [CrossRef]
- de Puelles, M.F.; Grás, D.; Hernández-León, S. Annual cycle of zooplankton biomass, abundance and species composition in the neritic area of the Balearic Sea, Western Mediterranean. Mar. Ecol. 2003, 24, 123–139. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Antonopoulou, E.; Stergiou, K.I. Spawning period of Mediterranean marine fishes. Rev. Fish Biol. Fisher. 2010, 20, 499–538. [Google Scholar] [CrossRef]
- Shan, X.J.; Xian, W.W.; Wu, Y.F. Progress of studies on ichthyoplankton ecology of Changjiang River Estuary. Trans. Oceanol. Limnol. 2004, 4, 87–93. [Google Scholar]
- Zhang, H.; Wang, Y.; Liang, C.; Liu, S.; Xian, W. Estuarine ichthyoplankton studies—A review. Front. Mar. Sci. 2022, 9, 794433. [Google Scholar] [CrossRef]
- Rice, J. Food web theory, marine food webs and what climate changes may do to northern marine fish populations. In Climate Change and Northern Fish Populations; Beamish, R.J., Ed.; Canadian Special Publication Fish Aquatic Science: Sidney, Australia, 1995; Volume 121, pp. 561–568. [Google Scholar]
- Bakun, A. Patterns in the Ocean: Ocean Processes and Marine Population Dynamics; California Sea Grant College System: La Jolla, CA, USA, 1996; p. 323. [Google Scholar]
- Cury, P.; Bakun, A.; Crawford, R.J.; Jarre, A.; Quinones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Koutrakis, E.T.; Kallianiotis, A.A.; Tsikliras, A.C. Temporal patterns of larval fish distribution and abundance in a coastal area of northern Greece. Sci. Mar. 2004, 68, 585–595. [Google Scholar] [CrossRef]
- Somarakis, S.; Palomera, I.; Garcia, A.; Quintanilla, L.; Koutsikopoulos, C.; Uriarte, A.; Motos, L. Daily egg production of anchovy in European waters. ICES J. Mar. Sci. 2004, 61, 944–958. [Google Scholar] [CrossRef]
- Sabatés, A.; Olivar, M.P.; Salat, J.; Palomera, I.; Alemany, F. Physical and biological processes controlling the distribution of fish larvae in the NW Mediterranean. Prog. Oceanogr. 2007, 74, 355–376. [Google Scholar] [CrossRef]
- Demir, N. Notes on the variations of the eggs of anchovy (Engraulis encrasicolus Cuv.) from Black, Marmara, Aegean and Mediterranean Seas. Istanb. Univ. Hidrobiyoloji Mecmuası 1959, 4, 180–187. [Google Scholar]
- Gordina, A.D.; Nikolskiy, V.N.; Niermann, U.; Bingel, F.; Subbotin, A.A. New data on the morphological differences of anchovy eggs (Engraulis encrasicolus L.) in the Black Sea. Fish. Res. 1997, 31, 139145. [Google Scholar] [CrossRef]
- Niermann, U.; Bingel, F.; Gorban, A.; Gordina, A.D.; Gücü, A.C.; Kideys, A.E.; Konsulov, A.; Radu, G.; Subbotin, A.A.; Zaika, V.E. Distribution of anchovy eggs and larvae (Engraulis encrasicolus Cuv.) in the Black Sea in 1991–1992. ICES J. Mar. Sci. 1994, 51, 395–406. [Google Scholar] [CrossRef]
- Kideys, A.E.; Gordina, A.D.; Bingel, F.; Niermann, U. The effect of environmental conditions on the distribution of eggs and larvae of anchovy (Engraulis encrasicolus L.) in the Black Sea. ICES J. Mar. Sci. 1999, 56, 58–64. [Google Scholar] [CrossRef]
- Somarakis, S.; Koutsikopoulos, C.; Machias, A.; Tsimenides, N. Applying the daily egg production method (DEPM) to small stocks in highly heterogeneous seas. Fish. Res. 2002, 55, 193–204. [Google Scholar] [CrossRef]
