The Efficiency of Chemical and Electrochemical Coagulation Methods for Pretreatment of Wastewater from Underground Coal Gasification
Abstract
1. Introduction
2. Materials and Methods
UCG Wastewater Characteristic
3. Analytical Methods
3.1. Determination of pH, Electrochemical Conductivity, and Redox Potential
3.2. Determination of COD
3.3. Determination of the Content of Phenols
3.4. Determination of the Content of Free Cyanides
3.5. Determination of the Content of Ammonium Nitrogen
3.6. Determination of the Content of Sulphides
3.7. Determination of the Content of Cations and Anions
3.8. Qualitative and Quantitative Analysis of Trace Elements
3.9. Qualitative and Quantitative Analysis of Volatile Organic Compounds (VOCs)
3.10. Qualitative and Quantitative Analysis of the Polyaromatic Hydrocarbons (PAHs)
4. Introduction of Cyanides and Sulphides to the UCG Wastewater
5. Calculation of Removal Efficiency/Reduction of the Value of Component i
6. Feedstocks
6.1. Coagulants
6.2. Electrodes
7. Chemical Reagents
8. Chemical Coagulation Setup
8.1. Sample Notation
8.2. Test Procedure
8.2.1. Coagulation Procedure
- pH correction;
- Measurement of basic parameters of raw wastewater: pH, conductivity, redox;
- Introducing 1000 cm3 of wastewater into each of the beakers;
- Measurement of preset doses of coagulant and their introduction into syringes;
- Start of the mixing and coagulation program;
- Measurement of basic parameters in the effluent after sedimentation is finished;
- Sampling and lab analyses of the supernatant of the treated wastewater.
8.2.2. Mixing Program
