Osmoregulatory Capacity and Non-Specific Food Preferences as Strengths Contributing to the Invasive Success of the Signal Crayfish Pacifastacus leniusculus: Management Implications
Abstract
1. Introduction
- (1)
- To determine the osmoregulatory pattern and osmoregulatory capacities of the signal crayfish in a range of salinities from 0 to 20 PSU.
- (2)
- To determine the food preferences of signal crayfish using two food items:
- −
- aquatic macrophyte, the Canadian pondweed Elodea canadensis (Michaux, 1803);
- −
- fish, the rainbow trout Oncorhynchus mykiss (Walbaum, 1792).
2. Materials and Methods
2.1. Collecting of the Material
- −
- temperature ranged between 11.6 and 15.6 °C;
- −
- salinity was equal to 0.1 PSU;
- −
- oxygenation reached 80.7%.
2.2. Acclimatization to the Laboratory Conditions
2.3. Laboratory Experiments on Osmoregulation
2.4. Laboratory Experiments on Food Preferences
2.5. Statistical Analysis
3. Results
3.1. Osmoregulation
3.1.1. Behaviour
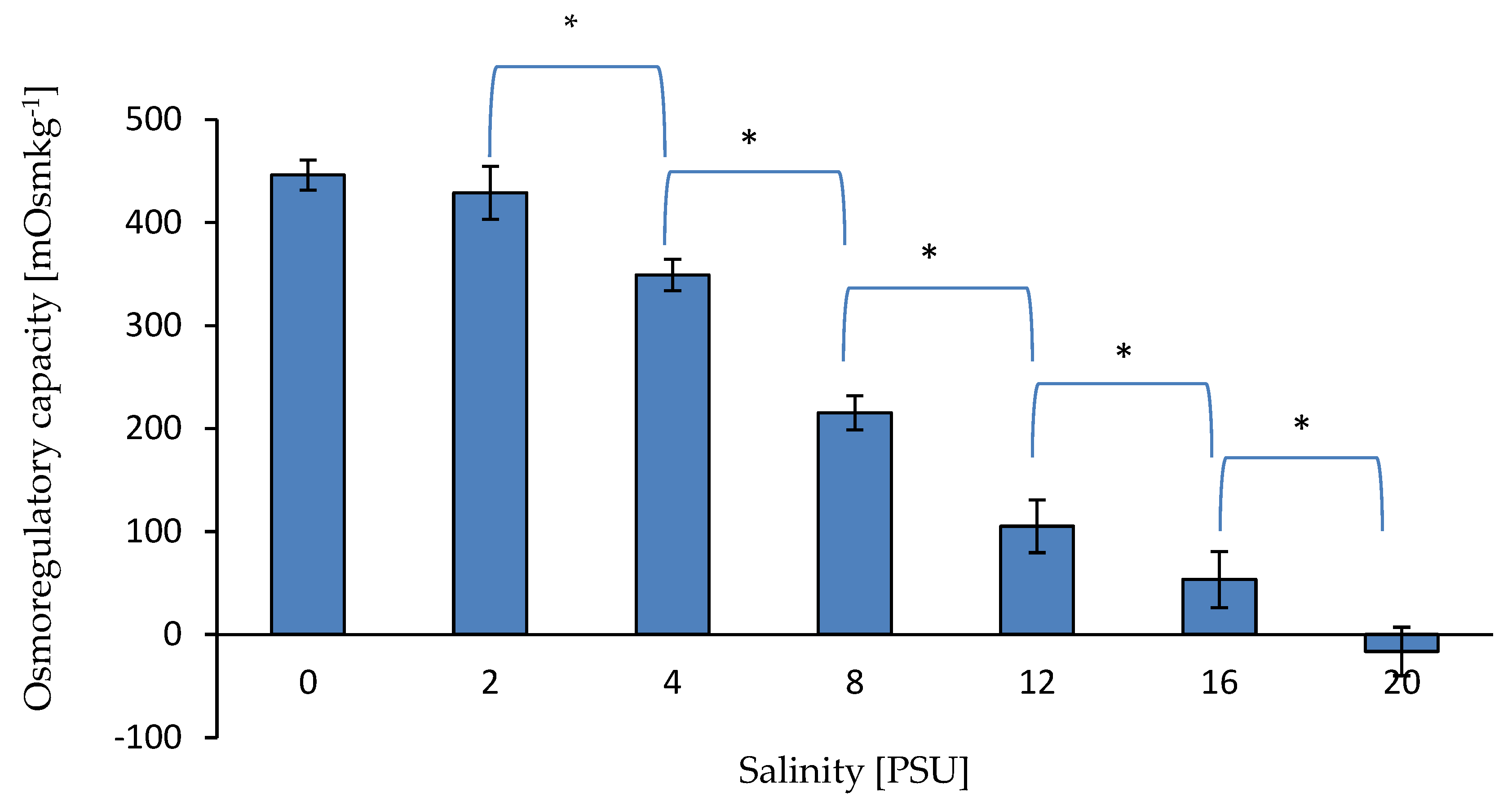
3.1.2. Osmotic Concentrations and Osmoregulatory Capacity
3.2. Food Preferences of the Signal Crayfish
4. Discussion
4.1. Osmoregulatory Capacity and Food Preferences
4.2. Implication to Management of the Signal Crayfish
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Early, R.; Bradley, B.; Dukes, J.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Invasive Alien Species: A Growing Problem for Environment and Health 2020. Available online: https://www.eea.europa.eu/highlights/invasive-alien-species-a-growing (accessed on 5 February 2024).
