Phytosorbents in Wastewater Treatment Technologies: Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
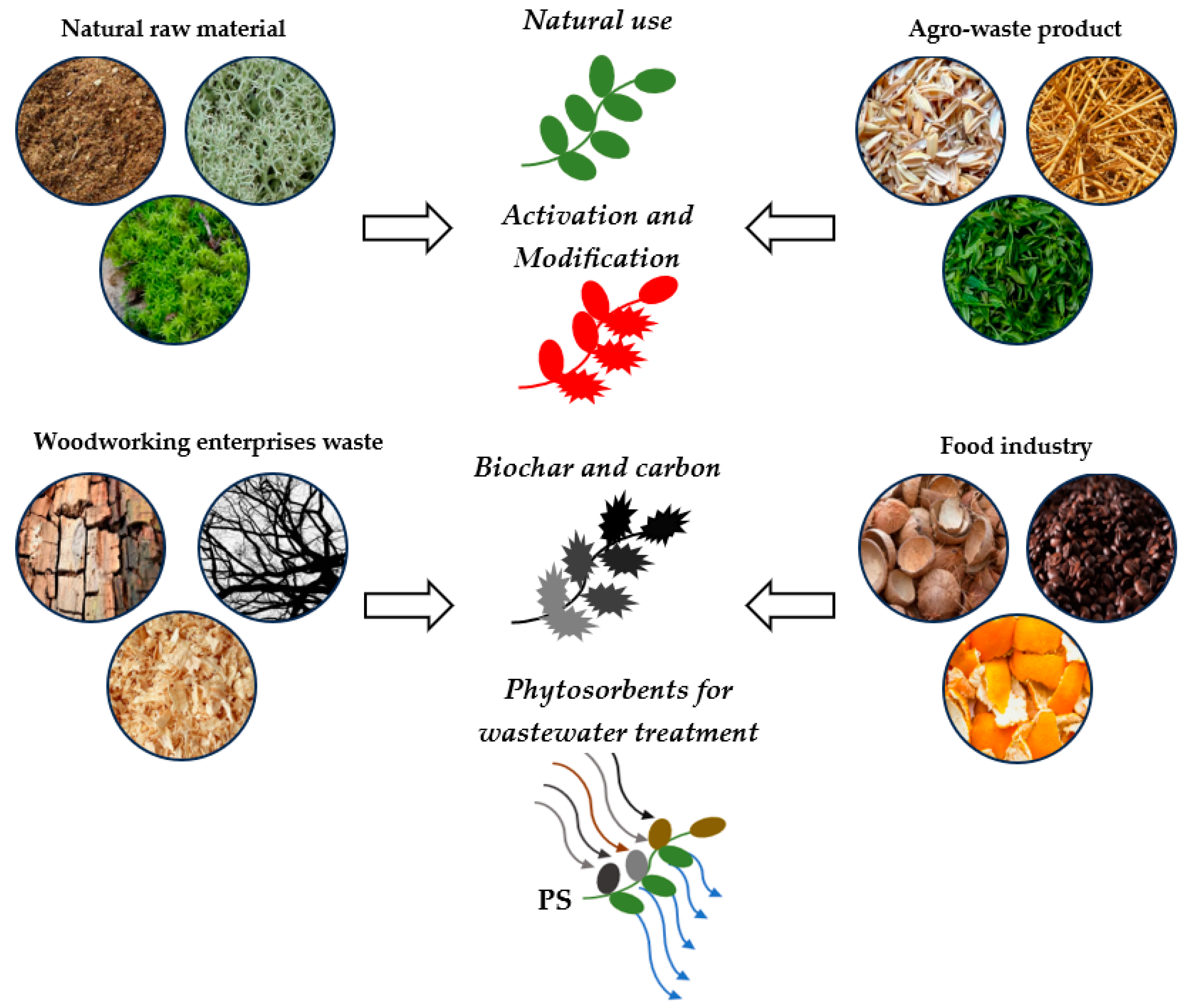
3.1. Kind of Raw Materials for the Phytosorbents
3.2. Methods of Obtaining Phytosorbents
3.3. Mechanism of the Sorption Process
3.4. Efficacy of Wastewater Treatment with Phytosorbents
3.4.1. Extraction of Metal Ions
3.4.2. Removing Dyes
3.4.3. Removing Crude Oil and Petroleum Products
3.4.4. Removing Other Harmful Pollutants
3.5. Regeneration and Disposal of Phytosorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Samonin, V.V.; Spiridonova, E.A.; Zotov, A.S.; Podvyaznikov, M.L.; Garabadzhiu, A.B. Chemical and Porous Structure, Sorption Properties of Adsorbents from Organic Technogenic Substrates. J. Gen. Chem. 2021, 91, 1284–1308. (In Russian) [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Chen, X.; Li, Y.; Jiang, Y. The Efficiency of Green Technology Innovation and Its Influencing Factors in Wastewater Treatment Companies. Separations 2022, 9, 263. [Google Scholar] [CrossRef]
- Shrivastava, P. Environment technologies and competitive advantage. Strateg. Manag. J. 1995, 16, 183–200. [Google Scholar] [CrossRef]
- Du, K.; Li, J. Towards a green world: How do green technology innovations affect total-factor carbon productivity. Energy Policy 2019, 131, 240–250. [Google Scholar] [CrossRef]
- Liu, M.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.; Zeng, G.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806, 150531. [Google Scholar] [CrossRef]
- Nasr, M.; Khan, N.; Sillanpää, M. Fourth Industrial Revolution of Wastewater Treatment with Adsorption. Hindawi Adsorpt. Sci. Technol. 2023, 2023, 9897865. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Li, X.; Cheng, J.; Yang, C. Cupric ions inducing dynamic hormesis in duckweed systems for swine wastewater treatment: Quantification, modelling and mechanisms. Sci. Total Environ. 2023, 866, 161411. [Google Scholar] [CrossRef]
- Reynel-Ávila, H.; Aguayo-Villarreal, I.; Diaz-Muñoz, L.; Moreno-Pérez, J.; Sánchez-Ruiz, F.; Rojas-Mayorga, C.; Mendoza-Castillo, D.; Bonilla-Petriciolet, A. A review of the modeling of adsorption of organic and inorganic pollutants from water using artificial neural networks. Adsorpt. Sci. Technol. 2022, 2022, 9384871. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Zhu, X.; Yin, L.; Yin, Z.; Li, X.; Zheng, W. Calculation of carbon emissions in wastewater treatment and its neutralization measures: A review. Sci. Total Environ. 2024, 912, 169356. [Google Scholar] [CrossRef]
- Alprol, A.E.; Mansour, A.T.; Ibrahim, M.E.E.-D.; Ashour, M. Artificial Intelligence Technologies Revolutionizing Wastewater Treatment: Current Trends and Future Prospective. Water 2024, 16, 314. [Google Scholar] [CrossRef]
- Valikhan Anaraki1, M.; Mahmoudian, F.; Nabizadeh Chianeh, F.; Farzin, S. Dye Pollutant Removal from Synthetic Wastewater: A New Modeling and Predicting Approach Based on Experimental Data Analysis, Kriging Interpolation Method, and Computational Intelligence Techniques. J. Environ. Inform. 2022, 40, 84–94. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Kang, T. Environmental Problems and Strategies Caused by Coal Mining. Adv. Mater. Res. 2012, 433–440, 2071–2076. [Google Scholar] [CrossRef]
- Vialkova, E.; Fugaeva, A. Wastewater Treatment with the Natural Sorbents from the Arctic. Water 2022, 14, 4009. [Google Scholar] [CrossRef]
- Voronov, A.A.; Maksimova, S.V.; Osipova, E.Y. Purification of urbanized melt water with plant sorbents. Vestn. Tomsk. Gos. Arkhitekturno-Stroit. Univ. J. Constr. Archit. 2021, 2, 105–117. (In Russian) [Google Scholar] [CrossRef]
- Malyshkina, E. Classification of the sorbents capable of removing the petroleum products from Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 042063. [Google Scholar] [CrossRef]
- Hu, Y.; Christensen, E.; Amin, H.; Smith, T.; Rein, G. Experimental study of moisture content effects on the transient gas and particle emissions from peat fires. Combust. Flame 2019, 209, 408–417. [Google Scholar] [CrossRef]
- Couillard, D. The use of peat in wastewater treatment. Water Res. 1994, 28, 1261–1274. [Google Scholar] [CrossRef]
- Perez, J.; Ramos, A.; Ordonez, J.; Gomes, M. Dual-stage peat beds in small community wastewater treatment. J. Environ. Sci. Health Part A 2007, 42, 1125–1130. [Google Scholar] [CrossRef]
- Bannova, E.A.; Kitaeva, N.K.; Merkov, S.M.; Muchkina, M.V.; Zaloznaya, E.P.; Martynov, P.N. The study of method of synthesis of the hydrophobic sorbent based on modified peat. Sorpt. Chromatogr. Process. 2013, 13, 60–68. Available online: http://www.chem.vsu.ru/sorbcr/images/pdf/2013/1/2013_01_09.pdf (accessed on 1 August 2024). (In Russian).
