Biomonitoring of Waters and Tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil
Abstract
1. Introduction
2. Materials and Methods
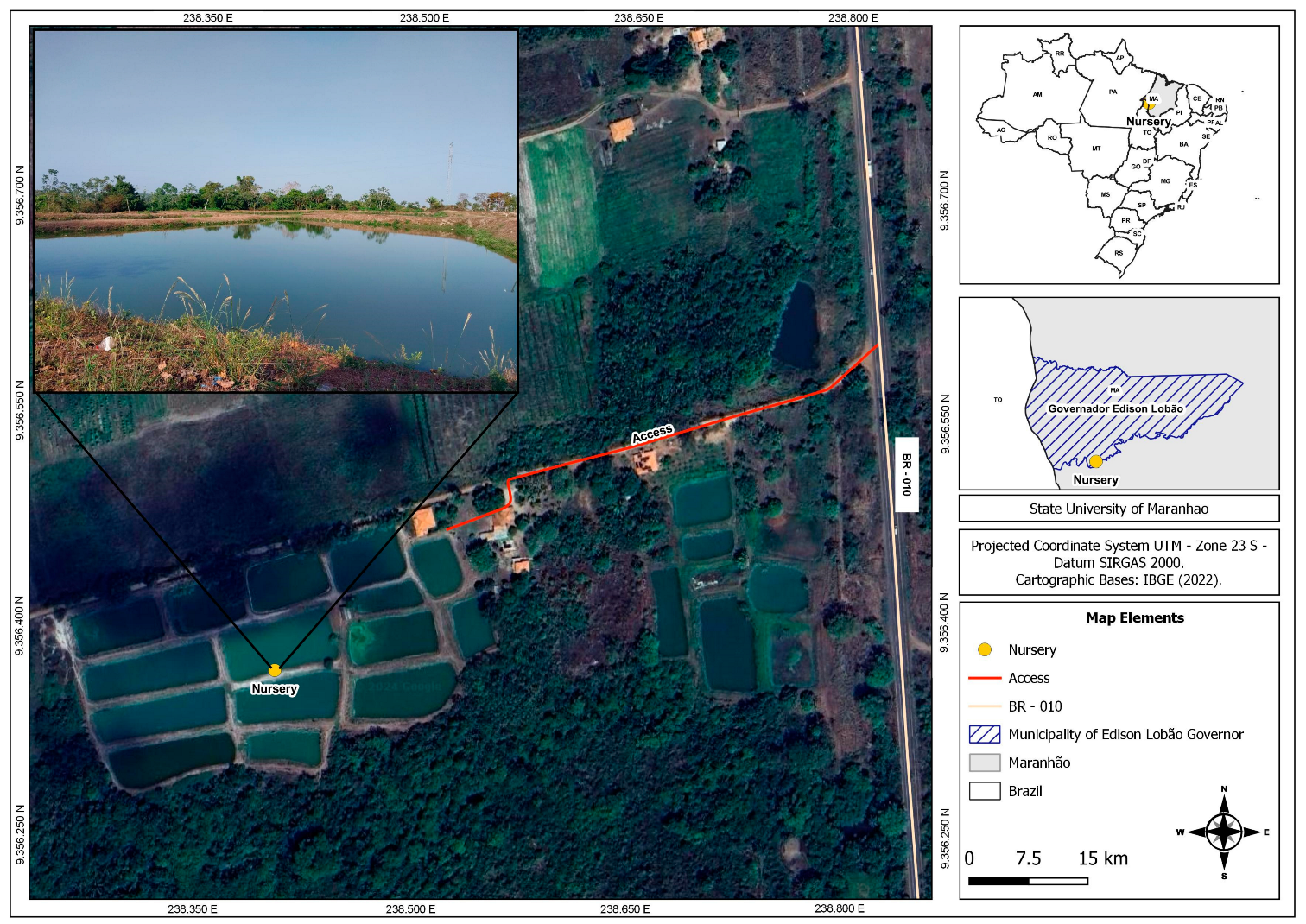
2.1. Study Area and Ethical Standards
2.2. Fish and Water Sampling
2.3. Water Physicochemical Analysis
2.4. E. coli Analysis of Fish Muscles
2.5. E. coli Analysis of Water
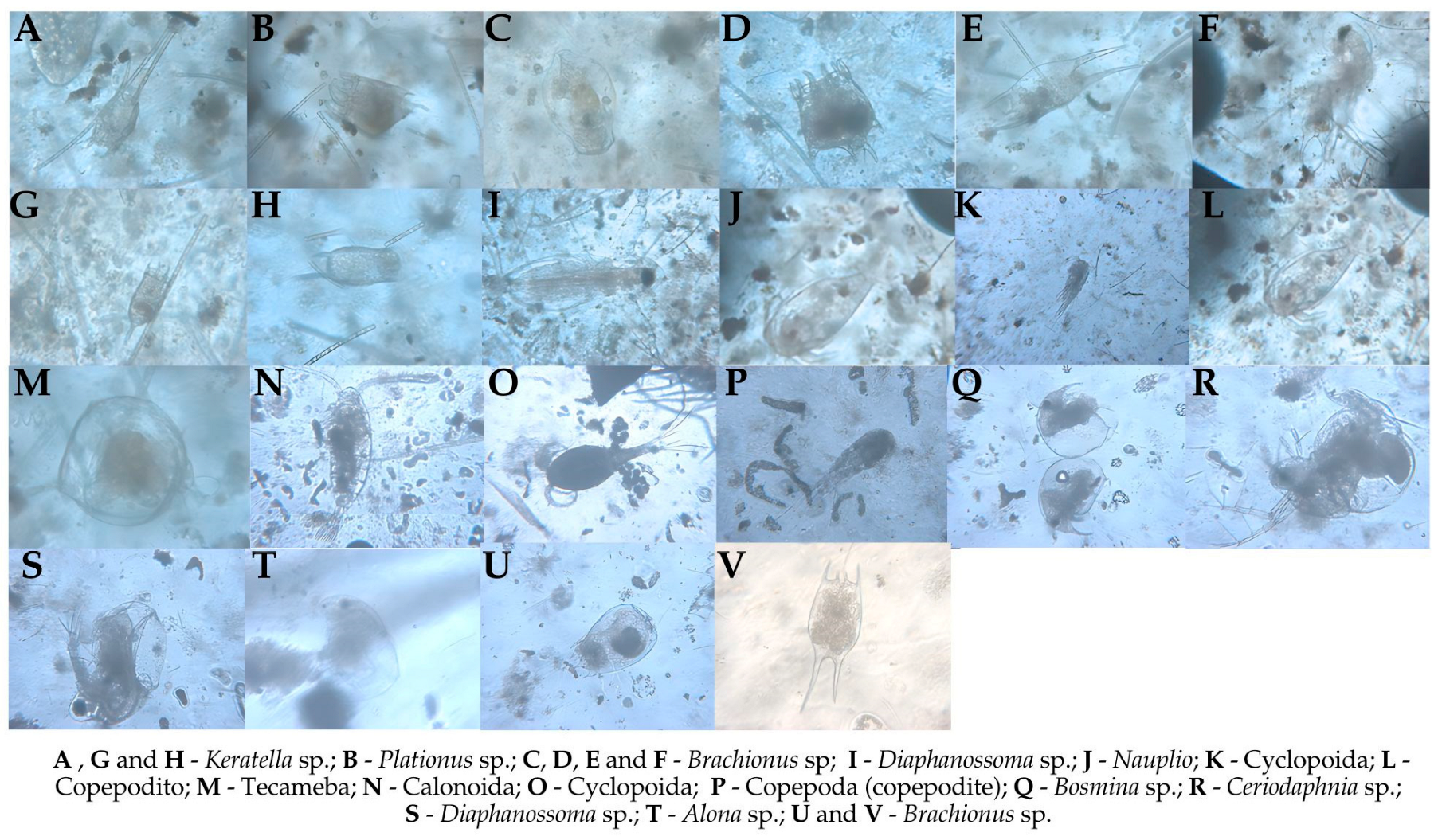
2.6. Qualitative Analysis of Zooplankton Community
2.7. Environmental Variables
2.8. Analysis of Data
3. Results
3.1. Water Physicochemical Parameters
3.2. E. coli Analysis of Fish Muscles, Water, and Environmental Variables
3.3. Zooplankton Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO no Brasil. Uma Produção Pesqueira e Aquícola Sem Precedentes Contribui Decisivamente Para a Segurança Alimentar Global. 2022. Available online: https://www.fao.org/brasil/noticias/detail-events/es/c/1585153/ (accessed on 10 December 2022).
