The Degradation of Polycyclic Aromatic Hydrocarbons by Biological Electrochemical System: A Mini-Review
Abstract
1. Introduction
2. Overview of Biological Electrochemical Systems
2.1. System Types of BES
2.2. Electron Transfer in BES
3. Electrochemical Bioremediation Technology for PAHs-Contaminated Sediments
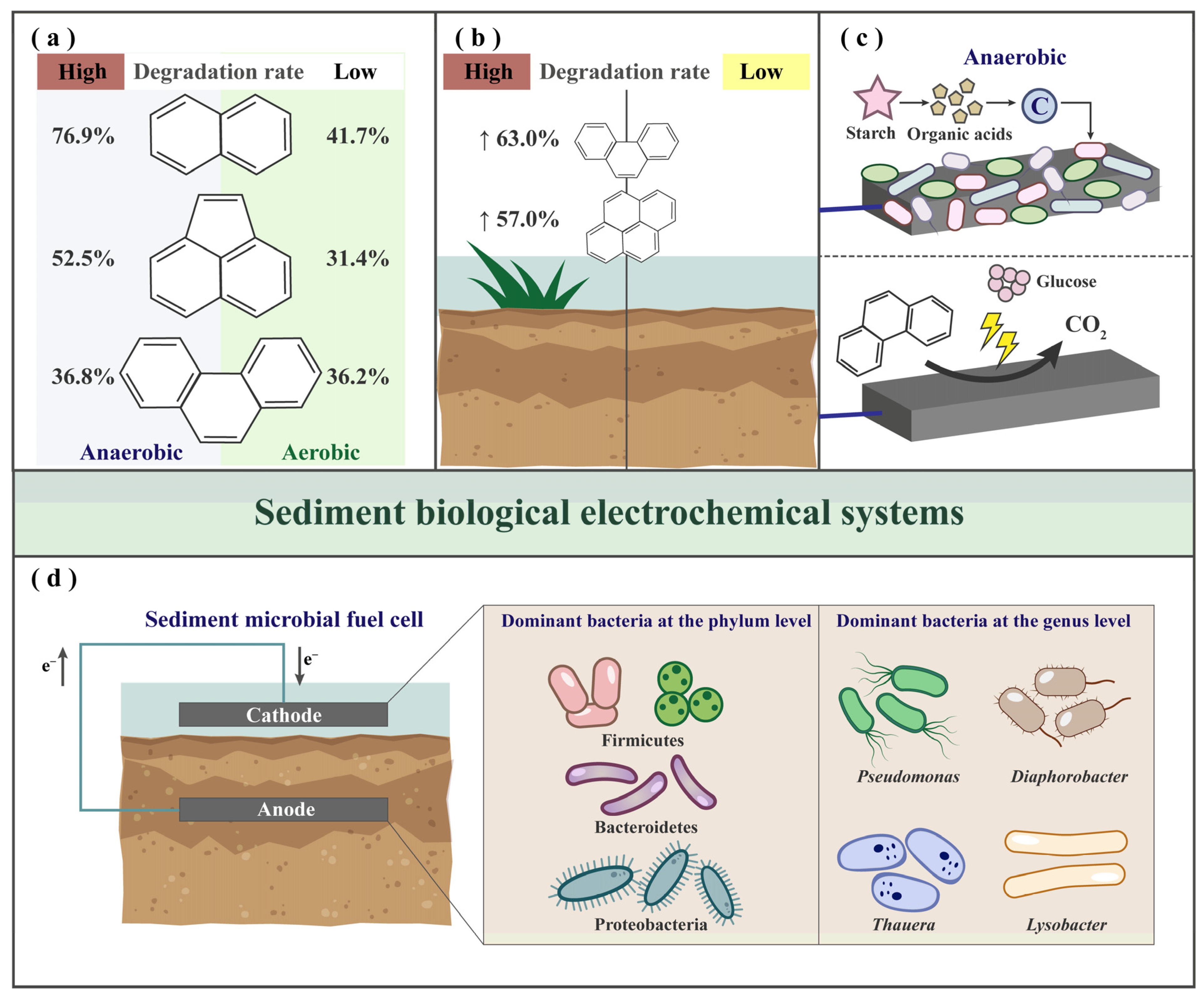
3.1. Degradation of PAHs by BES
| Sediment Source | Conditions; Temperature; Period | PAH Type; Concentration | Degradation Efficiency (%) | Notes | References |
|---|---|---|---|---|---|
| Macritchie reservoir | Aerobic; 27 °C; 45 d | Naphthalene; 50 ppm | 50 | [38] | |
| Aerobic; 27 °C; 45 d | Acenaphthene; 50 ppm | 31.4 | |||
| Aerobic; 27 °C; 45 d | Phenanthrene; 50 ppm | 36.2 | |||
| Anaerobic; 27 °C; 45 d | Naphthalene; 50 ppm | 76 | |||
| Anaerobic; 27 °C; 45 d | Acenaphthene; 50 ppm | 52.5 | |||
| Anaerobic; 27 °C; 45 d | Phenanthrene; 50 ppm | 36.8 | |||
| A large shallow lake | Anaerobic; 25 °C; 240 d | Phenanthrene; 10 mg/kg | 96.14 | [40] | |
| Anaerobic; 25 °C; 240 d | Pyrene; 5 mg/kg | 92.13 | |||
| Anaerobic; 25 °C; 240 d | Phenanthrene; 10 mg/kg | 99.47 | Amorphous ferric hydroxide (16 g wet weight) | ||
| Anaerobic; 25 °C; 240 d | Pyrene; 5 mg/kg | 94.79 | |||
| Urban river surface sediments | Anaerobic; 24 °C; 82 d | Phenanthrene; 1.38 mg/kg | 62.98 | [41] | |
| Anaerobic; 24 °C; 82 d | Pyrene; 1.28 mg/kg | 57.02 | |||
| Lake surface sediments | Anaerobic; 367 d | Pyrene; 1.28 mg/kg | 55.73 | [46] | |
| Anaerobic; 367 d | Benzo[a]pyrene; 1.28 mg/kg | 47.2 | |||
| Anaerobic; 367 d | Pyrene; 1.28 mg/kg | 87.18 | Macrophyte Acorus Calamus | ||
| Anaerobic; 367 d | Benzo[a]pyrene; 1.28 mg/kg | 76.4 | |||
| Aquaculture pond sediment | Anaerobic; 25 °C; 68 d | Naphthalene; 39.4–43.1 mg/kg | 39.2 | [44] | |
| Anaerobic; 25 °C; 68 d | Acenaphthene; 50.3–52.3 mg/kg | 23.4 | |||
| Anaerobic; 25 °C; 68 d | Pyrene; 50.4–53.6 mg/kg | 19.1 | |||
| Anaerobic; 25 °C; 68 d | Naphthalene | 69.9 | Starch (10 mg/g) | ||
| Anaerobic; 25 °C; 68 d | Acenaphthene | 55.6 | |||
| Anaerobic; 25 °C; 68 d | Pyrene | 46.8 |
3.2. Functional Microbial Communities
4. Electrochemical Bioremediation Technology for PAHs Contaminated Water
4.1. Degradation of PAHs by MFC
| PAHs Type; Concentration | Conditions; Temperature; Period | Degradation Efficiency (%) | Notes | References |
|---|---|---|---|---|
| Phenanthrene; 5 mg/L | Anaerobic; 30 °C; 40 h | 98.84 | [70] | |
| Phenanthrene; 10 mg/L | Anaerobic; 30 °C; 40 h | 98.77 | ||
| Phenanthrene; 5 mg/L | Anaerobic; 30 °C; 40 h | 95.3 | CuCo@NC 800 (3 mg/cm2) | |
| Phenanthrene; 10 mg/L | Anaerobic; 30 °C; 40 h | 98.37 | ||
| Phenanthrene; 5 mg/L | Anaerobic; 30 °C; 60 h | 97.05 | [69] | |
| Phenanthrene; 10 mg/L | Anaerobic; 30 °C; 70 h | 94.9 | ||
| Phenanthrene; 20 mg/L | Anaerobic; 30 °C; 80 h | 98.44 | ||
| Phenanthrene 0.17 mg/L | Anaerobic; 26 °C; 48 h | 93.6 | [71] | |
| Anthracene; 0.17 mg/L | Anaerobic; 26 °C; 48 h | 95.3 | ||
| Phenanthrene; 0.17 mg/L | Anaerobic; 26 °C; 48 h | 95.8 | nZVI modified carbon fiber felt electrode | |
| Anthracene; 0.17 mg/L | Anaerobic; 26 °C; 48 h | 96.5 | ||