- Zolgharnein, J.; Bagtash, M.; Feshki, S.; Zolgharnein, P.; Hammond, D. Crossed mixture process design optimization and adsorption characterization of multimetal (Cu(II), Zn(II) and Ni(II)) removal by modified Buxus sempervirens tree leaves. J. Taiwan Inst. Chem. Eng. 2017, 78, 104–117. [Google Scholar] [CrossRef]
- Shrestha, B.; Homagai, P.L.; Pokhrel, M.R.; Ghimire, K.N. Exhausted Tea Leaves—A low cost bioadsorbent for the removal of Lead (II) and Zinc (II) ions from their aqueous solution. J. Nepal Chem. Soc. 2012, 30, 123–129. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al Lawati, W.M.; Al Hoqani, U.H.A.; Al Aufi, E.; Al Hatmi, K.; Al Zadjali, J.S.; Rahman, N.; Nasir, M.; Rahman, H.; Khan, S.A. Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals 2022, 15, 760. [Google Scholar] [CrossRef]
- Khan, Q.; Zahoor, M.; Salman, S.M.; Wahab, M.; Talha, M.; Kamran, A.W.; Khan, Y.; Ullah, R.; Ali, E.A.; Shah, A.B. The Chemically Modified Leaves of Pteris vittata as Efficient Adsorbent for Zinc (II) Removal from Aqueous Solution. Water 2022, 14, 4039. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Galić, A.; Kosović, I.; Jakovljević, T.; Habuda-Stanić, M. From Waste to Biosorbent: Removal of Congo Red from Water by Waste Wood Biomass. Water 2021, 13, 279. [Google Scholar] [CrossRef]
- Khmylko, L.I.; Orekhova, S.E. Sorbents based on lignin and cellulose-containing materials. Sviridov Read. Minsk 2012, 8, 232. Available online: https://elib.belstu.by/handle/123456789/6452 (accessed on 1 August 2024). (In Russian).
- Asemave, K.; Thaddeus, L.; Tarhemba, P.T. Lignocellulosic-Based Sorbents: A Review. Sustain. Chem. 2021, 2, 271–285. [Google Scholar] [CrossRef]
- Malyshkina, E.S.; Vyalkova, E.I.; Osipova, E.Y. Water Purification with Natural Sorbents. Vestn. Tomsk. Gos. Arkhitekt. Univ. J. Constr. Arch. 2019, 21, 188–200. (In Russian) [Google Scholar] [CrossRef]
- Rahman, N.u.; Ullah, I.; Alam, S.; Khan, M.S.; Shah, L.A.; Zekker, I.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Vincevica-Gaile, Z.; et al. Activated Ailanthus altissima Sawdust as Adsorbent for Removal of Acid Yellow 29 from Wastewater: Kinetics Approach. Water 2021, 13, 2136. [Google Scholar] [CrossRef]
- Díaz-García, C.; Christianson, L.E. Batch-Mode Denitrifying Woodchip Bioreactors for Expanded Treatment Flexibility. Water 2024, 16, 206. [Google Scholar] [CrossRef]
- Denisova, T.R.; Shaikhiev, I.G.; Sippel, I.Y. Ash sawdust oil capacity increased by acid solution treatment. Vestn. Tekhnol. Univ. 2015, 18, 233–235. Available online: https://cyberleninka.ru/article/n/uvelichenie-nefteemkosti-opilok-yasenya-obrabotkoy-rastvorami-kislot (accessed on 1 August 2024). (In Russian).
- Velić, N.; Stjepanović, M.; Pavlović, S.; Bagherifam, S.; Banković, P.; Jović-Jovičić, N. Modified Lignocellulosic Waste for the Amelioration of Water Quality: Adsorptive Removal of Congo Red and Nitrate Using Modified Poplar Sawdust. Water 2023, 15, 3776. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R.; Halawani, R.F.; Al-Mur, B.A.; Seliem, M.K. Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations. Water 2022, 14, 3491. [Google Scholar] [CrossRef]
- Alrowais, R.; Bashir, M.T.; Khan, A.A.; Bashir, M.; Abbas, I.; Abdel Daiem, M.M. Adsorption and Kinetics Modelling for Chromium (Cr6+) Uptake from Contaminated Water by Quaternized Date Palm Waste. Water 2024, 16, 294. [Google Scholar] [CrossRef]
- Mikova, N.M.; Skvortsova, G.P.; Mazurova, E.V.; Chesnokov, N.V. Influence of the cross-linking effect on the properties of sorbents obtained from aspen and larch bark. J. Appl. Chem. 2019, 92, 1333–1343. (In Russian) [Google Scholar] [CrossRef]
- Ikenyiri, P.N.; Ukpaka, C.P. Overview on the Effect of Particle Size on the Performance of Wood Based Adsorbent. J. Chem. Eng. Process Technol. 2016, 7, 315. [Google Scholar] [CrossRef]
- Surma, H.H.; Paul, A. Turning Waste into Wealth: Exploring Strategies for Effective Agricultural Waste Management. Vigyan Varta 2024, 5, 322–330. Available online: https://www.researchgate.net/publication/381008204 (accessed on 1 August 2024).
