Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment
Abstract
1. Introduction
| Sea Areas | Dominant Algae Species | Refs. |
|---|---|---|
| Gulf of Mexico | Karenia brevis Dinophysis ovum | [6,7,8] |
| North Atlantic Ocean | Alexandrium spp. Pseudo-nitzschia spp. | [9,10,11] |
| Pacific Ocean | Karenia spp. Alexandrium spp. Dinophysis spp. | [12,13,14] |
| North Sea and Baltic Sea | Alexandrium spp. Dinophysis spp. | [15] |
| Indian Ocean | Cochlodinium spp. Noctiluca scintillans | [16,17,18] |
| Arctic Ocean | Karlodinium spp. Heterosigma spp. | [19] |
| Mediterranean Sea | Alexandrium spp. Karenia spp. | [20] |
| Caribbean Sea | Karenia brevis | [21] |
| South China Sea | Alexandrium spp. Cochlodinium spp. | [22,23] |
| Black Sea | Alexandrium spp. Dinophysis spp. | [24,25] |
| Bay of Bengal | Dinoflagellate spp. Noctiluca scintillans Karenia brevis | [18,26] |
| East China Sea | Alexandrium spp. Prorocentrum spp. | [23,27] |
| East Siberian Sea | Karlodinium spp. Heterosigma spp. | [28,29,30] |
| Barents Sea | Alexandrium spp. Dinophysis spp. | [31,32,33] |
| Southern Ocean | Phaeocystis spp. Dinophysis spp. | [34,35] |
| South Atlantic Ocean | Alexandrium spp. Karenia spp. | [36,37] |
| Norwegian Sea | Alexandrium spp. Dinophysis spp. | [32,38] |
| Adriatic Sea | Alexandrium spp. Pseudo-nitzschia spp. | [39,40] |
| Tyrrhenian Sea | Alexandrium spp. Dinophysis spp. | [41] |
| Monterey Bay | Pseudo-nitzschia australis | [42] |
2. Hazards of Marine Harmful Algal Blooms
2.1. Oxygen Depletion
2.2. Algal Toxin
| Toxins | Producers | Type | Refs. |
|---|---|---|---|
| Paralytic Shellfish Toxins | Alexandrium spp. Gymnodinium catenatum Pyrodinium bahamense | Neurotoxins | [100] |
| Amnesic Shellfish Toxins | Pseudo-nitzschia spp. | Neurotoxins | [101,102,103,104] |
| Neurotoxic Shellfish Toxins | Karenia brevis | Neurotoxins | [105,106,107] |
| Ciguatoxins | Gambierdiscus toxicus | Neurotoxins | [108,109] |
| Kalkitoxin | Lyngbya majuscula | Neurotoxins | [110,111] |
| Prymnesins | Prymnesium parvum | Hemolytic toxins and neurotoxins | [112] |
| Caulerpenyne | Caulerpa taxifolia | Cytotoxins | [113,114,115,116] |
| Aplysiatoxins | Lyngbya spp. | Cytotoxins | [112] |
| Pectenotoxins | Dinophysis spp. | Hepatotoxins and cytotoxins | [117,118] |
| Diarrhetic Shellfish Toxins | Dinophysis spp. Prorocentrum lima | Gastrointestinal toxins and cytotoxins | [107,119,120] |
| Azaspiracids | Azadinium spp. Mytilus edulis | Gastrointestinal toxins and cytotoxins | [121,122,123] |
| Yessotoxins | Protoceratium reticulatum Lingulodinium polyedrum Gonyaulax spinifera | Cardiovascular toxins | [124,125,126] |
| Azaspiracids | Azadinium spp. Mytilus edulis | Gastrointestinal toxins and cytotoxins | [121,122,123] |
| Cylindrospermopsin | Cylindrospermopsis raciborskii | Hepatotoxins and nephrotoxins | [127,128] |
| Microcystins | Microcystis spp., Anabaena spp., Planktothrix spp. | Hepatotoxins | [129,130,131] |
| Pectenotoxins | Dinophysis spp. | Hepatotoxins and cytotoxins | [132,133] |
2.3. Habitat Degradation
2.4. The Impact of Water Blooms on the Carbon Cycle and Carbon Accumulation Processes
3. Factors Leading to Marine Eutrophication
3.1. Sources of Nutrient Inputs
3.1.1. Agricultural Runoff
3.1.2. Aquaculture
3.1.3. Urban Runoff
3.2. CO2 Emissions
3.2.1. Rising Sea Temperatures
- (1).
- Enhanced Growth Rates of Warm-Water Algal Species
- (2).
- Altered Stratification of Water Columns
- (3).
- Changes in Nutrient Availability
- (4).
- Increased Toxicity of Certain Algal Species
- (5).
- Geographical Expansion of HABs
- (6).
- Impacts on Predator–Prey Dynamics
3.2.2. Ocean Acidification
3.3. The Influence of Alien Species
3.4. Geographical Distribution of HABs
4. Monitoring Techniques and Management Strategies
4.1. Field Sampling and Microscopy
4.2. Molecular Techniques
4.2.1. DNA/RNA Analysis
- (1)
- Partitioning: The sample is diluted and then partitioned into thousands or millions of tiny individual reactions. Each partition ideally contains either zero or one DNA molecule of the target sequence.
- (2)
- PCR Amplification: Each partition undergoes PCR amplification independently. If a partition contains the target DNA, it will produce a positive (fluorescent) signal after amplification; if it does not, it will remain negative.
- (3)
- Counting Positive Partitions: After amplification, the number of positive partitions (those that fluoresce) is counted.
- (4)
- Poisson Statistics: The proportion of positive partitions is used to estimate the absolute number of target molecules in the original sample using Poisson distribution. This calculation accounts for the fact that not all partitions will contain exactly one DNA molecule due to the random distribution.