| Pyrene; 5 mg/L | Anaerobic; 30 °C; 48 h | 44.8 | [75] | |
| Pyrene; 20 mg/L | Anaerobic; 30 °C; 48 h | 72.4 | ||
| Pyrene; 30 mg/L | Anaerobic; 30 °C; 48 h | 88.1 |
4.2. Degradation of PAHs by MECs
4.3. Functional Microbial Communities
5. Metabolite Analysis
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.C.; Farrington, J.W.; Reddy, C.M. Combustion-derived polycyclic aromatic hydrocarbons in the environment—A review. Environ. Forensics 2005, 6, 109–131. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yin, Z.; Zhao, X.; Zhang, J.; Zuo, R.; Wu, J.; Yang, J.; Teng, Y.; Wang, J. Polycyclic aromatic hydrocarbons (PAHs) in the environment of Beijing, China: Levels, distribution, trends and sources. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 137–157. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Margesin, R.; Moertelmaier, C.; Mair, J. Low-temperature biodegradation of petroleum hydrocarbons (n-alkanes, phenol, anthracene, pyrene) by four actinobacterial strains. Int. Biodeterior. Biodegrad. 2013, 84, 185–191. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef]
- Suess, M.J. The environmental load and cycle of polycyclic aromatic hydrocarbons. Sci. Total Environ. 1976, 6, 239–250. [Google Scholar] [CrossRef]
- Manzetti, S. Polycyclic Aromatic Hydrocarbons in the Environment: Environmental Fate and Transformation. Polycycl. Aromat. Compd. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. TRENDS Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, L.; Moghaddam, T.B.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, A.; Hassan, S.; Singh, T.; Mandal, R.; Chinnadurai, J.; Khan, H.A.; Hussain, M.A.; Brindhadevi, K.; Pugazhendhi, A. Bioremediation of polycyclic aromatic hydrocarbons: An updated microbiological review. Chemosphere 2023, 328, 138498. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rajput, V.D.; Sushkova, S.; Minkina, T. Microbial electrochemical system: An emerging technology for remediation of polycyclic aromatic hydrocarbons from soil and sediments. Environ. Geochem. Health 2023, 45, 9451–9467. [Google Scholar] [CrossRef] [PubMed]
- Gebregiorgis Ambaye, T.; Vaccari, M.; Franzetti, A.; Prasad, S.; Formicola, F.; Rosatelli, A.; Hassani, A.; Aminabhavi, T.M.; Rtimi, S. Microbial electrochemical bioremediation of petroleum hydrocarbons (PHCs) pollution: Recent advances and outlook. Chem. Eng. J. 2023, 452, 139372. [Google Scholar] [CrossRef]
- Shukla, V.; Siddiqui, K.A. Dosage Dependent Photocatalytic Degradation of NFT and Other Antibiotics and Energy Storage Application of Unprecedented Gd-doped Zinc-MOF Composite. J. Inorg. Organomet. Polym. Mater. 2024, 1–18. [Google Scholar] [CrossRef]
- Shukla, V.; Ahmad, M.; LaDuca, R.L.; Siddiqui, K.A. 6-connected Zn (II)-MOF: Efficient photocatalytic dye degradation and remarkable luminescent detection of biomolecules and hazardous ions. J. Mol. Struct. 2023, 1294, 136371. [Google Scholar] [CrossRef]
- Shukla, V.; Ahmad, M.; Siddiqui, K.A. Colorimetric recognition of a biomarker of trichloroethylene in human urine and photocatalytic dye degradation employing unprecedented Co (II) MOF luminescent probe. J. Mol. Struct. 2024, 1308, 138068. [Google Scholar] [CrossRef]
