Abstract
This study evaluates the preparation of novel ternary functional adsorbents based on polyaniline, zinc oxide nanoparticles, and moringa oleifera gum to produce zinc oxide/Moringa oleifera gum-grafted L-methionine-functionalized polyaniline bionanocomposites (ZM-g-Pani) and employed to sequestrate divalent metal ions (Cd2+, Hg2+ and Pb2+) from wastewater samples. The morphological and structural properties of ZM-g-Pani were exploited using FT-IR, FE-SEM/EDS, TEM, and XRD. FT-IR and FE-SEM studies show that the as prepared nanocomposite has an abundant number of reactive groups and a porous structure, thus demonstrating outstanding divalent metal cation removal. FT-IR study confirms that the attachment of L-methionine to polyaniline is facilitated by the C-S linkage. Both TEM and FE-SEM techniques confirmed the clustered granules of ZnO over the surface of polyaniline, which ultimately provided more surface area to adsorb metal ions. The study demonstrated that Cd2+, Hg2+ and Pb2+ ions could undergo physical sorption and chemisorption simultaneously during the adsorption process. The maximum adsorption capacity was 840.33, 497.51, and 497.51 mg/g for Cd2+, Hg2+, and Pb2+, respectively. The impact of co-existing ions, including NO3−, PO43−, SO42−, Cl−, Na+, Cu2+, and Al3+, showed that there were no notable alterations in the adsorption of the selected metal ions with ZM-g-Pani. ZM-g-Pani showed eight successive regeneration cycles for Cd2+, Hg2+, and Pb2+ with more than 85% removal efficiency.
1. Introduction
Water scarcity has been elevated to a major global issue for ecological sustainability. This problem will only worsen due to escalating water requirements and persistent water contamination brought on by a rapidly growing population and economic growth. Wastewater contains hazardous heavy metals that pose a major risk to the environment and public health. Divalent metal ions, such as cadmium (Cd2+), mercury (Hg2+), and lead (Pb2+) can be highly toxic to living organisms, including humans, animals, and plants, when their concentrations are higher than permissible limits [1]. When these metals enter the water supply, either through industrial or natural processes, they can accumulate in the tissues of organisms and cause health problems. Cadmium, for example, can cause kidney damage, nausea, anemia, and bone demineralization [2,3]. Mercury in its oxidized form, i.e., Hg2+, is bioaccumulated through the food chain in the human body and causes neurological damage, paralysis, etc. [4]. Lead can damage the nervous system, causing learning and developmental problems in children and cognitive impairment in adults. Both mercury and lead can also have harmful effects on the cardiovascular system and the immune system [2,5]. The effective elimination of these toxic divalent metal ions from water is, therefore, of great importance.
Various techniques, including oxidation, electrocoagulation, ion exchange, photocatalysis, and adsorption, have been utilized for the treatment of polluted and wastewater, which can remove both inorganic and organic pollutants [6,7,8,9,10]. Each of these techniques has its own advantages and limitations, and the selection of a particular technique depends on the nature and concentration of pollutants present in the wastewater, as well as the desired treatment objectives. Adsorption is a widely used technique in the field of environmental protection, especially for the sequestration of metal ions from wastewater or polluted water sources [1,5]. One of the main advantages of adsorption is its effectiveness at low concentrations of metal ions. This is because the process can remove even trace amounts of metal ions from water, making it a highly efficient technique [2,11]. Another advantage of adsorption is its ease of use, operation with relatively simple equipment, and modest design requirements, thus making it a cost-effective solution for wastewater treatment [12,13].
Many researchers have developed new adsorbents with enhanced properties for the abatement of toxic water pollutants. Some commonly used adsorbent materials are activated carbon [3,14], zeolites [15,16,17], metal oxides [18,19], clay minerals [14], chitosan [20,21], and various polymers [22,23,24,25,26]. However, these materials often suffer from limitations, such as a low adsorption capacity, slow kinetics, regeneration, and high cost. Functionalized polymer composite-based adsorbents are an effective and versatile solutions for metal removal from wastewater [27]. They offer improved adsorption capacity, selectivity, and efficiency compared to individual materials, making them a valuable tool in the treatment of contaminated water.
Zinc oxide nanoparticles (ZnO NPs) have garnered significant interest due to their distinctive characteristics, such as optical activity, photocatalytic activity, and antibacterial properties, thus finding potential applications in biomedical science and environmental remediation [28,29,30]. In addition, despite having a harmful impact at large ZnO dosages, chemically produced ZnO NPs do not have a detrimental influence on humans when exposed in low quantities. Furthermore, ZnO NPs have been found to be an effective adsorbent and have applications in water purification [31].
Natural gums, such as guar gum, tamarind gum, chitosan, alginate, and gum arabic, have been used for pollution clean-up due to their low cost, biodegradability, more active functional groups, and availability, especially for metal ions [7]. Commonly these gums are used in a modified form to enhance their adsorption capabilities. For example, functionalized Moringa oleifera gum has been used to sequestrate Hg2+ ions from wastewater [4]. In another study, gum ghatti-grafted poly (acrylamide-co-acrylonitrile) has been developed and used in separating Pb2+ and Cu2+ ions from wastewater [32].
Conducting polymers including polythiophene, polypyrrole, and polyaniline have been extensively employed in many different domains [33,34]. The distinctive properties of polyaniline, including its redox property, low cost, simple fabrication, bio-compatibility, and high content of active amine and imine groups in the polymeric chains related to the management of polluted water, have caused many scientists to use it for water purification [25,33].
For the functionalization of polymers, using amino acids is a safe, green, and environmentally friendly approach, as they are essential substances for living organisms [35]. Methionine has sulfhydryl and amino groups that can chelate with heavy metal ions [36]. In this research, we employed methionine (Meth) amino acid to functionalize polyaniline (Pani) and produced a modified form of the ‘Pani’ with an altered morphology and functional groups. In our previous studies, we have demonstrated changes in Pani morphology and functional groups after cysteine functionalization [31]. The high quantity of amino groups in the modified material also allows for additional chemical modifications, such as protonation and grafting, which can enhance the adsorption efficiency of the material.
The novelty of this work is to introduce a novel ternary functional adsorbent, ZM-g-Pani, combining polyaniline, zinc oxide nanoparticles, and Moringa oleifera gum for the sequestration of divalent metal ions (Cd2+, Hg2+, and Pb2+) from wastewater. The combination of zinc oxide nanoparticles with Moringa oleifera gum grafted onto L-methionine functionalized polyaniline is unprecedented. This synergy enhances the adsorbent’s capacity and selectivity for heavy metal ions. The attachment of L-methionine to polyaniline, facilitated by the C-S linkage, introduces additional functional groups that enhance the adsorption efficiency. This approach leverages the chelating properties of amino acids, which is a green and environmentally friendly modification. The excellent adsorption capacity, impressive regeneration performance, and interference resistance in the presence of common co-existing ions (NO3−, PO43−, SO42−, Cl−, Na+, Cu2+, and Al3+) highlight the high selectivity and robustness of the composite, and its potential for practical and sustainable applications in water purification.
2. Materials and Methods
2.1. Materials
All the chemicals, namely zinc acetate dihydrate, monoethanolamine, poly (ethylene glycol), L-Methionine, aniline, ammonium persulfate, hydrochloric acid (HCl), acetone, ethanol, sodium hydroxide (NaOH), cadmium nitrate tetrahydrate, lead (II) nitrate, and mercury (II) sulfate, were of analytical grade and were procured from Sigma-Aldrich Chemicals Private Limited, Bommasandra, Bangalore, Karnataka, India. Raw Moringa oleifera gum was purchased from the local market in New Delhi, India.
2.2. Preparation
2.2.1. Purification of Moringa Oleifera Gum (MGm)
Typically, 5 g of crushed powder of MGm was transferred in a 500 mL Erlenmeyer flask containing 200 mL of double distilled water (DDW). Then, the flask was put on a stirrer to agitate the raw powder of MGm in water for 6 h at 25 ± 3 °C. Thereafter, the solid residual of MGm was collected. Then, this residue was mixed with 400 mL of acetone under vigorous stirring. After the precipitation of pure MGm, it was washed using DDW and dehydrated under a vacuum at 40 °C [4].
2.2.2. Synthesis of ZnO NPs
In a typical reaction, zinc acetate (2 g) was added to a conical flask containing 70 mL of DDW and stirred with a magnetic stirrer at 800 rpm for 90 min. Then, monoethanolamine (10 mL) and 0.1% (w/v) polyethylene glycol as a surfactant were added gradually to the mixture and magnetically stirred for an additional 45 min until the mixture became transparent. Thereafter, the resulting mixture was transferred to an autoclave with a Teflon top and heated to 140 °C for 3 h. The product was then washed with DDW and ethanol using centrifugation (8000 rpm) followed by drying in an air oven for 12 h at 80 °C. Lastly, the dried product was calcined for 2 h at 400 °C in a muffle furnace to obtain the final white color product [31].