4.2.2. Metagenomics
| Harmful Algal Species | Location | Toxins | Application of Genomic/Metagenomic Technologies | Refs. |
|---|---|---|---|---|
| Karenia brevis | Florida, USA | Brevetoxins | Metagenomics for monitoring bloom dynamics, toxin production, and environmental influences. | [392,393] |
| Dinophysis spp. | Europe | Okadaic acid | High-throughput sequencing to monitor species abundance and predict bloom events impacting shellfish harvesting. | [394] |
| Alexandrium spp. | Gulf of Maine, USA | Saxitoxins | Genomic surveillance to detect genetic markers specific to Alexandrium spp., facilitating early warning and management of paralytic shellfish poisoning outbreaks. | [395] |
| Microcystis spp. | Global freshwater systems | Microcystins | Metagenomics for tracking bloom dynamics, toxin production, and ecological impacts on freshwater ecosystems. | [396] |
| Pseudo-nitzschia spp. | California, USA | Domoic acid | Application of metagenomics to monitor bloom occurrence, toxin levels in shellfish, and issuance of public health advisories. | [397] |
| Gambierdiscus spp. | Pacific Islands | Ciguatoxins | Metagenomic surveillance to monitor species distribution and abundance in coral reef ecosystems, informing safe fish consumption practices. | [398] |
| Akashiwo sanguinea | Asian coastal waters | Unknown toxins | Genomic approaches for understanding genetic diversity and environmental responses of A. sanguinea blooms. | [399] |
| Heterosigma akashiwo | Pacific Northwest, USA | Ichthyotoxins | Metagenomics to study genetic variability, bloom initiation, and environmental factors influencing bloom formation. | [400] |
| Dinoflagellate spp. | Baltic Sea | Saxitoxins, Okadaic acid | Utilization of metagenomics to analyze species composition, toxin production dynamics, and microbial interactions within blooms. | [401,402] |
4.3. Remote Sensing
| Location | Algal Species | Technology | Application | Ref. |
|---|---|---|---|---|
| Florida, USA | Karenia brevis | MODIS, Sentinel-3 | Detecting and monitoring red tides, tracking bloom dynamics, and issuing public health advisories. | [22] |
| Baltic Sea | Various dinoflagellates | MERIS, Sentinel-3 | Monitoring algal bloom extents, identifying species composition, and assessing environmental impacts. | [405] |
| California, USA | Pseudo-nitzschia spp. | MODIS, Landsat, Sentinel-2 | Tracking bloom development, assessing toxin levels in shellfish, and providing early warnings. | [406] |
| Gulf of Mexico | Karenia brevis | MODIS, VIIRS, Sentinel-3 | Detecting bloom onset, monitoring spatial distribution, and guiding management decisions for fisheries and tourism. | [407] |
| Great Lakes, USA | Microcystis spp. | MODIS, Sentinel-2, Landsat | Monitoring cyanobacterial blooms, assessing water quality, and informing drinking water treatment processes. | [408] |
| East China Sea | Karenia mikimotoi | MODIS, Sentinel-3, Hyperspectral sensors | Tracking bloom dynamics, assessing environmental conditions, and supporting fisheries management. | [409] |
| Chesapeake Bay, USA | Prorocentrum minimum | Landsat, Sentinel-2 | Monitoring bloom occurrence, species composition, and environmental influences. | [410] |
| Mediterranean Sea | Various dinoflagellates | MODIS, MERIS, Sentinel-3 | Assessing bloom extent, species diversity, and ecological impacts on marine ecosystems. | [411] |
| Arabian Gulf | Cochlodinium polykrikoides | MODIS, Sentinel-3, Landsat | Detecting fish-killing algal blooms, tracking bloom movement, and mitigating impacts on aquaculture. | [412] |
| Australia | Nodularia spumigena | MODIS, Sentinel-2 | Monitoring toxic cyanobacterial blooms, assessing water quality, and informing public health decisions. | [413] |
| Irish Coastal Waters | Karenia mikimotoi | MODIS, Sentinel-2, Landsat | Detecting bloom initiation, monitoring spatial distribution, and assessing impacts on marine life. | [414] |
| North Sea | Various dinoflagellates | Sentinel-3, MODIS | Monitoring bloom dynamics, species composition, and environmental influences. | [415] |
| South China Sea | Heterosigma akashiwo | MODIS, Hyperspectral sensors | Tracking bloom initiation and dynamics, assessing environmental conditions, and supporting fisheries management. | [22,23] |
| Gulf of Maine, USA | Alexandrium spp. | MODIS, Sentinel-2, Landsat | Detecting and monitoring blooms, assessing toxin production, and guiding management practices for shellfish harvesting. | [416] |
| Black Sea | Various algae | MODIS, Sentinel-3 | Monitoring bloom extents, species diversity, and environmental factors influencing bloom formation and persistence. | [417] |
4.4. Automated In Situ Sensors
4.5. Modeling and Forecasting
4.6. Comparison of Monitoring Techniques for Eutrophic Oceans
5. Treatment Technologies for Eutrophication of Seawater
5.1. Chemical Treatments
| Chemical Treatment | Mechanism | Efficacy | Cost | Specific Environmental Impact | Considerations | Refs. |
|---|---|---|---|---|---|---|
| Aluminum Sulfate | Forms insoluble aluminum phosphate | High | Moderate | Potential aluminum toxicity to aquatic organisms; increases water acidity. | Effective at low pH; potential aluminum toxicity in aquatic environments. | [472,473] |
| Ferric Chloride | Forms insoluble ferric phosphate | High | Low | Iron accumulation in sediments; potential to lower pH significantly. | Effective across a wide pH range; can cause iron accumulation in sediments. | [474] |
| Calcium Hydroxide | Forms insoluble calcium phosphate | Moderate | Low | Minimal impact; may increase water hardness and pH. | Safe for the environment but less effective in cold temperatures. | [475,476] |