- Shukla, V.; Ahmad, M.; Siddiqui, K.A. Synthesis of dual functional Zn (II) MOF for colorimetric detection of norfloxacin and photocatalytic degradation of ornidzole drugs in aqueous medium. Polyhedron 2024, 117078. [Google Scholar] [CrossRef]
- Sakshi; Haritash, A. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, J.; Ji, Y.; Zhang, W.; Fang, Y.; Xin, F.; Dong, W.; Wei, P.; Ma, J.; Jiang, M. Progress and prospects of bioelectrochemical systems: Electron transfer and its applications in the microbial metabolism. Front. Bioeng. Biotechnol. 2020, 8, 10. [Google Scholar] [CrossRef]
- Al-Sahari, M.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tian, J.; Feng, L. Remediation of PAH polluted soils using a soil microbial fuel cell: Influence of electrode interval and role of microbial community. J. Hazard. Mater. 2017, 336, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; SundarRajan, P.; Pavithra, K.G.; Priyadharsini, P.; Shyam, S.; Goutham, R.; Le, Q.H.; Pugazhendhi, A. New insights into microbial electrolysis cells (MEC) and microbial fuel cells (MFC) for simultaneous wastewater treatment and green fuel (hydrogen) generation. Fuel 2024, 355, 129530. [Google Scholar] [CrossRef]
- Addagada, L.; Goel, M.; Shahid, M.K.; Prabhu, S.V.; Chand, S.; Sahoo, N.K.; Rout, P.R. Tricks and tracks in resource recovery from wastewater using bio-electrochemical systems (BES): A systematic review on recent advancements and future directions. J. Water Process Eng. 2023, 56, 104580. [Google Scholar] [CrossRef]
- Ramírez-Moreno, M.; Rodenas, P.; Aliaguilla, M.; Bosch-Jimenez, P.; Borràs, E.; Zamora, P.; Monsalvo, V.; Rogalla, F.; Ortiz, J.M.; Esteve-Núñez, A. Comparative performance of microbial desalination cells using air diffusion and liquid cathode reactions: Study of the salt removal and desalination efficiency. Front. Energy Res. 2019, 7, 135. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, X.; Cai, W.; Cui, K.; Patil, S.A.; Guo, K. Recent progress on microbial electrosynthesis reactor designs and strategies to enhance the reactor performance. Biochem. Eng. J. 2023, 190, 108745. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Rózsenberszki, T.; Kalauz-Simon, V.; Bélafi-Bakó, K.; Nemestóthy, N. CO2-refinery through microbial electrosynthesis (MES): A concise review on design, operation, biocatalysts and perspectives. J. CO2 Util. 2023, 67, 102348. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ji, M.; Liang, B.; Zhao, Y.; Zhai, S.; Ma, Z.; Yang, Z. Bioelectrochemical degradation of monoaromatic compounds: Current advances and challenges. J. Hazard. Mater. 2020, 398, 122892. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Hägerhäll, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 2012, 4, 159–192. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires: A new paradigm for biological electron transfer and bioelectronics. ChemSusChem 2012, 5, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.M.; Alvarez, L.H. Application of redox mediators in bioelectrochemical systems. Biotechnol. Adv. 2018, 36, 1412–1423. [Google Scholar] [CrossRef]