- Cojocaru, A.L.; Liu, Y.; Smith, M.D.; Akpalu, W.; Chávez, C.; Dey, M.M.; Dresdner, J.; Kahui, V.; Pincinato, R.B.; Tran, N. The “seafood” system: Aquatic foods, food security, and the Global South. Rev. Environ. Econ. Policy 2022, 16, 306–326. [Google Scholar] [CrossRef]
- Bjorndal, T.; Dey, M.; Tusvik, A. Economic analysis of the contributions of aquaculture to future food security. Aquaculture 2024, 578, 740071. [Google Scholar] [CrossRef]
- Fong, C.R.; Gonzales, C.M.; Rennick, M.; Gardner, L.D.; Halpern, B.S.; Froehlich, H.E. Global yield from aquaculture systems. Rev. Aquac. 2024, 16, 1021–1029. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Viana, D.C. Current status of aquaculture in the world: COVID-19 first impacts. RSD 2020, 9, e462985798. [Google Scholar] [CrossRef]
- Silva, F.N.L.; Paes, A.C.; Mendonça, R.C.; Quadros, M.L.A.; Oliveira, L.C.; Silva, O.L.L. Challenges in the aquaculture production chain in Curralinho, Marajó archipelago, Pará, Brazil. Braz. J. Develop. 2020, 6, 27598–27616. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Guimarães, E.C.; Santos, J.P.; Costa, F.N.; Viana, D.C. Piscicultura no estado do Maranhão: Perspectivas para aceleração da produção de peixes nativos. Sci. Plena 2022, 18, 027401. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, D.; Jiang, Q. Chinese Freshwater aquaculture: A comparative analysis of the competitiveness on regional aquaculture industries. Aquac. Fish. 2022, 9, 860–870. [Google Scholar] [CrossRef]
- Yue, K.; e Shen, Y. An overview of disruptive technologies for aquaculture. Aquac. Fish. 2022, 7, 111–120. [Google Scholar] [CrossRef]
- Lal, J.; Vaishnav, A.; Kumar, D.; Jana, A.; Jayaswal, R.; Chakraborty, A.; Kumar, S.; Pavankalyan, M. Emerging Innovations in Aquaculture: Navigating towards Sustainable Solutions. Int. J. Environ. Clim. Chang. 2024, 14, 83–96. [Google Scholar] [CrossRef]
- Chan, H.L.; Cai, J.; Leung, P. Aquaculture production and diversification: What causes what? Aquaculture 2024, 583, 740626. [Google Scholar] [CrossRef]
- Yue, G.H.; Tay, Y.X.; Wong, J.; Shen, Y.; Xia, J. Aquaculture species diversification in China. Aquac. Fish. 2023, 9, 206–217. [Google Scholar] [CrossRef]
- Mridul, M.M.I.; Zeehad, M.S.K.; Aziz, D.; Salin, K.R.; Hurwood, D.A.; Rahi, M.L. Temperature induced biological alterations in the major carp, Rohu (Labeo rohita): Assessing potential effects of climate change on aquaculture production. Aquac. Rep. 2024, 35, 101954. [Google Scholar] [CrossRef]
- Mitra, S.; Khan, M.A.; Nielsen, R.; Kumar, G.; Rahman, M.T. Review of environmental challenges in the Bangladesh aquaculture industry. Environ. Sci. Pollut. Res. 2024, 31, 8330–8340. [Google Scholar] [CrossRef]
- Arruda, M.C.F. Avaliação Dos Indicadores da Política de Pesca do Programa Zona Franca Verde: Perspectivas Econômicas e Ambientais; Universidade Federal do Amazonas: Manaus, Brasil, 2017. [Google Scholar]
- Vasconcellos, A.C.S.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.M.; Lima, M.O.; Jesus, I.M.; Hacon, S.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.M.; Ferreira, A.H.C.; Santana Júnior, H.A.; Oliveira, A.P.A.; Santos, C.H.L.; Oliveira, L.T.S. Desempenho zootécnico de juvenis de tilápias do nilo (Oreochromis niloticus) alimentados com cepas probióticas e submetidos a desafio sanitário. Ciência Anim. Bras. 2019, 20, e-37348. [Google Scholar] [CrossRef]
- Nascimento, I.R.M.A.; Souza, A.C.F.; Silva, L.R.; Bezerra, C.A.M.; Sousa, R.R.; Abreu, A.S.; Sousa, D.S.; Serra, I.M.R.S.; Bezerra, N.P.C.; Cantanhede, S.P.D. Patógenos em peixes de ambientes naturais e de cultivo no Estado do Maranhão: Uma visão geral e perspectivas para pesquisa. Res. Soc. Dev. 2021, 10, e15910716284. [Google Scholar] [CrossRef]
- Medina-Morillo, M.; Sotil, G.; Arteaga, C.; Cordero, G.; Martins, M.L.; Murrieta-Morey, G.; Yunis-Aguinaga, J. Pathogenic Aeromonas spp. in Amazonian fish: Virulence genes and susceptibility in Piaractus brachypomus, the main native aquaculture species in Peru. Aquac. Rep. 2023, 33, 101811. [Google Scholar] [CrossRef]
- Timi, J.T.; Buchmann, K. A century of parasitology in fisheries and aquaculture. J. Helminthol. 2023, 97, e4. [Google Scholar] [CrossRef]