- Initiation of mixing; 1200 rpm for 1 min; very intensive mixing;
- Coagulant injection;
- 700 rpm for 5 min; medium intensity mixing;
- 400 rpm for 5 min;
- 200 rpm for 10 min; minimal intensity mixing, minimal agitation of the liquid;
- Cessation of mixing;
- Sedimentation—120 min.
8.3. Preliminary Tests on the Effect of Coagulant Dosage on the Change in Solution pH
9. Electrocoagulation Setup
Sample Notation
10. Results and Discussion
10.1. Chemical Coagulation of UCG Wastewater
10.1.1. FeCl2 as a Source of Fe Ions—PIX-100
10.1.2. FeSO4 as a Source of Fe Ions—PIX-100 COP
10.1.3. Fe2(SO4)3 as a Source of Fe Ions—PIX-113
10.1.4. FeCl3 as a Source of Fe Ions—PIX-116
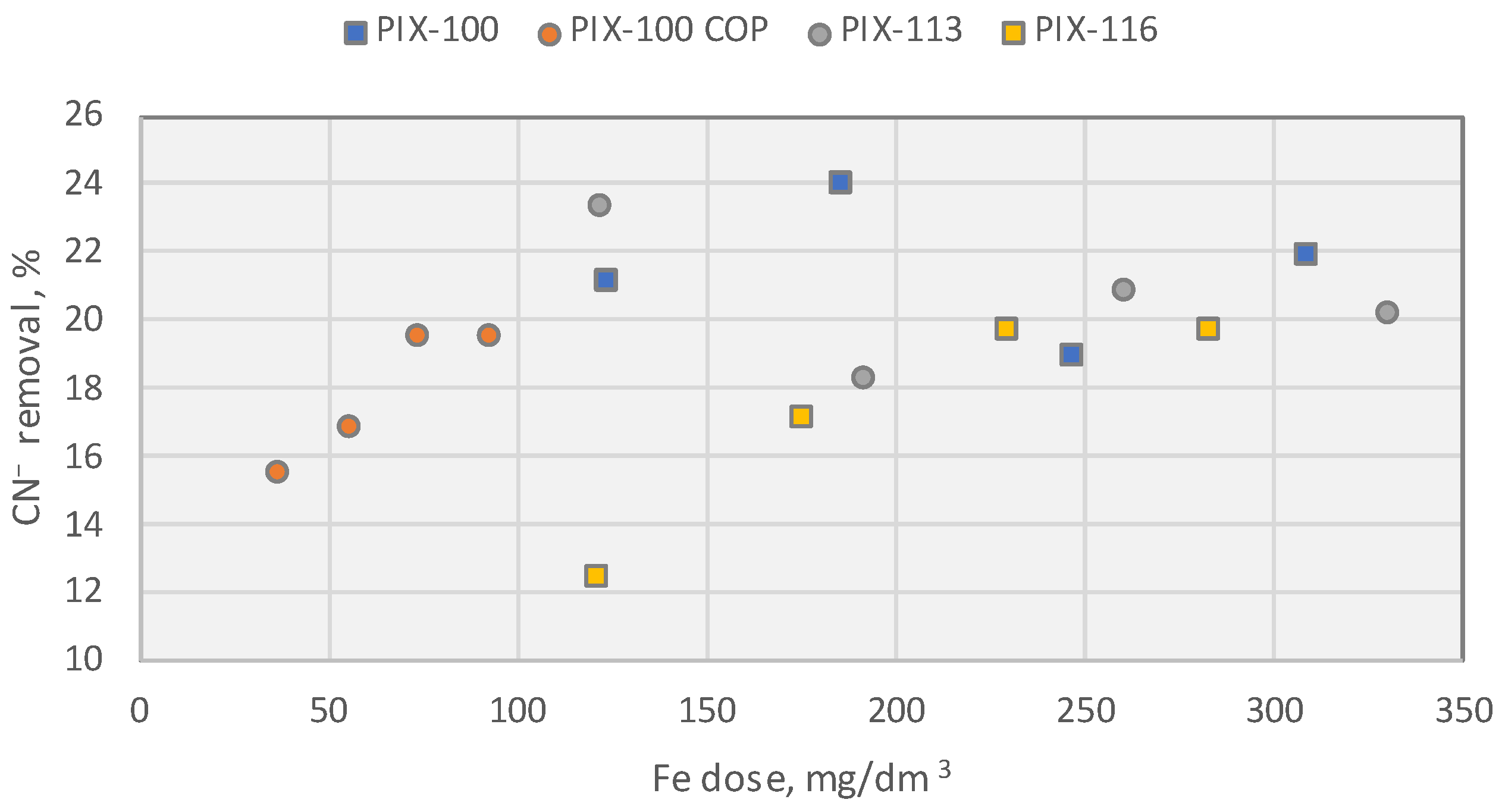
10.1.5. Effect of Coagulant on the Removal of Cyanide Ions
10.1.6. Effect of Coagulant on the Removal of Metals and BTX
10.2. Electrocoagulation of UCG Wastewater
10.2.1. Effect of the Dose of Fe
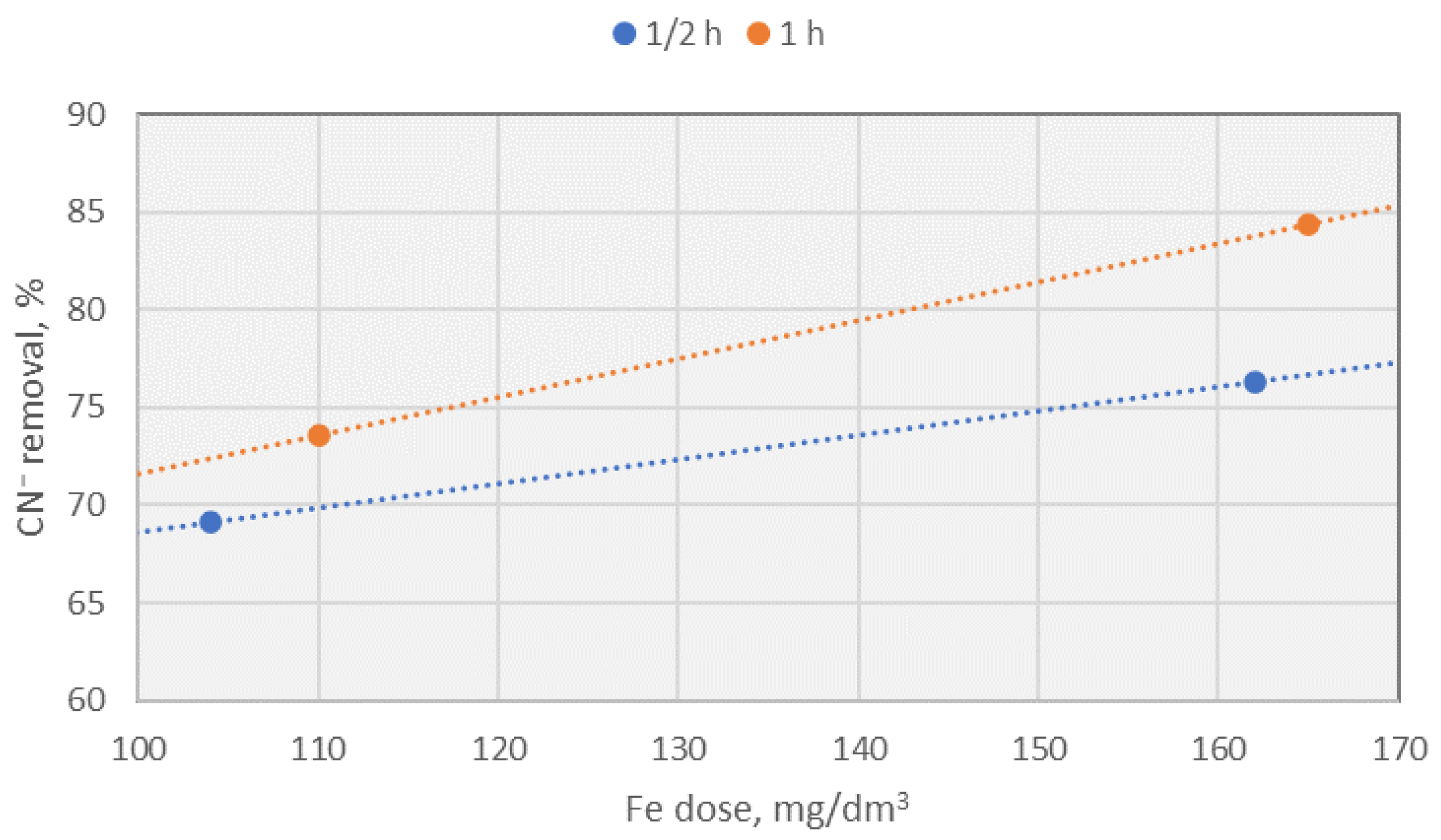
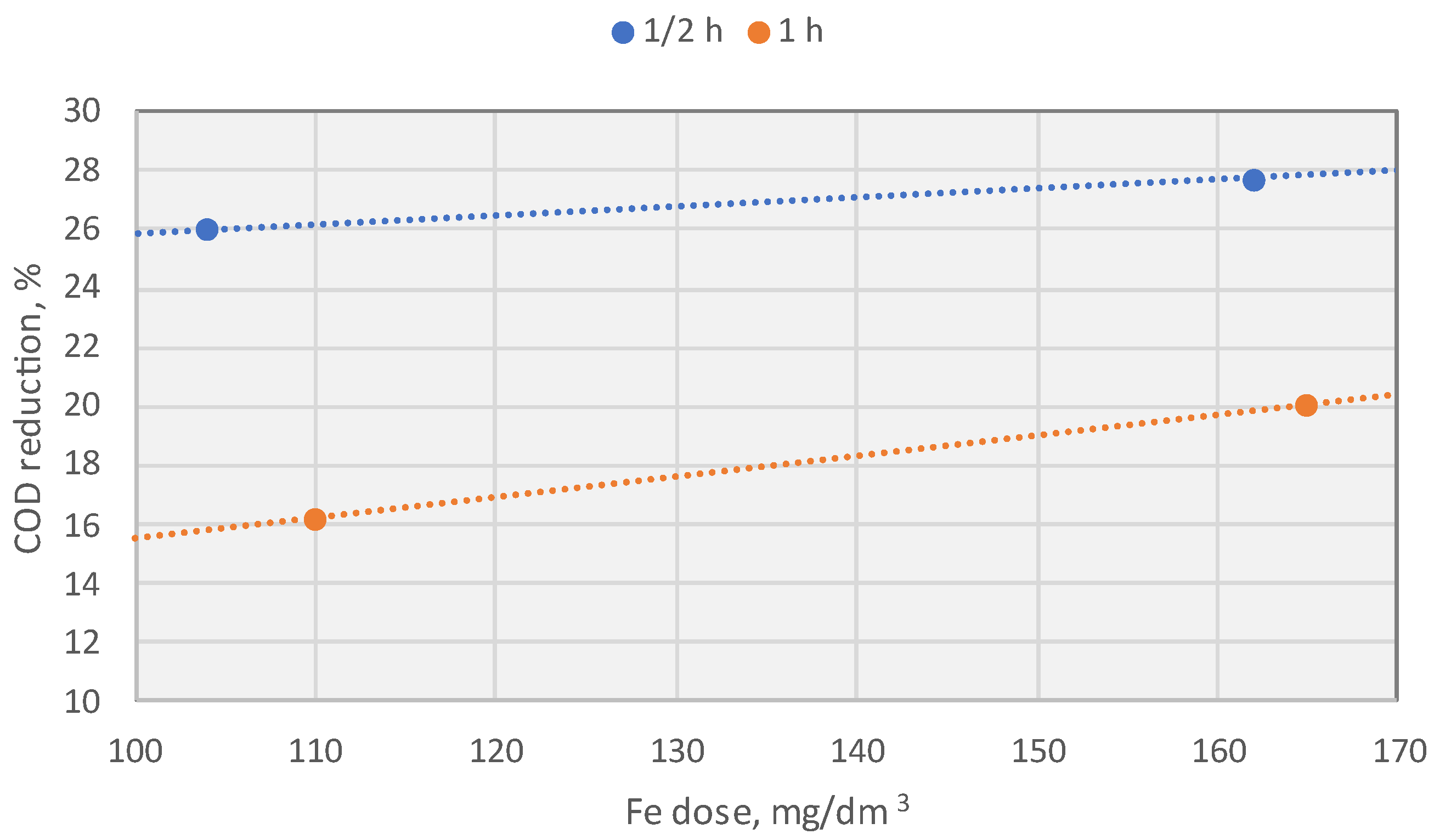
10.2.2. Effect of the Electrode Dissolution Time at a Constant Dose of Fe
10.2.3. Determination of Main Effects and Interactions between Time and Dose
10.2.4. Effect of Effluent pH
10.2.5. Efficiency of Electrocoagulation on Removal of Metals and BTX from Wastewater
11. Conclusions
11.1. Chemical Coagulation
- For all tested doses and forms of Fe used in the coagulation process, only a slight decrease in cyanide concentration was observed. The maximum reduction of 24% was achieved with PIX100 (FeCl2) at a dose of 185 mg Fe/dm3.