- Hayes, K.R.; Barry, S.C. Are there any consistent predictors of invasion success? Biol. Invasions 2008, 10, 483–506. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Graca, B. Laboratory study of the effect of salinity and ionic composition of water on the mortality and osmoregulation of the gammarid amphipod Dikerogammarus haemobaphes (Eichwald, 1841): Implications for understanding its invasive distribution pattern. Mar. Freshw. Behav. Physiol. 2014, 47, 227–238. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Lynn Kemp, J.; Fidalgo, M.L. Cold-tolerant traits that favour northwards movement and establishment of Mediterranean and Ponto-Caspian aquatic invertebrates. Aquat. Sci. 2022, 84, 47. [Google Scholar] [CrossRef]
- Piria, M.; Copp, G.H.; Dick, J.T.A.; Duplić, A.; Groom, Q.; Jelić, D.; Lucy, F.E.; Roy, H.E.; Sarat, E.; Simonović, P.; et al. Tackling invasive alien species in Europe II: Threats and opportunities until 2020. Manag. Biol. Invasions 2017, 8, 273–286. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Museum National d’Histoire Naturelle: Paris, France, 2006; 187p. [Google Scholar]
- CABI. CABI Compendium. 2024. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.70581 (accessed on 3 February 2024).
- McGriff, D. Growth, maturity and fecundity of the crayfish Pacifastacus leniusculus, from the Sacramento-San Joaquin Delta. Calif. Fish. Game 1983, 68, 227–242. [Google Scholar]
- Holdich, D.M. A review of astaculture—Freshwater crayfish farming. Aquat. Living Resour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- Ackefors, H.E.G. Freshwater crayfish farming technology in the 1990s: A European and global perspective. Fish Fish. 2000, 1, 337–359. [Google Scholar] [CrossRef]
- Krzywosz, T.; Chybowski, Ł.; Ulikowski, Ł. Rak sygnałowy w Polsce—Historia, stan obecny, perspektywy signal crayfish in Poland—History, actual state and prospects. Komun. Ryb. 1995, 24, 5–8. (In Polish) [Google Scholar]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 394, 39511. [Google Scholar] [CrossRef]
- Crawford, L.; Yeomans, W.E.; Adams, C.E. The impact of introduced signal crayfish Pacifastacus leniusculus on stream invertebrate communities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2006, 16, 611–621. [Google Scholar] [CrossRef]
- Galib, S.M.; Findlay, J.S.; Lucas, M.C. Strong impacts of signal crayfish invasion on upland stream fish and invertebrate communities. Freshw. Biol. 2021, 66, 223–240. [Google Scholar] [CrossRef]
- Peay, S.; Guthrie, N.; Spees, J.; Nilsson, E.; Bradley, P. The impact of signal crayfish (Pacifastacus leniusculus) on the recruitment of salmonid fish in headwater stream in Yorkshire, England. Knowl. Manag. Aquat. Ecosyst. 2009, 12, 394–395. [Google Scholar] [CrossRef]
- Griffiths, S.W.; Collen, P.; Armstrong, J.D. Competition for shelter among over-wintering signal crayfish and juvenile Atlantic salmon. J. Fish. Biol. 2004, 65, 436–447. [Google Scholar] [CrossRef]
- Kossakowski, J.; Mnich, M.; Kossakowski, G. The first introduction of the crayfish Pacifastacus leniusculus Dana into Polish waters. Freshw. Crayfish 1978, 4, 195. [Google Scholar]
- Wu, N.C.; Seebacher, F. Physiology can predict animal activity, exploration, and dispersal. Commun. Biol. 2022, 5, 109. [Google Scholar] [CrossRef]
- Pettersen, A.K.; Marshall, D.J.; White, C.R. Understanding variation in metabolic rate. J. Exp. Biol. 2018, 221, jeb166876. [Google Scholar] [CrossRef]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef]
