Short-Term Warming Induces Cyanobacterial Blooms and Antibiotic Resistance in Freshwater Lake, as Revealed by Metagenomics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Batch Reactor Experiments with Different Temperatures and ROS Levels
2.2. Chemical Analysis
2.3. DNA Extraction and Metagenomics Analysis
2.4. Bioinformatics Analysis
2.5. Statistical Analysis and Visualization
3. Results and Discussion
3.1. Nutrient Dynamics and DOM Analysis under Different Temperature and Oxidative Stress Conditions
3.2. Temperature Warming and Oxidative Stress Restructure Microbial Communities and Promote Cyanobacterial Proliferation
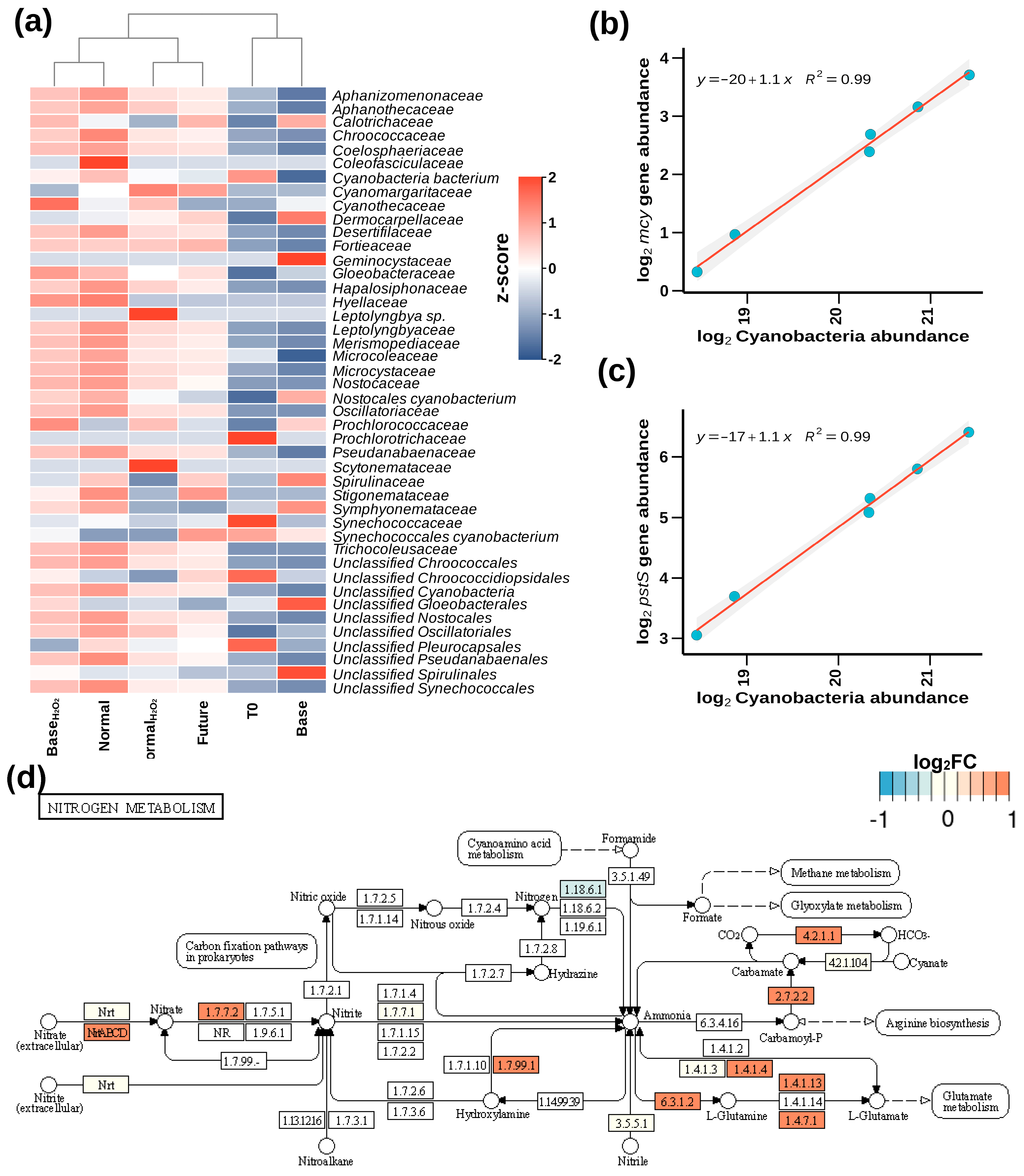
3.3. Proliferation of Toxin-Producing Cyanobacterial Families and Functional Adaptations under Warming
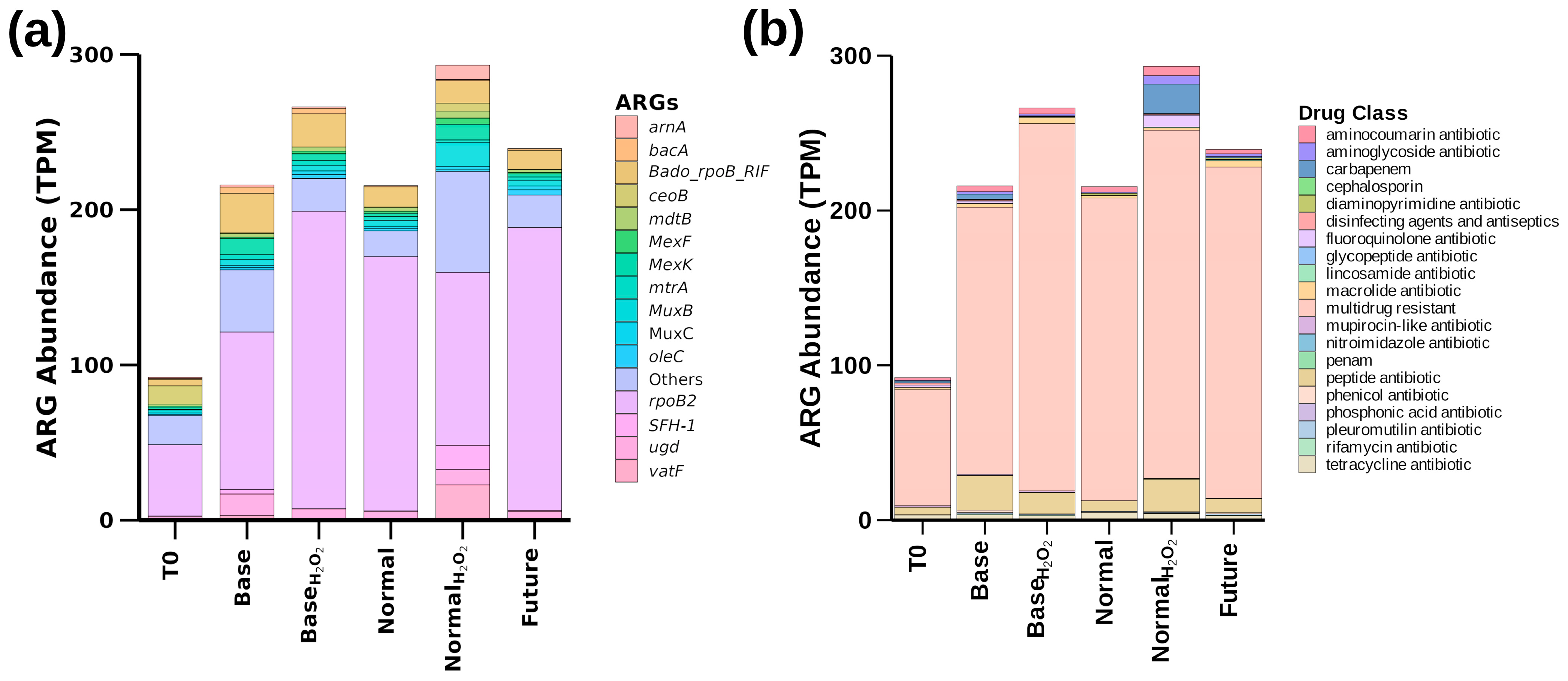
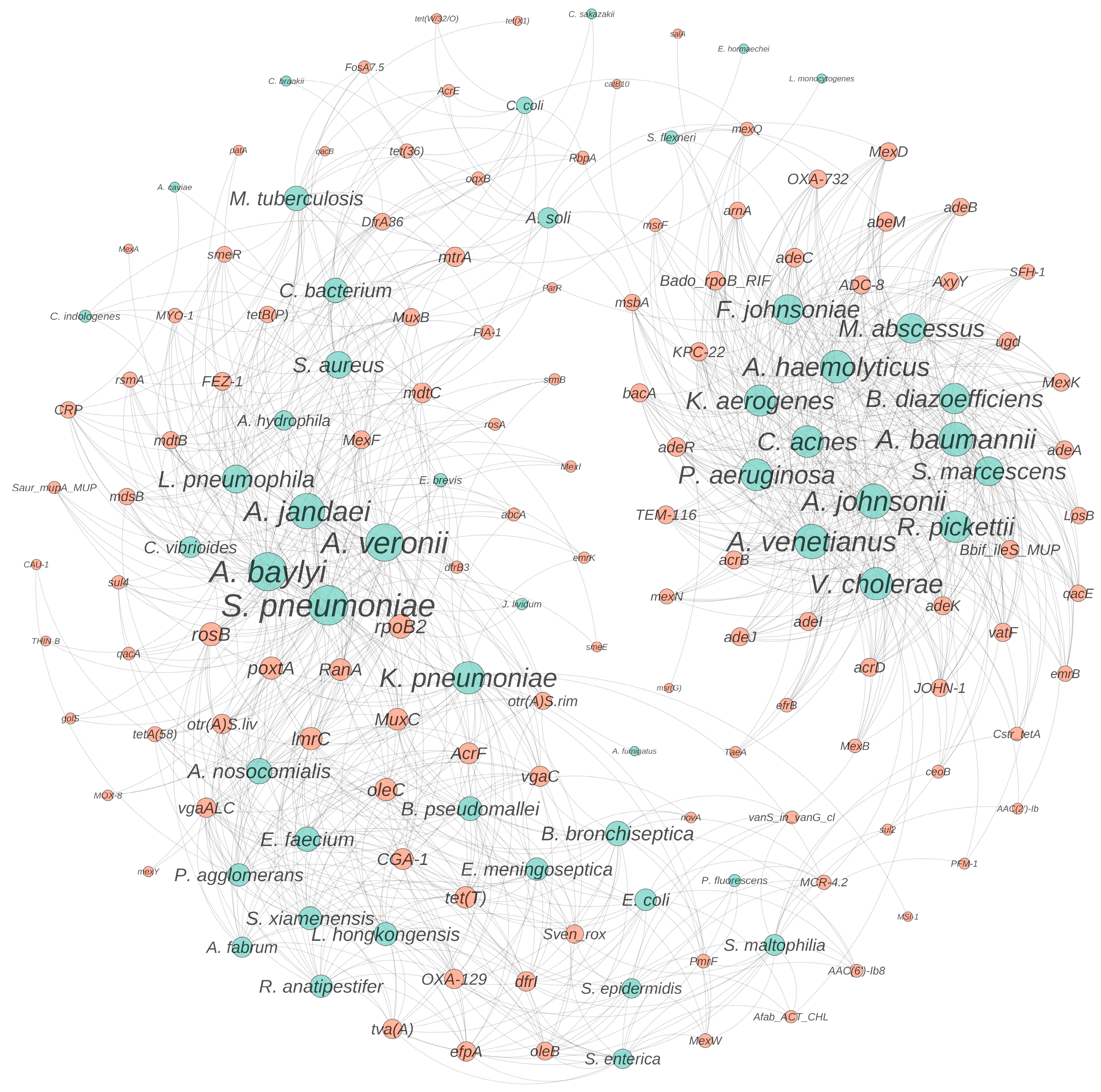
3.4. Temperature- and ROS-Induced Proliferation of Antibacterial Resistance
3.5. Study Limitations and Future Research Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Miao, C.; Hanel, M.; Borthwick, A.G.; Duan, Q.; Ji, D.; Li, H. Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ. Int. 2019, 128, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Hajat, S.; Murage, P.; Heaviside, C.; Macintyre, H.; Davies, M.; Wilkinson, P. The contribution of demographic changes to future heat-related health burdens under climate change scenarios. Environ. Int. 2023, 173, 107836. [Google Scholar] [CrossRef] [PubMed]
- IPOC Change. Climate Change 2021—The Physical Science Basis. Chem. Int. 2021, 43, 22–23. [Google Scholar] [CrossRef]
- WMO. WMO Global Annual to Decadal Climate Update: Target Years: 2023 and 2023–2027; WMO: Geneva, Switzerland, 2023. [Google Scholar]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2020, 50, 85–94. [Google Scholar] [CrossRef]
- Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Reddy, M.K.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of Climate Change and Anthropogenic Activities on Aquatic Ecosystem—A Review. Environ. Res. 2023, 238, 117233. [Google Scholar]