- Khalil, U.; Shakoor, M.B.; Ali, S.; Ahmad, S.R.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation. Water 2021, 13, 263. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Cuestas, J.; Guerrero, V.H.; Villamar-Ayala, C.A. Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns. Water 2024, 16, 197. [Google Scholar] [CrossRef]
- Fedotov, A.A.; Rudenko, E.Y. Production of adsorbents based on sunflower husks for removal of chromium (VI) from wastewater. Proc. Univ. Appl. Chem. Biotechnol. 2022, 12, 506–513. (In Russian) [Google Scholar] [CrossRef]
- Skorupa, A.; Worwąg, M.; Kowalczyk, M. Coffee Industry and Ways of Using By-Products as Bioadsorbents for Removal of Pollutants. Water 2023, 15, 112. [Google Scholar] [CrossRef]
- Taufik, S.H.; Ahmad, S.A.; Zakaria, N.N.; Shaharuddin, N.A.; Azmi, A.A.; Khalid, F.E.; Merican, F.; Convey, P.; Zulkharnain, A.; Abdul Khalil, K. Rice Straw as a Natural Sorbent in a Filter System as an Approach to Bioremediate Diesel Pollution. Water 2021, 13, 3317. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice Straw Biochar and Magnetic Rice Straw Biochar for Safranin O Adsorption from Aqueous Solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Alrowais, R.; Said, N.; Bashir, M.T.; Ghazy, A.; Alwushayh, B.; Daiem, M.M.A. Adsorption of Diphenolic Acid from Contaminated Water onto Commercial and Prepared Activated Carbons from Wheat Straw. Water 2023, 15, 555. [Google Scholar] [CrossRef]
- Gorbunov, G.I.; Rasulov, O.R. Rice Straw Recycling Problems. Proc. Mosc. State Univ. Civ. Eng. 2013, 7, 106–113. (In Russian) [Google Scholar] [CrossRef]
- Molaudzi, N.R.; Ambushe, A.A. Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples. Water 2022, 14, 3395. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Benalia, A.; Selama, Z.; Hammoud, A.; Derbal, K.; Panico, A.; Pizzi, A. Removal of Chromium (VI) from Water Using Orange peel as the Biosorbent: Experimental, Modeling, and Kinetic Studies on Adsorption Isotherms and Chemical Structure. Water 2024, 16, 742. [Google Scholar] [CrossRef]
- Azamzam, A.A.; Rafatullah, M.; Yahya, E.B.; Ahmad, M.I.; Lalung, J.; Alam, M.; Siddiqui, M.R. Enhancing the Efficiency of Banana Peel Bio-Coagulant in Turbid and River Water Treatment Applications. Water 2022, 14, 2473. [Google Scholar] [CrossRef]
- Nadew, T.T.; Keanab, M.; Sisayc, N.; Getyec, B. Synthesis of activated carbon from banana peels for dye removal of an aqueous solution in textile industries: Optimization, kinetics, and isotherm aspects. Water Pract. Technol. 2023, 18, 947–966. [Google Scholar] [CrossRef]
- Huang, C.; Wang, L.; Fan, L.; Chen, Y. Co-Pyrolysis of Fenton Sludge and Pomelo Peel for Heavy Metal Stabilization: Speciation Mechanism and Risk Evaluation. Water 2023, 15, 3733. [Google Scholar] [CrossRef]
- González-Delgado, A.D.; Villabona-Ortíz, A.; Tejada-Tovar, C. Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water 2022, 14, 408. [Google Scholar] [CrossRef]
- Flores Alarcón, M.A.D.; Revilla Pacheco, C.; Garcia Bustos, K.; Tejada Meza, K.; Terán-Hilares, F.; Pacheco Tanaka, D.A.; Colina Andrade, G.J.; Terán-Hilares, R. Efficient Dye Removal from Real Textile Wastewater Using Orange Seed Powder as Suitable Bio-Adsorbent and Membrane Technology. Water 2022, 14, 4104. [Google Scholar] [CrossRef]
- Arsenie, T.; Cara, I.G.; Popescu, M.-C.; Motrescu, I.; Bulgariu, L. Evaluation of the Adsorptive Performances of Rapeseed Waste in the Removal of Toxic Metal Ions in Aqueous Media. Water 2022, 14, 4108. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Bhatnagar, A.; Ehrampoush, M.H.; Amrollahi, A.; Jamshidi, B.; Dehvari, M.; Taghavi, M. Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: Kinetic modeling studies. Int. J. Environ. Sci. Technol. 2017, 14, 331–340. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; de Paula Menezes Barbosa, P.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. J. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.-G. Adsorption Characteristics of Spent Coffee Grounds as an Alternative Adsorbent for Cadmium in Solution. Environments 2020, 7, 24. [Google Scholar] [CrossRef]
- Stanković, V.; Bozić, D.; Gorgievski, M.; Bogdanovic, G. Heavy metal ions adsorption from mine waters by sawdust. Chem. Ind. Chem. Eng. Q. 2009, 15, 237–249. [Google Scholar] [CrossRef]
- Kim, A.N.; Mikhailov, A.V. Urban stormwater treatment on local passive systems. J. Water Ecol. 2017, 4, 40–52. (In Russian) [Google Scholar]
- Prodous, O.A.; Mikhailov, A.V. The experience of using peat filtration for surface runoff treatment. Water Supply Sanit. Tech. 2019, 3, 34–39. Available online: https://www.vstnews.ru/en/archives-all/2019/2019-3/7500-opyt-primeneniya (accessed on 1 August 2024). (In Russian).
- Veprikova, E.V.; Tereshchenko, E.A.; Chesnokov, N.V.; Shchipko, M.L.; Kuznetsov, B.N. Peculiarity of Water Purifying from Oil Products with Make Use of Oil Sorbents, Filtering Materials and Active Coals. J. Sib. Fed. Univ. Chem. 2010, 3, 285–304. Available online: https://elib.sfu-kras.ru/bitstream/handle/2311/2187/10_Veprikova.pdf?sequence=1 (accessed on 1 August 2024). (In Russian).
- Veprikova, E.V.; Ivanov, I.P. Structure and sorption properties of activated carbon based on pine bark carbonizats. Chem. Plant Mater. 2020, 4, 289–296. (In Russian) [Google Scholar] [CrossRef]
- Ruchkinova, O.I.; Romanova, N.A. Waste-based oil sorbents. Mod. Technol. Constr. Theory Pract. 2020, 1, 109–116. Available online: https://elibrary.ru/item.asp?id=42882117 (accessed on 1 August 2024). (In Russian).
- Awasthi, M.K. Engineered biochar: A multifunctional material for energy and environment. Environ. Pollut. 2022, 298, 118831. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Aziz, A.; Ouali, M.S.; Elandaloussi, E.H.; De Menorval, L.C.; Lindheimer, M. Chemically modified olive stone, A low-cost sorbent for heavy metals and basic dyes removal from aqueous solutions. J. Hazard. Mater. 2009, 163, 441–447. [Google Scholar] [CrossRef]
- Faizal, A.M.; Kutty, S.R.M.; Ezechi, E.H. Removal of oil from water by column adsorption method using microwave incinerated rice husk ash (MIRHA). In Proceedings of the International Civil and Infrastructure Engineering Conference 2014 (InCIEC 2014), Kota Kinabalu, Malaysia, 28 September–1 October 2014; pp. 963–971. [Google Scholar] [CrossRef]
- Bakhia, T.; Khamizov, R.K.; Bavizhev, M.D.; Konov, M.A. The effect of microwave treatment of clinoptilolite on its ion-exchange kinetic properties. Sorpt. Chromatogr. Process. 2016, 16, 803–812. Available online: https://journals.vsu.ru/sorpchrom/article/download/1410/1468/ (accessed on 1 August 2024).
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave irradiation in technologies of wastewater and wastewater sludge treatment: A review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Danilov, O.S.; Mikheyev, V.A.; Moskalenko, T.V. Research of electromagnetic microwave radiation influence on the solid fuels. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2011, 13, 1264–1267. Available online: https://sciup.org/148199846 (accessed on 1 August 2024).
- Hanif, A.; Ali, S.; Hanif, M.A.; Rashid, U.; Bhatti, H.N.; Asghar, M.; Alsalme, A.; Giannakoudakis, D.A. A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water 2021, 13, 3316. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Tang, S.; Zhang, L.; Zhang, J.; Zhao, Y.; Zhang, X.; Zhu, Y. Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template. Water 2022, 14, 1848. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Wu, T.; Ren, X.; Zhao, X. Research on the Preparation of Biochar from Waste and Its Application in Environmental Remediation. Water 2023, 15, 3387. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Thamilselvan, A.; Doong, R.; Pandit, B. Sustainable high-energy supercapacitors: Metal oxide-agricultural waste biochar composites paving the way for a greener future. J. Energy Storage 2024, 77, 109723. [Google Scholar] [CrossRef]
- Kong, F.; Liu, J.; Xiang, Z.; Fan, W.; Liu, J.; Wang, J.; Wang, Y.; Wang, L.; Xi, B. Degradation of Water Pollutants by Biochar Combined with Advanced Oxidation: A Systematic Review. Water 2024, 16, 875. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. Industrial Crops & Products The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Clean. Prod. 2018, 210, 1324–1342. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-garz, M.; Almudena, G.; Rodriguez, J.J. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; Herrera-Barros, A.; Cabrales-Sanjuan, D. Use of Sawdust (Aspidosperma polyneuron) in the Preparation of a Biocarbon-Type Adsorbent Material for Its Potential Use in the Elimination of Cationic Contaminants in Wastewater. Water 2023, 15, 3868. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Fitzsimmons, M.; Ross, A.B.; Mariner, T. Response Surface Modelling of Methylene Blue Adsorption onto Seaweed, Coconut Shell and Oak Wood Hydrochars. Water 2023, 15, 977. [Google Scholar] [CrossRef]
- Mukhina, I.M.; Dicke, C.; Lanza, G.; Kalderis, D.; Kern, J. The effect of different hydrochars on carbon dioxide, nitrous oxide emissions and plant growth. Agrophysics 2015, 4, 1–12. Available online: https://agrophys.ru/Media/Default/JournalAgrophysica/Agrophysica4-2015/Мухина.pdf (accessed on 1 August 2024). (In Russian).