- Koussoroplis, A.-M.; Pincebourde, S.; Wacker, A. Understanding and predicting physiological performance of organisms in fluctuating and multifactorial environments. Ecol. Monogr. 2017, 87, 178–197. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Graca, B. Effect of salinity on the distribution of Ponto–Caspian gammarids in a non-native area–environmental and experimental study. Mar. Biol. Res. 2018, 14, 183–190. [Google Scholar] [CrossRef]
- Occhi, T.V.T.; Vitule, J.R.S.; Metri, C.B.; Prodocimo, V. Use of osmoregulatory ability to predict invasiveness of the Indo-Pacific swimming crab Charybdis hellerii (A.Milne-Edwards, 1867) an invader in Southern Brazil. Nauplius 2019, 27, e2019014. [Google Scholar] [CrossRef]
- Nagelkerke, L.A.J.; van Onselen, E.; van Kessel, N.; Leuven, R.S.W. Functional feeding traits as predictors of invasive success of alien freshwater fish species using a food-fish mode. PLoS ONE 2018, 13, e0197636. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka-Krahel, A.; Skóra, M.E.; Raczyński, M.; Szaniawska, A. The signal crayfish Pacifastacus leniusculus—Distribution and invasion in the southern Baltic coastal river. Pol. J. Ecol. 2017, 65, 445–452. [Google Scholar] [CrossRef]
- Obernier, J.A.; Baldwin, R.L. Establishing an Appropriate Period of Acclimatization Following Transportation of Laboratory Animals. ILAR J. 2006, 47, 364–369. [Google Scholar] [CrossRef]
- Niu, J.; Hu, X.L.; Ip, J.C.H.; Ma, K.Y.; Tang, Y.; Wang, Y.; Qin, J.; Qiu, J.W.; Chan, T.F.; Chu, K.H. Multi-omic approach provides insights into osmoregulation and osmoconformation of the crab Scylla paramamosain. Sci. Rep. 2020, 10, 21771. [Google Scholar] [CrossRef]
- Havird, J.C.; Santos, S.R.; Henrt, R.P. Osmoregulation in the Hawaiian anchialine shrimp Halocaridina rubra (Crustacea: Atyidae): Expression of ion transporters, mitochondria-rich cell proliferation and hemolymph osmolality during salinity transfers. J. Exp. Biol. 2014, 217, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Holdich, D.M.; Harlioğliu, M.M.; Firkins, I. Salinity adaptations of crayfish in British waters with particular reference to Austropotamobius pallipes, Astacus leptodactylus and Pacifastacus leniusculus. Estuar. Coast. Shelf Sci. 1997, 44, 147–154. [Google Scholar] [CrossRef]
- Harlioğlu, M.M. Comparative Biology of the Signal Crayfish, Pacifastacus leniusculus (Dana) and the Narrow-Clawed Crayfish, Astacus leptodactylus (Eschscholtz). Ph.D. Thesis, University of Nottingham, Nottingham, UK, 1996. [Google Scholar]
- Staszak, K.; Szaniawska, A. Feeding Rates and Food Preferences of the Spiny-Cheek Crayfish Orconectes limosus at Two Different Temperatures. Freshw. Crayfish 2006, 15, 148–154. [Google Scholar]
- Klekowski, R.Z.; Fischer, Z. Bioenergetyka Ekologiczna Zwierząt Zmiennocieplnych; PAN Wydział II Nauk Biologicznych: Warszawa, Poland, 1993. [Google Scholar]
- Ramsay, J.A. A new method of freezing-point determination for small quantities. J. Exp. Biol. 1949, 26, 57–64. [Google Scholar] [CrossRef]
- Leppäkoski, E.; Olenin, S. Non-native species and rates of spread: Lessons from the brackish Baltic Sea. Biol. Invasions 2000, 2, 151–163. [Google Scholar] [CrossRef]
- Leppäkoski, E.; Gollasch, S.; Gruszka, P.; Ojaveer, H.; Olenin, S.; Panov, V. The Baltic—A sea of invaders. Can. J. Fish. Aquat. Sci. 2002, 59, 1175–1188. [Google Scholar] [CrossRef]
- Anonim. Signal crayfish in a fisherman’s net in the Paimio Bay. Turun Sanomat, 20 October 1999. (In Finnish) [Google Scholar]
- Riegel, J.A. The systematics and distribution of crayfish in California. Calif. Fish. Game 1959, 45, 29–50. [Google Scholar]
- Casellato, S.; Masiero, L. Does Procambarus clarkii (Girard, 1852) represent a threat for estuarine brackish ecosystems of Northeastern Adriatic Coast (Italy)? J. Life Sci. 2011, 5, 549–554. [Google Scholar]