2.2.3. Preparation of ZM-G-Pani Bionanocomposites
Firstly, 0.4 g of ZnO and 1 g of MGm were mixed in 100 mL of 2 M HCl solution in a 250 mL beaker and allowed to be agitated for half an hour. After that, 0.26 g of L-methionine was added, and the mixture was ultrasonically treated for one hour, subsequently, followed by the addition of aniline monomer. In another beaker, APS solution was prepared in 2 M HCl at 0 to 4 °C. After that, cooled APS solution was added dropwise in a beaker containing MGm and ZnO solution with continuous stirring for 10 h to initiate the in situ polymerization reaction. After the completion of the chain reaction, a green colored product, i.e., ZM-g-Pani nanocomposite, was obtained. Finally, the resultant green product was washed (with DDW, ethanol, and acetone), followed by drying in an oven at 70 °C overnight. Scheme 1 illustrates the preparation of the ZM-g-Pani bionanocomposites.
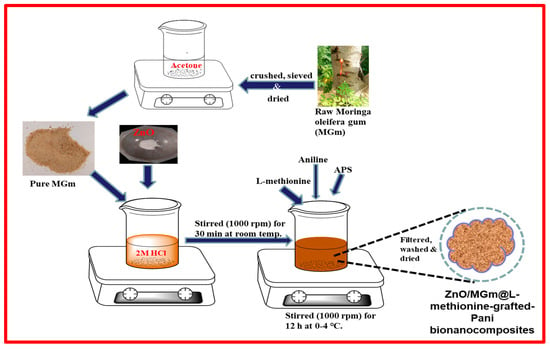
Scheme 1.
Schematic illustration of the preparation process of ZM-g-Pani bionanocomposites.
2.3. Characterizations
To investigate the chemical structural groups of the prepared samples, a Fourier transform infrared (Nicolet 6700, Thermo Fisher, Waltham, MA, USA) spectrometer was used. The spectra were recorded in the range of 4000–400 cm−1 at a resolution of 4 cm−1, with 32 scans per sample. An Ultima VI, Rigaku diffractometer (Tokyo, Japan) with Cu Kα radiation was used to carry out the XRD analysis of pristine ZnO, purified MGm, and ZM-g-Pani biocomposite. The surface morphology and percentage composition of ZM-g-Pani biocomposite were monitored using the FE-SEM/EDS technique (JEOL JSM-7800F, Frenchs Forest, Australia). The samples were coated with a thin layer of gold to improve conductivity. The images were captured at an accelerating voltage of 0.01 kV to 30 kV, with a magnification range from ×25 to ×1,000,000 to observe the composite’s surface morphology. To obtain more morphological insight, the pristine ZnO and ZM-g-Pani biocomposite were further studied by the TEM technique (2100F, JEOL, Tokyo, Japan) at an accelerating voltage of 200 kV. ICP-OES was employed to quantify the remaining concentrations of Cd2⁺, Hg2⁺, and Pb2⁺ ions after adsorption. The measurements were performed using an AVIO 200, Perkin Elmer (Waltham, MA, USA), with the following wavelengths used for detection: 214.43 nm for Cd, 194.2 nm for Hg, and 220.25 nm for Pb. The samples were introduced into the plasma at a flow rate of 12 L/min, and the emission intensity was recorded to determine the metal ion concentrations.
2.4. Adsorption Experiments
To obtain the artificial wastewater solutions, a separate stock solution was prepared for each metal ion using double distilled water at a concentration of 1 g per liter (1 g/L). The desired concentration for each metal ion was achieved by making necessary dilutions from the respective stock solutions. In an Erlenmeyer flask (100 mL), adsorption experiments were performed; the flasks contained 25 mL of distilled water with 40 ppm Cd2+, Hg2+, and Pb2+ solution each, in which approximately 0.1 g of ZM-g-Pani composite was added. The system was kept under constant agitation at regular intervals over a period of 120 min. A portion of the aliquot was analyzed with inductive coupled plasma–optical emission spectroscopy (ICP-OES) to determine the concentrations of Cd2+, Hg2+, and Pb2+ in the solution. Effects of various adsorption factors were explored as a function of the ZM-g-Pani dose (0.01 to 0.1 g), pH (2.0–10.0), temperature (298.15 to 328.15 K), and initial Cd2+, Hg2+, and Pb2+ concentrations (5–400 mg/L). All measurements were conducted three times. The removal efficiency RE(%) and adsorption capacities (qe) were determined using the following Equations (1) and (2), respectively:
where qe represents the equilibrium adsorption capacity of ZM-g-Pani in milligrams per gram, Ci, Cr, and Ce represent the initial, residual, and equilibrium concentrations of the adsorbate (Cd2+, Hg2+, and Pb2+) in the solution in milligrams per liter, respectively, V is the volume of the adsorbate solution in liters, and m is the mass of the ZM-g-Pani in grams.
3. Results and Discussions
3.1. Characterization
3.1.1. FT-IR Study
The purified MGm was analyzed using spectroscopy, showing characteristic peaks in its infrared (IR) spectrum. The prominent broad bands observed at 3290 and 2923 cm−1 are attributed to the presence of O–H stretching of sugars and aldehydes and –CH symmetric stretching of purified MGm (Figure 1A) [4,37]. The emergence of absorption peaks at 1601 and 1420 cm−1 are due to –COO– vibrations of –COOH groups of uronic acid. Specifically, the peak observed at 1015 cm−1 is credited to –CH vibrations of methyl groups, while the small peak at 840 cm−1 is associated with –OH group bending vibrations [38]. The presence of a peak at 1508 cm−1 has also been noted, which can be correlated with the carboxylic acid groups found in ketonic sugars of MGm [39]. Taken together, these findings provide strong evidence for the polysaccharide structure of MGm.
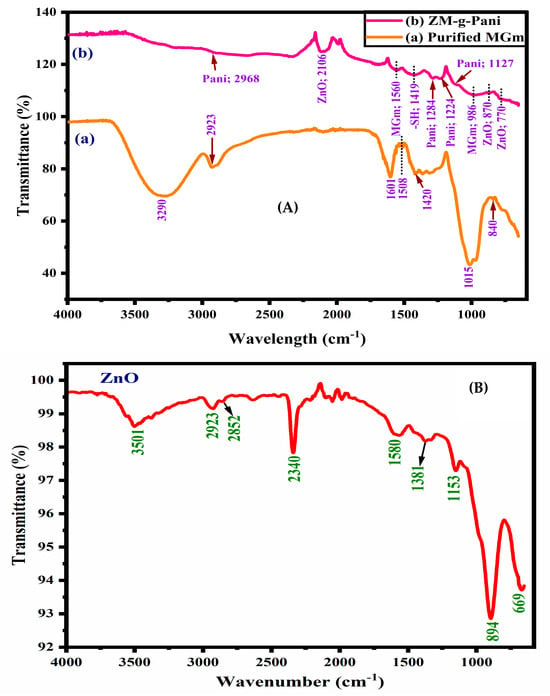
Figure 1.
FT-IR spectra of (A) purified MGm (a) and ZM-g-Pani bionanocomposites (b); (B) ZnO NPs.
The inset in Figure 1 shows the FT-IR spectra of pristine ZnO NPs. The peak at 3501 cm−1 observed in the high frequency range could be attributed to the H-bonding between the adsorbed water molecules and the hydroxyl groups present on the surface of the ZnO nanoparticles. The absorption peaks at about 2852 and 2923 cm−1 exhibit the stretching vibration of C–H groups [40,41]. The strong absorption peak at 2340 cm−1 can be attributed to the H–O–H vibration of water molecules. The peaks at 1580 and 1381 cm−1 can be attributed to the asymmetric and symmetric stretching modes of C=O group of zinc acetate [42]. The band at 1153 cm−1 corresponds to the symmetric bending vibration mode of ZnO–OH [30]. In the fingerprint region, the peak at 669 cm−1 represents the hexagonal phase of the ZnO lattice structure [31], and the peak at 894 cm−1 is the characteristic absorption peak of the Zn–O bond [30]. In the biocomposite (Figure 1B), the bands of Pani, ZnO, and MGm match well with the literature. The bands at 2968, 1284, and 1224 cm−1 are due to aromatic C–H stretching, C–N and C=N stretching, and C–N stretching for the benzenoid rings of Pani, respectively [31,33,43]. One more significant absorption band seen at 1127 cm−1 refers to in-plane C–H bending of Pani. The thiol group (–SH) grafting on Pani of ZM-g-Pani composite is confirmed by the emergence of bands around 1419 cm−1, attributed to a C–S vibration [44]. The FT-IR of ZM-g-Pani bionanocomposites shows a substantial distinctive peak at about 870 cm−1, which suggests that para-coupling, or polymerization, occurs at the 1–4 position due to out-of-plane –CH bending vibrations [45]. It confirms that ZnO NPs become successfully adhered to the polymer matrix. The band at 770 cm−1 signifies peculiar identity of the Zn–O bond [30]. The 2106 cm−1 peak may be caused by metallic cations absorbing CO2 from the atmosphere. Furthermore, spectral changes were also found for ZM-g-Pani bionanocomposites (Figure 1A), and significant displacement of such peaks took place: (MGm) from 1601 cm−1 (–COO) to 1560 cm−1, and 1015 cm−1 (–OH) to 986 cm−1, showing that the interaction of MGm and the Pani polymer was successful. In addition, the disappearance of the characteristic peaks of MGm, such as 3920, 2923, and 840 cm−1 in the ZM-g-Pani, indicates the full participation of –OH and –CH functional groups in the interaction of MGm with the Pani polymer. These FT-IR spectra results for ZnO and MGm validate the efficacious formulation of ZM-g-Pani nanocomposite.