| Lanthanum-Modified Clay | Binds phosphate to form insoluble compound | Very High | High | Minimal environmental risks; low mobility of lanthanum in aquatic systems. | High selectivity for phosphate; expensive but highly effective. | [477,478] |
| Activated Alumina | Adsorbs phosphate ions | High | High | Disposal of spent media can lead to aluminum leaching; moderate environmental risk. | High adsorption capacity; disposal of spent material needs careful handling. | [479,480] |
| Zeolites | Adsorbs ammonium and phosphate ions | Moderate | Moderate | Low risk; naturally occurring, can be reused. | Environmentally friendly, reusable, but with moderate phosphate removal capacity. | [481] |
| Ferric Sulfate | Forms insoluble ferric phosphate | High | Low | Can cause iron overload in aquatic systems; potential for acidification. | Similar to ferric chloride; potential for iron overload in treated waters. | [482] |
| Poly Aluminum Chloride | Forms insoluble aluminum phosphate | High | Moderate | Potential for aluminum accumulation in water bodies; moderate sludge production. | Effective at low pH, lower sludge volume compared to alum; risk of aluminum accumulation. | [483] |
| Chitosan | Binds with phosphate ions | Moderate | High | Biodegradable with low environmental impact; safe for aquatic life. | Biodegradable, but less effective at high phosphorus concentrations. | [484] |
| Biochar | Adsorbs phosphorus and nitrogen | Low to Moderate | Low | Carbon-neutral; potential for organic contaminants based on feedstock. | Sustainable, but effectiveness varies depending on feedstock and activation. | [485,486] |
| Lanthanum Chloride | Forms insoluble lanthanum phosphate | Very High | High | Low environmental risk; high selectivity for phosphate; stable in water. | Highly effective but expensive; mainly used in sensitive environments like lakes. | [487,488] |
| Iron(III) Chloride | Forms insoluble iron phosphate | High | Low | Potential for iron accumulation and acidification; can harm aquatic life. | Common and cost-effective; environmental risks include iron build-up. | [489,490] |
| Calcium Nitrate | Increases oxygen levels, reducing phosphorus release | Moderate | Moderate | Low risk; dual function in promoting plant growth and phosphate binding. | Dual function as both nutrient and phosphorus binder; environmentally safe. | [491,492] |
| Magnesium Hydroxide | Precipitates phosphate as magnesium phosphate | Moderate | Low | Minimal impact; can increase water pH, making it more alkaline. | Safe and cost-effective but limited by pH dependency. | [475,493] |
| Ferric Aluminum Sulfate | Forms insoluble phosphorus compounds | High | Moderate | Increased sludge production; potential iron and aluminum toxicity. | Combines benefits of ferric and alum salts; increased sludge production. | [472] |
| Sodium Sulfate | Forms insoluble sodium phosphate | Low | Low | Least effective; minimal direct environmental impact. | Least effective for phosphorus removal; often used in conjunction with other chemicals. | [494] |
5.2. Biological Treatments
| Treatment | Mechanism | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Biomanipulation | Altering food web to control algae | Environmentally friendly; sustainable | Requires careful management; potential ecological imbalance | [498] |
| Bioaugmentation | Adding nutrient-degrading microorganisms | Effective in targeted applications; quick results | Potential introduction of non-native species; cost of microbes | [499,500] |
| Phytoremediation | Using plants to absorb nutrients | Cost-effective; easy to implement | Potential plant disposal issues; variable effectiveness | [501,502] |
5.2.1. Biomanipulation
5.2.2. Bioaugmentation
5.2.3. Phytoremediation
| Species | Mechanism | Usage Scenarios | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|
| Zostera marina | Uptake and store nutrients in their tissues | Effective in shallow coastal waters | Enhances biodiversity, provides habitats | Sensitive to water quality changes | [531] |
| Rhizophora spp. | Absorb nutrients through root systems, stabilize sediments | Suitable for intertidal zones | Stabilizes shorelines, reduces erosion | Requires large areas, slow growth | [532] |
| Spartina alterniflora | Uptake nutrients, improve sediment quality | Effective in estuarine and coastal environments | Improves water quality, supports diverse fauna | Limited to specific habitats | [533,534] |
| Ulva spp. | Rapidly uptake nutrients through fronds | Suitable for nutrient-rich coastal waters | Fast-growing, easy to harvest | Can become invasive, requires management | [535,536] |
| Chlorella spp. | Absorb nutrients through cellular uptake, can be cultured easily | Effective in controlled environments | High nutrient uptake efficiency, potential biofuel source | Requires controlled conditions, potential for algal blooms | [537,538] |
| Phaeodactylum tricornutum | Absorb nutrients through frustules | Suitable for controlled environments | High nutrient uptake, potential biofuel source | Requires specific cultivation conditions | [539] |
| Anabaena spp. | Fix nitrogen, uptake phosphorus | Effective in nutrient-rich environments | High growth rate, potential biofertilizer source | Can produce harmful toxins, needs careful management | [540,541] |
| Eichhornia crassipes | Uptake nutrients through roots and leaves | Effective in various water bodies | High biomass production, enhances water quality | Can become invasive, requires management | [542,543] |
| Laminaria spp. | Uptakes nutrients and provides habitats for marine life | Effective in temperate marine environments | High growth rates and nutrient absorption capacity | Requires attachment substrate | [544] |
| Plant | Allelopathic Substances | Habitat | Principle of Algae Removal | Refs. |