- Alves, L.S.A.; Oliveira, L.B.; Santos, K.F.S.; Jesus, G.S.; Sousa, G.A.P.; Bastos, L.S.; Bezerra, D.C.; Serra, I.M.R.S.; Cantanhede, S.P.D.; Bezerra, N.P.C. Qualidade Microbiológica da Água Para Fins de Aquicultura no Estado do Maranhão: Levantamento das Análises Realizadas em Laboratório de Controle da Qualidade no Período de 2015 a 2021. Tecnol. Microbiol. Sob Perspect. Segurança Aliment. 2022, 2, 140–150. [Google Scholar] [CrossRef]
- Yunis-Aguinaga, J.; Sotil, G.; Morey, G.A.M.; Fernandez-Espinel, C.; Flores-Dominick, V.; Rengifo-Marin, G.; Claudiano, G.S.; Medina-Morillo, M. Susceptibility of the cultured Amazonian fish, Colossoma macropomum, to experimental infection with Aeromonas species from ornamental fish. Microb. Pathog. 2024, 186, 106461. [Google Scholar] [CrossRef]
- Instituto Nacional de Pesquisas Espaciais (INPE). Desflorestamento; Instituto Nacional de Pesquisas Espaciais (INPE): Sao Paulo, Brazil, 1997. [Google Scholar]
- Russo, M.R.; Leal, F.C.; Mendes, S.G.F.; Souza, E.C.V. A Aquicultura Sustentável Como Alternativa de Geração de Renda; Mauad, J.C., Mussuri, R.M., Eds.; Centro de Desenvolvimento Rural do Itamarati: Relatos e Vivências; Seriema: Dourados, Brazil, 2021; pp. 221–234. [Google Scholar]
- Barroso, G.R.; Pinto, C.C.; Gomes, L.N.L.; Oliveira, S.C. Assessment of water quality based on statistical analysis of physical-chemical, biomonitoring and land use data: Manso River supply reservoir. Sci. Total Environ. 2024, 912, 169554. [Google Scholar] [CrossRef]
- Kadadou, D.; Tizani, L.; Alsafar, H.; Hasan, S.W. Analytical methods for determining environmental contaminants of concern in water and wastewater. MethodsX 2024, 12, 102582. [Google Scholar] [CrossRef]
- Ktari, N.; Kalfat, R. Water Contamination in Fish Farms: Electrochemical Contribution. In Clean Water: Next Generation Technologies; Springer International Publishing: Cham, Switzerland, 2024; pp. 95–106. [Google Scholar]
- Tavechio, W.L.G.; Guidelli, G.; Portz, L. Alternativas para a prevenção e o controle de patógenos em piscicultura. Bol. Inst. Pesca 2018, 35, 335–341. [Google Scholar]
- Mustafa, R.A.; Rather, S.A.; Kousar, R.; Ashraf, M.V.; Shah, A.A.; Ahmad, S.; Khan, M.H. Comprehensive review on parasitic infections reported in the common fish found in UT of Jammu and Kashmir, India. J. Parasit. Dis. 2024, 1–26. [Google Scholar] [CrossRef]
- Kanwal, S.; Noureen, A.; Hayat, S.; Tahir, M.A.A.; Mahmood, S.; Suleman, S. Microbial and Parasitic Infection in Fish: Microbial and Parasitic Infection in Fish. Markhor J. Zool. 2023, 2–11. [Google Scholar] [CrossRef]
- Santos, E.F.; Tavares-Dias, M.; Pinheiro, D.A.; Neves, L.R.; Marinho, R.G.B.; Dias, M.K.R. Fauna parasitária de tambaqui Colossoma macropomum (Characidae) cultivado em tanque-rede no estado do Amapá, Amazônia oriental. Acta Amaz. 2013, 43, 105–112. [Google Scholar] [CrossRef]
- Araújo, K.S.S.; Neres, H.G.C.; Mendes, J.A.C.; Costa, J.F.; Silva, M.L.; Barbosa, L.A.; Viana, D.C. Monogenoidea parasites of tambacu from the cultivation system in the tocantine region of Maranhão. Ciência Anim. 2024, 34, 30–38. [Google Scholar]
- Ishikawa, M.M.; Queiroz, J.F.; Nascimento, J.L.; Pádua, S.B.; Martins, M.L. Uso de Biomarcadores em Peixe e Boas Práticas de Manejo Sanitário Para a Piscicultura. Jaguariúna; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2020. [Google Scholar]
- Gagneten, A.M.; Marchese, M.R. Ambientes Acuáticos de la Província de Santa Fe: Protocolos de Monitoreo con Perspectiva Socioecológica, 1st ed.; Santa Fe: Ediciones UNL; Universidad Nacional del Litoral: Santa Fe, Brazil, 2022; ISBN 978-987-749-332-0. [Google Scholar]
- BRASIL, 2005. Resolução CONAMA n° 357, de 17 de Março de 2005. Dispõe Sobre a Classificação dos Corpos de Água e Diretrizes Ambientais para o Seu Enquadramento, Bem Como Estabelece as Condições e Padrões de Lançamento de Efluentes, e dá Outras Providências. Diário Oficial da República Federativa do Brasil, Brasília, 18 mar., 27 p. Available online: http://www.siam.mg.gov.br/sla/download.pdf?idNorma=2747 (accessed on 15 August 2022).