- Scalici, M.; Chiesa, S.; Scuderi, S.; Celauro, D.; Gibertini, G. Population structure and dynamics of Procambarus clarkii (Girard, 1852) in a Mediterranean brackish wetland (Central Italy). Biol. Invasions 2010, 12, 1415–1425. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Scalici, M.; Caldaroni, B.; Magara, G.; Scoparo, M.; Goretti, E.; Elia, A.C. Salinity tolerance of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852). Hydrobiologia 2020, 847, 2065–2081. [Google Scholar] [CrossRef]
- Nota, A.; Santovito, A.; Gattelli, R.; Tiralongo, F. From Fresh to Salt Waters: First Reports of the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) in Mediterranean Marine Waters. Hydrobiology 2024, 3, 1–10. [Google Scholar] [CrossRef]
- Szaniawska, A.; Dobrzycka-Krahel, A.; Jaszczołt, J. Spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817) on its way to the open coastal waters of the Baltic Sea. Oceanol. Hydrobiol. Stud. 2017, 46, 451–463. [Google Scholar] [CrossRef]
- Jaszczołt, J.; Szaniawska, A. The spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817) as an inhabitant of the Baltic Sea—Experimental evidences for its invasion of brackish waters. Ocean. Hydrobiol. Stud. 2011, 40, 52–60. [Google Scholar] [CrossRef]
- Śmietana, P.; Bonk, M.; Solarz, W. Karta Informacyjna Gatunku—Rak Marmurkowy. Generalna Dyrekcja Ochrony Środowiska. 2018. Available online: https://www.gov.pl/web/gdos/procambarus-fallax-f-virginalis---rak-marmurkowy (accessed on 1 June 2024). (In Polish)
- Veselý, L.; Hrbek, V.; Kozák, P.; Buřič, M.; Sousa, R.; Kouba, A. Salinity tolerance of marbled crayfish Procambarus fallax f. virginalis. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 21. [Google Scholar] [CrossRef]
- Sarver, R.G.; Flynn, M.A.; Holliday, C.W. Renal Na, K-ATPase and osmoregulation in the crayfish, Procambarus clarkia. Comp. Biochem. Physiol. 1994, 107, 349–356. [Google Scholar] [CrossRef]
- Śmietana, P. Orconectes limosus (Rafinesque, 1817). In Obce Gatunki w Faunie Polski; Alien Species in the Fauna of Poland; Okarma, H., Pawłowski, J., Glowaciński, Z., Solarz, W., Eds.; Instytut Ochrony PrzyrodyPAN: Kraków, Poland, 2011; pp. 201–205. [Google Scholar]
- Veselý, L.; Buřič, M.; Kouba, A. Hardy exotics species in temperate zone: Can “warm water” crayfish invaders establish regardless of low temperatures? Sci. Rep. 2015, 5, 16340. [Google Scholar] [CrossRef] [PubMed]
- Kaldre, K.; Meženin, A.; Paaver, T.; Kawai, T. A preliminary study on the tolerance of marble crayfish Procambarus fallax f. virginalis to low temperature in Nordic climate: 54–62. W. In Freshwater Crayfish: A Global Overview; Kawai, T., Faulkes, Z., Scholtz, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Normant, M.; Lamprecht, I. Does scope for growth change as a result of salinity stress in the amphipod Gammarus oceanicus? J. Exp. Mar. Biol. Ecol. 2006, 334, 158–163. [Google Scholar] [CrossRef]
- Normant, M.; Gibowicz, M. Salinity induced changes in haemolymph osmolality and total metabolic rate of the mud crab Rhithropanopeus harrisii Gould, 1841 from the Baltic coastal waters. J. Exp. Mar. Biol. Ecol. 2008, 355, 145–152, 349. [Google Scholar] [CrossRef]
- Strużyński, W. Raki; Wydawnictwo Klubu Przyrodników: Swiebodzin, Poland, 2007; 123p. (In Polish) [Google Scholar]
- Stenroth, P.; Holmqvist, N.; Nyström, P.; Berglund, O.; Larsson, P.; Granéli, W. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): The influence of tissue, sample treatment, and season. Can. J. Fish. Aquat. Sci. 2006, 63, 821–831. [Google Scholar] [CrossRef]