|---|---|---|---|---|
| Sargassum spp. | Phlorotannins, polysaccharides | Marine coastal areas, tropical and subtropical waters | Inhibits algal growth by reducing nutrient availability and altering the microenvironment. | [522] |
| Ulva spp. | Ulvan, polysaccharides | Coastal marine environments, intertidal zones | Competes for nutrients and modifies the local chemical environment. | [522,523] |
| Zostera marina | Terpenoids, phenolic compounds | Subtidal areas, seagrass meadows | Reduces light penetration and competes for nutrients. | [524,525] |
| Cymodocea nodosa | Phenolic compounds, saponins | Seagrass meadows, coastal regions | Alters nutrient availability and creates unfavorable growth conditions. | [526] |
| Gracilaria spp. | Phycobiliproteins, polysaccharides | Tropical and subtropical waters, coral reefs | Inhibits algae through nutrient competition and altering local chemical environment. | [527,528] |
| Padina spp. | Phlorotannins, polysaccharides | Tropical and subtropical marine environments | Reduces algal growth by competing for nutrients and releasing inhibitory substances. | [545] |
| Fucus vesiculosus | Phlorotannins, polysaccharides | Temperate coastal regions | Alters the nutrient dynamics and inhibits algal growth through chemical release. | [546] |
| Chaetomorpha spp. | Algal acids, polysaccharides | Coastal and estuarine environments | Competes for nutrients and modifies the chemical environment to inhibit algal growth. | [547] |
| Ruppia maritima | Phenolic compounds, flavonoids | Shallow coastal and estuarine waters | Reduces nutrient availability and light penetration, affecting algae growth. | [548,549,550] |
| Caulerpa spp. | Caulerpenyne, polysaccharides | Tropical and subtropical marine environments | Competes for space and nutrients and releases toxins that inhibit algae. | [529,530] |
| Syringodium isoetifolium | Terpenoids, flavonoids | Seagrass meadows, coastal waters | Reduces nutrient availability and light penetration, inhibiting algae growth. | [551,552] |
| Enteromorpha spp. | Ulvan, phenolic compounds | Coastal and estuarine environments | Competes for nutrients and releases inhibitory substances affecting algae. | [523,553] |
| Corallina spp. | Calcified polysaccharides, phenolics | Coral reefs, rocky intertidal zones | Alters the chemical environment and reduces nutrient availability to inhibit algae. | [522] |
| Laminaria spp. | Fucoidan, phlorotannins | Temperate coastal areas, kelp forests | Reduces nutrient levels and alters the microenvironment to inhibit algal growth. | [554,555] |
5.3. Physical Treatments
5.4. Constructed Wetlands
5.5. Advanced Technologies
5.6. Resource Processing of Algae
5.7. Comparison of Treatments for Eutrophic Oceans
- (1)
- Comprehensive Monitoring Systems
- (2)
- Nutrient Management Practices
- (3)
- Physical and Chemical Treatment Methods
- (4)
- Ecosystem Restoration and Biomanipulation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Nixon, S.W. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 1995, 41, 199–219. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Bricker, S.B.; Longstaff, B.; Dennison, W.; Jones, A.; Boicourt, K.; Wicks, C.; Woerner, J. Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae 2008, 8, 21–32. [Google Scholar] [CrossRef]
- Landsberg, J.; Flewelling, L.; Naar, J. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae 2009, 8, 598–607. [Google Scholar] [CrossRef]
- Ulloa, M.J.; Álvarez-Torres, P.; Horak-Romo, K.P.; Ortega-Izaguirre, R. Harmful algal blooms and eutrophication along the mexican coast of the Gulf of Mexico large marine ecosystem. Environ. Dev. 2017, 22, 120–128. [Google Scholar] [CrossRef]
- Harred, L.B.; Campbell, L. Predicting harmful algal blooms: A case study with Dinophysis ovum in the Gulf of Mexico. J. Plankton Res. 2014, 36, 1434–1445. [Google Scholar] [CrossRef]
- Watson, S.B.; Molot, L. Harmful Algal Blooms. In Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013; pp. 575–596. [Google Scholar]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- Edwards, M.; Johns, D.G.; Leterme, S.C.; Svendsen, E.; Richardson, A.J. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef]
- Baldrich, Á.M.; Díaz, P.A.; Rosales, S.A.; Rodríguez-Villegas, C.; Álvarez, G.; Pérez-Santos, I.; Díaz, M.; Schwerter, C.; Araya, M.; Reguera, B. An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia. Toxins 2024, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Caruana, A.M.; Le Gac, M.; Hervé, F.; Rovillon, G.-A.; Geffroy, S.; Malo, F.; Abadie, E.; Amzil, Z. Alexandrium pacificum and Alexandrium minutum: Harmful or environmentally friendly? Mar. Environ. Res. 2020, 160, 105014. [Google Scholar] [CrossRef]
- Ayache, N.; Bill, B.D.; Brosnahan, M.L.; Campbell, L.; Deeds, J.R.; Fiorendino, J.M.; Gobler, C.J.; Handy, S.M.; Harrington, N.; Kulis, D.M. A survey of Dinophysis spp. and their potential to cause diarrhetic shellfish poisoning in coastal waters of the United States. J. Phycol. 2023, 59, 658–680. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. United Kingd. 2016, 96, 61–91. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Tholkapiyan, M.; Shanmugam, P.; Suresh, T. Monitoring of ocean surface algal blooms in coastal and oceanic waters around India. Environ. Monit. Assess. 2014, 186, 4129–4137. [Google Scholar] [CrossRef]
- D’Silva, M.S.; Anil, A.C.; Naik, R.K.; D’Costa, P.M. Algal blooms: A perspective from the coasts of India. Nat. Hazards 2012, 63, 1225–1253. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7 (Suppl. S2), S4. [Google Scholar] [CrossRef] [PubMed]