- Mukhin, V.M.; Kurilkin, A.A.; Voropaeva, N.L.; Leksyukova, K.V.; Uchanjv, P.V. A position of active carbons in the ecology and economy, new technologies of their production. Sorpt. Chromatogr. Process. 2016, 16, 346–353. Available online: https://journals.vsu.ru/sorpchrom/article/view/1357 (accessed on 1 August 2024). (In Russian).
- Qin, F.; Wen, B.; Shan, X.-Q.; Xie, Y.-N.; Liu, T.; Zhang, S.-Z.; Khan, S.U. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006, 144, 669–680. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.R. Effects of pH on Isotherms Modeling for Cu (II) Ions Adsorption Using Maple Wood Sawdust. Chem. Eng. J. 2009, 149, 273–280. [Google Scholar] [CrossRef]
- Glagoleva, L.E.; Rodionova, N.S.; Korneeva, O.S.; Shuvaeva, G.P. Investigation of the sorption of metals vegetable sorbents. Proc. Voronezh State Univ. Eng. Technol. 2012, 1, 141–143. (In Russian) [Google Scholar]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Gholami, P.; Kalderis, D.; Pachatouridou, E.; Konsolakis, M. Preparation of novel CeO2 biochar nanocomposite for sonocatalytic degradation of a textile dye. Ultrason. Sonochem. 2018, 41, 503–513. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, Y.; Wei, W.; Shi, J.; Jin, H. Preparation of biochar adsorption material from walnut shell by supercritical CO2 pretreatment. Biochar 2024, 6, 11. [Google Scholar] [CrossRef]
- Boyko, Y.N.; Agoshkov, A.I.; Gul’kov, A.N.; Solomennik, S.F.; Gul’kova, S.G.; Mayss, N.A. Natural sorbents used for water purification from oil and products of its processing. Min. Inf. Anal. Bull. (Sci. Tech. J.) 2013, 22, 12–17. Available online: https://cyberleninka.ru/article/n/prirodnye-sorbenty-ispolzuyuschiesya-dlya-ochistki-vod-ot-nefti-i-produktov-ee-pererabotki (accessed on 1 August 2024). (In Russian).
- Kahramanly, Y.N. Foamed Polymeric Petroleum Sorbents. Environmental Problems and Their Solutions; ELM: Baku, Azerbaijan, 2012; 305p, Available online: https://anl.az/el_ru/kniqi/2013/1-753193.pdf (accessed on 1 August 2024). (In Russian)
- Alyoshina, L.A.; Gurtova, V.A.; Melekh, N.V. Structure and Physico-Chemical Properties of Celluloses and Nanocomposites Based on Them; PetrGU: Petrozavodsk, Russia, 2014; 240p, Available online: http://journal.asu.ru/public/doc/cell-2014.pdf (accessed on 1 August 2024). (In Russian)
- Bordunov, V.V.; Bordunov, S.V.; Leonenko, V.V. Purification of water from oil and petroleum products. Ecol. Ind. Russ. 2005, 8, 8–11. Available online: https://www.elibrary.ru/download/elibrary_11714436_88535439.pdf (accessed on 1 August 2024). (In Russian).
- Yakubovsky, S.F.; Oshchepkova, N.V.; Bulavka, Y.A.; Pisareva, S.S.; Popkova, L.A. Features of the microstructure of dry pine debarking waste as a raw material for the production of petroleum sorbents. Bull. Polotsk State Univ. 2011, 11, 154–158. Available online: https://cyberleninka.ru/article/n/osobennosti-mikrostruktury-othodov-suhoy-okorki-sosny-kak-syrya-dlya-polucheniya-neftyanyh-sorbentov (accessed on 1 August 2024). (In Russian).
- Li, L.; Li, Y.; Liu, Y.; Ding, L.; Jin, X.; Lian, H.; Zheng, J. Preparation of a Novel Activated Carbon from Cassava Sludge for the High-Efficiency Adsorption of Hexavalent Chromium in Potable Water: Adsorption Performance and Mechanism Insight. Water 2021, 13, 3602. [Google Scholar] [CrossRef]
- Shvartseva, O.; Skripkina, T.; Gaskova, O.; Podgorbunskikh, E. Modification of Natural Peat for Removal of Copper Ions from Aqueous Solutions. Water 2022, 14, 2114. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Lee, Y.-G.; Chon, K. Enhanced Adsorptive Removal of Dyes Using Mandarin Peel Biochars via Chemical Activation with NH4Cl and ZnCl2. Water 2021, 13, 1495. [Google Scholar] [CrossRef]
- Ashfaq, A.; Nadeem, R.; Bibi, S.; Rashid, U.; Hanif, A.; Jahan, N.; Ashfaq, Z.; Ahmed, Z.; Adil, M.; Naz, M. Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water 2021, 13, 3344. [Google Scholar] [CrossRef]
- Sawalha, H.; Bader, A.; Sarsour, J.; Al-Jabari, M.; Rene, E.R. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes 2022, 10, 2039. [Google Scholar] [CrossRef]
- Roy, H.; Sarkar, D.; Pervez, M.N.; Paul, S.; Cai, Y.; Naddeo, V.; Firoz, S.H.; Islam, M.S. Synthesis, Characterization and Performance Evaluation of Burmese Grape (Baccaurea ramiflora) Seed Biochar for Sustainable Wastewater Treatment. Water 2023, 15, 394. [Google Scholar] [CrossRef]
- Guediri, A.; Bouguettoucha, A.; Tahraoui, H.; Chebli, D.; Zhang, J.; Amrane, A.; Khezami, L.; Assadi, A.A. The Enhanced Adsorption Capacity of Ziziphus jujuba Stones Modified with Ortho-Phosphoric Acid for Organic Dye Removal: A Gaussian Process Regression Approach. Water 2024, 16, 1208. [Google Scholar] [CrossRef]
- Vialkova, E.I. Extraktion of petrolium product from waste water by natural sorbents of the Artic. Urban Constr. Archit. 2022, 12, 25–33. (In Russian) [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; VillabonaOrtíz, Á.; González-Delgado, Á.D.; Herrera-Barros, A.; Ortega-Toro, R. Selective and Binary Adsorption of Anions onto Biochar and Modified Cellulose from Corn Stalks. Water 2023, 15, 1420. [Google Scholar] [CrossRef]
- Lugo-Arias, J.; Vargas, S.B.; Maturana, A.; González-Álvarez, J.; Lugo-Arias, E.; Rico, H. Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective. Water 2024, 16, 1543. [Google Scholar] [CrossRef]
- Kayiwa, R.; Kasedde, H.; Lubwama, M.; Kirabira, J.B. Active Pharmaceutical Ingredients Sequestrated from Water Using Novel Mesoporous Activated Carbon Optimally Prepared from Cassava Peels. Water 2022, 14, 3371. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Abdulzahra, M.A.; Hussein, T.S.; Shlakaa, A.S.; Karhib, M.M.; Meiczinger, M.; Grmasha, R.A.; Al-Juboori, R.A.; Somogyi, V.; Domokos, E.; et al. Removal of Pharmaceuticals from Water Using Laccase Immobilized on Orange Peels Waste-Derived Activated Carbon. Water 2023, 15, 3437. [Google Scholar] [CrossRef]
- Behnood, R.; Anvaripour, B.; Jaafarzade, N.; Fard, H.; Farasati, M. Application of Natural Sorbents in Crude Oil Adsorption. Iran. J. Oil Gas Sci. Technol. 2013, 2, 1–11. [Google Scholar] [CrossRef]
| Section | Direction of PS Research | Ref. |
|---|---|---|
| 3.1 | Kind of raw materials for the phytosorbents | [14,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| 3.2 | Methods of obtaining phytosorbents | [33,52,53,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| 3.3 | Mechanism of sorption processes | [14,23,28,41,49,52,57,58,86,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| 3.4 | Efficacy of wastewater treatment with phytosorbents | |
| 3.4.1 | Extraction of metal ions | [21,22,24,34,38,40,46,47,50,53,54,58,64,68,87,96,97,98,100] |
| 3.4.2 | Removing dyes | [20,43,49,51,52,66,82,83,91,99,101,102,103] |