- Bezerra, C.A.M.; Sousa, A.L.; Viana, D.C. Histopathologic alterations of gill tissue in Siluriformes and Characiformes from the Middle Tocantins River in the Brazilian Amazon. Arq. Bras. Med. Veterinária E Zootec. 2020, 72, 285–289. [Google Scholar] [CrossRef]
- Bezerra, C.A.M.; Cohen, S.C.; Meneses, Y.C.; Neres, H.G.C.; Viana, D.C.; Justo, M.C.N. Two new species of Curvianchoratus (Monogenoidea, Dactylogyridae) parasitizing Psectrogaster amazonica (Characiformes, Curimatidae) and a new record for Curvianchoratus singularis in the Tocantins River, Maranhão, Brazil. ZooKeys 2023, 1172, 101–116. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Antoniassi, N.A.B.; Silva, F.G.; Povh, J.A.; Po-Tença, A.; Moraes, T.C.H.; Silva, T.K.S.T.; Abreu, J.S. Características histomorfométricas do intestino de juvenis de tambaqui após uso de probiótico na dieta e durante transporte. Pesq. Vet. Bras. 2014, 34, 1258–1264. [Google Scholar] [CrossRef][Green Version]
- Timi, J.T.; Mackenzie, K. Parasites in fisheries and mariculture. Parasitology 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Aquicultura com Sanidade Programa Nacional de Sanidade de Animais Aquáticos de Cultivo: Manual Orientado aos Órgãos Executores de Sanidade Agropecuária; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2020; ISBN 978-65-86803-27-3. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Produção da Pecuária Municipal (v.45). 2020. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9107-producao-da-pecuaria-municipal.html?=&t=o-que-e (accessed on 7 May 2021).
- Ndashe, K.; Hang’ombe, B.M.; Changula, K.; Yabe, J.; Samutela, M.T.; Songe, M.M.; Kefi, A.S.; Njobvu Chilufya, L.; Sukkel, M. An Assessment of the risk factors associated with disease outbreaks across tilapia farms in Central and Southern Zambia. Fishes 2023, 8, 49. [Google Scholar] [CrossRef]
- Aly, S.M.; ElBanna, N.I.; Fathi, M. Chlorella in aquaculture: Challenges, opportunities, and disease prevention for sustainable development. Aquac. Int. 2024, 32, 1559–1586. [Google Scholar] [CrossRef]
- Samsing, F.; Barnes, A.C. The rise of the opportunists: What are the drivers of the increase in infectious diseases caused by environmental and commensal bacteria? Rev. Aquac. 2024, 16, 1787–1797. [Google Scholar] [CrossRef]
- Abdel-Galil, M.A.; Shehata, S.; Mohamed, R.A. Impact of Parasitic Infection and Water Quality on the Bagrid Fish, Bagrus bajad, Inhabiting Ismailia Canal Waters, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 405. [Google Scholar] [CrossRef]
- Opiyo, M.; Mziri, V.; Musa, S.; Kyule, D.; Hinzano, S.; Wainaina, M.; Magondu, E.; Werimo, K.; Ombwa, V. Fish disease management and biosecurity systems. State Aquac. Kenya 2020, 97–126. Available online: https://www.researchgate.net/publication/351050775_Fish_Disease_Management_and_Biosecurity_Systems (accessed on 10 July 2024).