- The collected experimental data indicate that the upper limit of cyanide removal efficiency from UCG wastewater for all tested coagulants lies in the range of 20–24%.
- None of the tested process configurations were able to reduce the concentration of cyanides in the solution to below 10 mg/dm3.
- The optimum pH range for the use of all tested coagulants was determined to be 4–9.
- For the maximum tested doses of coagulants, effective coagulation, flocculation, and sedimentation were observed, resulting in residual Fe concentrations in the solution below the limit of detection (LDL).
- All tested coagulants led to a decrease in Al and Sb concentrations to below LDL; however, they also introduced secondary contamination with Ni and Mn. Additionally, PIX100 introduced Cu into the solution, while PIX116 introduced Zn.
- Higher trace element removal efficiency was observed with the sulphate coagulants.
- None of the tested coagulants demonstrated a clear effect on BTX removal.
- One of the risks associated with chemical coagulation for the removal of cyanide or sulphide is the need to apply very high doses of the coagulant. This not only introduces secondary pollutants but also counter ions into the wastewater. It is important to note that the sulphates and chlorides introduced along with the iron can lead to concentrations that exceed permissible limits, necessitating further treatment before the effluent can be safely released.
11.2. Electrocoagulation
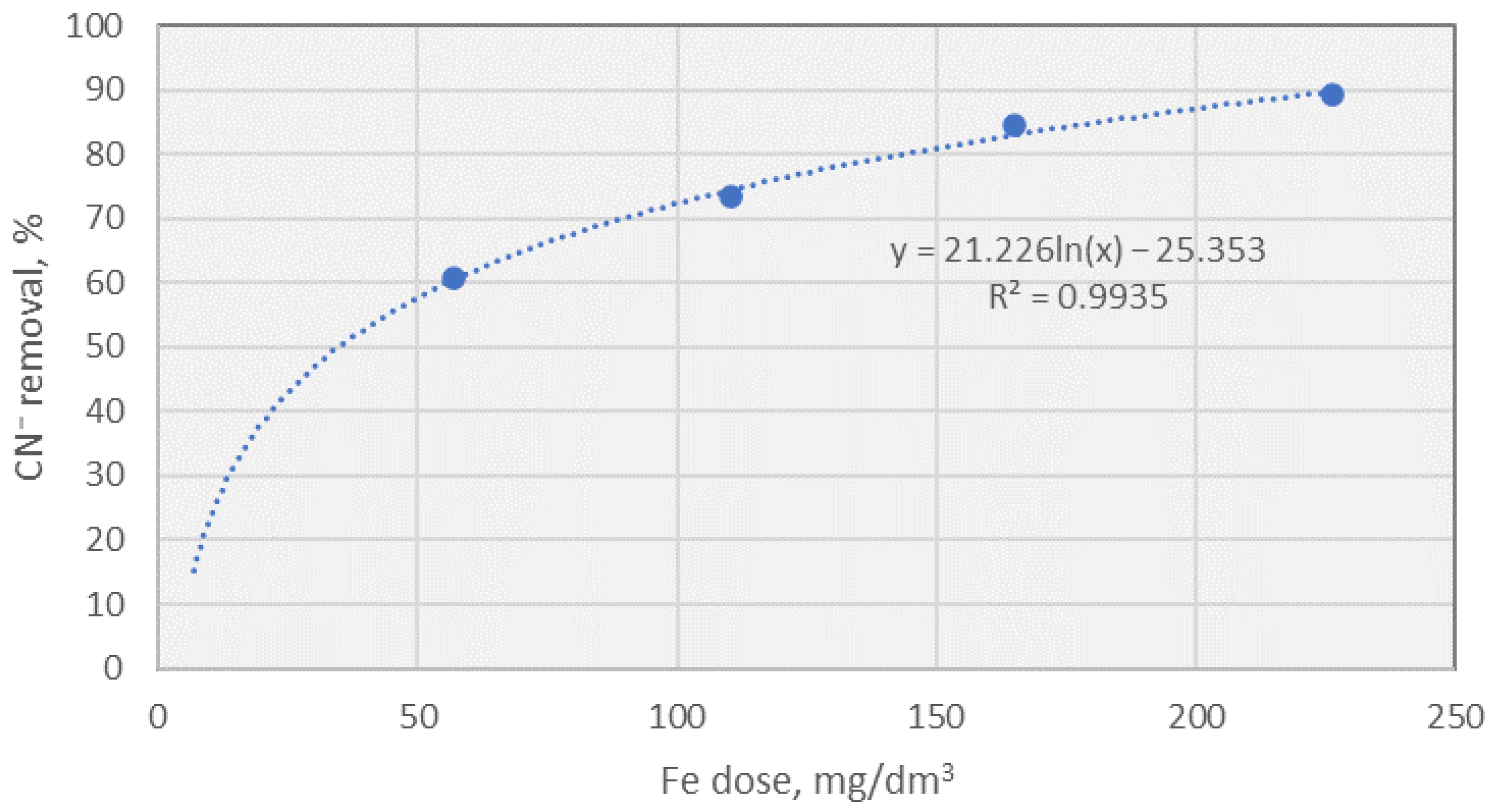
- A dose as low as 60 mg Fe/dm3 led to over 60% cyanide reduction and more than 98% sulphide removal efficiency.