- Li, Z.; Sun, A.; Liu, X.; Chen, Q.-L.; Bi, L.; Ren, P.-X.; Shen, J.-P.; Jin, S.; He, J.-Z.; Hu, H.-W.; et al. Climate warming increases the proportions of specific antibiotic resistance genes in natural soil ecosystems. J. Hazard. Mater. 2022, 430, 128442. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Q.; Su, W.; Wang, S.; Wang, X.; Han, Q.; Li, H. Metagenomics reveals that temperature predicts a small proportion of antibiotic resistomes and mobile genetic elements in polluted water. Environ. Pollut. 2023, 317, 120793. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, M.; Liu, L.; Liu, X.; Chen, H.; Yang, J. The antibiotic resistome of free-living and particle-attached bacteria under a reservoir cyanobacterial bloom. Environ. Int. 2018, 117, 107–115. [Google Scholar] [CrossRef]
- Tomitani, A.; Knoll, A.H.; Cavanaugh, C.M.; Ohno, T. The evolutionary diversification of cyanobacteria: Molecular–phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. USA 2006, 103, 5442–5447. [Google Scholar] [CrossRef]
- Bullerjahn, G.S.; Post, A.F. Physiology and molecular biology of aquatic cyanobacteria. Front. Microbiol. 2014, 5, 359. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology—Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, C.; Tarran, G.A.; Schuback, N.; Woodward, E.M.S.; Arístegui, J.; Marañón, E. Phytoplankton responses to changing temperature and nutrient availability are consistent across the tropical and subtropical Atlantic. Commun. Biol. 2022, 5, 1035. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; Zhu, X.; Sotto, P.; Paulino, Y. Oral exposure to environmental cyanobacteria toxins: Implications for cancer risk. Environ. Int. 2021, 148, 106381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef]
- Wood, R. Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environ. Int. 2016, 91, 276–282. [Google Scholar] [CrossRef]
- Filatova, D.; Picardo, M.; Núñez, O.; Farré, M. Analysis, Levels and Seasonal Variation of Cyanotoxins in Freshwater Eco-systems. Trends Environ. Anal. Chem. 2020, 26, e00091. [Google Scholar] [CrossRef]