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Dar, G.H.; Bhat, R.A.; Kamili, A.N.; Chishti, M.Z.; Qadri, H.; Dar, R.; Mehmood, M.A. Correlation between pollution trends of freshwater bodies and bacterial disease of fish fauna. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 51–67. [Google Scholar]
- Matvienko, N.; Levchenko, A.; Danchuk, O.; Kvach, Y. Assessment of the occurrence of microorganisms and other fish parasites in the freshwater aquaculture of Ukraine in relation to the ambient temperature. Acta Ichthyol. Piscat. 2020, 50, 333–348. [Google Scholar] [CrossRef]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Hardy, R.W.; Kaushik, S.J.; Mai, K.; Bai, S.C. Fish nutrition—History and perspectives. In Fish Nutrition; Academic Press: Cambridge, MA, USA, 2022; pp. 1–16. [Google Scholar]
- Jongjaraunsuk, R.; Taparhudee, W.; Suwannasing, P. Comparison of Water Quality Prediction for Red Tilapia Aquaculture in an Outdoor Recirculation System Using Deep Learning and a Hybrid Model. Water 2024, 16, 907. [Google Scholar] [CrossRef]
- Arias, N.M.O.; Martínez, M.E.S.; Chaupe, N.N.S.; Sousa, A.L.; Morey, G.A.M. Intestinal endoparasitism in Corydoras multiradiatus (Siluriformes: Callichthyidae) in Iquitos, Loreto-Peru. Rev. Ciências Agroveterinárias 2023, 22, 631–639. [Google Scholar] [CrossRef]
- Negreiros, L.P.; Tavares-Dias, M.; Pereira, F.B. Monogeneans of the catfish Pimelodus blochii Valenciennes (Siluriformes: Pimelodidae) from the Brazilian Amazon, with a description of a new species of Ameloblastella Kritsky, Mendoza-Franco & Scholz, 2000 (Monogenea: Dactylogyridae). Syst. Parasitol. 2019, 96, 399–406. [Google Scholar] [CrossRef]
- Poulin, R. Parasite biodiversity revisited. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef]
- Cohen, S.C.; Justo, M.C.N.; Kohn, A. Parasitas Monogenoidea da América do Sul de Peixes, Anfíbios e Répteis; FioCruz: Rio de Janeiro, Brazil, 2013; 662p. [Google Scholar]
- Silva, A.L.S.; Cohen, S.C.; Santos-Clapp, M.D.; Brasil-Sato, M.C.; Costa, A.P.; Justo, M.C.N. Two new species of Anacanthorus (Monogenoidea, Dactylogyridae) parasitizing serrasalmid fish in Brazil. Braz. J. Vet. Parasitol. 2024, 33, e017623. [Google Scholar] [CrossRef]
- Morey, G.A.M.; Rojas, C.A.T.; Dávila, G.V.; Chu, L.A.R.; Pina, C.A.V. New species of Demidospermus (Monogenoidea: Dactylogyridae) from the gills of Pseudoplatystoma punctifer (Siluriformes: Pimelodidae) collected in the Peruvian Amazonia. Syst. Parasitol. 2024, 101, 3. [Google Scholar] [CrossRef]
- Lima, T.A.; Pimentel, S.C.R.; Soares, M.P.; Guimarães, V.A.A.C.; Ribeiro, L.S.; Queiroz, J.F.; Ishikawa, M.M. Avaliação de biomarcadores hematológicos em Tilápia mantida em diferentes sistemas de aquários experimentais. Rev. Obs. De La Econ. Latinoam. Curitiba 2023, 21, 16044–16060. [Google Scholar]
- Lopes, J.M.; Santos, M.D.C.; Gomes, A.M.N.; Pinto, F.E.N.; Sousa, A.W.S.; Marques, N.C. Caracterização da Piscicultura Familiar na Região Do Baixo Parnaíba—Araioses/Ma. Extensio Rev. Eletr. Extensão 2020, 17, 41–60. [Google Scholar] [CrossRef]
- Ribeiro, E.B.; Bastos, L.S.; Galeno, L.S.; Mendes, R.S.; Garinojr, F.; Carvalho-Neta, R.N.F.; Costa, F.N. Integrated assessment of biomarker responses and microbiological analysis of oysters from São Luís Island, Brazil. Mar. Pollut. Bull. 2016, 113, 182–186. [Google Scholar] [CrossRef]
- Santos, E.J.R.; Galeno, L.S.; Bastos, L.S.; Costa, T.F.; Carvalho, I.A.; Costa, F.N. Sanitary hygienic quality of tambaqui (Colossoma macropomum) marketed in the city of São Luís—MA. Cienc. Anim. Bras. 2019, 20, e-46537. [Google Scholar]
- Milijasevic, M.; Veskovic-Moracanin, S.; Milijasevic, J.B.; Petrovic, J.; Nastasijevic, I. Antimicrobial Resistance in Aquaculture: Risk Mitigation within the One Health Context. Foods 2024, 13, 2448. [Google Scholar] [CrossRef]
- CONCEA nº 37/2018, Resolução Normativa CONCEA nº 37, de 15.02.2018. Anexo—Resolução Normativa CONCEA nº 37/2018—Diretriz da Prática de Eutanásia do Conselho Nacional de Controle de Experimentação Animal. Available online: https://antigo.mctic.gov.br/mctic/opencms/legislacao/outros_atos/resolucoes/Resolucao_CONCEA_n_37_de_15022018.html (accessed on 2 February 2021).