- Hao, D.-C.; Li, X.-J.; Xiao, P.-G.; Wang, L.-F. The utility of electrochemical systems in microbial degradation of polycyclic aromatic hydrocarbons: Discourse, diversity and design. Front. Microbiol. 2020, 11, 557400. [Google Scholar] [CrossRef] [PubMed]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhai, H.; Liu, B.; Ji, M.; Li, J. Carbon nanomaterial-modified graphite felt as an anode enhanced the power production and polycyclic aromatic hydrocarbon removal in sediment microbial fuel cells. Sci. Total Environ. 2020, 713, 136483. [Google Scholar] [CrossRef]
- Yan, Z.; Song, N.; Cai, H.; Tay, J.-H.; Jiang, H. Enhanced degradation of phenanthrene and pyrene in freshwater sediments by combined employment of sediment microbial fuel cell and amorphous ferric hydroxide. J. Hazard. Mater. 2012, 199, 217–225. [Google Scholar] [CrossRef]
- Liu, B.; Zhai, H.; Liang, Y.; Ji, M.; Wang, R. Increased power production and removal efficiency of polycyclic aromatic hydrocarbons by plant pumps in sediment microbial electrochemical systems: A preliminary study. J. Hazard. Mater. 2019, 380, 120896. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Jiang, H.; Cai, H.; Zhou, Y.; Krumholz, L.R. Complex interactions between the macrophyte Acorus calamus and microbial fuel cells during pyrene and benzo[a]pyrene degradation in sediments. Sci. Rep. 2015, 5, 10709. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, Q.; Chen, P.; Ouyang, F.; Hu, J.; Feng, C.; Sun, J. Effect and mechanism of Fe-based biochar combined with bioelectrochemical technology for in situ remediation of Pb-polycyclic aromatic hydrocarbons contaminated sediment. Chin. J. Ecol. 2023, 42, 504–512. [Google Scholar] [CrossRef]
- Zhang, H.; Chao, B.; Gao, X.; Cao, X.; Li, X. Effect of starch-derived organic acids on the removal of polycyclic aromatic hydrocarbons in an aquaculture-sediment microbial fuel cell. J. Environ. Manag. 2022, 311, 114783. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Xiao, L.; Zhou, Y.; Zhao, G. Spontaneous fermentation tunes the physicochemical properties of sweet potato starch by modifying the structure of starch molecules. Carbohydr. Polym. 2019, 213, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, H.Z.; Salam, D.A. Ferric iron stimulation in marine SMFCs: Impact on the microbial structure evolution in contaminated sediments with low and high molecular weight PAHs. J. Environ. Manag. 2021, 280, 111636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Hu, Y.; Huang, W.; Niu, Z.; Sun, J. Bacterial community shift and incurred performance in response to in situ microbial self-assembly graphene and polarity reversion in microbial fuel cell. Bioresour. Technol. 2017, 241, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Cao, W.; Yuan, J.; Wang, Y.; Guo, Y.; Ding, A.; Zhu, Y.; Dou, J. Microbial diversity and co-occurrence patterns in deep soils contaminated by polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol. Environ. Saf. 2020, 203, 110931. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Meng, Q.; Liu, Y.; Tuyiringire, D.; Chen, Z.; Liang, S. Effects of trichloroethylene stress on the microbiological characteristics of Mollisol. Ecotoxicol. Environ. Saf. 2019, 184, 109595. [Google Scholar] [CrossRef]