- The highest sulphide removal efficiency achieved was over 99.7%, with a residual S2⁻ concentration of 0.103 mg/dm3, at a dose of 240 mg Fe/dm3.
- Performing EC at a starting pH higher than 8.5 resulted in reduced removal efficiency for S2⁻ and CN⁻, likely due to decreased availability of free Fe ions in solution.
- Increasing the dose of Fe from 60 mg/dm3 to 240 mg/dm3 improved cyanide removal efficiency from 60% to 90%, resulting in a residual concentration of 1.03 mg/dm3.
- For COD, the highest removal efficiency achieved was only 26%.
- During batch EC experiments, the treated wastewater became enriched with Ni and Sn, which may be related to the components of the electrodes used. Other trace elements remained at levels below LDL.
- EC was effective in reducing Zn, Al, and Mn present in UCG wastewater. Notably, Mn was one of the contaminants introduced by chemical coagulants. Doses higher than 220 mg Fe/dm3 led to the complete removal of Zn.
- EC resulted in an increase in Fe content in the solution, which was dose dependent. The maximum concentrations of Fe in the treated effluent were measured at 2–3 mg/dm3. Given the production of insoluble iron hydroxides, effective flocculation and filtration should help manage this contamination effectively.
- For BTX, reductions of up to 50% were observed in benzene, toluene, and ethylbenzene. These results were more consistent than those achieved with chemical coagulants, although the residual concentrations of organics in UCG wastewater remained very low.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTX | benzene, toluene, xylene |
| COD | chemical oxygen demand |
| EC | electrocoagulation |
| GIG-PIB | Główny Instytut Górnictwa—Państwowy Instytut Badawczy (Central Minining Institute—National Research Institute)—Katowice, Poland |
| LDL | lower detection limit |
| ME | main effect |
| PAH | polyaromatic hydrocarbons |
| UCG | underground coal gasification |
References
- Dvornikova, E.V. The Role of Groundwater as an Important Component in Underground Coal Gasification. In Underground Coal Gasification and Combustion; Blinderman, M.S., Klimenko, A.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–281. ISBN 978-0-08-100313-8. [Google Scholar]
- Kapusta, K.; Stańczyk, K. Pollution of Water during Underground Coal Gasification of Hard Coal and Lignite. Fuel 2011, 90, 1927–1934. [Google Scholar] [CrossRef]
- Kapusta, K.; Stańczyk, K.; Wiatowski, M. Comparison of the Contaminants in the Wastewater Produced in the Ex Situ Underground Ortho- and Meta-Lignite Gasification. Water Air Soil Pollut. 2019, 230, 200. [Google Scholar] [CrossRef]
- Jiang, J.-Q. The Role of Coagulation in Water Treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Kamizela, T.; Worwąg, M.; Kowalczyk, M. Environmentally Safe Method for Conditioning and Dewatering Sewage Sludge Using Iron Coagulant, Cellulose and Perlite. Energies 2023, 17, 134. [Google Scholar] [CrossRef]
- Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. The Treatment Effect of Chemical Coagulation Process in South African Brewery Wastewater: Comparison of Polyamine and Aluminum-Chlorohydrate Coagulants. Water 2022, 14, 2495. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W.; Yang, Q.; Chen, W. Chemical Coagulation Process for the Removal of Heavy Metals from Water: A Review. Desalination Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Rathilal, S.; Tetteh, E.K. Coagulation Treatment of Wastewater: Kinetics and Natural Coagulant Evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Alazaiza, M.; Albahnasawi, A.; Ali, G.; Bashir, M.; Nassani, D.; Al Maskari, T.; Amr, S.; Abujazar, M. Application of Natural Coagulants for Pharmaceutical Removal from Water and Wastewater: A Review. Water 2022, 14, 140. [Google Scholar] [CrossRef]
- Khor, C.M.; Wang, J.; Li, M.; Oettel, B.A.; Kaner, R.B.; Jassby, D.; Hoek, E.M.V. Performance, Energy and Cost of Produced Water Treatment by Chemical and Electrochemical Coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al-Kilani, M.R. A Comparison between Iron and Mild Steel Electrodes for the Treatment of Highly Loaded Grey Water Using an Electrocoagulation Technique. Arab. J. Chem. 2023, 16, 105199. [Google Scholar] [CrossRef]
- Mahmad, M.K.N.; Rozainy, M.A.Z.M.R.; Abustan, I.; Baharun, N. Electrocoagulation Process by Using Aluminium and Stainless Steel Electrodes to Treat Total Chromium, Colour and Turbidity. Procedia Chem. 2016, 19, 681–686. [Google Scholar] [CrossRef]
- Chafi, M.; Gourich, B.; Essadki, A.H.; Vial, C.; Fabregat, A. Comparison of Electrocoagulation Using Iron and Aluminium Electrodes with Chemical Coagulation for the Removal of a Highly Soluble Acid Dye. Desalination 2011, 281, 285–292. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, J.; Ren, X.; He, C.; Han, J.; Zhang, Z. A Comparative Study of Electrocoagulation Treatment with Iron, Aluminum and Zinc Electrodes for Selenium Removal from Flour Production Wastewater. Chemosphere 2022, 303, 135249. [Google Scholar] [CrossRef]