- Tsikoti, C.; Genitsaris, S. Review of harmful algal blooms in the coastal Mediterranean Sea, with a focus on Greek waters. Diversity 2021, 13, 396. [Google Scholar] [CrossRef]
- Hoagland, P.; Anderson, D.M.; Kaoru, Y.; White, A.W. The economic effects of harmful algal blooms in the United States: Estimates, assessment issues, and information needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Wang, S.; Tang, D.; He, F.; Fukuyo, Y.; Azanza, R.V. Occurrences of harmful algal blooms (HABs) associated with ocean environments in the South China Sea. Hydrobiologia 2008, 596, 79–93. [Google Scholar] [CrossRef]
- Tang, D.; Di, B.; Wei, G.; Ni, I.-H.; Oh, I.S.; Wang, S. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia 2006, 568, 245–253. [Google Scholar] [CrossRef]
- Turkoglu, M.; Koray, T. Algal blooms in surface waters of the Sinop Bay in the Black Sea, Turkey. Pak. J. Biol. Sci. 2004, 7, 1577–1585. [Google Scholar]
- Taylor, T.; Longo, A. Valuing algal bloom in the Black Sea Coast of Bulgaria: A choice experiments approach. J. Environ. Manag. 2010, 91, 1963–1971. [Google Scholar] [CrossRef]
- Naik, R.K.; Hegde, S.; Anil, A.C. Dinoflagellate community structure from the stratified environment of the Bay of Bengal, with special emphasis on harmful algal bloom species. Environ. Monit. Assess. 2011, 182, 15–30. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J. Occurrence and potential risks of harmful algal blooms in the East China Sea. Sci. Total Environ. 2009, 407, 4012–4021. [Google Scholar] [CrossRef]
- Bondur, V.; Zamshin, V.; Chvertkova, O.; Matrosova, E.; Khodaeva, V. Detection and analysis of the causes of intensive harmful algal bloom in Kamchatka based on satellite data. J. Mar. Sci. Eng. 2021, 9, 1092. [Google Scholar] [CrossRef]
- Walsh, J.J.; Dieterle, D.A.; Chen, F.R.; Lenes, J.M.; Maslowski, W.; Cassano, J.J.; Whitledge, T.E.; Stockwell, D.; Flint, M.; Sukhanova, I.N. Trophic cascades and future harmful algal blooms within ice-free Arctic Seas north of Bering Strait: A simulation analysis. Prog. Oceanogr. 2011, 91, 312–343. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.J.; Yang, E.-J.; Cho, K.-H.; Jung, J.; Kang, S.-H.; Lee, K.-E.; Cho, S.; Kim, D.; on behalf of the Collaborative Working Group. Temporal and spatial variations in particle fluxes on the Chukchi Sea and East Siberian Sea slopes from 2017 to 2018. Front. Mar. Sci. 2021, 7, 609748. [Google Scholar] [CrossRef]
- Dong, K.; Kvile, K.Ø.; Stenseth, N.C.; Stige, L.C. Associations among temperature, sea ice and phytoplankton bloom dynamics in the Barents Sea. Mar. Ecol. Prog. Ser. 2020, 635, 25–36. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Islas, A.M.; Rember, R.D.; Mordy, C.W.; Wu, J. Sea ice-derived dissolved iron and its potential influence on the spring algal bloom in the Bering Sea. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Boyd, P.W.; Watson, A.J.; Law, C.S.; Abraham, E.R.; Trull, T.; Murdoch, R.; Bakker, D.C.E.; Bowie, A.R.; Buesseler, K.O.; Chang, H.; et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 2000, 407, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Nodder, S.D.; Charette, M.A.; Waite, A.M.; Trull, T.W.; Boyd, P.W.; Zeldis, J.; Buesseler, K.O. Particle transformations and export flux during an in situ iron-stimulated algal bloom in the Southern Ocean. Geophys. Res. Lett. 2001, 28, 2409–2412. [Google Scholar] [CrossRef]
- González, J.M.; Simó, R.; Massana, R.; Covert, J.S.; Casamayor, E.O.; Pedrós-Alió, C.; Moran, M.A. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 2000, 66, 4237–4246. [Google Scholar] [CrossRef]
- Bermejo, P.; Helbling, E.W.; Durán-Romero, C.; Cabrerizo, M.J.; Villafañe, V.E. Abiotic control of phytoplankton blooms in temperate coastal marine ecosystems: A case study in the South Atlantic Ocean. Sci. Total Environ. 2018, 612, 894–902. [Google Scholar] [CrossRef]
- Dundas, I.; Johannessen, O.; Berge, G.; Heimdal, B. Toxic algal bloom in Scandinavian waters, May–June 1988. Oceanography 1989, 2, 9–14. [Google Scholar] [CrossRef]
- Roselli, L.; Caroppo, C.; Bevilacqua, S.; Ciciriello, P.C.; Ungaro, N.; Vadrucci, M.R. Harmful algae and pressure-impact relationship: Noxious blooms and toxic microalgae occurrence from coastal waters of the Apulia region (Adriatic and Ionian Seas, Mediterranean). Mar. Environ. Res. 2023, 183, 105791. [Google Scholar] [CrossRef]
- Penna, N.; Capellacci, S.; Ricci, F. The influence of the Po River discharge on phytoplankton bloom dynamics along the coastline of Pesaro (Italy) in the Adriatic Sea. Mar. Pollut. Bull. 2004, 48, 321–326. [Google Scholar] [CrossRef]
- Zingone, A.; Siano, R.; D’Alelio, D.; Sarno, D. Potentially toxic and harmful microalgae from coastal waters of the Campania region (Tyrrhenian Sea, Mediterranean Sea). Harmful Algae 2006, 5, 321–337. [Google Scholar] [CrossRef]
- Ryan, J.; Kudela, R.; Birch, J.; Blum, M.; Bowers, H.; Chavez, F.; Doucette, G.; Hayashi, K.; Marin III, R.; Mikulski, C. Causality of an extreme harmful algal bloom in Monterey Bay, California, during the 2014–2016 northeast Pacific warm anomaly. Geophys. Res. Lett. 2017, 44, 5571–5579. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Dortch, Q.; Wiseman, W.J.; Gupta, B.K.S.; Justic, D. Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf. Estuaries 1996, 19, 386–407. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Bodeanu, N. Algal blooms and development of the main phytoplanktonic species at the Romanian Black Sea littoral in conditions of intensification of the eutrophication process. In Marine Coastal Eutrophication; Elsevier: Amsterdam, The Netherlands, 1992; pp. 891–906. [Google Scholar]
- Coffin, M.R.; Courtenay, S.C.; Pater, C.C.; Heuvel, M.R.v.D. An empirical model using dissolved oxygen as an indicator for eutrophication at a regional scale. Mar. Pollut. Bull. 2018, 133, 261–270. [Google Scholar] [CrossRef]