- De Chaves, M.G.; Silva, G.G.Z.; Rossetto, R.; Edwards, R.A.; Tsai, S.M.; Navarrete, A.A. Acidobacteria subgroups and their metabolic potential for carbon degradation in sugarcane soil amended with vinasse and nitrogen fertilizers. Front. Microbiol. 2019, 10, 1680. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Geng, S.; Cao, W.; Zuo, R.; Teng, Y.; Ding, A.; Fan, F.; Dou, J. Vertical distribution characteristics and interactions of polycyclic aromatic compounds and bacterial communities in contaminated soil in oil storage tank areas. Chemosphere 2022, 301, 134695. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, K.; Ouyang, H.; Li, M.K.; Luo, Z.; Li, Y.; Chen, C.; Yang, X.; Shao, Z.; Yan, D.Y. Simultaneous PAHs degradation, odour mitigation and energy harvesting by sediment microbial fuel cell coupled with nitrate-induced biostimulation. J. Environ. Manag. 2021, 284, 112045. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qiao, Y.-J.; Zou, L.; Ma, C.-X.; Liu, J.-H. Real-time monitoring of phenazines excretion in Pseudomonas aeruginosa microbial fuel cell anode using cavity microelectrodes. Bioresour. Technol. 2015, 198, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, M.; Trably, E.; Bernet, N.; Patureau, D. Biodegradation of polycyclic aromatic hydrocarbons: Using microbial bioelectrochemical systems to overcome an impasse. Environ. Pollut. 2017, 231, 509–523. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, J.; Jiang, T.; Gao, W.; Ma, Y.; Wei, D. Characterisation of a thermostable catechol-2, 3-dioxygenase from phenanthrene-degrading Pseudomonas sp. strain ZJF08. Ann. Microbiol. 2007, 57, 503–508. [Google Scholar] [CrossRef]
- Xia, M.; Wang, S.; Chen, B.; Qiu, R.; Fan, G. Enhanced Solubilization and Biodegradation of HMW-PAHs in Water with a Pseudomonas mosselii-Released Biosurfactant. Polymers 2023, 15, 4571. [Google Scholar] [CrossRef] [PubMed]
- Naloka, K.; Kuntaveesuk, A.; Muangchinda, C.; Chavanich, S.; Viyakarn, V.; Chen, B.; Pinyakong, O. Pseudomonas and Pseudarthrobacter are the key players in synergistic phenanthrene biodegradation at low temperatures. Sci. Rep. 2024, 14, 11976. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Jin, J.; Wang, T.; Wang, J.; Jiang, B. A high-efficiency phenanthrene-degrading Diaphorobacter sp. isolated from PAH-contaminated river sediment. Sci. Total Environ. 2020, 746, 140455. [Google Scholar] [CrossRef]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.-J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and naphthalene degrading bacterial communities in an oil sands tailings pond. Front. Microbiol. 2017, 8, 272817. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Liu, P.; Tang, H.; Zhang, A.; Liu, Z.; Li, Z. Optimization and regulation effects of microbial community on the efficient degradation of aromatic hydrocarbons. J. Water Process Eng. 2024, 59, 105020. [Google Scholar] [CrossRef]