- Bondar, C.A.; Bottriell, K.; Zeron, K.; Richardson, J.S. Does trophic position of the omnivorous signal crayfish (Pacifastacus leniusculus) in a stream food web vary with life history stage or density? Can. J. Fish. Aquat. Sci. 2005, 62, 2632–2639. [Google Scholar] [CrossRef]
- Popelka, P.; Marcinčák, S.; Maskal’ová, I.; Guothová, L.; Čertík, M. Comparison of the chemical composition and nutritional values of fresh and frozen rainbow trout. Slov. Vet. Res. 2014, 51, 73–80. [Google Scholar]
- Gorham, E.; Sanger, J.E. Caloric values of organic matter in woodland, swamp, and lake soils energy flow. Ecology 1967, 48, 492–494. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Diéguez-Uribeondo, J.; Tricarico, E. Managing Invasive Crayfish: Is There a Hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Stebbing, P.; Longshaw, M.; Taylor, N.; Norman, R.; Lintott, R.; Pearce, F.; Scott, A. Review of Methods for the Control of Invasive Crayfish in Great Britain; CEFAS Contract-Final Report C5471; Fisheries Aquaculture Science: Suffolk, UK, 2012; Volume 1, pp. 1–106. [Google Scholar]
- Stebbing, P.; Longshaw, M.; Scott, A. Review of methods for the management of non-indigenous crayfish, with particular reference to Great Britain. Ethol. Ecol. Evol. 2014, 26, 204–231. [Google Scholar] [CrossRef]
- Manfrin, C.; Souty-Grosset, C.; Anastácio, P.M.; Reynolds, J.; Giulianini, P.G. Detection and Control of Invasive Freshwater Crayfish: From Traditional to Innovative Methods. Diversity 2019, 11, 5. [Google Scholar] [CrossRef]
- Green, N.; Bentley, M.; Stebbing, P.; Andreou, D.; Britton, R. Trapping for invasive crayfish: Comparisons of efficacy and selectivity of baited traps versus novel artificial refuge traps. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 15. [Google Scholar] [CrossRef]
- Curti, J.N.; Fergus, C.E.; De Palma-Dow, A.A. State of the ART: Using artificial refuge traps to control invasive crayfish in southern California streams. Freshw. Sci. 2021, 40, 494–507. [Google Scholar] [CrossRef]
- Peay, S.; Dunn, A.M.; Kunin, W.E.; McKimm, R.; Harrod, C. A method test of the use of electric shock treatment to control invasive signal crayfish in streams. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 874–880. [Google Scholar] [CrossRef]
- Frings, R.M.; Vaeßen, S.C.K.; Groß, H.; Roger, S.; Schüttrumpf, H.; Hollert, H. A fish-passable barrier to stop the invasion of non-indigenous crayfish. Biol. Conserv. 2013, 159, 521–529. [Google Scholar] [CrossRef]
- Krieg, R.; Zenker, A. A Review of the Use of Physical Barriers to Stop the Spread of Non-Indigenous Crayfish Species. Rev. Fish. Biol. Fish. 2020, 30, 423–435. [Google Scholar] [CrossRef]
- Krieg, R.; King, A.; Zenker, A. Measures to Control Invasive Crayfish Species in Switzerland: A Success Story? Front. Environ. Sci. 2020, 8, 252. [Google Scholar] [CrossRef]
- Peay, S.; Dunn, A.M. The behavioural response of the invasive signal crayfish Pacifastacus leniusculus to experimental dewatering of burrows and its implications for eradication treatment and management of ponds with crayfish. Ethol. Ecol. Evol. 2014, 26, 277–298. [Google Scholar] [CrossRef]
- Chadwick, D.D.A.; Pritchard, E.G.; Bradley, P.; Sayer, C.D.; Chadwick, M.A.; Eagle, L.J.B.; Axmacher, J.C. A novel ‘triple drawdown’ method highlights deficiencies in invasive alien crayfish survey and control techniques. J. Appl. Ecol. 2021, 58, 316–326. [Google Scholar] [CrossRef]
- Peay, S.; Hiley, P.D.; Collen, P.; Martin, I. Biocide Treatment of Ponds in Scotland to Eradicate Signal Crayfish. Bull. Pêche Piscic. 2006, 380–381, 1363–1379. [Google Scholar] [CrossRef]