3.1.2. FE-SEM/EDS
In a typical FE-SEM study, a spike-like morphology in ZM-g-Pani bionanocomposites has been observed, as shown in Figure 2a. Such a type of morphology may increase the surface area of ZM-g-Pani bionanocomposites, ultimately enhancing the adsorption capacity of an adsorbent material. The micrograph of the ZM-g-Pani nanocomposite shows highly clustered granular particles. The clustering of the particles means that they are firmly coupled to one another and that they have enough binding energy to unite with nearby molecules. In addition, the FE-SEM micrograph of ZM-g-Pani also shows some small and larger pores and cavities, which may be due to the functionalization of amino acid, i.e., L-methionine [27,31], hence facilitating extra active sites to adsorb water pollutants. Moreover, the FE-SEM studies also indicated that the ZM-g-Pani nanocomposite has a porous structure. This porosity enhances the diffusion of metal ions into the internal structure of the adsorbent, promoting both physical sorption (due to the porous network) and chemisorption (through reactive sites within the pores). Figure 2c shows the EDS spectrum of the ZM-g-Pani ternary system, which reveals that the composite is mainly composed of C, N, O, Zn, and S. The appearance of sulfur confirms the presence of L-methionine grafted Pani in the composite.
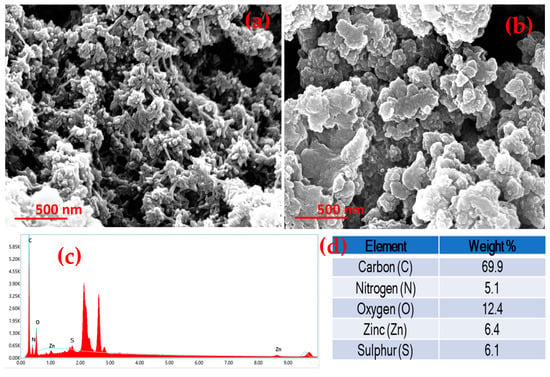
Figure 2.
FE-SEM image of ZM-g-Pani bionanocomposites before adsorption (a) and after adsorption (b); EDS of ZM-g-Pani (c) and its composition (d).
After sorption of the metal ions in Figure 2b, all the pores and cavities of ZM-g-Pani nanocomposite become occupied with targeted metal ion pollutants, demonstrating the accomplishment of sorption of metal ions over the surface of ZM-g-Pani. Obviously, after adsorption, cationic metal ions fill the open sites on the ZM-g-Pani. Hence, it can be inferred from the FE-SEM images that the ZM-g-Pani nano-adsorbent has sufficient morphology for selective metal ion adsorption.
3.1.3. TEM
The morphology of pristine ZnO and ZM-g-Pani bionanocomposites was also recorded by the TEM technique, presented in Figure 3. The TEM micrograph in Figure 3a shows ZnO NPs of almost spherical shape (dark regions) embedded on MGm/Pani copolymer (grey region). Based on the estimated data using ImageJ software (version 1.53t), the ZnO particle size distribution (PSD) is shown in histogram (Figure 3c). The ZnO average particle size was estimated about 58.11 nm. Figure 3b presents morphological evidence of ZM-g-Pani bionanocomposites. It is revealed that the MGm and Pani polymers are strongly adhered to each other, forming flat layered structure (in grey color), hence proving to be functionalized. In addition, ZnO particles are found to be spread all over the surface of ZM-g-Pani bionanocomposites. While ZnO is highly clustered at some places (encircled with red dotted circle) over the exterior surface of ZM-g-Pani which is also evident by FE-SEM study. Thus, this study confirms successful formulation of ZM-g-Pani bionanocomposites.
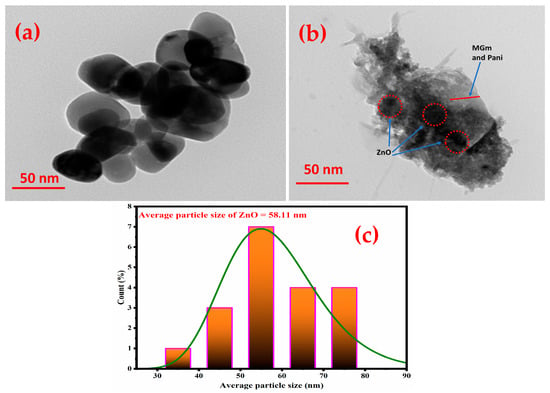
Figure 3.
TEM images of the ZnO NPs (a), ZM-g-Pani bionanocomposites (b), and PSD Of the ZM-g-Pani bionanocomposites (c).
3.1.4. XRD Investigation
The X-ray diffraction (XRD) patterns of purified MGm and ZM-g-Pani composites are presented in Figure 4. The purified MGm shows a broad peak within a range of a 10–30° 2θ value, confirming its amorphous nature [46] (Figure 4a). Figure 4b indicates the semicrystalline nature of ZM-g-Pani composites, having few sharp peaks, with the intensity lying within the parenthesis, at 2θ values of 15.1°, 20.56°, and 25.3°. The peak at 2θ = 25.3° is observed for the pure Pani chain. This peak is assigned to the periodicity parallel to the Pani chain [47]. A small hump at 2θ = 15.1° confirms that the Pani in the composite is in an emeraldine form [33,48]. The 2θ = 20.56° in Figure 4b with diminished intensity represents the presence of MGm in the as prepared ZM-g-Pani composites. Noticeably, the ZnO peak becomes insignificant in the XRD pattern of ZM-g-Pani composite (Figure 4b). This phenomenon was also observed by Deb et al. [49]. They explained that the dense coating of Pani on the ZnO nanoparticles made the peaks of ZnO negligible in the composite.
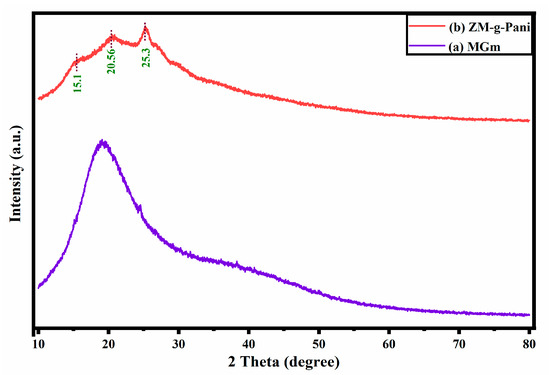
Figure 4.
X-ray diffraction patterns of purified MGm (a) and ZM-g-Pani nanocomposite (b).
3.2. Adsorption Studies
3.2.1. Removal Efficiency of Pristine ZnO, Pristine MGm, and ZM-G-Pani Nanocomposites towards Cd2+, Pb2+, and Hg2+ Adsorption
For comparative studies, the efficacy of pristine ZnO, pristine MGm, and ZM-g-Pani nanocomposites towards Cd2+, Pb2+, and Hg2+ removal has been investigated in an aqueous neutral media (Figure 5). For this, 0.015 g of adsorbent material was added in separate beakers containing 40 mg/L of Cd2+, Pb2+, and Hg2+ ions, agitated using shaker at 400 rpm at 25 ± 3 °C for 24 h. After the completion of the sorption process, the RE(%) of each adsorbent materials were calculated using Equation (1). The trend of the RE(%) of adsorbent materials towards chosen metal ions was recorded as follows: ZM-g-Pani > ZnO > MGm. The RE(%) of the ZM-g-Pani nanocomposite is more because of the occurrence of active functional groups, such as carboxyl, hydroxyl, amines, imines, and thiol groups, as well as a more active surface area in comparison with pristine ZnO NPs and pristine MGm.
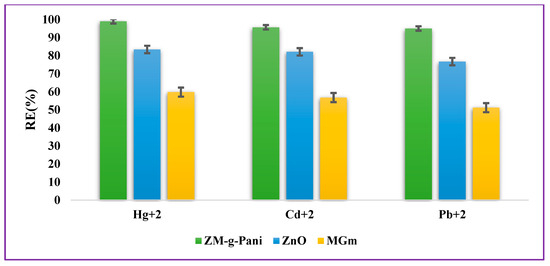
Figure 5.
Removal efficacy of adsorbent materials for Cd2+, Pb2+, and Hg2+ ion adsorption in an aqueous solution at a neutral pH.
3.2.2. Point of Zero Charge and Effect of pH
The point of zero charge (pHpzc) was determined to assess the charge characteristics of the ZM-g-Pani nanocomposite surface. At pH values above the pHpzc, the composite surface becomes negatively charged, while at pH values below the pHpzc, it becomes positively charged [50]. The pHpzc of the ZM-g-Pani nanocomposite, determined using the solid addition method [31], was found to be at pH 5.1 (Figure 6a). The sorption experiments for the selected divalent ions were conducted at pH values above the pHpzc, suggesting that the composite surface is negatively charged due to the deprotonation of functional groups at higher pH levels.
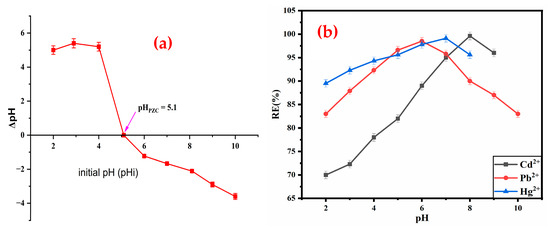
Figure 6.