- Wang, B.; Teng, Y.; Li, R.; Meng, K.; Xu, Y.; Liu, S.; Luo, Y. Exploring the PAHs dissipation and indigenous bacteria response in soil amended with two different microbial inoculants. Sci. Total Environ. 2023, 859, 160186. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Huang, Y.; Wang, W.; Zhang, L.; Xu, J.; Li, Z.; Xu, P.; Tang, H. A novel Diaphorobacter sp. strain isolated from saponification wastewater shows highly efficient phenanthrene degradation. Environ. Res. 2022, 214, 114047. [Google Scholar] [CrossRef]
- Yao, K.; Xie, Z.; Zhi, L.; Wang, Z.; Qu, C. Polycyclic aromatic hydrocarbons in the water bodies of Dong Lake and Tangxun Lake, China: Spatial distribution, potential sources and risk assessment. Water 2023, 15, 2416. [Google Scholar] [CrossRef]
- Min, Z.; Rui, T.; Yu, L. A combination of microbial electrolysis cells and bioaugmentation can effectively treat synthetic wastewater containing polycyclic aromatic hydrocarbon. Water Sci. Technol. 2024, 89, 2716–2731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous reduction of vanadium (V) and chromium (VI) with enhanced energy recovery based on microbial fuel cell technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. Enhanced biodegradation of phenanthrene using different inoculum types in a microbial fuel cell. Eng. Life Sci. 2014, 14, 218–228. [Google Scholar] [CrossRef]
- Hua, T.; Wang, H.; Li, S.; Chen, P.; Li, F.; Wang, W. Electrochemical performance and response of bacterial community during phenanthrene degradation in single-chamber air-cathode microbial fuel cells. Environ. Sci. Pollut. Res. 2021, 28, 22705–22715. [Google Scholar] [CrossRef]
- Huang, S.; Xia, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Cu, Co embedded N-enriched mesoporous carbon cathode catalyst for the efficient bioelectrochemical removal of phenanthrene in microbial fuel cell. Appl. Surf. Sci. 2022, 599, 153759. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Li, Q.; Bai, H.; Zhu, C.; Weng, B.; Yan, D.; Bai, J. Bioenergy generation and degradation pathway of phenanthrene and anthracene in a constructed wetland-microbial fuel cell with an anode amended with nZVI. Water Res. 2019, 150, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Yang, B.; Xu, H.; Wang, J.; Song, X.; Wang, Y.; Li, F.; Tian, Q.; Ma, C.; Wang, D.; Bai, J. Bacterial and archaeal community distribution and stabilization of anaerobic sludge in a strengthen circulation anaerobic (SCA) reactor for municipal wastewater treatment. Bioresour. Technol. 2017, 244, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Gambino, E.; Toscanesi, M.; Del Prete, F.; Flagiello, F.; Falcucci, G.; Minutillo, M.; Trifuoggi, M.; Guida, M.; Nastro, R.; Jannelli, E. Polycyclic aromatic hydrocarbons (PAHs) degradation and detoxification of water environment in single-chamber air-cathode microbial fuel cells (MFCs). Fuel Cells 2017, 17, 618–626. [Google Scholar] [CrossRef]