- Silva, N.; Junqueira, V.C.A.; Silveira, N.F.A.; Taniwaki, M.H.; Gomes, R.A.R.; Okazaki, M.M. Manual de Métodos de Análise Microbiológica de Alimentos e Água, 5th ed.; Blucher: São Paulo, Brazil, 2017; 535p, ISBN 13 978-8521212256. [Google Scholar]
- Gagneten, A.M. Effects of Contamination by Heavy Metals and Eutrophication on Zooplankton, and their possible effects on the Throphic Webs of Freshwater Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control, 1st ed.; Ansari, A.A., Singh Gill, S., Lanza, G.R., Rast, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; 394p, ISBN 97890-481-9624-1. [Google Scholar]
- Arias, M.J.; Vaschetto, P.A.; Marchese, M.; Regaldo, L.; Gagneten, A.M. Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina. Sustainability 2022, 14, 4045. [Google Scholar] [CrossRef]
- Paz, F.A.Z.E.; Bercini, M.A. Emerging and Reemerging Diseases in the Context of Public Health. Bol. Saúde: Porto Alegre | v. 23 | n. 1 | p. 9-13 | jan./jun. 2009, INFLUENZA (01). p6. ISSN 01021001. Available online: http://www.boletimdasaude.rs.gov.br/conteudo/3224/corpo-editorial (accessed on 20 July 2024).
- Acioly, T.M.S.; Silva, M.F.; Barbosa, L.A.; Iannacone, J.; Viana, D.C. Lev-els of Potentially Toxic and Essential Elements in Water and Estimation of Human Health Risks in a River Located at the Interface of Brazilian Savanna and Amazon Biomes (Tocantins River). Toxics 2024, 12, 444. [Google Scholar] [CrossRef]
- Nascimento, B.L.M.; Gomes, D.R.C.S.; Costa, G.P.; Araújo, S.S.; Santos, L.C.A.; Oliveira, J.D. Behavior and evaluation of potentially toxic metals (Cu(II), Cr(III), Pb(II) and Fe(III)) in surface waters of streams Capivara and streams Bacuri Imperatriz-MA, Brazil. Eng. Sanit. Ambient. 2015, 20, 369–378. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Slingenbergh, J. World Livestock 2013: Changing Disease Landscapes; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; p. 2. [Google Scholar]
- Seben, D.; Toebe, M.; Wastowski, A.D.; Rosa Genésio, M.; Prestes, O.D.; Zanella, R.; Golombieski, J.I. Association Patterns among Physical, Chemical and Microbiological Indicators of Springs in Rio Grande do Sul, Brazil. Water 2022, 14, 3058. [Google Scholar] [CrossRef]
- Jeamsripong, S.; Thaotumpitak, V.; Anuntawirun, S.; Roongrojmongkhon, N.; Atwill, E.R. Meteorological and Water Quality Factors Associated with Microbial Diversity in CoastalWater from Intensified Oyster Production Areas of Thailand. Water 2022, 14, 3838. [Google Scholar] [CrossRef]
- Mahagamage, M.G.Y.L.; Pathirage, M.V.S.C.; Manage, P.M. Contamination Status of Salmonella spp., Shigella spp. and Campylobacter spp. in Surface and Groundwater of the Kelani River Basin, Sri Lanka. Water 2020, 12, 2187. [Google Scholar] [CrossRef]
- Rondón-Espinoza, J.; Gavidia, C.M.; González, R.; Ramos, D. Water Quality and Microbiological Contamination across the Fish Marketing Chain: A Case Study in the Peruvian Amazon (Lagoon Yarinacocha). Water 2022, 14, 1465. [Google Scholar] [CrossRef]
- Oliveira, F.S.L.; Targa, M.S.; Balduíno, R.; Catelani, C.S.; Castro, M.P. Análise das ações antrópicas na bacia hidrográfica do Riacho Bacuri no município de Imperatriz—MA. Rev. Tec. Ciências Ambient. 2021, 5, 1–9. [Google Scholar]
- Kumar, V.; Sharma, A.; Kumar, R.; Bhardwaj, R.; Kumar Thukral, A.; Rodrigo-Comino, J. Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1–16. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, A.; Shukla, P. Estimation of heavy metal contamination in the Hindon River, India: An environmetric approach. Appl. Water Sci. 2021, 11, 2. [Google Scholar] [CrossRef]
- Wilczynski, W.; Babkiewicz, E.; Pukos, S.; Wawrzeńczak, J.; Zebrowski, M.L.; Banasiak, Ł.; Kudriashov, M.; Maszczyk, P. The Effects of Hypoxia on Threshold Food Concentrations in Different Daphnia Species. Water 2022, 14, 3213. [Google Scholar] [CrossRef]
- Carpitella, S.; Olmo, G.D.; Izquierdo, J.; Husband, S.; Boxall, J.; Douterelo, I. Decision-Making Tools to Manage the Microbiology of Drinking Water Distribution Systems. Water 2020, 12, 1247. [Google Scholar] [CrossRef]
- Acioly, T.M.S.; Silva, M.F.; Iannacone, J.; Viana, D.C. Levels of potentially toxic and essential elements in Tocantins River sediment: Health risks at Brazil’s Savanna-Amazon interface. Sci. Rep. 2024, 14, 18037. Available online: https://www.nature.com/articles/s41598-024-66570-4#citeas (accessed on 7 August 2024).