(a) Zero-point charge of ZM-g-Pani nanocomposites at different pH values, and (b) effect of pH for the adsorption of Cd2+, Pb2+, and Hg2+ ion from aqueous solutions.
The experiments aimed to explore the impact of pH levels (ranging from 2 to 10 for Pb2+, 2 to 6 for Hg2+, and 2 to 9 for Cd2+) on the adsorption of Cd2+, Pb2+, and Hg2+ on the ZM-g-Pani nanocomposite, with an initial concentration of 40 mg/L for each metal cation, A ZM-g-Pani dose of 0.015 g for each metal ions was kept in a shaker for 120 min to achieve equilibrium. The outcomes presented in Figure 6b demonstrated that the adsorption of Cd2+ and Pb2+ is slightly boosted with increasing pH, while the adsorption of Hg2+ remains largely unaffected. The point zero charge (pHzpc) of the prepared ZM-g-Pani nanocomposite was found at 5.1 pH. The surface of ZM-g-Pani will be negatively charged at a pH above 5.1 and will be positively charged at a pH below 5.1. When the pH is very acidic, large amounts of protons would compete with heavy metal cations for adsorption onto ZM-g-Pani nanocomposite, which would lead to reduced adsorption at lower pH levels. However, the impact is negligible for Hg2+ due to its strong attraction towards thiol groups (provided by L-methionine functionalization) on the ZM-g-Pani nanocomposite. The observation of such a phenomenon can also be explained by considering the solubility products (Ksp) of CdS (7.1 × 10−27), PbS (9.3 × 10−28), and HgS (4 × 10−53) [51].
3.2.3. Effect of Contact Time and Kinetic Studies
The impact of contact time on the uptake of Cd2+, Pb2+, and Hg2+ ions onto the ZM-g-Pani nanocomposite is shown in Figure 7. The equilibrium time required by the ZM-g-Pani to capture the metal ions was determined by adding 0.015 g/25 mL of composite into a solution of 40 mg/L of each metal ions for different time periods (5–180 min) at a pre-optimized pH. The RE(%) for Cd2+ and Hg2+ ions increases dramatically in the first 60 min, while for Pb2+, it takes 110 min. When further increasing the contact time, the RE(%) does not increase, which means equilibria have been achieved for the adsorption process. Initially, the RE(%) is higher due to the accessibility of free active functional groups on the exterior surface of ZM-g-Pani nanocomposites for the uptake of Cd2+, Pb2+, and Hg2+ ions in aqueous media [2]. When increasing the contact time, the RE(%) remains constant, suggesting that all adsorbent sites are fully occupied by adsorbate, i.e., Cd2+, Pb2+, and Hg2+ ions. This indicates that ZM-g-Pani has no further functional groups and active sites available to interact with Cd2+, Pb2+, and Hg2+ ions.
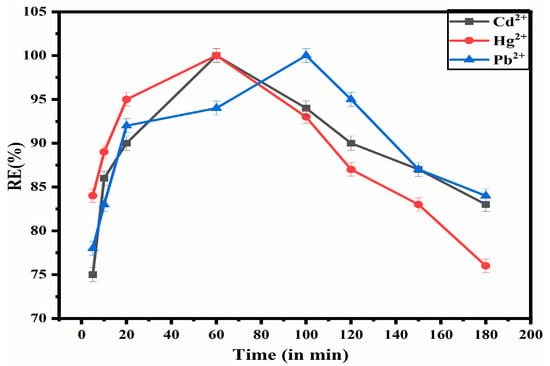
Figure 7.
Effect of contact time for the adsorption of Cd2+, Pb2+ and Hg2+ ions from aqueous solutions.
To investigate the adsorption kinetics mechanism, three models were employed in this study, namely the pseudo-first order, pseudo-second order, and intraparticle diffusion models, represented by the following Equation (3), Equation (4), and Equation (5), respectively:
where the adsorption capacity at equilibrium (qe in mg/g) and at a given time ‘t’ (in minutes), denoted by qt (in mg/g), were determined. K1 (in/min) and K2 (in g/mg/min) are the rate constants for the pseudo-first order and pseudo-second order models, respectively. Additionally, the intraparticle diffusion rate constant is represented as Kid, the square root of contact time as t1/2, and a constant as C.
Figure 8 shows the kinetics for the adsorption process of Cd2+, Hg2+, and Pb2+ onto the ZM-g-Pani nanocomposite. The aqueous solution of metal ions with an initial concentration (40 mg/L for each metal ions), ZM-g-Pani dose (0.015 g for each metal ions), and pH (Cd2+: 8, Hg2+: 7, and Pb2+: 6) was taken, and the residual concentration was measured at a time period from 0 to 180 min. Table 1 displays the comparison between the experimental and theoretical adsorption capacities of ZM-g-Pani, as well as the computed results derived from Equations (3)–(5). The calculated equilibrium adsorption value (qecal) obtained from the pseudo-first order kinetic model were 19.10, 10.82, and 17.11 mg/g, respectively, which were greatly different from the experimental data (qeexp). Moreover, the theoretical values of adsorption capacity (qecal: 101.21, 99.30, and 100.1 mg/g for Cd2+, Hg2+ and Pb2+, respectively) closely matched the experimental values (qeexp: 100, 98.7, and 98.9 mg/g for Cd2+, Hg2+ and Pb2+, respectively) when using the pseudo-second order kinetics model. Also, negative values of K1 suggested that the pseudo-first order model does not appropriately describe the kinetics for chosen metal ions. However, a positive K2 value in the pseudo-second order model confirms that the adsorption process is chemisorption, with stronger binding between the metal ions and the adsorbent. This model typically provides a better fit for systems where chemical interactions dominate, which seems consistent with our findings. The chemisorption plays a crucial role in controlling the rate of Cd2+, Hg2+, and Pb2+ adsorption by exchanging the electrons between the adsorbate and adsorbent [52,53]. Overall, for all the chosen metal ions, such as Cd2+, Hg2+, and Pb2+, the pseudo-first order model fits poorly with the experimental data, while the pseudo-second order model displays a very good fit with higher regression coefficients. This implies that the adsorption process is dependent on both the adsorbent (ZM-g-Pani nanocomposites) and adsorbate (Cd2+, Hg2+ and Pb2+). These findings are consistent with previous research [51,54].
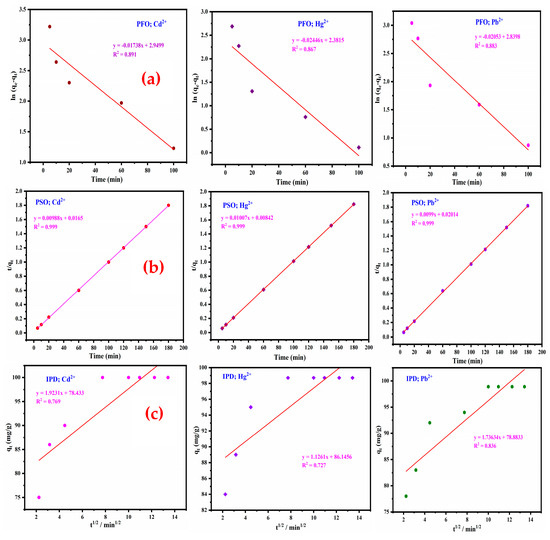
Figure 8.
Pseudo-first order kinetics (a), Pseudo-second order kinetics (b), and intraparticle diffusion (c) linear fitting for the adsorption of Cd2+, Hg2+, and Pb2+ on ZM-g-Pani nanocomposites.

Table 1.
Kinetics parameters obtained for the adsorption of Cd2+, Hg2+, and Pb2+ using ZM-g-Pani nanocomposites.
To explain the diffusion mechanism, the intraparticle diffusion model is utilized. The process of metal ion sorption involves several steps: (a) metal ion diffusion through the solution to the outer surface of the adsorbent (film diffusion), (b) metal ion sorption on the outer surface of the adsorbent, (c) metal ion diffusion from the surface into the interior of the adsorbent (intraparticle diffusion), and (d) metal ion sorption onto the active centers on the interior surface of the adsorbent [55,56]. The obtained plots in Figure 8 indicate linearity, suggesting that multiple steps occur during the adsorption process. At room temperature, the straight line representing the intraparticle region does not intersect the origin, suggesting that intraparticle diffusion alone is not the rate-controlling step. The deviation from the origin may be attributed to variations in mass transfer during the initial and final stages of the adsorption process. The intraparticle diffusion rate constant (kid) and the boundary layer thickness effect (C) were determined from the plots of qt versus t1/2 and are presented in Table 1. The intercept length is maximum, and adsorption is more boundary layer-controlled.