- Wang, H.; Chen, P.; Zhang, S.; Jiang, J.; Hua, T.; Li, F. Degradation of pyrene using single-chamber air-cathode microbial fuel cells: Electrochemical parameters and bacterial community changes. Sci. Total Environ. 2022, 804, 150153. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.O.; Ibrahim, M.N.M.; Noh, N.A.M.; Yaqoob, A.A.; Hussin, M.H.; Shukri, I.A.M.; Hamidon, T.S. Simultaneous naphthalene degradation and electricity production in a biowaste-powered microbial fuel cell. Chemosphere 2023, 340, 139985. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vargas, C.A.; Prado, A.; Arias, C.A.; Carvalho, P.N.; Esteve-Núñez, A.; Brix, H. Microbial electrochemical technologies for wastewater treatment: Principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water 2018, 10, 1128. [Google Scholar] [CrossRef]
- Ding, P.; Wu, P.; Jie, Z.; Cui, M.-H.; Liu, H. Damage of anodic biofilms by high salinity deteriorates PAHs degradation in single-chamber microbial electrolysis cell reactor. Sci. Total Environ. 2021, 777, 145752. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Wu, P.; Cao, Q.; Liu, H.; Chen, C.; Cui, M.-H.; Liu, H. Advantages of residual phenol in coal chemical wastewater as a co-metabolic substrate for naphthalene degradation by microbial electrolysis cell. Sci. Total Environ. 2023, 901, 166342. [Google Scholar] [CrossRef]
- Kanaly, R.A.; Harayama, S. Advances in the field of high-molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria. Microb. Biotechnol. 2010, 3, 136–164. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Z.; Wang, H. Metagenomic analysis exhibited the co-metabolism of polycyclic aromatic hydrocarbons by bacterial community from estuarine sediment. Environ. Int. 2019, 129, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Gupta, S.; Tripathi, V.; Chauhan, A.; Parashar, D.; Shankar, P.; Kashyap, V. Microbiome based approaches for the degradation of polycyclic aromatic hydrocarbons (PAHs): A current perception. Chemosphere 2023, 341, 139951. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Das, S. Potential and prospects of Actinobacteria in the bioremediation of environmental pollutants: Cellular mechanisms and genetic regulations. Microbiol. Res. 2023, 273, 127399. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Rene, E.R.; Chen, Z.; Ma, W. Fate of PAHs in treated wastewater reused as irrigation water: Environmental risks in water-soil-ryegrass multimedia system. J. Hazard. Mater. 2022, 424, 127500. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, D.S.; Bakir, U.; Phillips, S.; Mcpherson, M.; Ögel, Z.B. Purification, characterization and identification of a novel bifunctional catalase-phenol oxidase from Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 2009, 79, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Rathore, D.S.; Sheikh, M.; Singh, S.P. Marine Actinobacteria: New horizons in bioremediation. In Recent Developments in Microbial Technologies; Springer: Singapore, 2021; pp. 425–449. [Google Scholar] [CrossRef]