- Federigi, I.; Salvadori, R.; Lauretani, G.; Leone, A.; Lippi, S.; Marvulli, F.; Pagani, A.; Verani, M.; Carducci, A. Wastewater Treatment Plants Performance for Reuse: Evaluation of Bacterial and Viral Risks. Water 2024, 16, 1399. [Google Scholar] [CrossRef]
- European Union (EU), Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse, Official Journal of the European Union (L 177/3). Available online: https://eur-lex.europa.eu/eli/reg/2020/741/oj (accessed on 9 August 2024).
- Principais Dados de Saneamento. Available online: https://conteudo.clp.org.br/saneamento-basico-e-eleicoes-maranhao (accessed on 10 August 2024).
- Maranhão é o 3° Estado do País Com os Piores Indicadores de Coleta de Esgoto, Aponta Instituto Trata Brasil. Available online: https://g1.globo.com/ma/maranhao/noticia/2023/11/24/maranhao-e-o-3-estado-do-pais-com-os-piores-indicadores-de-coleta-de-esgoto-aponta-instituto-trata-brasil.ghtml (accessed on 10 August 2024).
- Maranhão Apresenta Índices Muito Alto em Perdas de Água e Baixo Acesso ao Saneamento Básico. Available online: https://tratabrasil.org.br/maranhao-apresenta-indices-muito-alto-em-perdas-de-agua-e-baixo-acesso-ao-saneamento-basico/ (accessed on 10 August 2024).
- Chernova, E.N.; Lysenko, E.V. The content of metals in organisms of various trophic levels in freshwater and brackish lakes on the coast of the Sea of Japan. Environ. Sci. Pollut. Res. 2019, 26, 20428–20438. [Google Scholar] [CrossRef]
- Brasil. Resolução CONAMA nº 430, de 13 de maio de 2011. Publicada no Diário Oficial nº 92 em 16 de Maio de 2011. Composição e Classificação dos Esgotos Sanitários. Available online: https://www.legisweb.com.br/legislacao/?id=114770 (accessed on 10 August 2024).

| Physicochemical Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Turbidity (NTU) | Chlorophyll a (µg/L) | pH (UNITS) | TDS (g/L) | SAL (PPT) | COND (µS/CM) | LDO (mg/L) | Temp (°C) |
| 35.03 ± 2.5 | 58.8 ± 6.93 | 6.77 ± 0.76 | 0.04 ± 0.01 | 0.02 ± 0.01 | 66.37 ± 9.67 | 18.61 ± 3.48 | 26.57 ± 0.76 |
| Seasons | Environmental Variables | E. coli Variables in Fish Muscles (NMP/g) | E. coli Variables in Water (NMP/100 mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T air (°C) | Air Humidity | Rainfall (mm) | Ctotal | Cterm | E. coli | CTotal | Cterm | E. coli | |
| Dry/2021 | 29.41 | 78.03 | 1.36 | 166.25 | 65.65 | 24.71 | 71.73 | 71.07 | 4.97 |
| Rainy/2022 | 30.14 | 75.60 | 0.71 | 836.33 | 675.67 | 416.85 | 562.53 | 558.40 | 264.00 |
| Dry/2023 | 29.84 | 77.37 | 8.39 | 1035.40 | 521.40 | 338.27 | 948.67 | 506.53 | 193.87 |
| Rainy/2024 | 29.54 | 78.54 | 9.91 | 1189.33 | 241.50 | 38.33 | 756.00 | 84.33 | 39.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, K.S.d.S.; Acioly, T.M.d.S.; Nascimento, I.O.; Costa, F.N.; Corrêa, F.; Gagneten, A.M.; Viana, D.C. Biomonitoring of Waters and Tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil. Water 2024, 16, 2588. https://doi.org/10.3390/w16182588
Araújo KSdS, Acioly TMdS, Nascimento IO, Costa FN, Corrêa F, Gagneten AM, Viana DC. Biomonitoring of Waters and Tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil. Water. 2024; 16(18):2588. https://doi.org/10.3390/w16182588
Chicago/Turabian StyleAraújo, Karuane Saturnino da Silva, Thiago Machado da Silva Acioly, Ivaneide Oliveira Nascimento, Francisca Neide Costa, Fabiano Corrêa, Ana Maria Gagneten, and Diego Carvalho Viana. 2024. "Biomonitoring of Waters and Tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil" Water 16, no. 18: 2588. https://doi.org/10.3390/w16182588
APA StyleAraújo, K. S. d. S., Acioly, T. M. d. S., Nascimento, I. O., Costa, F. N., Corrêa, F., Gagneten, A. M., & Viana, D. C. (2024). Biomonitoring of Waters and Tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil. Water, 16(18), 2588. https://doi.org/10.3390/w16182588