| 3.4.3 | Removing crude oil and petroleum products | [14,15,20,28,42,67,104] |
| 3.4.4 | Removing others harmful pollutants | [20,48,72,75,105,106,107,108] |
| 3.5 | Regeneration and disposal of phytosorbents | [14,15,16,17,36,61,109] |
| Kind of Raw Materials for the Phytosorbents | Production Waste | Origin |
|---|---|---|
| Peat | No | Peat is a sedimentary loose rock that is an ancient accumulation of dead remains of marsh plants |
| Leaves and stems (fern, moss, lichen, reindeer moss, tea, bamboo, swamp plants, sugarcane bagasse) | No/Yes | These are plants growing in natural clusters/waste from tea production, sugar, or fodder preparations for animals |
| Sawdust and bark (pine, poplar, ash, palm) | Yes | Sawdust and bark are waste products that are generated in woodworking enterprises |
| Branches (maple, birch) | No/Yes | Dry tree branches that can be specially pruned/waste from municipal services of a city which is generated during the pruning of trees |
| Husk (rice, sunflower, coffee) | Yes | The husks of some plants are an agro-waste product |
| Straw (rice, wheat, corn) | Yes | Straw is a waste product of crop production at harvest time |
| Peel (citrus fruits, bananas, cassava, potato) | Yes | The peel is the outer protective layer of a fruit or vegetable that can be removed; this is the waste of the processing agricultural industry, canneries, food industry |
| Fibrous husk (coconut) | Yes | Fibrous husk is a protective layer of a coconut that can be removed; this is the waste of the processing agricultural industry |
| Pulp (coffee berries) | Yes | Waste from coffee processing plants |
| Seed, stones (citrus fruits, grape, dates, rapeseed, pomegranate, olive, ziziphus jujuba) | Yes | This is the waste of the processing agricultural industry, canneries, food industry |
| Sorbent; Country | Metal Ions Removed from Water | Sorbent Preparation Process | Sorption Conditions | Description of Results | Ref. |
|---|---|---|---|---|---|
| Buxus sempervirens tree leaves (BSTLs); Iran | Copper, Cu (II); Zinc, Zn (II); Nickel, Ni (II) | BSTLs were washed with distilled water and dried in shadow; then, they were ground well and sieved from 0.297 to 1.0 mm; 20 g of leaf powder was soaked in 250 mL NaOH solution for 4 h; it was filtered and washed with double-distilled water to remove excess NaOH, then dried at room temperature and stored in a plastic bag. | The 0.1 g BSTL mixture was loaded, along with 10 mL of metal ion (Cu(II), Ni(II), and Zn(II)) solutions of 100–450 mg/L, into 250 mL Erlenmeyer flasks, and these were shaken at a rate of 300 rpm for 2–90 min at room temperature; pH = 2–5. | The MAC for Cu(II), Zn(II), and Ni(II) on BSTLs in a single system were found to be 19.7, 22.1, and 23.4 mg/g, respectively; pH = 5. | [21] |
| Aminated tea leaves (ATLs); Nepal | Zinc, Zn(II); Lead, Pb(II) | Tea leaves were washed with boiling water and dried in an oven; powdered and sieved through a 212 µ sieve; charred with concentrated H2SO4; washed thoroughly with distilled water, and then, dried in an oven; and chemically modified by using hydrazine monohydrate. | 20 mL of metal solution with different concentrations (25–800 mg/L) was mixed with 25 mg of ATLs and shaken for 24 hrs at 150 rpm at 250 °C in a mechanical shaker; pH = 1–6. | The MAC of the adsorbent was found to be 120.8 mg/g for Pb(II) and 79.76 mg/g for Zn(II). | [22] |
| Chemically modified Pteris vittata leaves (CMPVL); Pakistan | Zinc, Zn(II) | The leaves were washed, dried, and heated for a while in an oven set to 50 °C; then, 100 g of ground leaf was immersed in a 0.1 M HNO3 solution for 24 h; for the neutralization process, 0.1 M NaOH was used; then, leaves were baked in an oven at 100 °C; for activation of biosorbent, the whole mass was added to a 0.1 M solution of calcium chloride and desiccated in an oven. | Zinc sulphate heptahydrate (ZnSO4·7H2O) was used to prepare the initial solutions; for the batch experiment, 0.1 g of CMPVL was stirred with Zn(II) solution (50 mL) for 2 h; the effect of parameters was investigated: T = 20–50 °C, IC = 20–300 mg/L, and SD = 0.01–0.12 g, pH = 2–8, as well as CT = 10–140 min. | The MAC of Zn(II) was 84.74 mg/g; the optimal conditions were IC = 100 mg/L, CT = 2 h, pH = 6, SD = 0.1 g, and T = 30 °C. | [24] |
| Date palm waste bark (DPW); Saudi Arabia | Chromium, Cr(VI) | DPW was washed with hot water, followed by rinsing with acetone, sun-dried for a period of 4–5 days, and ground (0.25 to 0.5 mm). Further, it was mercerized with 40 wt.% KOH for a duration of 2 h; then, rinsed with distilled water and dried (24 h) at 60 °C in an oven. | A stock solution of chromium was prepared by dissolving 2.8 g of K2Cr2O7 in one liter of distilled water; pH was changed from 2 to 9 by the addition of 0.1 n solution alkali; the ICs were 25, 50, 75, 100, 125, and 150 mg/L. | Optimal chromium Cr(VI) uptake was achieved at a solution pH of 6.5 over two hours, with removal efficiency of 88% and MAC = 22.26 mg/g. | [34] |
| Rice husk; Pakistan | Chromium Cr(VI) | The rice husk was collected from a rice mill; it was sun-dried and oven-dried (65 °C) for 2 and 3 days, respectively; then, it was ground thoroughly after drying and sieved (size < 250 µm). | To conduct the isotherm study, IC was varied from 10 to 250 mg/L with CT = 2 h and SD = 0.6 g/L. | Equilibrium time was achieved in 2 h and maximum Cr(VI) adsorption was 78.6% at pH 5.2 and IC = 120 mg/L; MAC= 379.63 mg/g. | [38] |
| Sunflower husks; Russia | Chromium, Cr(VI) | Sunflower husks were treated with a 2.5 M solution of sulfuric acid at a temperature of 60 °C for 30 min, washed with distilled water, and dried at a temperature of 105 °C to a constant weight. | IC of model solution Cr(VI) was 10 mg/L; the flasks were shaken for 2 h on an ES 20/60 orbital shaker–incubator at room temperature; then, the chromium concentration in the filtered sample was measured. | MAC = 0.44–0.85 mg/g. | [40] |
| Sugarcane bagasse (SCB) and orange peels (OPs); South Africa | Lead, Pb(II) | The SCB and OPs were washed, air-dried in a clean room for 7–10 days, ground using an electric grinder, and sieved using a sieve with a pore size of 5 µm to obtain particles of uniform size. | The IC of Pb(II) was changed from 10 to 50 mg/L; SD = 0.01–0.35 g/L; pH = 2–12; CT = 60–120 min. | Optimum experimental conditions could achieve up to 100% removal efficiencies for 10–20 mg/L of Pb(II). | [46] |
| Orange peels; Algeria, Italy, France | Chromium, Cr(VI) | Orange peels were washed, dried in the sun, and crushed; before use, they were dried at T = 105 °C to a constant weight. | IC of Cr(VI) was changed from 10 to 80 mg/L; SD = 10 g/L. | The highest removal efficiency was 99.2%, and it was achieved in an acidic solution (pH = 2) after 5–15 min. | [47] |