- Rosales, D.; Ellett, A.; Jacobs, J.; Ozbay, G.; Parveen, S.; Pitula, J. Investigating the Relationship between nitrate, total dissolved nitrogen, and phosphate with abundance of pathogenic Vibrios and harmful algal blooms in Rehoboth Bay, Delaware. Appl. Environ. Microbiol. 2022, 88, e00356-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, F.; Hua, H.; Yuan, Z. Identifying hotspots based on high-resolution emission inventory of volatile organic compounds: A case study in China. J. Environ. Manag. 2021, 288, 112419. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerril, D.U.; Alonso-Rodríguez, R.; Álvarez-Góngora, C.; Barón-Campis, S.A.; Ceballos-Corona, G.; Herrera-Silveira, J.; Meave del Castillo, M.E.; Juárez-Ruíz, N.; Merino-Virgilio, F.; Morales-Blake, A.; et al. Toxic and harmful marine phytoplankton and microalgae (HABs) in Mexican Coasts. J. Environ. Sci. Health Part A 2007, 42, 1349–1363. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Skrobek, I.; Ziułkiewicz, M.; Adamski, M.; Kaminski, A.; Żmudzki, P. Persistent cyanobacteria blooms in artificial water bodies—An effect of environmental conditions or the result of anthropogenic change. Int. J. Environ. Res. Public Health 2022, 19, 6990. [Google Scholar] [CrossRef]
- Telesh, I.; Schubert, H.; Skarlato, S. Abiotic stability promotes dinoflagellate blooms in marine coastal ecosystems. Estuar. Coast. Shelf Sci. 2021, 251, 107239. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Bravo, I.; Zapata, M.; Marañón, E. Review of the main ecological features affecting benthic dinoflagellate blooms. Cryptogam. Algol. 2012, 33, 171–179. [Google Scholar] [CrossRef]
- Kong, X.; Seewald, M.; Dadi, T.; Friese, K.; Mi, C.; Boehrer, B.; Schultze, M.; Rinke, K.; Shatwell, T. Unravelling winter diatom blooms in temperate lakes using high frequency data and ecological modeling. Water Res. 2021, 190, 116681. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shen, H.; Xie, P. Can hydrodynamics change phosphorus strategies of diatoms?—Nutrient levels and diatom blooms in lotic and lentic ecosystems. Microb. Ecol. 2012, 63, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, D.H.; Sorrell, B.K.; Sejr, M.K.; Andersen, P.; Rysgaard, S.; Hansen, P.J.; Skyttä, A.; Lemcke, S.; Lund-Hansen, L.C. An under-ice bloom of mixotrophic haptophytes in low nutrient and freshwater-influenced Arctic waters. Sci. Rep. 2021, 11, 2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Kong, F.-Z.; Geng, H.-X.; Zhang, Q.-C.; Yuan, Y.-Q.; Yu, R.-C. CHEMTAX analysis of phytoplankton assemblages revealed potential indicators for blooms of haptophyte Phaeocystis globosa. Ecol. Indic. 2021, 131, 108177. [Google Scholar] [CrossRef]
- Bearon, R.N.; Grünbaum, D.; Cattolico, R.A. Effects of salinity structure on swimming behavior and harmful algal bloom formation in Heterosigma akashiwo, a toxic raphidophyte. Mar. Ecol. Prog. Ser. 2006, 306, 153–163. [Google Scholar] [CrossRef]
- Jeong, H.J.; Du Yoo, Y.; Lim, A.S.; Kim, T.-W.; Lee, K.; Kang, C.K. Raphidophyte red tides in Korean waters. Harmful Algae 2013, 30, S41–S52. [Google Scholar] [CrossRef]
- Cheng, B.; Xia, R.; Zhang, Y.; Yang, Z.; Hu, S.; Guo, F.; Ma, S. Characterization and causes analysis for algae blooms in large river system. Sustain. Cities Soc. 2019, 51, 101707. [Google Scholar] [CrossRef]
- Lee, M.; Kim, B.; Kwon, Y.; Kim, J. Characteristics of the marine environment and algal blooms in Gamak Bay. Fish. Sci. 2009, 75, 401–411. [Google Scholar] [CrossRef]
- Brush, M.J.; Giani, M.; Totti, C.; Testa, J.M.; Faganeli, J.; Ogrinc, N.; Kemp, W.M.; Umani, S.F. Eutrophication, harmful algae, oxygen depletion, and acidification. Coast. Ecosyst. Transit. A Comp. Anal. North. Adriat. Chesap. Bay 2020, 75–104. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Yan, T.; Song, J.; Yu, R.; Zhou, M. Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom. J. Oceanol. Limnol. 2021, 39, 500–507. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Glibert, P.M.; Burford, M.A. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 2017, 30, 58–69. [Google Scholar] [CrossRef]
- Paul, V.J. Global warming and cyanobacterial harmful algal blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 239–257. [Google Scholar]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Hansen, L.S.; Blackburn, T.H. Effect of algal bloom deposition on sediment respiration and fluxes. Mar. Biol. 1992, 112, 147–152. [Google Scholar] [CrossRef]
- Vargas, G.; Donoso-Bravo, A.; Vergara, C.; Ruiz-Filippi, G. Assessment of microalgae and nitrifiers activity in a consortium in a continuous operation and the effect of oxygen depletion. Electron. J. Biotechnol. 2016, 23, 63–68. [Google Scholar] [CrossRef]
- Altieri, A.H.; Diaz, R.J. Dead zones: Oxygen depletion in coastal ecosystems. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 453–473. [Google Scholar]
- Marinho, P.R.; Moreira, A.P.B.; Pellegrino, F.L.P.C.; Muricy, G.; Bastos, M.d.C.d.F.; Santos, K.R.N.d.; Giambiagi-deMarval, M.; Laport, M.S. Marine Pseudomonas putida: A potential source of antimicrobial substances against antibiotic-resistant bacteria. Mem. Inst. Oswaldo Cruz 2009, 104, 678–682. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Chen, L.; Liu, D. Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Appl. Microbiol. Biotechnol. 2012, 93, 1305–1314. [Google Scholar] [CrossRef]
- Velupillaimani, D.; Muthaiyan, A. Potential of Bacillus subtilis from marine environment to degrade aromatic hydrocarbons. Environ. Sustain. 2019, 2, 381–389. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.; Fu, Q.; Sun, J.; An, Z.Y.; Ding, R.; Li, Y.; Zhao, X.D. Effect of Bacillus subtilis on corrosion behavior of 10MnNiCrCu steel in marine environment. Sci. Rep. 2020, 10, 5744. [Google Scholar] [CrossRef]