- Bote, M.E. Studies on Electrode Combination for COD Removal from Domestic Wastewater Using Electrocoagulation. Heliyon 2021, 7, e08614. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A Critical Review on Lead Removal from Industrial Wastewater: Recent Advances and Future Outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Solís-Rodríguez, R.; Pérez-Garibay, R.; Alonso-González, O.; Mendieta-George, D.; Alvarado-Gómez, A. Vanadium Removal by Electrocoagulation with Anodes of Zinc. J. Environ. Chem. Eng. 2022, 10, 108082. [Google Scholar] [CrossRef]
- Gong, C.; Shen, G.; Huang, H.; He, P.; Zhang, Z.; Ma, B. Removal and Transformation of Polycyclic Aromatic Hydrocarbons during Electrocoagulation Treatment of an Industrial Wastewater. Chemosphere 2017, 168, 58–64. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.-S.Z. Electrochemical Removal of Phenol from Oil Refinery Wastewater. J. Hazard. Mater. 2009, 163, 711–716. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy Metal Ions Removal from Metal Plating Wastewater Using Electrocoagulation: Kinetic Study and Process Performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Zazou, H.; Afanga, H.; Akhouairi, S.; Ouchtak, H.; Addi, A.A.; Akbour, R.A.; Assabbane, A.; Douch, J.; Elmchaouri, A.; Duplay, J.; et al. Treatment of Textile Industry Wastewater by Electrocoagulation Coupled with Electrochemical Advanced Oxidation Process. J. Water Process Eng. 2019, 28, 214–221. [Google Scholar] [CrossRef]
- Mamelkina, M.A.; Cotillas, S.; Lacasa, E.; Sáez, C.; Tuunila, R.; Sillanpää, M.; Häkkinen, A.; Rodrigo, M.A. Removal of Sulfate from Mining Waters by Electrocoagulation. Sep. Purif. Technol. 2017, 182, 87–93. [Google Scholar] [CrossRef]
- Kadier, A.; Al-Qodah, Z.; Akkaya, G.K.; Song, D.; Peralta-Hernández, J.M.; Wang, J.-Y.; Phalakornkule, C.; Bajpai, M.; Niza, N.M.; Gilhotra, V.; et al. A State-of-the-Art Review on Electrocoagulation (EC): An Efficient, Emerging, and Green Technology for Oil Elimination from Oil and Gas Industrial Wastewater Streams. Case Stud. Chem. Environ. Eng. 2022, 6, 100274. [Google Scholar] [CrossRef]
- Akkaya, G.K. Treatment of Petroleum Wastewater by Electrocoagulation Using Scrap Perforated (Fe-Anode) and Plate (Al and Fe-Cathode) Metals: Optimization of Operating Parameters by RSM. Chem. Eng. Res. Des. 2022, 187, 261–275. [Google Scholar] [CrossRef]
- Alam, R.; Sheob, M.; Saeed, B.; Khan, S.U.; Shirinkar, M.; Frontistis, Z.; Basheer, F.; Farooqi, I.H. Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges. Water 2021, 13, 2105. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Kim, S.; So, Y.; Yoon, Y.; Park, C. Comprehensive Analysis of the Integrated Electro-Coagulation and Membrane Filtration Process for Semiconductor Wastewater Treatment. J. Water Process Eng. 2023, 56, 104468. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A State-of-the-Art Review of the Electrocoagulation Technology for Wastewater Treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.; Roa-Morales, G.; Ávila-Córdoba, L.; Pavón-Silva, T.; Bilyeu, B. Electrochemical Treatment Applied to Food-Processing Industrial Wastewater. Ind. Eng. Chem. Res. 2006, 45, 34–38. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Application of Electrocoagulation Process for the Treatment of Dairy Wastewater: A Mini Review. Mater. Today Proc. 2023, 77, 117–124. [Google Scholar] [CrossRef]
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability 2022, 14, 1985. [Google Scholar] [CrossRef]
- Sediqi, S.; Bazargan, A.; Mirbagheri, S.A. Consuming the Least Amount of Energy and Resources in Landfill Leachate Electrocoagulation. Environ. Technol. Innov. 2021, 22, 101454. [Google Scholar] [CrossRef]
- Ika Pratiwi, N.; Mukimin, A.; Zen, N.; Septarina, I. Integration of Electrocoagulation, Adsorption and Wetland Technology for Jewelry Industry Wastewater Treatment. Sep. Purif. Technol. 2021, 279, 119690. [Google Scholar] [CrossRef]
- An, B.-H.; Xu, D.-M.; Geng, R.; Cheng, Y.; Qian, R.-B.; Tang, X.-C.; Fan, Z.-Q.; Chen, H.-B. The Pretreatment Effects of Various Target Pollutant in Real Coal Gasification Gray Water by Coupling Pulse Electrocoagulation with Chemical Precipitation Methods. Chemosphere 2023, 311, 136898. [Google Scholar] [CrossRef]
- Rusdianasari, R.; Rachman, S.; Ibrahim, E.; Ngudiantoro, N. Reduction of Metal Contents in Coal Stockpile Wastewater Using Electrocoagulation. Appl. Mech. Mater. 2013, 391, 29–33. [Google Scholar] [CrossRef]
- Rusdianasari, R.; Bow, Y.; Taqwa, A. Treatment of Coal Stockpile Wastewater by Electrocoagulationusing Aluminum Electrodes. Adv. Mater. Res. 2014, 896, 145–148. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Strugała-Wilczek, A.; Stańczyk, K.; Castro-Muñiz, A.; Suárez-García, F.; Paredes, J.I. Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products. Energies 2023, 16, 4455. [Google Scholar] [CrossRef]
- Borgulat, J.; Ponikiewska, K.; Jałowiecki, Ł.; Strugała-Wilczek, A.; Płaza, G. Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes? Energies 2022, 15, 4419. [Google Scholar] [CrossRef]
- Strugała-Wilczek, A.; Stańczyk, K.; Bebek, K. Comparison of Metal Adsorption from Aqueous Solutions on Coal and Char Remaining After In-situ Underground Coal Gasification (UCG). Mine Water Environ. 2020, 39, 369–379. [Google Scholar] [CrossRef]
- ISO 11885:2007 (en); Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11885:ed-2:v1:en (accessed on 5 August 2024).