| Pomelo peel; China | Lead, zinc, chromium, copper | Co-pyrolysis of Fenton sludge (FS) and pomelo peel (PP) at different temperatures (300–600 °C) was performed (the mass ratio of 1:1). | Modified PP was used to recover metals from sewage sludge after oxidation in co-pyrolysis. | The recovery rates of Cu, Zn, Cr, and Pb were 71.77–86.85%, 84.0–95.8%, 89.78–104.25%, and 87.24–101.90% respectively. | [50] |
| Rapeseed waste biomass (RWB); Romania | Lead, Pb(II); mercury, Hg(II) | Rapeseeds were washed, dried for 6 h at 105 °C, ground, and sieved; the obtained rapeseed biomass was then subjected to an oil extraction process, using n-hexane as solvent, to obtain rapeseed waste biomass (RWB); then RWB was air-dried for 3 days at room temperature. | To obtain a stock solution of metal ions (10−2 mol/L), lead nitrate and mercuric nitrate were used; pH = 1.0–6.5; the concentration of metal ions was kept constant 40 mg/L; SD = 4.0–20.0 g/L; CT = 3 h; T = 10–55 °C. | The MAC was higher in the case of Pb(II) (61.97 mg/g) than in the case of Hg(II) (51.32 mg/g); optimal pH = 6.5 for Pb(II) and pH = 4.0 for Hg(II); SD = 4.0 g/L; CT = 3 h; T = 25 °C. | [53] |
| Pomegranate seed waste; Iran | Chromium, Cr(VI) | The pomegranate seed powder was separated and rinsed with deionized water; after boiling in water for 2 h, the seeds were dried at a temperature between 100 and 105 °C in an air oven for 24 h, then milled; standard sieves with 40–100 mesh sizes were used to sieve the adsorbent. | The adsorption studies were conducted at room temperature (25 ± 2 °C); CT = 15–180 min; pH = 2–6 and IC = 10 mg/L; SD = 0.2–0.6 g/100 mL. | MAC = 3.313–1.6 mg/g. | [54] |
| Linden and poplar sawdust; Serbia | Iron, Fe(II); manganese, Mn(II); zinc, Zn(II); copper, Cu(II) | The sawdust was sieved through a set of laboratory sieves with a sieve fraction < 0.4 mm. | Sorbent samples (1 g) and 50 mL of metal ion solution were placed in beakers equipped with magnetic stirrers; the contact time was ensured by the stirring. | MAC is achieved at 3.5 < pH < 5. It was found that poplar and linden sawdust have almost equal MACs against copper ions. Removal efficiency of Cu was about 80%; Fe, above 10%; Zn and Mn were between these two. | [58] |
| Treated olive stone (TOS); Algeria, France | Cadmium, Cd | TOS material was prepared by treatment of olive stones (after washing, drying, and grinding) with concentrated sulfuric acid at room temperature followed by a subsequent neutralization with 0.1 M NaOH aqueous solution. | An aqueous solution of safranine was used. The sorption process occurred in less than 15 min of contact time. | MAC of cadmium was 128.2 mg/g. | [64] |
| Heat-inactivated hybrid biosorbent from date seed waste (HI HB); Pakistan | Lead, Pb(II) | The pre-washed, oven-dried (60 °C) date seed waste was autoclaved for 15 min at 121 °C; then, 5.0 ± 0.025 mL of Ganoderma lucidum mycelium suspension was added and agitated at 100 rpm, for 7 days at 30 °C (HB); the HB was autoclaved at 121 °C for 15 min, and then, oven dried for 72 h at 70 °C to obtain heat-inactivated hybrid biosorbent (HI HB). | The effects of pH = 2–4.5, SD = 0.05–0.3 g/L, IC = 25–400 ppm, and T = 30–70 °C were checked by varying one parameter while keeping the other parameters constant. The effects of the presence of metal ions (Mg, Al, Cu, and Zn) on the MAC of the immobilized HI HB in a binary system were also studied. | MAC of immobilized HI HB was 365.9 mg/g at IC = 100 mg/L, with the Langmuir isotherm model presenting the best fit. | [68] |
| Maple wood sawdust; Canada | Copper, Cu(II) | The maple wood sawdust samples were sieved through 20–50 mesh and was used directly for adsorption experiments without any physical or chemical treatments. | The required concentrations of Cu(II) were obtained from the 1000 mg/L reference solution by diluting with distilled deionized water to concentrations of 5.0–100 mg/L. | The isotherms studies revealed that an MAC of 9.51 mg/g for maple wood sawdust was obtained at pH = 6.0. The experimental MAC value was only 6.1 mg/g. | [87] |
| Spent coffee grounds (SCGs); Korea | Cadmium, Cd(II) | The collected SCGs were air-dried for two weeks, and then, passed through a 0.5 mm sieve; the sieved SCGs were stored in polyethylene bottles until used and were not subjected to any physical or chemical pretreatment prior to use. | The SCGs (1 g) were reacted with 40 mL of Cd(II) solution (IC = 0.1–120 mM) prepared in a 2 mM Ca(NO3)2 solution, using a shaker for 2 h. The amount of ions in the 0.45 µm filtrate was determined using ICP-OES after acidifying with 2% HNO3. | The rate of Cd(II) removal remained constant, at 71.19%; pH = 4–8. MAC =19.32 mg/g. | [96] |
| Cassava; China | Chromium, Cr(VI) | Activated carbon (ACDCS) based on dewatered cassava sludge (DCS) and ZnCl2 was obtained. The activated DCS was pyrolyzed and carbonized at T = 673–973 K for a time of 30–120 min. After cooling, the obtained samples were fully pickled with 1.0 mol/L HCl solution, and washed. | K2Cr2O7 (2.829 g) was dissolved in 1 L of ultrapure water to prepare a Cr(VI) stock solution (1000 mg/L). The IC of Cr (VI) was 1–100 mg/L. The effects of SD = 0.2–2.5 g, pH = 2–13, CT = 0–180 min, and T = 283–323 K on Cr(VI) removal were investigated. | MAC= 8.01 mg/g; CT = 3 h; SD = 1 g/L; pH = 2. | [97] |
| Peat; Russia | Copper, Cu(II) | Used: (1) natural peat (NP); (2) mechanically activated peat in a planetary mill (MAP); (3) modified peat with mechanochemical activation by dry sodium percarbonate (MCAP). | The adsorption of copper ions by NP, MAP, and MCAP was studied for IC = 10–150 mg/L with a CT of 0.25–12 h. | MAC = 24.1 mg/g (for NP); MAC = 42.1 mg/g (for MAP); and MAC = 16.0 mg/g (for MCAP). | [98] |
| Raw Materials; Country | Dye Type | Sorbent Preparation Process | Sorption Conditions | Description of Results | Ref. |
|---|---|---|---|---|---|
| Peat; Russia | Methylene blue (MB) | Washing, drying (T = 20 °C), and microwave treatment of the peat samples. | With microwave power from 60 to 600 W for 60 min. | With increasing power, the adsorption of MB decreased by 2 times: from 55 to 28 mg/g. | [20] |
| Banana peels; Ethiopia | Reactive blue 19 | The banana peels were washed, dried, and crushed. Further, 20 g of the sample was subjected to 350 °C at a rate of 10 °C/min for 3 h in a furnace under an N2 environment; then, samples were treated by sulfuric acid solution at T = 50–90 °C. | CT = 20–140 min, pH = 1.0–7.0, SD = 1–4 g/L, and IC = 20–80 mg/L. | The removal efficiency of reactive blue 19 achieved was 70–94%; CT = 60 min, pH = 3, SD = 2 g/L, and IC = 40 mg/L. | [49] |