- Noguera, D.R.; Brusseau, G.A.; Rittmann, B.E.; Stahl, D.A. A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol. Bioeng. 1998, 59, 732–746. [Google Scholar] [CrossRef]
- Anderson, K.L.; Apolinario, E.E.; Sowers, K.R. Desiccation as a Long-Term Survival Mechanism for the Archaeon Methanosarcina barkeri. Appl. Environ. Microbiol. 2012, 78, 1473–1479. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Loesgen, S. Bioprospecting Chemical Diversity and Bioactivity in a Marine Derived Aspergillus terreus. Chem. Biodivers. 2016, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Wang, J.; He, Y.; Zhang, J.; Li, F.; Qi, C.; Zhu, H.; Xue, Y.; Hu, Z.; et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorganic Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef]
- Tran, N.T.; Li, Z.; Ma, H.; Zhang, Y.; Zheng, H.; Gong, Y.; Li, S. Clostridium butyricum: A promising probiotic confers positive health benefits in aquatic animals. Rev. Aquac. 2020, 12, 2573–2589. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Santos, L.; Silva, B.M.V.; Abreu, A.C.; Vicente, T.F.L.; Esteves, A.C.; Alves, A. Biodiversity of Penicillium species from marine environments in Portugal and description of Penicillium lusitanum sp. nov., a novel species isolated from sea water. Int. J. Syst. Evol. Microbiol. 2019, 69, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cravo-Laureau, C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. Fems Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Bouskill, N.J.; Eveillard, D.; Chien, D.; Jayakumar, A.; Ward, B.B. Environmental factors determining ammonia-oxidizing organism distribution and diversity in marine environments. Environ. Microbiol. 2011, 14, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.O.; Sampaio, E.; Santos, C.P.; Rosa, R. Impacts of low oxygen on marine life: Neglected, but a crucial priority for research. Biol. Bull. 2022, 243, 104–119. [Google Scholar] [CrossRef]
- Cocco, V.; Joos, F.; Steinacher, M.; Frölicher, T.L.; Bopp, L.; Dunne, J.; Gehlen, M.; Heinze, C.; Orr, J.; Oschlies, A. Oxygen and indicators of stress for marine life in multi-model global warming projections. Biogeosciences 2013, 10, 1849–1868. [Google Scholar] [CrossRef]
- Bianchi, D.; Galbraith, E.D.; Carozza, D.A.; Mislan, K.A.S.; Stock, C.A. Intensification of open-ocean oxygen depletion by vertically migrating animals. Nat. Geosci. 2013, 6, 545–548. [Google Scholar] [CrossRef]
- Riedel, B.; Pados, T.; Pretterebner, K.; Schiemer, L.; Steckbauer, A.; Haselmair, A.; Zuschin, M.; Stachowitsch, M. Effect of hypoxia and anoxia on invertebrate behaviour: Ecological perspectives from species to community level. Biogeosciences 2014, 11, 1491–1518. [Google Scholar] [CrossRef]
- Breitburg, D.L. Episodic hypoxia in Chesapeake Bay: Interacting effects of recruitment, behavior, and physical disturbance. Ecol. Monogr. 1992, 62, 525–546. [Google Scholar] [CrossRef]
- Gray, J.S.; Wu, R.S.-s.; Or, Y.Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 2002, 238, 249–279. [Google Scholar] [CrossRef]
- Leduc, A.O.H.C.; Munday, P.L.; Brown, G.E.; Ferrari, M.C.O. Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: A synthesis. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120447. [Google Scholar] [CrossRef]
- Anderson, C.R.; Moore, S.K.; Tomlinson, M.C.; Silke, J.; Cusack, C.K. Living with harmful algal blooms in a changing world: Strategies for modeling and mitigating their effects in coastal marine ecosystems. In Coastal and Marine Hazards, Risks, and Disasters; Elsevier: Amsterdam, The Netherlands, 2015; pp. 495–561. [Google Scholar]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.C.v.D.; Nunes, P.A.; Dotinga, H.M.; Kooistra, W.H.; Vrieling, E.G.; Peperzak, L. Exotic harmful algae in marine ecosystems: An integrated biological–economic–legal analysis of impacts and policies. Mar. Policy 2002, 26, 59–74. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Roelke, D.; Greene, R.M. Health and ecological impacts of harmful algal blooms: Risk assessment needs. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1329–1345. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef]
- Lopes, V.M.; Costa, P.R.; Rosa, R. Effects of harmful algal bloom toxins on marine organisms. In Ecotoxicology of Marine Organisms; CRC Press: Boca Raton, FL, USA, 2019; pp. 42–88. [Google Scholar]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef]
- Costa, P.R. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish. 2016, 17, 226–248. [Google Scholar]
- Burkholder, J.M.; Shumway, S.E.; Glibert, P.M. Food web and ecosystem impacts of harmful algae. In Harmful Algal Blooms: A Compendium Desk Reference; Wiley: Hoboken, NJ, USA, 2018; pp. 243–336. [Google Scholar]
- Riebesell, U.; Aberle-Malzahn, N.; Achterberg, E.P.; Algueró-Muñiz, M.; Alvarez-Fernandez, S.; Arístegui, J.; Bach, L.T.; Boersma, M.; Boxhammer, T.; Guan, W.; et al. Toxic algal bloom induced by ocean acidification disrupts the pelagic food web. Nat. Clim. Change 2018, 8, 1082–1086. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.M.; Parsons, M.L.; Rensel, J.E.J.; Townsend, D.W.; et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef]
- Aboualaalaa, H.; El Kbiach, M.L.; Leblad, B.R.; Hervé, F.; Hormat-Allah, A.; Baudy, L.; Ennaskhi, I.; Hammi, I.; Ibghi, M.; Elmortaji, H.; et al. Development of harmful algal blooms species responsible for lipophilic and amnesic shellfish poisoning intoxications in southwestern Mediterranean coastal waters. Toxicon 2022, 219, 106916. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Soliño, L.; Bravo, I.; Rodríguez, F.; Casero, M.V.M. Paralytic and amnesic shellfish toxins impacts on seabirds, analyses and management. Toxins 2021, 13, 454. [Google Scholar] [CrossRef]