3.2.4. Effect of Initial Metal Ion Concentration and Isotherm Studies
The impact of the initial concentrations of Cd2+, Hg2+, and Pb2+ ions on the RE(%) of the ZM-g-Pani nanocomposite has been investigated within the range of 5.0–400 mg/L, with a pre-optimized pH and ZM-g-Pani dosage of 0.01 g for 120 min to achieve equilibrium at 25 ± 3 °C. As shown in Figure 9, the RE(%) for all three metal ions demonstrated a significant increase as the initial concentration was raised from 5 mg/L to 200 mg/L for Cd2+, 5 mg/L to 250 mg/L for Hg2+, and 5 mg/L to 160 mg/L for Pb2+. This behavior can be explained by the sufficiency of the active binding sites on the fixed adsorbent dosage. At lower concentrations, the metal ions in the solution occupy the binding sites efficiently, leading to a higher RE(%). However, at higher concentrations, saturation of the limited binding sites occurs, resulting in the presence of unadsorbed metal ions and a consequent decrease in RE(%).
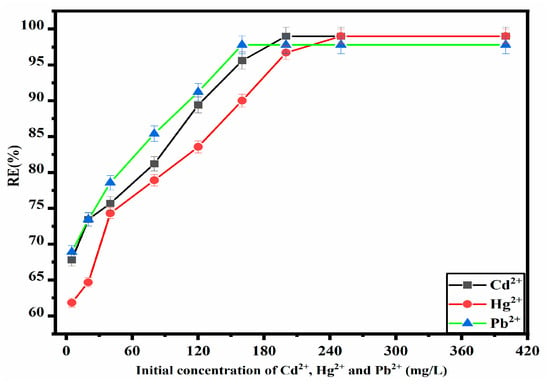
Figure 9.
Optimization of initial Cd2+, Hg2+, and Pb2+ ion concentrations for the adsorption onto ZM-g-Pani nanocomposites.
The present study focuses on the investigation of isotherm studies, specifically the Langmuir, Freundlich, and Temkin isotherm models, for the adsorption of Cd2+, Hg2+, and Pb2+ ions onto the surface of ZM-g-Pani nanocomposites. Figure 10 shows the adsorption isotherms of Cd2+, Hg2+, and Pb2+ ions onto the ZM-g-Pani nano-adsorbent at 25 ± 3 °C after a period of time (12 h). The Langmuir isotherm model postulates that the adsorption process takes place on a single layer and enables the determination of the maximum adsorption capacity. Therefore, to evaluate the isothermal adsorption of Cd2+, Hg2+, and Pb2+ ions onto the ZM-g-Pani nanocomposites, the Langmuir isotherm has been employed. The Langmuir model utilizes the following Equation (6) as a basis for analysis [27]:
where qmax (mg/g) is the maximum adsorption capacity, and KL (L/mg) is the Langmuir constant which is associated with the adsorption binding energy. qe (mg/g) and Ce (mg/L) are the equilibrium amounts of the Cd2+, Hg2+, and Pb2+ ions’ adsorption and the equilibrium concentrations of Cd2+, Hg2+, and Pb2+ ions in an aqueous phase, respectively.
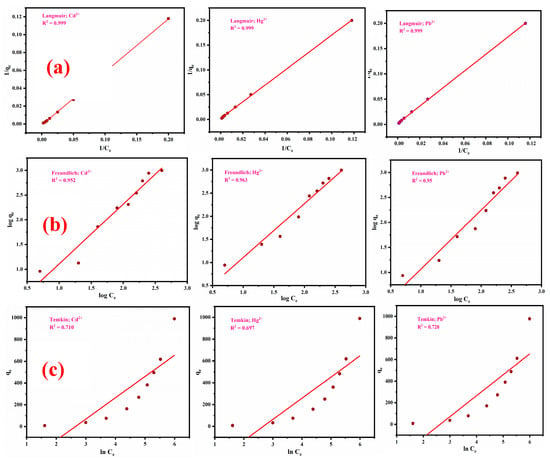
Figure 10.
Langmuir model (a), Freundlich model (b), and Temkin model (c) applied for the sequestration of Cd2+, Hg2+, and Pb2+ ions onto ZM-g-Pani nano-adsorbents.
By utilizing the separator factor RL, the Langmuir isotherm’s primary attribute can be employed to anticipate the adsorption nature between the metal ion and the adsorbent material, which is defined by the following Equation (7) [32]:
where Ci represents the highest initial concentration of the Cd2+, Hg2+, and Pb2+ ions. The RL values are categorized as follows: 0 < RL < 1 denotes favorable adsorption, RL > 1 indicates unfavorable adsorption, RL = 0 suggests irreversible adsorption, and RL = 1 represents linear adsorption.
In general, a higher KL value suggests a more advantageous adsorption process. The fitting results of the Langmuir isotherm model to the adsorption data for Cd2+, Hg2+, and Pb2+ onto the ZM-g-Pani nanocomposites are presented in Figure 10a, and the corresponding adsorption isotherm constants, including qmax and KL, are presented in Table 2. It is noteworthy that all of the regression coefficients (R2) values for the Langmuir isotherm model are above 0.99, indicating that the Langmuir isotherm model is effective in explaining the adsorption isotherm data. Langmuir model results show that the maximum adsorption capacities of Cd2+, Hg2+, and Pb2+ onto the ZM-g-Pani were 840.33, 497.51, and 497.51 mg/g, respectively. Also, the RL values of the Langmuir model for all studied metal ions lie in the range of 0–1, evincing promising sorption of Cd2+, Hg2+, and Pb2+ metal ions by ZM-g-Pani nano-adsorbents. Table S1 compares the maximum sorption capacities of ZM-g-Pani nanocomposites with values from the literature.

Table 2.
Isotherm parameters obtained for Cd2+, Hg2+, and Pb2+ removal using ZM-g-Pani nanocomposites.
Furthermore, the Freundlich model has also been employed to exemplify the adsorption isotherm using the following Equation (8) [57]:
The Freundlich constant, denoted by kf, and the heterogeneous factor, represented by 1/n, are associated with the surface heterogeneity of the adsorbent material. The degree of adsorption was indicated by parameter ‘n’, which reflected the intensity of the driving force for adsorption. A higher value of ‘n’ implied greater feasibility for adsorption. The Freundlich isotherm model, which accounts for both single layer and multilayer adsorption in an adsorption system, considers both chemical and physical adsorption. Cd2+, Hg2+, and Pb2+ adsorption data on ZM-g-Pani were fitted to the Freundlich isotherm model, with the isotherm constants summarized in Table 2 and presented in Figure 10b. Although the results in Table 2 show that the R2 value for Freundlich isotherm is marginally lower than that of Langmuir isotherm, it is even within acceptable range. Overall, Cd2+, Hg2+, and Pb2+ binding on ZM-g-Pani does not involve entirely monolayer adsorption, but rather a combination of monolayer and heterogeneous adsorption, where the driving force arises from chemical adsorption. Additionally, a value of 1/n greater than 1.0 for Cd2+, Hg2+, and Pb2+ suggests that changes in the adsorbed metal concentration are more significant than changes in the solute concentration [2,58].
In addition, the Temkin isotherm model has also been applied to investigate the sorption of Cd2+, Hg2+, and Pb2+ ions on the surface of ZM-g-Pani nanocomposites, using the following Equation (9) [5]:
where RT/bT represents the heat of adsorption in J/mol, bT is the Temkin isotherm constant, and AT represents the Temkin equilibrium constant associated with the maximum binding energy, in L/g. According to the Temkin model, the adsorption layer’s heat of adsorption for a molecule decreases linearly instead of logarithmically, also accounting for the interactions between the adsorbent and adsorbate. T is an absolute temperature in Kelvin. R is a universal gas constant (8.314 J/mol/K). From Figure 10c, it is clearly shown that the R2 value for the Temkin isotherm is far away from linearity. Also, the value of the binding constant AT (from Table 2) is less for the sorption of Cd2+, Hg2+, and Pb2+ onto the ZM-g-Pani nano-adsorbents. Conclusively, it is significant to record that the Langmuir and Freundlich (based on R2) isotherms are better suited for the chosen metal ions using ZM-g-Pani nanocomposites.
3.2.5. Adsorption Thermodynamics
Adsorption runs were carried out at 298.15, 308.15, 318.15, 328.15, and 338.15 K for 120 min to find out the effect of temperature on the adsorption efficiency of Cd2+, Hg2+, and Pb2+ (40 mg/L each) on the ZM-g-Pani nanocomposites (0.01 g). The outcomes associated with different temperatures are illustrated in Table 3. As witnessed in Figure 11a, an increase in the temperature has led to an increase in the RE(%) of Cd2+, Hg2+, and Pb2+. Therefore, Cd2+, Hg2+, and Pb2+ adsorption on the ZM-g-Pani nano-adsorbent is endothermic [4]. Polymer-based adsorbents have been found to yield comparable outcomes in the elimination of dicationic metal (M2+), as reported by several studies [4,59,60].

Table 3.
Thermodynamic parameters obtained for Cd2+, Hg2+, and Pb2+ on the ZM-g-Pani adsorbent.
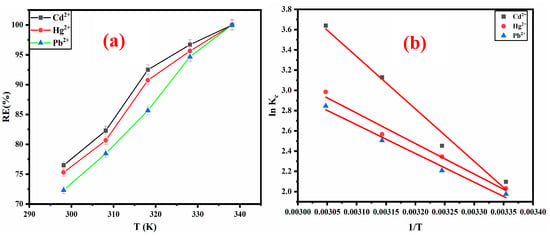
Figure 11.