- Hjort, J. Fluctuations in the great fisheries of northern Europe. Rapp. Proces Verbaux La Comm. Int. Pour L’exploration Sci. La Mer 1914, 20, 1–228. [Google Scholar]
- Houde, E.D. Recruitment variability. In Fish Reproductive Biology. Implications for Assessment and Management, 1st ed.; Jakobsen, T., Fogarty, M.J., Megrey, B.A., Moksness, E., Eds.; Wiley Blackwell UK: West Sussex, UK, 2009; pp. 91–171. [Google Scholar]
- Mavruk, S.; Avşar, D. Distribution dynamics of ichthyoplankton and recruitment hypotheses. EU J. Fish. Aquat. Sci. 2017, 34, 355–361. [Google Scholar] [CrossRef]
- Somarakis, S.; Schismenou, E.; Siapatis, A.; Giannoulaki, M.; Kallianiotis, A.; Machias, A. High variability in the daily egg production method parameters of an eastern Mediterranean anchovy stock: Influence of environmental factors, fish condition and population density. Fish. Res. 2012, 117, 12–21. [Google Scholar] [CrossRef]
- Zarrad, R.; Alemany, F.; Rodriguez, J.M.; Jarboui, O.; Lopez-Jurado, J.L.; Balbin, R. Influence of summer conditions on the larval fish assemblage in the eastern coast of Tunisia (Ionian Sea, Southern Mediterranean). J. Sea Res. 2013, 76, 114–125. [Google Scholar] [CrossRef]
- Patti, B.; Zarrad, R.; Jarboui, O.; Cuttitta, A.; Basilone, G.; Aronica, S.; Placenti, F.; Tranchida, G.; Armeri, G.M.; Buffa, G.; et al. Anchovy (Engraulis encrasicolus) early life stages in the Central Mediterranean Sea: Connectivity issues emerging among adjacent sub-areas across the Strait of Sicily. Hydrobiologia 2018, 821, 25–40. [Google Scholar] [CrossRef]
- Mavruk, S.; Bengil, F.; Yüksek, A.; Özyurt, C.E.; Kiyağa, V.B.; Avşar, D. Intra-annual patterns of coastal larval fish assemblages along environmental gradients in the northeastern Mediterranean. Fish. Oceanogr. 2018, 27, 232–245. [Google Scholar] [CrossRef]
- Şahin, C.; Hacımurtazaoğlu, N. Abundance and distribution of eggs and larvae of anchovy (Engraulis encrasicolus, Linnaeus, 1758) and horse mackerel (Trachurus mediterraneus, Steindachner, 1868) on the coasts of the eastern Black Sea. Turk. J. Zool. 2013, 37, 773–781. [Google Scholar] [CrossRef]
- Palomera, I.; Sabatés, A. Co-occurrence of Engraulis encrasicolus and Sardinella aurita eggs and larvae in the western Mediterranean. Sci. Mar. 1990, 54, 61–67. [Google Scholar]
- Palomera, I. Spawning of anchovy Engraulis encrasicolus in the Northwestern Mediterranean relative to hydrographic features in the region. Mar. Ecol. Prog. Ser. 1992, 79, 215–223. [Google Scholar] [CrossRef]
- Olivar, M.P.; Salat, J.; Palomera, I. Comparative study of spatial distribution patterns of the early stages of anchovy and pilchard in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2001, 217, 111–120. [Google Scholar] [CrossRef]
- Whitfield, A.K. Ichthyofaunal assemblages in estuaries: A South African case study. Rev. Fish. Biol. Fish. 1999, 9, 151–186. [Google Scholar] [CrossRef]