- Verspagen, J.M.H.; Van de Waal, D.B.; Finke, J.F.; Visser, P.M.; Van Donk, E.; Huisman, J. Rising CO2 Levels Will Intensify Phytoplankton Blooms in Eutrophic and Hypertrophic Lakes. PLoS ONE 2014, 9, e104325. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Jang, M.-H.; Ha, K.; Takamura, N. Reciprocal allelopathic responses between toxic cyanobacteria (Microcystis aeruginosa) and duckweed (Lemna japonica). Toxicon 2007, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial Toxins as Allelochemicals with Potential Appli-cations as Algaecides, Herbicides and Insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.; Monserrat, J. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ. Int. 2010, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.M.; Sienkiewicz, N.; Struewing, I.; Stelzer, E.A.; Graham, J.L.; Lu, J. Metagenomic mapping of cyanobacteria and potential cyanotoxin producing taxa in large rivers of the United States. Sci. Rep. 2023, 13, 2806. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 531–549. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Qamar, M.U. Aatika Impact of Climate Change on Antimicrobial Resistance Dynamics: An Emerging One Health Challenge. Future Microbiol. 2023, 18, 535–539. [Google Scholar] [CrossRef]
- Yu, Q.; Han, Q.; Shi, S.; Sun, X.; Wang, X.; Wang, S.; Yang, J.; Su, W.; Nan, Z.; Li, H. Metagenomics reveals the response of antibiotic resistance genes to elevated temperature in the Yellow River. Sci. Total Environ. 2023, 859, 160324. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Hozzein, W.N.; Mousa, A.S.M.; Rabie, W.; Alkhalifah, D.H.M.; Selim, S.; AbdElgawad, H. Heat stress as an innovative approach to enhance the antioxidant production in Pseudooceanicola and Bacillus isolates. Sci. Rep. 2020, 10, 15076. [Google Scholar] [CrossRef]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef] [PubMed]
- Tignat-Perrier, R.; van de Water, J.A.J.M.; Guillemain, D.; Aurelle, D.; Allemand, D.; Ferrier-Pagès, C. The Effect of Thermal Stress on the Physiology and Bacterial Communities of Two Key Mediterranean Gorgonians. Appl. Environ. Microbiol. 2022, 88, e0234021. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Jonker, M.J.; Teichmann, L.; Wortel, M.; ter Kuile, B.H. The influence of oxygen and oxidative stress on de novo acquisition of antibiotic resistance in E. coli and Lactobacillus lactis. BMC Microbiol. 2023, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pucher, M.; Wünsch, U.; Weigelhofer, G.; Murphy, K.; Hein, T.; Graeber, D. staRdom: Versatile Software for Analyzing Spectroscopic Data of Dissolved Organic Matter in R. Water 2019, 11, 2366. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2013, 6, 658–661. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Raes, E.J.; Karsh, K.; Sow, S.L.S.; Ostrowski, M.; Brown, M.V.; van de Kamp, J.; Franco-Santos, R.M.; Bodrossy, L.; Waite, A.M. Metabolic pathways inferred from a bacterial marker gene illuminate ecological changes across South Pacific frontal boundaries. Nat. Commun. 2021, 12, 2213. [Google Scholar] [CrossRef]
- Tamames, J.; Puente-Sánchez, F. SqueezeMeta, A Highly Portable, Fully Automatic Metagenomic Analysis Pipeline. Front. Microbiol. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.-L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef]
- Luo, C.; Rodriguez-R, L.M.; Konstantinidis, K.T. MyTaxa: An advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res. 2014, 42, e73. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; García-García, N.; Tamames, J. SQMtools: Automated processing and visual analysis of’ omics data with R and anvi’o. BMC Bioinform. 2020, 21, 358. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [PubMed]
- Peschel, S.; Müller, C.L.; von Mutius, E.; Boulesteix, A.-L.; Depner, M. NetCoMi: Network construction and comparison for microbiome data in R. Briefings Bioinform. 2020, 22, bbaa290. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the 3rd International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- R Core Team. R Core Team 2021 R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-Project.Org/ (accessed on 1 January 2024).
- Xia, Y.; Yu, E.; Li, Z.; Yu, D.; Wang, G.; Xie, J.; Gong, W.; Zhang, K. Bacterial Community Composition Associated with Freshwater Cyanobacterial Blooms of Intensive Culture Ponds. Nat. Environ. Pollut. Technol. 2017, 16, 1059–1066. [Google Scholar]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef]