Variation in RE% with temperature for Cd2+, Hg2+, and Pb2+ adsorption (a). ln Kc versus 1/T for the adsorption of Cd2+, Hg2+, and Pb2+ on the ZM-g-Pani adsorbent (b).
To find out the mechanism for Cd2+, Hg2+, and Pb2+ sorption onto ZM-g-Pani, the adsorption free energy (ΔG°), standard enthalpy (ΔH°), and standard entropy (ΔS°) have been calculated from the adsorption data of Cd2+, Hg2+, and Pb2+ on ZM-g-Pani at various temperatures (Figure 11b). The relationship between ΔG°, ΔH°, and ΔS° have been shown as the following Equations (10) and (11) [61]:
where R refers to the universal gas constant (8.314 J/mol/K); T is the solution temperature in Kelvin.
According to the outcomes presented in Table 3, it has been confirmed that the adsorption process of ZM-g-Pani/M2+ occurred with an endothermic nature and has a solid-phase interface with randomness. This has been indicated by the positive values of ΔH° and ΔS°, respectively. The negative values of ΔG° indicates that the uptake of M2+ (Cd2+, Hg2+, and Pb2+) onto ZM-g-Pani is a spontaneous process. Additionally, the values of ΔG° being less than −20 kJ/mol suggests that the physisorption mechanism is more favorable for the adsorption of Cd2+, Hg2+, and Pb2+ ions onto ZM-g-Pani [33]. Based on the results in Table 3, it can be concluded that the adsorption of Cd2+, Hg2+, and Pb2+ ions is caused by physisorption at low temperatures and chemisorption at high temperatures, as the values of ΔG° enhanced with temperature. Therefore, it can be inferred that the adsorption process suggested for Cd2+, Hg2+, and Pb2+ ions onto the surface of the ZM-g-Pani nano-adsorbent is thermodynamically viable.
3.2.6. Regeneration and the Effect of Competitive Ions
The regeneration of the nanocomposite is a crucial element in the removal process for saving energy and reducing costs. To address this, a specific amount of newly synthesized ZM-g-Pani is used in a continuous cycle of eight adsorption–desorption cycles. After each cycle, the adsorbate (Cd2+, Hg2+, and Pb2+) is extracted using 0.1 M HCl for 20 min. The results, as illustrated in Figure 12, demonstrate that RE(%) remained high at more than 85% for all the chosen metal ions after eight cycles without any substantial decline in removal efficiency. The impact of co-existing ions on the efficiency of adsorption has been evaluated separately for solutions containing 40 mg/L of Cd2+ at pH 8, 40 mg/L of Pb2+ at pH 6, and 40 mg/L of Hg2+ at pH 7. The solution also contains various ions, including NO3−, PO43−, SO42−, Cl−, Na+, Cu2+, and Al3+ at a concentration of 250 mg/L. The results of the removal study indicate that there are no substantial alterations in the uptake of the selected metal ions with ZM-g-Pani (R% = 85.53%) in the presence of the chosen anions and cations. However, the adsorption yield is lower compared to that of the wastewater (R% > 95%), which is due to the exhaustion of the functional sites of the ZM-g-Pani with external anions and cations. Additionally, there is close rivalry for adsorption between the co-existing Cd2+, Pb2+, and Hg2+ ions over the ZM-g-Pani, leading to a reduction in the adsorption yield.
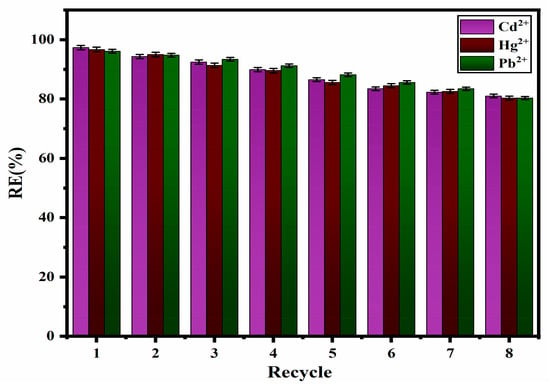
Figure 12.
Regeneration of ZM-g-Pani after adsorption of Cd2+, Hg2+, and Pb2+ ions.
4. Conclusions
In this study, zinc oxide-coated amino acid-functionalized polyaniline copolymerized with moringa oleifera gum has been studied for the removal of Cd2+, Pb2+, and Hg2+ ions from aqueous solutions. FTR-IR study shows that the carboxyl, hydroxyl, amines, imines, and thiol groups are the main reactive groups. Prepared bionanocomposites showed selectivity for heavy metal ions in the order of Cd2+ > Hg2+ = Pb2+ with individual adsorption capacities of 840.33, 497.51, and 497.51 mg/g, and there was no significant response for common interfering anionic (NO3−, PO43−, SO42−, Cl−) and cationic ions (Na+, Cu2+, and Al3+). According to the Langmuir isotherm, the sorption sites’ count remains almost constant at room temperature. In contrast, the Freundlich isotherm suggests that the sorbent’s surface is heterogeneous. The data on adsorption kinetics, depicting the adsorption of divalent metal ions by ZM-g-Pani, adhere to a pseudo-second order model. This observation implies that chemisorption determines the adsorption rate-limiting step. Furthermore, the thermodynamic studies prove that Cd2+, Pb2+, and Hg2+ adsorption onto the polyaniline-based nano-adsorbent is endothermic and spontaneous. Overall, combined with the porous polyaniline matrix and L-methionine grafting, ZnO NPs provided a high surface area, which enhances adsorption capacity and allows efficient ion diffusion, thereby improving overall adsorption effectiveness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16182576/s1, Table S1: Comparison of maximum adsorption capacity for various polymer-based nanocomposites. References [1,23,36,59,62,63,64,65,66,67,68,69,70,71,72] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.S.T.; Data curation, Z.I.; Investigation, A.M.R. and M.A.; Methodology, M.S.T.; Project administration, M.A.; Resources, Z.I.; Software, A.M.R.; Supervision, M.A.; Validation, M.S.T.; Writing—original draft, M.S.T.; Writing—review and editing, A.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are not publicly available due to confidentiality agreements or privacy concerns.
Acknowledgments
Mohd S. Tanweer is thankful to the University Grants Commission (UGC) for the Non-NET Fellowship.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rostami, M.; Jahed-khaniki, G.; Aghaee, E.M.-; Shariatifar, N.; Sani, M.A.; Azami, M.; Rezvantalab, S.; Ramezani, S.; Ghorbani, M. Polycaprolactone/Polyacrylic Acid/Graphene Oxide Composite Nanofibers as a Highly Efficient Sorbent to Remove Lead Toxic Metal from Drinking Water and Apple Juice. Sci. Rep. 2024, 14, 4372. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tanweer, M.S.; Alam, M. Kinetic, Isothermal, Thermodynamic and Adsorption Studies on Mentha Piperita Using ICP-OES. Surf. Interfaces 2020, 19, 100516. [Google Scholar] [CrossRef]
- Rind, I.K.; Memon, N.; Khuhawar, M.Y.; Soomro, W.A.; Lanjwani, M.F. Modeling of Cadmium(II) Removal in a Fixed Bed Column Utilizing Hydrochar-Derived Activated Carbon Obtained from Discarded Mango Peels. Sci. Rep. 2022, 12, 8001. [Google Scholar] [CrossRef] [PubMed]
- Ranote, S.; Ram, B.; Kumar, D.; Chauhan, G.S.; Joshi, V. Functionalization of Moringa Oleifera Gum for Use as Hg2+ Ions Adsorbent. J. Environ. Chem. Eng. 2018, 6, 1805–1813. [Google Scholar] [CrossRef]
- Rabiee Abyaneh, M.; Nabi Bidhendi, G.; Daryabeigi Zand, A. Pb(ΙΙ), Cd(ΙΙ), and Mn(ΙΙ) Adsorption onto Pruning-Derived Biochar: Physicochemical Characterization, Modeling and Application in Real Landfill Leachate. Sci. Rep. 2024, 14, 3426. [Google Scholar] [CrossRef]
- Pandey, L.M.; Hasan, A. Nanoscale Engineering of Biomaterials: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–704. [Google Scholar] [CrossRef]
- Ahmad, S.; Tanweer, M.S.; Mir, T.A.; Alam, M.; Ikram, S.; Sheikh, J.N. Antimicrobial Gum Based Hydrogels as Adsorbents for the Removal of Organic and Inorganic Pollutants. J. Water Process Eng. 2023, 51, 103377. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent Advances in Adsorptive Removal of Wastewater Pollutants by Chemically Modified Metal Oxides: A Review. J. Water Process Eng. 2022, 46, 102641. [Google Scholar] [CrossRef]
- Tanweer, M.S.; Chauhan, H.; Alam, M. Advanced 2D Nanomaterial Composites: Applications in Adsorption of Water Pollutants and Toxic Gases. In 2D Nanomaterials for Energy and Environmental; Springer Nature: Singapore, 2022; pp. 97–124. [Google Scholar] [CrossRef]
- Tanweer, M.S.; Alam, M. Novel 2D Nanomaterial Composites Photocatalysts: Application in Degradation of Water Contaminants. In 2D Nanomaterials for Energy and Environmental Sustainability; Springer Nature: Singapore, 2022; pp. 75–96. [Google Scholar] [CrossRef]