| Coconut shells (CS), coconut cellulose (CC), and treated coconut cellulose (MCC); Colombia | Anionic dye— Congo red (CR) | Coconut shells were rinsed, dried (60 °C), and ground to a size of 0.8–0.35 mm (CS); CS submerged in 4% NaOH solution, then mixed (80 °C) for 2 h (CC); CC contacted with 10% NaClO2 solution and liquid CH3COOH added and mixed for 24 h (MCC). | 5 mL of the solution was set in contact with the adsorbent at 250 rpm at room temperature for 24 h; the final RC concentration was measured by spectrophotometer; the ICs were 25, 50, 75, 100, 125, and 150 mg/L. | MCC achieved a removal efficiency for CR of 99.9%. CS showed slow kinetics in the initial stages, whereas CC and MCC achieved 78% and 99.98% removal at CT = 120 min, respectively; an equilibrium was reached at 480 and 20 min, respectively. MCC, CC, and CS achieved MACs of 256.12 mg/g, 121.62 mg/g, and 17.76 mg/g, respectively. | [51] |
| Orange seed (OS) powder; Peru | Dyes from real textile waste- water (TW) | Seeds were washed with distilled water, dried (at 60 °C), and ground using a hand mill. The obtained powder was submitted to fat extraction for 6 h in a Soxhlet using n-hexane. | The ranges were pH = 2–6, SD = 0.5–2.5 g/L, stirring speed = 80–160 rpm, T = 25–35 °C, and CT = 60–120 min. All of the experiments were carried out using 0.05 L of TW. | An adsorption process using fat-free orange seed powder successfully removed (92%) the dyes from TW; optimal CT = 30 min. | [52] |
| Treated olive stone (TOS); Algeria, France | Safranine | TOS material was prepared by treatment of olive stones with concentrated sulfuric acid at room temperature followed by a subsequent neutralization with 0.1 M NaOH aqueous solution. | An aqueous solution of safranine was used. The sorption process occurred in less than 15 min of CT. | MAC = 526.3 mg/g. | [66] |
| Aspidosperma polyneuron sawdust; Colombia | Methylene blue (MB) | The sawdust was washed, dried in the sun for 6 h, and then, in an oven at 70 °C for 8 h. The dry sawdust was introduced into a muffle with a heating rate of 5 °C/min up to 250 °C. Activation by sonication with H3PO4 and functionalization with urea (6 M). | To investigate the effect at IC = 60 ppm; pH = 7. | The obtained MAC was 12.4 mg/g due to its favorable physico-chemical properties derived from sonication, activation with phosphoric acid, and functionalization. | [82] |
| Coconut shell (CS-HC) and oak wood hydrochars (oak-HC); UK | Methylene blue (MB) | About 192 g of the biomass was mixed with 798 mL of distilled water in a 2-L batch autoclave; HC was conducted by heating the biomass–water mixture in the sealed autoclave at three different temperatures of 200, 220, and 250 °C for a residence time of 2 h. | The IC of MB was 50–300 mg/L; CT = 0–240 min; and pH = 2–12. | Efficiency of extracting MB from water on hydrochars: for CS-HC it was 80.22–95.01%, for oak-HC it was 90.18–93.42%. | [83] |
| Rice straw biochar (RSB) and magnetic rice straw biochar (MRSB); Vietnam | Organic dye— Safranin O (SO) | RSB was made by pyrolysis in a furnace at 500 °C, using a heating rate of 10 °C/min for 2 h in an oxygen-limited environment; the MRSB was produced via the chemical precipitation of Fe2+ and Fe3+ (pH = 10). | To determine the optimal conditions, a series of preliminary tests were performed with various solutions: pH = 2–10, SD = 1–5 g/L, IC = 10–200 mg/L, and CT = 1–720 min. | The MAC of MRSB was found to be 41.59 mg/g; for RSB the MAC was 31.06 mg/g. | [43] |
| Walnut shell (WS); China | Masterbatch dye (MB) | Supercritical carbon dioxide (SC-CO2) pretreatment technology was developed to prepare porous biochar from WS (200–400 °C); next, the biochar was activated with KOH solution, followed by heat treatment (700 °C) and washing with hydrochloric acid. | IC = 0.1–5 mg/L; activated and non-activated sorbents were tested. | The MAC was from 129.5 to 540 mg/g depending on sample preparation. | [91] |
| Mandarin peel; Korea | Methyl orange (MO) and fast green (FG) | The mandarin peel biochar (M-biochar) and chemical activated biochars by NH4Cl (MN-biochar) and ZnCl2 (MZ-biochar) were used. The peels were washed, dried, crushed, and heated to T = 700 °C using N2 gas. | The SD was 0.1–3 g/L; pH = 7; 25 mL of solution (MO or FG) was added; the concentration of each dye was 10 mg/L. | The best results were shown by the MZ-biochar, which extracted 93–99% of the MO and 87–99% of the FG. Efficiency of M-biochar was only 1–8%, and of MN-biochar 7–24%. | [99] |
| Coffee grains, almond shells, pistachio shells, date pits, jute sticks, sunflower shells, peanut shells, and grapevine sticks; Palestine | Methylene blue (MB) | 8 samples of biochar (BC) and activated carbon (AC) were prepared from the biomass of plant raw materials: grinding, drying, and washing of raw materials; activation of raw materials with a solution of ZnCl2 during heat treatment at T = 380 °C (in case of AC); production of BC and AC by pyrolysis with T = 700 °C for 1 h. | To investigate the effect, IC = 25–300 mg/L was tested. In these experiments, the adsorption tests were conducted with SD of 0.5% w/v, at a pH of 7.0 for an experimental time of 24 h. | The efficiency of MB removal with biochars was 77.2–99.94%, with the lowest results for almond shells (89%) and date pits (77.2%), for other sorbents it was 90% and higher. The efficiency of dye removal with activated carbon turned out to be excellent, up to 100%, while it was lowest for coffee beans (80%). | [101] |
| Burmese grape seed (BGS); Bangladesh, Italy | Methylene blue (MB) | The collected BGSs were chopped, air-dried for 2 days, mixed with a solution of H3PO4 (40%) in a weight ratio of 1:2, followed by drying in an air oven at 80 °C for 3 h; then, mixed with 50% (w/v) KOH solution. Then, this was dried at 100 °C for 2 h. Finally, the particles were carbonized at T = 500 °C for 3 h to be converted into biochar. | The SD for each experiment was 5 mg. The influence of pH on adsorption was investigated for pH between 3.0 and 9.0. Kinetic tests were performed using an MB dye with IC = 60 mg/L at T = 27 °C and examined at a time ranging from 5 to 90 min. | The maximum removal percentage was ~85%, and MAC = 166.30 mg/g. | [102] |
| Ziziphus jujuba stones (ZJS); Algeria | Methylene blue (MB) | The stones were crushed and sieved to obtain a powder, which was washed, and dried at 50 °C for 24 h before undergoing treatment; 1 g of the ZJS powder was mixed with 1 g of the solution of H3PO4 (1 M) and stirred with a magnetic stirrer for 24 h at room temperature. | IC = 50–500 mg/L; 50 mg ZJS mixed with 50 mL solution; CT = 24 h, and pH = 2–12. | The H3PO4 treatment significantly and positively enhanced adsorption performance, with the MAC increasing from 62.25 mg/g for untreated ZJS to 160.85 mg/g for H3PO4-treated ZJS. A 100% efficiency for MB removal was achieved at pH = 10. | [103] |
| Raw Materials; Country | Pollutant | Sorbent Preparation Process | Sorption Conditions | Description of Results | Ref. |