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J., Jr.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate Change Impacts on Harmful Algal Blooms in U.S. Freshwaters: A Screening-Level Assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Marshall, I.P.G.; Starnawski, P.; Cupit, C.; Cáceres, E.F.; Ettema, T.J.G.; Schramm, A.; Kjeldsen, K.U. The novel bacterial phylum Calditrichaeota is diverse, widespread and abundant in marine sediments and has the capacity to degrade detrital proteins. Environ. Microbiol. Rep. 2017, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kublanov, I.V.; Sigalova, O.M.; Gavrilov, S.N.; Lebedinsky, A.V.; Rinke, C.; Kovaleva, O.; Chernyh, N.A.; Ivanova, N.; Daum, C.; Reddy, T.; et al. Genomic Analysis of Caldithrix abyssi, the Thermophilic Anaerobic Bacterium of the Novel Bacterial Phylum Calditrichaeota. Front. Microbiol. 2017, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; Thomson-Laing, G.; Wood, S.A. Microcystins in New Zealand: A review of occurrence, congener diversity and cell quotas. N. Z. J. Bot. 2018, 57, 93–111. [Google Scholar] [CrossRef]
- Wood, S.A.; Maier, M.Y.; Puddick, J.; Pochon, X.; Zaiko, A.; Dietrich, D.R.; Hamilton, D.P. Trophic state and geographic gradients influence planktonic cyanobacterial diversity and distribution in New Zealand lakes. FEMS Microbiol. Ecol. 2016, 93, fiw234. [Google Scholar] [CrossRef]
- Liu, D.; Song, H.; Li, Y.; Huang, R.; Liu, H.; Tang, K.; Jiao, N.; Liu, J. The Transcriptional Repressor PerR Senses Sulfane Sulfur by Cysteine Persulfidation at the Structural Zn2+ Site in Synechococcus sp. PCC7002. Antioxidants 2023, 12, 423. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Liu, S.; Liu, B.; Gao, Y.; Wu, Z. Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere 2011, 85, 977–982. [Google Scholar] [CrossRef]
- Erratt, K.J.; Creed, I.F.; Lobb, D.A.; Smol, J.P.; Trick, C.G. Climate change amplifies the risk of potentially toxigenic cyanobacteria. Glob. Chang. Biol. 2023, 29, 5240–5249. [Google Scholar] [CrossRef]
- Padovan, A.; Kennedy, K.; Gibb, K. A microcystin synthesis mcyE/ndaF gene assay enables early detection of microcystin production in a tropical wastewater pond. Harmful Algae 2023, 127, 102476. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, C.; Struewing, I.; Li, X.; Allen, J.; Lu, J. Cyanotoxin-encoding genes as powerful predictors of cyanotoxin production during harmful cyanobacterial blooms in an inland freshwater lake: Evaluating a novel early-warning system. Sci. Total Environ. 2022, 830, 154568. [Google Scholar] [CrossRef]