- Rajendran, H.K.; Deen, M.A.; Ray, J.P.; Singh, A.; Narayanasamy, S. Harnessing the Chemical Functionality of Metal–Organic Frameworks Toward Removal of Aqueous Pollutants. Langmuir 2024, 40, 3963–3983. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Comparative Study for Adsorption of Congo Red and Methylene Blue Dye on Chitosan Modified Hybrid Nanocomposite. Process Biochem. 2021, 108, 90–102. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Novel In-Situ Fabrication of L-Methionine Functionalized Bionanocomposite for Adsorption of Amido Black 10B Dye. Process Biochem. 2022, 119, 48–57. [Google Scholar] [CrossRef]
- Duan, F.; Zhu, Y.; Liu, Y.; Mu, B.; Wang, A. Green Fabrication of Porous Adsorbent with Structural Evolution of Mixed-Dimension Attapulgite Clay for Efficient Removal of Methylene Blue and Sustainable Utilization. ACS Sustain. Resour. Manag. 2024, 1, 670–680. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Ivanov, N.P.; Balanov, M.I.; Dran’kov, A.N.; Shkuratov, A.L.; Zarubina, N.V.; Fedorets, A.N.; Mayorov, V.Y.; Lembikov, A.O.; et al. Study of Adsorption and Immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ Ions on Na-Faujasite Zeolite Transformed in Solid State Matrices. Sep. Purif. Technol. 2024, 332, 125662. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Bu, R.; Cai, X.; Sun, X. Purification of Runoff Pollution Using Porous Asphalt Concrete Incorporating Zeolite Powder. Constr. Build. Mater. 2024, 411, 134740. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Y.; Chen, M.; Wang, Y.; Ding, J.; Ma, B.; Wang, Q.; Lu, S. One-Step High Efficiency Synthesis of Zeolite from Fly Ash by Mechanochemical Method as a Low-Cost Adsorbent for Cadmium Removal. J. Environ. Chem. Eng. 2024, 12, 111877. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Reduced Graphene Oxide-Modified Spinel Cobalt Ferrite Nanocomposite: Synthesis, Characterization, and Its Superior Adsorption Performance for Dyes and Heavy Metals. ACS Omega 2023, 8, 6376–6390. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Simple One-POT Hydrothermal Synthesis of CTAB-Assisted Spinel Manganese Ferrite Nanoparticles for Dye Removal: Kinetic and Isotherm Studies. In Recent Advances in Nanomaterials; Springer: Singapore, 2024; Volume 27, pp. 319–324. [Google Scholar] [CrossRef]
- Akl, M.A.; Mostafa, A.G.; Abdelaal, M.Y.; Nour, M.A.K. Surfactant Supported Chitosan for Efficient Removal of Cr(VI) and Anionic Food Stuff Dyes from Aquatic Solutions. Sci. Rep. 2023, 13, 15786. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Sun, L.; Zhang, J.; Han, Z.; Xu, S.; Cheng, Z. Adsorption of Heavy Metals and Antibacterial Activity of Silicon-Doped Chitosan Composite Microspheres Loaded with ZIF-8. Sep. Purif. Technol. 2024, 328, 124969. [Google Scholar] [CrossRef]
- Gomez-Suarez, M.; Chen, Y.; Zhang, J. Porous Organic Polymers as a Promising Platform for Efficient Capture of Heavy Metal Pollutants in Wastewater. Polym. Chem. 2023, 14, 4000–4032. [Google Scholar] [CrossRef]
- Kumarage, S.; Munaweera, I.; Sandaruwan, C.; Weerasinghe, L.; Kottegoda, N. Electrospun Amine-Functionalized Silica Nanoparticles–Cellulose Acetate Nanofiber Membranes for Effective Removal of Hardness and Heavy Metals (As(V), Cd(II),Pb(II)) in Drinking Water Sources. Environ. Sci. Water Res. Technol. 2023, 9, 2664–2679. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhou, Z.; Feng, J.; Li, M.; Chen, J.; Yan, W. Selective Adsorption Behavior of Sulfuric Acid Oxidized and Doped Conjugated Microporous Poly(Aniline)s toward Lead Ions in an Aqueous Environment. Langmuir 2024, 40, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Dewa, L.; Tichapondwa, S.M.; Mhike, W. Adsorption of Hexavalent Chromium from Wastewater Using Polyaniline-Coated Microcrystalline Cellulose Nanocomposites. RSC Adv. 2024, 14, 6603–6616. [Google Scholar] [CrossRef] [PubMed]
- Tanweer, M.S.; Iqbal, Z.; Alam, M. Fabrication of Electrospun PVA-Aloe Vera Hybrid Nanofibers: Dye Removal Ability from Wastewater. In Recent Advances in Nanomaterials; Springer: Singapore, 2024; Volume 27, pp. 457–464. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; El Ouardi, M.; Ajmal, Z.; Lakhmiri, R.; Boukherroub, R.; Albourine, A. Synthesis and Characterization of Arginine-Doped Polyaniline/Walnut Shell Hybrid Composite with Superior Clean-up Ability for Chromium (VI) from Aqueous Media: Equilibrium, Reusability and Process Optimization. J. Mol. Liq. 2020, 316, 113832. [Google Scholar] [CrossRef]
- Ahmar Rauf, M.; Oves, M.; Ur Rehman, F.; Rauf Khan, A.; Husain, N. Bougainvillea Flower Extract Mediated Zinc Oxide’s Nanomaterials for Antimicrobial and Anticancer Activity. Biomed. Pharmacother. 2019, 116, 108983. [Google Scholar] [CrossRef]
- Rauf, M.A.; Owais, M.; Rajpoot, R.; Ahmad, F.; Khan, N.; Zubair, S. Biomimetically Synthesized ZnO Nanoparticles Attain Potent Antibacterial Activity against Less Susceptible S. Aureus Skin Infection in Experimental Animals. RSC Adv. 2017, 7, 36361–36373. [Google Scholar] [CrossRef]
- Saruchi; Sharma, M.; Hatshan, M.R.; Kumar, V.; Rana, A. Sequestration of Eosin Dye by Magnesium (II)-Doped Zinc Oxide Nanoparticles: Its Kinetic, Isotherm, and Thermodynamic Studies. J. Chem. Eng. Data 2021, 66, 646–657. [Google Scholar] [CrossRef]
- Bir, R.; Tanweer, M.S.; Singh, M.; Alam, M. Multifunctional Ternary NLP/ZnO@ l -Cysteine- Grafted-PANI Bionanocomposites for the Selective Removal of Anionic and Cationic Dyes from Synthetic and Real Water Samples. ACS Omega 2022, 7, 44850. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Sinha Ray, S. The Adsorption of Pb2+ and Cu2+ onto Gum Ghatti-Grafted Poly(Acrylamide- Co -Acrylonitrile) Biodegradable Hydrogel: Isotherms and Kinetic Models. J. Phys. Chem. A 2015, 119, 2026–2039. [Google Scholar] [CrossRef]
- Tanweer, M.S.; Iqbal, Z.; Alam, M. Experimental Insights into Mesoporous Polyaniline-Based Nanocomposites for Anionic and Cationic Dye Removal. Langmuir 2022, 38, 8837–8853. [Google Scholar] [CrossRef]
- Ayad, M.; El-Hefnawy, G.; Zaghlol, S. Facile Synthesis of Polyaniline Nanoparticles; Its Adsorption Behavior. Chem. Eng. J. 2013, 217, 460–465. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, W.; Xie, Z.; Song, Y.; Zheng, Q. L-Cysteine-Reduced Graphene Oxide/Poly(Vinyl Alcohol) Ultralight Aerogel as a Broad-Spectrum Adsorbent for Anionic and Cationic Dyes. J. Mater. Sci. 2017, 52, 5807–5821. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, S.; Xia, M.; Wang, F.; Lei, W. Methionine-Montmorillonite Composite—A Novel Material for Efficient Adsorption of Lead Ions. Adv. Powder Technol. 2020, 31, 708–717. [Google Scholar] [CrossRef]
- Gupta, S.; Kachhwaha, S.; Kothari, S.L.; Bohra, M.K.; Jain, R. Surface Morphology and Physicochemical Characterization of Thermostable Moringa Gum: A Potential Pharmaceutical Excipient. ACS Omega 2020, 5, 29189–29198. [Google Scholar] [CrossRef]