- Yu, X.; Xu, R.; Wei, C.; Wu, H. Removal of Cyanide Compounds from Coking Wastewater by Ferrous Sulfate: Improvement of Biodegradability. J. Hazard. Mater. 2016, 302, 468–474. [Google Scholar] [CrossRef]
- Jałowiecki, Ł.; Strugała-Wilczek, A.; Ponikiewska, K.; Borgulat, J.; Płaza, G.; Stańczyk, K. Constructed Wetland as a Green Remediation Technology for the Treatment of Wastewater from Underground Coal Gasification Process. PLoS ONE 2024, 19, e0300485. [Google Scholar] [CrossRef]
- Jałowiecki, Ł.; Borgulat, J.; Strugała-Wilczek, A.; Płaza, G.; Glaser, M. Searching of Phenol-Degrading Bacteria in Raw Wastewater from Underground Coal Gasification Process as Suitable Candidates in Bioaugmenation Approach. J. Ecol. Eng. 2024, 25, 62–71. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| pH [-] | 8.193 |
| Conductivity [mS/cm] | 1600.5 |
| Redox, [mV] | −113.3 |
| COD [mg dm3] | 189.03 |
| CN− [mg/dm3] | 0.971 |
| S2− [mg/dm3] | 0.215 |
| Trace elements | |
| Al [mg/kg] | 2.520 |
| As [mg/kg] | <0.02 |
| Cd [mg/kg] | <0.02 |
| Co [mg/kg] | <0.05 |
| Cr [mg/kg] | <0.025 |
| Cu [mg/kg] | <0.025 |
| Fe [mg/kg] | 0.179 |
| Mn [mg/kg] | 0.333 |
| Mo [mg/kg] | <0.05 |
| Ni [mg/kg] | 0.492 |
| Pb [mg/kg] | <0.05 |
| Sb [mg/kg] | 0.080 |
| Ti [mg/kg] | <0.02 |
| Zn [mg/kg] | 0.213 |
| Sum [mg/kg] | 3.817 |
| BTX | |
| Benzene [mg/dm3] | 0.210 |
| Toluene [mg/dm3] | 0.080 |
| Ethylbenzene [mg/dm3] | 0.004 |
| m-xylene [mg/dm3] | 0.004 |
| p-xylene [mg/dm3] | 0.010 |
| Isopropylbenzene [mg/dm3] | 0.002 |
| o-xylene [mg/dm3] | 0.010 |
| Sum [mg/dm3] | 0.320 |
| Mean | Min | Max | St. dev. | |
|---|---|---|---|---|
| CN−, [mg/Nm3] | 14.65 | 13.70 | 15.80 | 0.79 |
| Mean | Min | Max | St. dev. | |
|---|---|---|---|---|
| CN−, [mg/Nm3] | 14.19 | 13.40 | 15.30 | 0.71 |
| S2−, [mg/Nm3] | 37.92 | 29.85 | 45.15 | 5.39 |
| Trade Name | Chemical Specie | Density, kg/dm3 | Total Fe Content, % w/w |
|---|---|---|---|
| PIX-100 | FeCl2 | 1.2659 | 9.76 |
| PIX-100 COP | FeSO4 | 1.0552 | 1.75 |
| PIX-113 | Fe2(SO4)3 | 1.5262 | 11.39 |
| PIX-116 | FeCl3 | 1.3143 | 10.26 |
| Notation of the Tests and Samples | Coagulant_Dose [mg Fe/dm3]_pH before Coagulation |
|---|---|
| example | PIX100_10.0_6.3 |
| Test and Sample Notation | el_Dose [mg Fe/dm3]_Current [mA]_Time [min]_pH before the Process |
|---|---|
| example | el_d60_i58_t60_pH 8.5 |
| PIX100_1.0_7.9 | PIX100_1.5_7.9 | PIX100_2.0_7.9 | PIX100_2.5_7.9 | |
|---|---|---|---|---|
| Dose, [mg Fe/dm3] | 123.55 | 185.33 | 247.10 | 308.88 |
| pH after coag., [-] | 5.88 | 5.73 | 5.41 | 3.70 |
| Conductivity, [mS/cm] | 2.003 (−25.15%) | 2.241 (−40.02%) | 2.573 (−60.76%) | 2.894 (−80.82%) |
| Redox, [mV] | 24 (−121.18%) | 36 (−131.76%) | 52 (−145.88%) | 152 (−234.12%) |
| CN−, [mg/dm3] | 10.8 (21.17%) | 10.4 (24.09%) | 11.1 (18.98%) | 10.7 (21.9%) |
| COD, [mg/dm3] | 186.2 (2.62%) | 194.2 (−1.57%) | 204.2 (−6.8%) | 216.2 (−13.08%) |
| PIX100 COP_2.0_8.2 | PIX100 COP_3.0_8.2 | PIX100 COP_4.0_8.2 | PIX100 COP_5.0_8.2 | |
|---|---|---|---|---|
| Dose, [mg Fe/dm3] | 36.93 | 55.40 | 73.86 | 92.33 |
| pH after coag., [-] | 7.10 | 6.74 | 6.44 | 6.43 |
| Conductivity, [mS/cm] | 1.6931 (−5.79%) | 1.7402 (−8.73%) | 1.7625 (−10.12%) | 1.7563 (−9.73%) |
| Redox, [mV] | −46 (−59.41%) | −25 (−77.94%) | −7 (−93.82%) | −7 (−93.82%) |
| CN−, [mg/dm3] | 12.5 (15.54%) | 12.3 (16.89%) | 11.9 (19.59%) | 11.9 (19.59%) |
| COD, [mg/dm3] | 181.2 (5.72%) | 181.2 (5.72%) | 180.2 (6.24%) | 180.2 (6.24%) |
| PIX113_0.7_8.3 | PIX113_1.1_10.0 | PIX113_1.5_10.0 | PIX113_1.9_10.0 | |
|---|---|---|---|---|
| Dose, [mg Fe/dm3] | 121.68 | 191.22 | 260.75 | 330.28 |
| pH after coag., [-] | 5.46 | 8.88 | 7.71 | 6.28 |
| Conductivity, [mS/cm] | 1.7646 (−10.25%) | 2.383 (−48.89%) | 2.696 (−68.45%) | 2.847 (−77.88%) |
| Redox, [mV] | 50 (−144.12%) | −149 (−31.47%) | −76 (−32.94%) | 2 (−101.76%) |
| CN−, [mg/dm3] | 12.1 (16.55%) | 12.9 (18.35%) | 12.5 (20.89%) | 12.6 (20.25%) |
| COD, [mg/dm3] | 177.2 (7.32%) | 175.2 (3.31%) | 169.2 (6.62%) | 169.2 (6.62%) |
| PIX116_0.9_10 | PIX116_1.3_10 | PIX116_1.7_10 | PIX116_2.1_10 | |