- Yesankar, P.; Pal, M.; Patil, A.; Qureshi, A. Microbial exopolymeric substances and biosurfactants as ‘bioavailability enhancers’ for polycyclic aromatic hydrocarbons biodegradation. Int. J. Environ. Sci. Technol. 2023, 20, 5823–5844. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, R.; Singh, S.B.; Nain, L. Bioremediation of PAH by Streptomyces sp. Bull. Environ. Contam. Toxicol. 2011, 86, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Garbini, G.L.; Barra Caracciolo, A.; Grenni, P. Electroactive bacteria in natural ecosystems and their applications in microbial fuel cells for bioremediation: A review. Microorganisms 2023, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Xu, Q.; Yang, Q.-W.; Tian, R.-R.; Li, B.; Yan, S.; Zhang, X.-Y.; Zhou, J.; Yong, X.-Y. Enhancing extracellular electron transfer through selective enrichment of Geobacter with Fe@ CN-modified carbon-based anode in microbial fuel cells. Environ. Sci. Pollut. Res. 2023, 30, 28640–28651. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Han, X.; Shan, Y.; Li, F.; Shi, L. Biofilm biology and engineering of Geobacter and Shewanella spp. for energy applications. Front. Bioeng. Biotechnol. 2021, 9, 786416. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Q.; Fan, M.; Xu, Y.; Chen, Y. Highly efficient anaerobic co-degradation of complex persistent polycyclic aromatic hydrocarbons by a bioelectrochemical system. J. Hazard. Mater. 2020, 381, 120945. [Google Scholar] [CrossRef]
- Coates, J.D.; Woodward, J.; Allen, J.; Philp, P.; Lovley, D.R. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl. Environ. Microbiol. 1997, 63, 3589–3593. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Mouttaki, H. Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr. Opin. Biotechnol. 2011, 22, 406–414. [Google Scholar] [CrossRef]
- Estelmann, S.; Blank, I.; Feldmann, A.; Boll, M. Two distinct old yellow enzymes are involved in naphthyl ring reduction during anaerobic naphthalene degradation. Mol. Microbiol. 2015, 95, 162–172. [Google Scholar] [CrossRef]
- Safinowski, M.; Meckenstock, R.U. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ. Microbiol. 2006, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Zamir, S.M.; Vahabzadeh, F. Cometabolic degradation of ethyl mercaptan by phenol-utilizing Ralstonia eutropha in suspended growth and gas-recycling trickle-bed reactor. J. Environ. Manag. 2016, 165, 53–61. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, R.; Ji, M.; Tian, R.; Wang, R.; Zhang, B.; Wang, S.; Liu, L. The Degradation of Polycyclic Aromatic Hydrocarbons by Biological Electrochemical System: A Mini-Review. Water 2024, 16, 2424. https://doi.org/10.3390/w16172424
Tian Y, Wang R, Ji M, Tian R, Wang R, Zhang B, Wang S, Liu L. The Degradation of Polycyclic Aromatic Hydrocarbons by Biological Electrochemical System: A Mini-Review. Water. 2024; 16(17):2424. https://doi.org/10.3390/w16172424
Chicago/Turabian StyleTian, Yu, Rumeng Wang, Min Ji, Ruimin Tian, Renjie Wang, Bo Zhang, Shaopo Wang, and Lingjie Liu. 2024. "The Degradation of Polycyclic Aromatic Hydrocarbons by Biological Electrochemical System: A Mini-Review" Water 16, no. 17: 2424. https://doi.org/10.3390/w16172424
APA StyleTian, Y., Wang, R., Ji, M., Tian, R., Wang, R., Zhang, B., Wang, S., & Liu, L. (2024). The Degradation of Polycyclic Aromatic Hydrocarbons by Biological Electrochemical System: A Mini-Review. Water, 16(17), 2424. https://doi.org/10.3390/w16172424