|---|---|---|---|---|---|
| Moss, reindeer moss | Crude oil, oil product | Washing, drying (T = 20 °C), and microwave treatment of the moss and reindeer moss samples. | With microwave power of 600 W for 1 min for initial concentrations of oil products dissolved in water of 250 mg/L. | Sorption capacity of oil products increased by 10–15%; the MAC was reached at 326–338 mg/g. | [14] |
| Peat | Crude oil, oil product | Washing, drying (T = 20 °C), and microwave treatment of the peat samples. | With microwave power of 600 W for 1 min for initial concentrations of oil products dissolved in water of 250 mg/L. | Peat sorption capacity of oil products increased by 7.5%; the MAC was reached at 408.1 mg/g. | [14] |
| With microwave power from 60 to 600 W for 60 min. | MAC = 2.5–2.73 g/g. | [20] | |||
| Maple and birch branches | Petroleum product | Grinding, washing, drying (T = 105 °C), and microwave treatment of the samples. | With microwave power of 600 W for 1 min. | Increase in the MAC for petroleum products by 7.2–30%. | [15] |
| Pine sawdust | Petroleum product | Grinding, washing, drying (T = 105 °C), and microwave heating of pine sawdust. | With microwave power of 600 W for 1–2 min; initial concentration of model solutions was from 5 to 35 mg/L. | Increase in the MAC for petroleum products by 3.7–4 times for IC of less than 5 mg/L and by 1.2–1.3 times for IC = 16–35 mg/L. | [15,28] |
| Rice husk | Petroleum product | Combustion in a microwave furnace. | Treatment at T = 500–800 °C for 288–384 h. | The efficiency of petroleum product removal from water was 78–98%. | [67] |
| Rice straw (RS); Malaysia | Diesel | The RS was cut to 4–5 cm in length from the base throughout the length of the stalk; it was washed twice thoroughly with tap water to remove debris and sun-dried for 7 d (5 h per day) until it reached a constant mass; heat pretreatment (90–140 °C, 10–70 min), and diesel concentration 5–30%. | 12 g of RS sample was placed in the holder, which was then placed in a plastic bottle (T = 25 °C); the mixture of diesel (40 mL) and seawater (400 mL) was then poured into the bottle; the mass of each RS sample was measured after 10 min contact time. | The pretreated (at T = 120 °C) RS samples displayed the highest level of MAC (2.3 g/g) and also the most efficient oil absorption (51.67%). | [42] |
| Raw Materials; Country | Pollutant | Sorbent Preparation Process | Sorption Conditions | Description of Results | Ref. |
|---|---|---|---|---|---|
| Peat | Iodine | Washing, drying (T = 20 °C), and microwave treatment of the peat samples. | With microwave power from 60 to 600 W for 60 min. | With increasing power, the MAC of iodine increased by 1.2–1.4 times (from 115 to 150 mg/g). | [20] |
| With microwave power of 900 W for 12 min until 450 °C. | Iodine adsorption activity increased from 11.4% to 19.1%. | [75] | |||
| Banana peels; Malaysia, Korea, Saudi Arabia | Turbidity | Modified banana peel powder was prepared using a green approach, consisting of microwave treatment at a power of 800 W for 0.5 min. | For all of the experiments, synthetic turbid water with kaolin clay was used. NaCl was also added to the water for intensification of coagulation. | The optimum sorbent dose was found to be 0.4 g/L for modified banana peel, with turbidity removal of up to 90%. | [48] |
| Composite based on bamboo side shoots; China | Arsenic (As) | Porous α-Fe2O3/Fe3O4/C composite with the bamboo bio-template (PC-Fe/C-B compo-site). Wastewater was filtered through the fixed-bed column. | Influent flow was 5.136 mL/min, pH = 3, As(V) concentration was 20 mg/L, adsorbent particle size < 0.149 mm, adsorption temperature was 35 °C, PC-Fe/C-B dose was 0.5 g, and breakthrough time was 50 min. | MAC = 21.0 mg/g. | [72] |
| Modified cellulose from corn stalks; Colombia | Nitrate and phosphate | Cellulose was obtained from the CS, and dried for 3 h at 60 °C; it was mixed with 100 mM CTAC; it was rinsed and dried. The biochar was prepared by impregnating the biomass for 24 h with H2SO4 diluted at 50% v/v; the carbonization was performed at 520 °C for 30 min. | Equilibrium experiments were conducted using five different concentrations of the anions from 20 to 100 mg/L for 24 h, using a volume of 100 mL and 2 g/L of adsorbent (T = 25–45 °C). In the study of adsorption kinetics, the contact time changed within the limits of 5–1440 min. | The best MACs obtained for nitrate and phosphate were 15.8 and 23.2 mg/g, respectively. | [105] |
| Rice and corn husk; Colombia | Nitrate and phosphate | Rice husk biochar with/without activation used; corn straw biochar chemically modified with chemical activation. | No data. | It was found that 95–99% of nitrogen and phosphorus can be removed with biosorbents made from rice husks and corn residues. | [106] |
| Cassava peels; Uganda | Pharmaceuticals: carbamazepine (CBZ), clarithromycin (CLN), and trimethoprim (TRM) | Dry peels of the cassava were pulverized and soaked in 150 mL of 4.0% w/v NaOH; it was placed in a platinum crucible and heated to 400–900 °C for 20 to 180 min; thus, an activated carbon CPAC was obtained. | Initial concentration of 20 mg/L for all pharmaceuticals and a CPAC dosage of 2.0 g/L were used. | The MACs were 25.907, 84.034, and 1.487 mg/g for CBZ, TRM, and CLN, respectively. | [107] |
| Orange peels; Hungary, Iraq, and others | Pharmaceuticals: carbamazepine (CAR) and diclofenac (DIC) | The orange peels were washed, dried, and crushed. Then, at a temperature of 550 °C, pyrolysis was performed. Next, activated carbon (MOP) was obtained using 5 m solutions of sulfuric and nitric acid. Then, it was washed to pH = 7 and dried. Also, LMOP was obtained by immobilization of laccase. | Initial concentration of 25 mg/L for two pharmaceuticals and sorbent dosages of 50 mg mixed with 20 mL aqueous solution, were used. | MOPs revealed removal efficiencies of 73.34% and 82.51% for CAR and DIC, respectively. The LMOPs had 81.98% and 90.53% removal percentages for CAR and DIC, respectively. | [108] |
| Advantages | Problem Areas |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vialkova, E.; Korshikova, E.; Fugaeva, A. Phytosorbents in Wastewater Treatment Technologies: Review. Water 2024, 16, 2626. https://doi.org/10.3390/w16182626
Vialkova E, Korshikova E, Fugaeva A. Phytosorbents in Wastewater Treatment Technologies: Review. Water. 2024; 16(18):2626. https://doi.org/10.3390/w16182626
Chicago/Turabian StyleVialkova, Elena, Elena Korshikova, and Anastasiya Fugaeva. 2024. "Phytosorbents in Wastewater Treatment Technologies: Review" Water 16, no. 18: 2626. https://doi.org/10.3390/w16182626
APA StyleVialkova, E., Korshikova, E., & Fugaeva, A. (2024). Phytosorbents in Wastewater Treatment Technologies: Review. Water, 16(18), 2626. https://doi.org/10.3390/w16182626