|---|---|---|---|---|
| Dose, [mg Fe/dm3] | 121.36 | 175.30 | 229.24 | 283.18 |
| pH after coag., [-] | 8.97 | 8.59 | 7.30 | 5.84 |
| Conductivity, [mS/cm] | 2.381 (−48.77%) | 2.623 (−63.89%) | 2.93 (−83.07%) | 3.068 (−91.69%) |
| Redox, [mV] | −154 (−35.88%) | −132 (−16.47%) | −57 (−49.71%) | 27 (−123.82%) |
| CN−, [mg/dm3] | 13.3 (12.5%) | 12.6 (17.11%) | 12.2 (19.74%) | 12.2 (19.74%) |
| COD, [mg/dm3] | 177.2 (6.83%) | 173.2 (8.94%) | 175.2 (7.89%) | 174.2 (8.41%) |
| PIX100_1.5_7.9 | PIX100 CPO_4.0_8.2 | PIX113_1.5_10.0 | PIX116_2.1_10.0 | |
|---|---|---|---|---|
| Dose, [mg Fe/dm3] | 185.33 | 73.86 | 260.75 | 283.18 |
| CN−, [mg/dm3] | 10.4 (24.09%) | 11.9 (19.59%) | 12.5 (20.89%) | 12.2 (19.74%) |
| COD, [mg/dm3] | 194.2 (−1.57%) | 180.2 (6.24%) | 169.2 (6.62%) | 174.2 (8.41%) |
| Trace elements | ||||
| Al, [mg/kg] | <0.125 (>95.04%) | <0.125 (>95.04%) | <0.125 (>95.04%) | <0.125 (>95.04%) |
| Fe, [mg/kg] | 75.9 (−42,302.23%) | <0.125 (>30.17%) | <0.125 (>30.17%) | <0.125 (>30.17%) |
| Mn, [mg/kg] | 1.15 (−245.35%) | 0.452 (−35.74%) | 0.103 (69.07%) | 1.63 (−389.49%) |
| Ni, [mg/kg] | 0.545 (−10.77%) | 0.526 (−6.91%) | 0.546 (−10.98%) | 0.566 (−15.04%) |
| Sb, [mg/kg] | >0.02 (>75%) | >0.02 (>75%) | >0.02 (>75%) | >0.02 (>75%) |
| Zn, [mg/kg] | 0.871 (−308.92%) | 0.079 (62.91%) | 0.014 (93.43%) | 3.7 (−1637.09%) |
| Sum of metals, [mg/kg] | 78.677 (−1961.23%) | 1.327 (65.23%) | 0.933 (75.56%) | 6.257 (−63.92%) |
| BTX | ||||
| Benzene, [mg/dm3] | 0.16 (23.81%) | 0.75 (−257.14%) | 0.06 (71.43%) | 0.66 (−214.29%) |
| Toluene, [mg/dm3] | 0.13 (−62.5%) | 0.15 (−87.5%) | - | - |
| Ethylbenzene, [mg/dm3] | 0.41 (−10,150%) | 0.18 (−4400%) | 0.01 (−150%) | 0.02 (−400%) |
| m-xylene, [mg/dm3] | 0.05 (−1150%) | 0.02 (−400%) | - | - |
| p-xylene, [mg/dm3] | 0.05 (−400%) | 0.38 (−3700%) | 0.01 (0%) | - |
| Isopropylbenzene, [mg/dm3] | 0.04 (−1900%) | 0.06 (−2900%) | - | - |
| o-xylene, [mg/dm3] | 0.04 (−300%) | 0.51 (−5000%) | 0.01 (0%) | 0.22 (−2100%) |
| Sum of BTX, [mg/dm3] | 0.88 (−700%) | 2.05 (−1763.64%) | 0.09 (18.18%) | 1.11 (−909.09%) |
| el_d60_i58_t60 _pH 8.5 | el_d120_i115_t60_pH 8.5 | el_d180_i172_t60_pH 8.5 | el_d240_i230_t60_pH 8.5 | |
|---|---|---|---|---|
| Current, [mA] | 58 | 115 | 172 | 230 |
| Voltage min–max, [V] | 2.79 | 4.51 | 6.46–7.25 | 7.82–8.97 |
| Time, [s] | 3600 | 3600 | 3600 | 3600 |
| Measured Fe dose/difference from Faraday’s law, [mg Fe/dm3/%] | 56.8/6.25 | 110.6/7.93 | 165.2/8.05 | 226.2/5.85 |
| Parameters of the effluent after electrocoagulation | ||||
| pH, [-] | 8.74 | 8.91 | 9.06 | 9.18 |
| Conductivity, [mS/cm] | 1.8339 (−14.58%) | 1.7657 (−10.32%) | 1.6886 (−5.5%) | 1.5938 (0.42%) |
| Redox, [mV] | −142 (−25.29%) | −151 (−33.24%) | −160 (−41.18%) | −166 (−46.47%) |
| CN−, [mg/dm3] | 3.74 (60.59%) | 2.51 (73.55%) | 1.48 (84.4%) | 1.03 (89.15%) |
| S2−, [mg/dm3] | 0.395 (98.96%) | 0.405 (98.93%) | 0.306 (99.19%) | 0.217 (99.43%) |
| COD, [mg/dm3] | 247.2 (13.02%) | 238.2 (16.19%) | 227.2 (20.06%) | 222.2 (21.82%) |
| el_d120_i230_ t30_pH 8.7 | el_d120_i153_ t45_pH 8.5 | el_d120_i115_ t60_pH 8.5 | el_d120_i77_ t90_pH 8.6 | el_d120_i57_ t120_pH 8.7 | |
|---|---|---|---|---|---|
| Current, [mA] | 230 | 153 | 115 | 77 | 57 |
| Voltage min–max, [V] | 8.50–9.86 | 5.50–5.93 | 4.51 | 3.48–3.69 | 2.63–2.81 |
| Time, [s] | 1800 | 2700 | 3600 | 5400 | 7200 |
| Measured Fe dose/difference from Faraday’s law, mg Fe/dm3 | 104.2/13.26% | 109/9.06% | 110.6/7.93% | 113.7/5.76% | 114.9/3.51% |
| Parameters of the effluent after electrocoagulation | |||||
| pH, [-] | 8.99 | 9.04 | 8.91 | 9.02 | 9.03 |
| Conductivity, [mS/cm] | 1.7945 (−12.12%) | 1.7643 (−10.23%) | 1.7657 (−10.32%) | 1.7537 (−9.57%) | 1.7891 (−11.78%) |
| Redox, [mV] | −156 (−37.65%) | −157 (−38.53%) | −151 (−33.24%) | −157 (−38.53%) | −158 (−39.41%) |
| CN−, [mg/dm3] | 2.93 (69.13%) | 3.38 (64.38%) | 2.51 (73.55%) | 3.62 (61.85%) | 4.14 (56.38%) |
| S2−, [mg/dm3] | 0.771 (97.97%) | 0.563 (98.52%) | 0.405 (98.93%) | 0.595 (98.43%) | 0.996 (97.37%) |