- Fuiman, L.A. Special consideration of fish eggs and larvae. In Fishery Science: The Unique Contributions of Early Life Stages; Fuiman, L.A., Werner, R.G., Eds.; Blackwell Science: Hoboken, NJ, USA, 2003; pp. 1–32. [Google Scholar]
- Feng, Y.; Yao, L.; Zhao, H.; Yu, J.; Zhao, L. Environmental effects on the spatiotemporal variability of fish larvae in the western Guangdong waters, china. J. Mar. Sci. Eng. 2021, 9, 316. [Google Scholar] [CrossRef]
- Sabatés, A.N.A.; Martín, P.; Lloret, J.; Raya, V. Sea warming and fish distribution: The case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob. Chang. Biol. 2006, 12, 2209–2219. [Google Scholar] [CrossRef]
- Li, F.; Zhu, M.; Chen, W.; Su, B.; Yang, Y.; Wang, B. Interannual variation of ichthyoplankton community structure in the Yellow River estuary, China. Water 2023, 15, 1040. [Google Scholar] [CrossRef]
- Demirel, N. Ichthyoplankton dynamics in a highly urbanized estuary. Mar. Biol. Res. 2015, 11, 677–688. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Bruno, J.F.; Gaines, S.D.; Halpern, B.S.; Lester, S.E.; Kinlan, B.P.; Weiss, J.M. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Shen, X.Q.; Li, L.; Quan, W.M. Annual variability of ichthyoplankton in the Yangtze River estuary of China from August 2002 to 2009. Oceanol. Hydrobiol. Stud. 2013, 42, 59–69. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Ferreira, G.V.B.; Barletta, M. Estuarine ecocline function and essential habitats for fish larvae in tropical south western Atlantic Estuaries. Mar. Environ. Res. 2019, 151, 104786. [Google Scholar] [CrossRef]
- Jiang, M.; Shen, X.Q.; Chen, L.F. Relationship between with abundance distribution of fish eggs, larvae and environmental factors in the Changjiang Estuary and vicinity waters in spring. Mar. Environ. Sci. 2006, 25, 37–39. [Google Scholar] [CrossRef]
- Uygun, O.; Hoşsucu, B. The abundance and distribution of the early life stages of Sardine (Sardina pilchardus) in central of the Aegean Sea (Sığacık Bay) and their interactions with certain environmental factors. Thalassas 2020, 36, 507–515. [Google Scholar] [CrossRef]
- Lamia, D.; Soukri, A.; Abdelouahab, H.; Mohamed, M.; Baibai, T. The Moroccan ichtyoplankton—A general overview. Annu. Res. Rev. Biol. 2020, 35, 89–94. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Rogers, L.A.; Brodeur, R.D.; Thompson, A.R.; Auth, T.D.; Deary, A.L.; Duffy-Anderson, J.T.; Galbraith, M.; Koslow, J.A.; Perry, R.I. Responses of ichthyoplankton assemblages to the recent marine heatwave and previous climate fluctuations in several northeast pacific marine ecosystems. Glob. Chang. Biol. 2020, 27, 506–520. [Google Scholar] [CrossRef]


| Family | Species | Code | Mean Ab. (Ind.10 m−2) | D% | FO % | E/L/B | Months (Peak) |
|---|---|---|---|---|---|---|---|
| Blenniidae | Parablennius tentacularis | Pten | 8 | 0.2 | 16.7 | L | Jul |
| Salaria pavo | Spav | 8 | 0.2 | 16.7 | L | Jun | |
| Callionymidae | Callionymus spp. | Calsp | 25 | 0.5 | 33.3 | B | Jul |
| Carangidae | Trachurus mediterraneus | Tmed | 119 | 2.4 | 83.3 | B | Jul–Sep (Aug) |
| Engraulidae | Engraulis encrasicolus | Eenc | 3601 | 72.6 | 100.0 | B | May–Sep (Aug) |