- Ballantyne, L.; Baum, D.; Bean, C.W.; Long, J.; Whitaker, S. Successful eradication of signal crayfish (Pacifastacus leniusculus) using a non-specific biocide in a small isolated water body in Scotland. In Proceedings of the Island Invasives 2017 Conference Co-Hosted by the University of Dundee and the South Georgia Heritage Trust, Dundee, Scotland, 10–14 July 2017; 2019 IUCN Occasional Paper SSC no. 62. IUCN: Gland, Switzerland. 734p. [Google Scholar]
- Peay, S.; Johnsen, S.; Bean, C.; Dunn, A.; Sandodden, R.; Edsman, L. Biocide Treatment of Invasive Signal Crayfish: Successes, Failures and Lessons Learned. Diversity 2019, 11, 29. [Google Scholar] [CrossRef]
- Musseau, C.; Boulenger, C.; Crivelli, A.J.; Lebel, I.; Pascal, M.; Boulêtreau, S.; Santoul, F. Native European eels as a potential biological control for invasive crayfish. Freshw. Biol. 2015, 60, 636–645. [Google Scholar] [CrossRef]
- Johović, I.; Verrucchi, C.; Inghilesi, A.F.; Scapini, F.; Tricarico, E. Managing the Invasive Crayfish Procambarus clarkii: Is Manual Sterilisation the Solution? Freshw. Biol. 2020, 65, 621–631. [Google Scholar] [CrossRef]
- Hudina, S.; Maguire, I.; Dragičević, P.; Galic, N. Evaluating the Efficacy of Approaches to Control Invasive Populations: A Conceptual Model Development for the Signal Crayfish. Ecologies 2022, 3, 78–95. [Google Scholar] [CrossRef]
- Larson, C.E.; Bo, T.; Candiotto, A.; Fenoglio, S.; Doretto, A. Predicting invasive signal crayfish (Pacifastacus leniusculus) spread using a traditional survey and river network simulation. River Res. Appl. 2022, 38, 1424–1435. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Melzer, M.; Majkowski, W. Range extension of Dikerogammarus villosus (Sowinsky, 1894) in Poland (the Baltic Sea basin) and its ability to osmoregulate in different environmental salinities. Ocean. Hydrobiol. Stud. 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Vaeßen, S.; Hollert, H. Impacts of the North American signal crayfish (Pacifastacus leniusculus) on European ecosystems. Environ. Sci. Eur. 2015, 27, 33. [Google Scholar] [CrossRef]
- Kouba, A.; Oficialdegui, F.J.; Cuthbert, R.N.; Kourantidou, M.; South, J.; Tricarico, E.; Gozlan, R.E.; Courchamp, F.; Haubrock, P.J. Identifying economic costs and knowledge gaps of invasive aquatic crustaceans. Sci. Total Environ. 2022, 813, 152325. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzycka-Krahel, A.; Skóra, M.E.; Raczyński, M.; Magdoń, K. Osmoregulatory Capacity and Non-Specific Food Preferences as Strengths Contributing to the Invasive Success of the Signal Crayfish Pacifastacus leniusculus: Management Implications. Water 2024, 16, 2657. https://doi.org/10.3390/w16182657
Dobrzycka-Krahel A, Skóra ME, Raczyński M, Magdoń K. Osmoregulatory Capacity and Non-Specific Food Preferences as Strengths Contributing to the Invasive Success of the Signal Crayfish Pacifastacus leniusculus: Management Implications. Water. 2024; 16(18):2657. https://doi.org/10.3390/w16182657
Chicago/Turabian StyleDobrzycka-Krahel, Aldona, Michał E. Skóra, Michał Raczyński, and Katarzyna Magdoń. 2024. "Osmoregulatory Capacity and Non-Specific Food Preferences as Strengths Contributing to the Invasive Success of the Signal Crayfish Pacifastacus leniusculus: Management Implications" Water 16, no. 18: 2657. https://doi.org/10.3390/w16182657
APA StyleDobrzycka-Krahel, A., Skóra, M. E., Raczyński, M., & Magdoń, K. (2024). Osmoregulatory Capacity and Non-Specific Food Preferences as Strengths Contributing to the Invasive Success of the Signal Crayfish Pacifastacus leniusculus: Management Implications. Water, 16(18), 2657. https://doi.org/10.3390/w16182657