| COD, [mg/dm3] | 256.2 (26%) | 254.2 (16.44%) | 238.2 (16.19%) | 261.2 (19.43%) | 266.2 (23.11%) |
| Effect | Removal of CN | Removal of COD |
|---|---|---|
| MEtime | 4.42 | −9.81 |
| MEdose | 7.16 | 1.73 |
| Interaction.Eff. | 3.69 | 2.14 |
| el_d240_i230_t60_pH 8.5 | el_d240_i230_t60_pH 9.5 | el_d240_i230_t60_pH 10.5 | - | |
|---|---|---|---|---|
| Current, [mA] | 230 | 230 | 230 | - |
| Voltage min–max, [V] | 7.82–8.97 | 8.78–10.61 | 7.50–10.25 | - |
| Time, [s] | 3600 | 3600 | 3600 | - |
| Measured Fe dose/difference from Faraday’s law, [mg Fe/dm3/%] | 226.2/5.85 | 213.7/11.05 | 215.5/10.3 | - |
| Parameters of the effluent after electrocoagulation | ||||
| pH, [-] | 9.18 | 10.07 | 11.01 | - |
| Conductivity, [mS/cm] | 1.5938 (0.42%) | 1.7268 (−7.89%) | 2.384 (−48.95%) | - |
| Redox, [mV] | −166 (−46.47%) | −219 (−93.24%) | −272 (−140%) | - |
| CN−, [mg/dm3] | 1.03 (89.15%) | 4.56 (51.95%) | 4.89 (48.47%) | - |
| S2−, [mg/dm3] | 0.217 (99.43%) | 2.578 (93.2%) | 4.038 (89.35%) | - |
| COD, [mg/dm3] | 222.2 (21.82%) | 239.2 (26.22%) | 244.2 (24.68%) | - |
| el_d240_i230_t60_pH8.5 | el_d240_i306_t45_pH 8.5 | el_d180_i345_t30_pH 8.7 | - | |
|---|---|---|---|---|
| Current, [mA] | 230 | 306 | 345 | - |
| Voltage min–max, [V] | 7.82–8.97 | 10.12–11.97 | 11.64–12.65 | - |
| Time, [s] | 3600 | 2700 | 1800 | - |
| Measured Fe dose/difference from Faraday’s law, [mg Fe/dm3/%] | 226.2/5.85 | 227/5.31 | 162.2/9.98 | - |
| Parameters of the effluent after electrocoagulation | ||||
| pH, [-] | 9.18 | 9.22 | 9.16 | - |
| Conductivity, [mS/cm] | 1.5938 (0.42%) | 1.6005 (0%) | 1.7334 (−8.3%) | - |
| Redox, [mV] | −166 (−46.47%) | −168 (−48.24%) | −166 (−46.47%) | - |
| CN−, [mg/dm3] | 1.03 (89.15%) | 2.43 (74.39%) | 2.25 (76.29%) | - |
| S2−, [mg/dm3] | 0.217 (99.43%) | 0.103 (99.73%) | 0.647 (98.29%) | - |
| COD, [mg/dm3] | 222.2 (21.82%) | 229.2 (24.65%) | 250.2 (27.73%) | - |
| Trace elements | ||||
| Al, [mg/kg] | <0.125 (>95.04%) | <0.125 (>95.04%) | <0.125 (>95.04%) | - |
| Fe, [mg/kg] | 1.94 (−983.8%) | 2.55 (−1324.58%) | 2.99 (−1570.39%) | - |
| Mn, [mg/kg] | <0.2 (>39.94%) | <0.2 (>39.94%) | <0.2 (>39.94%) | - |
| Ni, [mg/kg] | 0.748 (−52.03%) | 0.77 (−56.5%) | 0.686 (−39.43%) | - |
| Sb, [mg/kg] | 0.085 (−6.25%) | 0.084 (−5%) | 0.096 (−20%) | - |
| Zn, [mg/kg] | <0.02 (>90.61%) | <0.02 (>90.61%) | <0.02 (>90.61%) | - |
| Sum of metals, [mg/kg] | 3.118 (18.31%) | 3.749 (1.78%) | 4.117 (−7.86%) | - |
| BTX | ||||
| Benzene, [mg/dm3] | 0.204 (2.86%) | 0.1 (52.38%) | 0.2 (4.76%) | - |
| Toluene, [mg/dm3] | 0.269 (−236.25%) | 0.07 (12.5%) | 0.04 (50%) | - |
| Ethylbenzene, [mg/dm3] | 0.01 (−150%) | 0.002 (50%) | - | - |
| m-xylene, [mg/dm3] | 0.02 (−400%) | 0.03 (−650%) | - | - |
| p-xylene, [mg/dm3] | 0.02 (−100%) | 0.01 (0%) | 0.02 (−100%) | - |
| Isopropylbenzene, [mg/dm3] | 0.01 (−400%) | - | 0.009 (−350%) | - |
| o-xylene, [mg/dm3] | 0.03 (−200%) | 0.09 (−800%) | 0.04 (−300%) | - |
| Sum of BTX, [mg/dm3] | 0.563 (−411.82%) | 0.302 (−174.55%) | 0.309 (−180.91%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szul, M.; Rychlewska, K.; Iluk, T.; Billig, T. The Efficiency of Chemical and Electrochemical Coagulation Methods for Pretreatment of Wastewater from Underground Coal Gasification. Water 2024, 16, 2540. https://doi.org/10.3390/w16172540
Szul M, Rychlewska K, Iluk T, Billig T. The Efficiency of Chemical and Electrochemical Coagulation Methods for Pretreatment of Wastewater from Underground Coal Gasification. Water. 2024; 16(17):2540. https://doi.org/10.3390/w16172540
Chicago/Turabian StyleSzul, Mateusz, Katarzyna Rychlewska, Tomasz Iluk, and Tomasz Billig. 2024. "The Efficiency of Chemical and Electrochemical Coagulation Methods for Pretreatment of Wastewater from Underground Coal Gasification" Water 16, no. 17: 2540. https://doi.org/10.3390/w16172540
APA StyleSzul, M., Rychlewska, K., Iluk, T., & Billig, T. (2024). The Efficiency of Chemical and Electrochemical Coagulation Methods for Pretreatment of Wastewater from Underground Coal Gasification. Water, 16(17), 2540. https://doi.org/10.3390/w16172540