- Velu, M.; Balasubramanian, B.; Velmurugan, P.; Kamyab, H.; Ravi, A.V.; Chelliapan, S.; Lee, C.T.; Palaniyappan, J. Fabrication of Nanocomposites Mediated from Aluminium Nanoparticles/Moringa Oleifera Gum Activated Carbon for Effective Photocatalytic Removal of Nitrate and Phosphate in Aqueous Solution. J. Clean. Prod. 2021, 281, 124553. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and Functional Characteristics of Purified Gum from Australian Chia Seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Largani, S.H.; Mohammad, P.A. The Effect of Concentration Ratio and Type of Functional Group on Synthesis of CNT–ZnO Hybrid Nanomaterial by an in Situ Sol–Gel Process. Int. Nano Lett. 2016, 7, 25–33. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Wang, X.J.; Li, J.; Xu, G. Bin Synthesis and Performance of ZnO Quantum Dots Water-Based Fluorescent Ink for Anti-Counterfeiting Applications. Sci. Rep. 2021, 11, 5841. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mallakpour, S.; Borandeh, S. Preparation, Characterization and Surface Morphology of Novel Optically Active Poly(Ester-Amide)/Functionalized ZnO Bionanocomposites via Ultrasonication Assisted Process. Appl. Surf. Sci. 2011, 257, 6725–6733. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Mohamad, S.; Atika Baharin, S.N. Synthesis and Characterization of Co3O4 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Methyl Orange Adsorption from Aqueous Solution. RSC Adv. 2016, 6, 43388–43400. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Sree Vidya, S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Rani, N.; Saxena, P.; Bhandari, H.; Dhawan, S.K. Development of Polyaniline/Zinc Oxide Nanocomposite Impregnated Fabric as an Electrostatic Charge Dissipative Material. Polym. Int. 2015, 64, 1096–1103. [Google Scholar] [CrossRef]
- Ahmad, S.; Manzoor, K.; Purwar, R.; Ikram, S. Morphological and Swelling Potential Evaluation of Moringa Oleifera Gum/Poly(Vinyl Alcohol) Hydrogels as a Superabsorbent. ACS Omega 2020, 5, 17955–17961. [Google Scholar] [CrossRef] [PubMed]
- Majhi, D.; Patra, B.N. Polyaniline and Sodium Alginate Nanocomposite: A PH-Responsive Adsorbent for the Removal of Organic Dyes from Water. RSC Adv. 2020, 10, 43904–43914. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qin, Z.; Liang, B.; Tian, F.; Zhao, J.; Liu, N.; Zhu, M. Morphology-Dependent Capacitive Properties of Three Nanostructured Polyanilines through Interfacial Polymerization in Various Acidic Media. Electrochim. Acta 2015, 177, 343–351. [Google Scholar] [CrossRef]
- Deb, A.; Kanmani, M.; Debnath, A.; Bhowmik, K.L.; Saha, B. Ultrasonic Assisted Enhanced Adsorption of Methyl Orange Dye onto Polyaniline Impregnated Zinc Oxide Nanoparticles: Kinetic, Isotherm and Optimization of Process Parameters. Ultrason. Sonochem. 2019, 54, 290–301. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, Y.; Rao, W.; Liu, T.; Liao, L. Study on the Adsorption Properties of Methyl Orange by Natural One-Dimensional Nano-Mineral Materials with Different Structures. Sci. Rep. 2021, 11, 10640. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, L.W.; Zhao, X.; Liu, S.; Li, D. Removal of Pb(II), Cd(II) and Hg(II) from Aqueous Solution by Mercapto-Modified Coal Gangue. J. Environ. Manag. 2019, 231, 391–396. [Google Scholar] [CrossRef]
- Masoumi, A.; Hemmati, K.; Ghaemy, M. Structural Modification of Acrylonitrile–Butadiene–Styrene Waste as an Efficient Nanoadsorbent for Removal of Metal Ions from Water: Isotherm, Kinetic and Thermodynamic Study. RSC Adv. 2014, 5, 1735–1744. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Polyacrylamide-Grafted Actinidia Deliciosa Peels Powder (PGADP) for the Sequestration of Crystal Violet Dye: Isotherms, Kinetics and Thermodynamic Studies. Appl. Water Sci. 2020, 10, 195. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Ahmad, M.N.; Walker, G.; Leahy, J.J.; Kwapinski, W. Batch and Continuous Systems for Zn, Cu, and Pb Metal Ions Adsorption on Spent Mushroom Compost Biochar. Ind. Eng. Chem. Res. 2019, 58, 7296–7307. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nano Zerovalent Iron Particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Mansour, R.A.E.G.; Simeda, M.G.; Zaatout, A.A. Removal of Brilliant Green Dye from Synthetic Wastewater under Batch Mode Using Chemically Activated Date Pit Carbon. RSC Adv. 2021, 11, 7851–7861. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Liu, J.; Li, X.; Zhao, L.; Su, Y.; Yue, Q.; Gao, B. Adsorption Behavior and Mechanism of Heavy Metal Ions by Chicken Feather Protein-Based Semi-Interpenetrating Polymer Networks Super Absorbent Resin. RSC Adv. 2016, 6, 83234–83243. [Google Scholar] [CrossRef]
- Nematidil, N.; Sadeghi, M.; Nezami, S.; Sadeghi, H. Synthesis and Characterization of Schiff-Base Based Chitosan-g-Glutaraldehyde/NaMMTNPs-APTES for Removal Pb2+ and Hg2+ Ions. Carbohydr. Polym. 2019, 222, 114971. [Google Scholar] [CrossRef]
- Ukani, H.; Mehra, S.; Parmar, B.; Kumar, A.; Khan, I.; El Seoud, O.A.; Malek, N. Metal-Organic Framework-Based Aerogel: A Novel Adsorbent for the Efficient Removal of Heavy Metal Ions and Selective Removal of a Cationic Dye from Aqueous Solution. Ind. Eng. Chem. Res. 2022, 62, 5002–5014. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Chemically Treated Lawsonia Inermis Seeds Powder (CTLISP): An Eco-Friendly Adsorbent for the Removal of Brilliant Green Dye from Aqueous Solution. Groundw. Sustain. Dev. 2020, 11, 100417. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Rezaul Karim, M.; Rahman, M.M.; Khaleque, M.A. Efficient Detection and Adsorption of Cadmium(II) Ions Using Innovative Nano-Composite Materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Abdelmonem, H.A.; Hassanein, T.F.; Sharafeldin, H.E.; Gomaa, H.; Ahmed, A.S.A.; Abdel-lateef, A.M.; Allam, E.M.; Cheira, M.F.; Eissa, M.E.; Tilp, A.H. Cellulose-Embedded Polyacrylonitrile/Amidoxime for the Removal of Cadmium (II) from Wastewater: Adsorption Performance and Proposed Mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 684, 133081. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Al Harby, N.; El Batouti, M.; Abd El-Monaem, E.M. Engineering a Sustainable Cadmium Sulfide/Polyethyleneimine-Functionalized Biochar/Chitosan Composite for Effective Chromium Adsorption: Optimization, Co-Interfering Anions, and Mechanisms. RSC Adv. 2024, 14, 22266–22279. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, I.; Soltanolkottabi, F.; Hosseini, F.; Jafari, H. Investigation of Cadmium Adsorption Factors from Water by Synthesis of Chitosan/Polyvinyl Alcohol/Modified FDU-12 Nanocomposite. J. Chin. Chem. Soc. 2024, 71, 197–208. [Google Scholar] [CrossRef]
- Wang, Y.; Nakano, T.; Chen, X.; Xu, Y.L.; He, Y.J.; Wu, Y.X.; Zhang, J.Q.; Tian, W.; Zhou, M.H.; Wang, S.X. Studies on Adsorption Properties of Magnetic Composite Prepared by One-Pot Method for Cd(II), Pb(II), Hg(II), and As(III): Mechanism and Practical Application in Food. J. Hazard. Mater. 2024, 466, 133437. [Google Scholar] [CrossRef]
- Mondal, H.; Karmakar, M.; Dutta, A.; Mahapatra, M.; Deb, M.; Mitra, M.; Roy, J.S.D.; Roy, C.; Chattopadhyay, P.K.; Singha, N.R. Tetrapolymer Network Hydrogels via Gum Ghatti-Grafted and N-H/C-H-Activated Allocation of Monomers for Composition-Dependent Superadsorption of Metal Ions. ACS Omega 2018, 3, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.A.A.M.; Alayyafi, A.A.A.; El-Desouky, M.G.; El-Bindary, A.A. Chitosan-Nano CuO Composite for Removal of Mercury (II): Box-Behnken Design Optimization and Adsorption Mechanism. Int. J. Biol. Macromol. 2024, 261, 129769. [Google Scholar] [CrossRef]
- Ghumman, A.S.M.; Shamsuddin, R.; Alothman, Z.A.; Waheed, A.; Aljuwayid, A.M.; Sabir, R.; Abbasi, A.; Sami, A. Amine-Decorated Methacrylic Acid-Based Inverse Vulcanized Polysulfide for Effective Mercury Removal from Wastewater. ACS Omega 2024, 9, 4831–4840. [Google Scholar] [CrossRef]
- Jemli, S.; Lütke, S.F.; Chamtouri, F.; Ben Amara, F.; Bejar, S.; Oliveira, M.L.S.; Knani, S.; Silva, L.F.O.; Dotto, G.L. A Novel Cartoon Crosslinked β-Cyclodextrin (C-β-CD) Polymer for Effective Uptake of Hg from Aqueous Solutions: Kinetics, Equilibrium, Thermodynamics, and Statistical Physics Approach. Sep. Purif. Technol. 2024, 330, 125578. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Fakhre, N.A.; Jalhoom, M.G.; Qader, I.N.; Shareef, H.Y.; Jalal, A.F. Removal of Lead Ions from Aqueous Solutions by Modified Cellulose. Environ. Technol. 2024, 45, 2335–2347. [Google Scholar] [CrossRef]
- Du, J.; Sun, J.; Zhang, D. Preparation of Modified Zeolite/Chitosan Composites and Study on the Adsorption Performance of Pb2+. Polym. Eng. Sci. 2024, 64, 196–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).