- Omata, T. Structure, Function and Regulation of the Nitrate Transport System of the Cyanobacterium Synechococcus sp. PCC7942. Plant Cell Physiol. 1995, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.E.; Flores, E.; Herrero, A. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1997, 179, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Xia, J.; Sun, H.; Zhang, X.; Ye, L. Phenolic compounds promote the horizontal transfer of antibiotic resistance genes in activated sludge. Sci. Total Environ. 2021, 800, 149549. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mecha-nisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Lu, J.; Chen, Z.; Nguyen, S.H.; Mao, L.; Li, J.; Yuan, Z.; Guo, J. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ. Int. 2018, 120, 421–430. [Google Scholar] [CrossRef]
- Lira, F.; Vaz-Moreira, I.; Tamames, J.; Manaia, C.M.; Martínez, J.L. Metagenomic analysis of an urban resistome before and after wastewater treatment. Sci. Rep. 2020, 10, 8174. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manna, B.; Jay, E.; Zhang, W.; Zhou, X.; Lyu, B.; Thomas, G.M.; Singhal, N. Short-Term Warming Induces Cyanobacterial Blooms and Antibiotic Resistance in Freshwater Lake, as Revealed by Metagenomics Analysis. Water 2024, 16, 2655. https://doi.org/10.3390/w16182655
Manna B, Jay E, Zhang W, Zhou X, Lyu B, Thomas GM, Singhal N. Short-Term Warming Induces Cyanobacterial Blooms and Antibiotic Resistance in Freshwater Lake, as Revealed by Metagenomics Analysis. Water. 2024; 16(18):2655. https://doi.org/10.3390/w16182655
Chicago/Turabian StyleManna, Bharat, Emma Jay, Wensi Zhang, Xueyang Zhou, Boyu Lyu, Gevargis Muramthookil Thomas, and Naresh Singhal. 2024. "Short-Term Warming Induces Cyanobacterial Blooms and Antibiotic Resistance in Freshwater Lake, as Revealed by Metagenomics Analysis" Water 16, no. 18: 2655. https://doi.org/10.3390/w16182655
APA StyleManna, B., Jay, E., Zhang, W., Zhou, X., Lyu, B., Thomas, G. M., & Singhal, N. (2024). Short-Term Warming Induces Cyanobacterial Blooms and Antibiotic Resistance in Freshwater Lake, as Revealed by Metagenomics Analysis. Water, 16(18), 2655. https://doi.org/10.3390/w16182655