| Clupeidae | Sprattus sprattus | Sspr | 382 | 7.7 | 100.0 | B | Nov–Apr (Jan) |
| Sparidae | Diplodus annularis | Dann | 85 | 1.7 | 83.3 | B | Jun–Aug (Jul) |
| Spicara spp. | Ssma | 8 | 0.2 | 16.7 | L | Jul | |
| Labridae | Symphodus tinca | Stin | 8 | 0.2 | 16.7 | L | Jul |
| Symphodus ocellatus | Symsp | 8 | 0.2 | 16.7 | L | Jun | |
| Lotidae | Gaidropsarus mediterraneus | Gmed | 178 | 3.6 | 100.0 | E | Oct–Feb (Nov) |
| Gadidae | Merlangius merlangus | Mmer | 25 | 0.5 | 50.0 | E | Sep |
| Gobiesocidae | Gobiesocidae spp. | Lepsp | 25 | 0.5 | 50.0 | L | Jul, Sep (Sep) |
| Gobiidae | Gobius cobitis | Gcob | 8 | 0.2 | 16.7 | L | Jun |
| Gobius niger | Gnig | 195 | 3.9 | 100.0 | L | May–Oct (Sep) | |
| Gobius spp. | Gobsp | 17 | 0.3 | 33.3 | L | Jun, Sep | |
| Pomatoschistus minutus | Pmin | 8 | 0.2 | 16.7 | L | May | |
| Mugilidae | Mugil cephalus | Mcep | 8 | 0.2 | 16.7 | L | Aug |
| Chelon saliens | Csal | 8 | 0.2 | 16.7 | E | Sep | |
| Mullidae | Mullus barbatus | Mbar | 51 | 1.0 | 50.0 | B | Jul–Sep (Aug) |
| Ophidiidae | Ophidion rochei | Oroc | 25 | 0.5 | 33.3 | B | Sep |
| Ammodytidae | Gymnammodytes cicerelus | Gcic | 8 | 0.2 | 16.7 | L | Nov |
| Scorpaenidae | Scorpaena porcus | Spor | 17 | 0.3 | 33.3 | E | Jul–Aug |
| Serranidae | Serranus scriba | Sscr | 17 | 0.3 | 16.7 | E | Aug |
| Trachinidae | Trachinus draco | Tdra | 8 | 0.2 | 16.7 | E | Sep |
| Bothidae | Arnoglossus laterna | Alat | 51 | 1.0 | 16.7 | B | Jul |
| Soleidae | Pegusa lascaris | Plas | 8 | 0.2 | 16.7 | L | Sep |
| Sciaenidae | Sciaena umbra | Sumb | 42 | 0.9 | 50.0 | E | Jul–Sep |
| Environmental Factors | Adj. R2 | SS(trace) | Pseudo-F | p | % | Cumulative |
|---|---|---|---|---|---|---|
| Temperature | 0.379 | 28,025 | 13.828 | 0.001 ** | 0.409 | 0.409 |
| Salinity | 0.455 | 6739.6 | 3.789 | 0.016 * | 0.098 | 0.507 |
| Dissolved oxygen | 0.483 | 3463.8 | 2.055 | 0.126 | 0.051 | 0.558 |
| Chlorophyll-a | 0.513 | 3288.1 | 2.067 | 0.119 | 0.048 | 0.606 |
| Zooplankton biomass | 0.532 | 2625.1 | 1.720 | 0.191 | 0.038 | 0.644 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uygun, O.; Üstün, F. Ichthyoplankton Assemblages from the Coasts of Hamsilos Nature Park, Sinop, Southern Black Sea: Biodiversity, Abundance, and Relationships with Environmental Variables. Water 2024, 16, 2670. https://doi.org/10.3390/w16182670
Uygun O, Üstün F. Ichthyoplankton Assemblages from the Coasts of Hamsilos Nature Park, Sinop, Southern Black Sea: Biodiversity, Abundance, and Relationships with Environmental Variables. Water. 2024; 16(18):2670. https://doi.org/10.3390/w16182670
Chicago/Turabian StyleUygun, Orçin, and Funda Üstün. 2024. "Ichthyoplankton Assemblages from the Coasts of Hamsilos Nature Park, Sinop, Southern Black Sea: Biodiversity, Abundance, and Relationships with Environmental Variables" Water 16, no. 18: 2670. https://doi.org/10.3390/w16182670
APA StyleUygun, O., & Üstün, F. (2024). Ichthyoplankton Assemblages from the Coasts of Hamsilos Nature Park, Sinop, Southern Black Sea: Biodiversity, Abundance, and Relationships with Environmental Variables. Water, 16(18), 2670. https://doi.org/10.3390/w16182670