- Farabegoli, F.; Blanco, L.; Rodríguez, L.P.; Vieites, J.M.; Cabado, A.G. Phycotoxins in marine shellfish: Origin, occurrence and effects on humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef]
- Leal, J.F.; Cristiano, M.L.S. Marine paralytic shellfish toxins: Chemical properties, mode of action, newer analogues, and structure–toxicity relationship. Nat. Prod. Rep. 2022, 39, 33–57. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.-Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Costa, P.R. Differential toxin profiles of ciguatoxins in marine organisms: Chemistry, fate and global distribution. Toxicon 2018, 150, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; Liu, Y.; Coothankandaswamy, V.; Mahdi, F.; Jekabsons, M.B.; Gerwick, W.H.; Valeriote, F.A.; Zhou, Y.-D.; Nagle, D.G. Kalkitoxin inhibits angiogenesis, disrupts cellular hypoxic signaling, and blocks mitochondrial electron transport in tumor cells. Mar. Drugs 2015, 13, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Okino, T.; Nogle, L.M.; Marquez, B.L.; Williamson, R.T.; Sitachitta, N.; Berman, F.W.; Murray, T.F.; McGough, K.; Jacobs, R.; et al. Structure, Synthesis, and Biological Properties of Kalkitoxin, a Novel Neurotoxin from the Marine Cyanobacterium Lyngbya m ajuscula. J. Am. Chem. Soc. 2000, 122, 12041–12042. [Google Scholar] [CrossRef]
- Nagai, H.; Yasumoto, T.; Hokama, Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. Toxicon 1996, 34, 753–761. [Google Scholar] [CrossRef]
- Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Caulerpenyne from Caulerpa taxifolia has an antiproliferative activity on tumor cell line SK-N-SH and modifies the microtubule network. Life Sci. 2001, 70, 415–429. [Google Scholar] [CrossRef]
- Pesando, D.; Lemée, R.; Ferrua, C.; Amade, P.; Girard, J.-P. Effects of caulerpenyne, the major toxin from Caulerpa taxifolia on mechanisms related to sea urchin egg cleavage. Aquat. Toxicol. 1996, 35, 139–155. [Google Scholar] [CrossRef]
- Nagai, H.; Sato, S.; Iida, K.; Hayashi, K.; Kawaguchi, M.; Uchida, H.; Satake, M. Oscillatoxin I: A new aplysiatoxin derivative, from a marine cyanobacterium. Toxins 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-H.; Zhang, X.-K.; Si, R.-R.; Shen, S.-C.; Liang, T.-T.; Fan, T.-T.; Chen, W.; Xu, L.-H.; Han, B.-N. Chemical and biological study of novel aplysiatoxin derivatives from the marine cyanobacterium Lyngbya sp. Toxins 2020, 12, 733. [Google Scholar] [CrossRef]
- Dhanji-Rapkova, M.; O’Neill, A.; Maskrey, B.H.; Coates, L.; Alves, M.T.; Kelly, R.J.; Hatfield, R.G.; Rowland-Pilgrim, S.J.; Lewis, A.M.; Algoet, M.; et al. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysis toxins and pectenotoxins. Harmful Algae 2018, 77, 66–80. [Google Scholar] [CrossRef]
- Boundy, M.J.; Harwood, D.T.; Kiermeier, A.; McLeod, C.; Nicolas, J.; Finch, S. Risk assessment of pectenotoxins in New Zealand bivalve molluscan shellfish, 2009–2019. Toxins 2020, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Igarashi, T.; Yamamoto, M.; Yasuda, M.; Sekiguchi, R.; Watai, M.; Tanno, K.; Yasumoto, T. Quantitative determination of marine toxins associated with diarrhetic shellfish poisoning by liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2001, 907, 181–189. [Google Scholar] [CrossRef]
- Aune, T.; Yndestad, M. Diarrhetic shellfish poisoning. Algal Toxins Seaf. Drink. Water. 1993, 87–104. [Google Scholar] [CrossRef]
- Furey, A.; O’Doherty, S.; O’Callaghan, K.; Lehane, M.; James, K.J. Azaspiracid poisoning (AZP) toxins in shellfish: Toxicological and health considerations. Toxicon 2010, 56, 173–190. [Google Scholar] [CrossRef]
- Satake, M.; Ofuji, K.; Naoki, H.; James, K.J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar] [CrossRef]
- Vilariño, N. Marine toxins and the cytoskeleton: Azaspiracids. FEBS J. 2008, 275, 6075–6081. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Dell’Ovo, V.; Sosa, S.; Florio, C. Yessotoxins: A toxicological overview. Toxicon 2010, 56, 163–172. [Google Scholar] [CrossRef]
- Rhodes, L.; McNabb, P.; De Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobó, P.; Franco, J.M.; Fernández, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Pinheiro, C.; Azevedo, J.; Campos, A.; Loureiro, S.; Vasconcelos, V. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 2013, 705, 27–42. [Google Scholar] [CrossRef]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Umehara, A.; Takahashi, T.; Komorita, T.; Orita, R.; Choi, J.-W.; Takenaka, R.; Mabuchi, R.; Park, H.-D.; Tsutsumi, H. Widespread dispersal and bio-accumulation of toxic microcystins in benthic marine ecosystems. Chemosphere 2017, 167, 492–500. [Google Scholar] [CrossRef]
- Chen, D.Z.; Boland, M.P.; Smillie, M.A.; Klix, H.; Ptak, C.; Andersen, R.J.; Holmes, C.F. Identification of protein phosphatase inhibitors of the microcystin class in the marine environment. Toxicon 1993, 31, 1407–1414. [Google Scholar] [CrossRef]
- Butler, S.C.; Miles, C.O.; Karim, A.; Twiner, M.J. Inhibitory effects of pectenotoxins from marine algae on the polymerization of various actin isoforms. Toxicol. Vitr. 2012, 26, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Rubiolo, J.A. Marine toxins and the cytoskeleton: Pectenotoxins, unusual macrolides that disrupt actin. FEBS J. 2008, 275, 6082–6088. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef]
- Gibble, C.M.; Peacock, M.B.; Kudela, R.M. Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health. Harmful Algae 2016, 59, 59–66. [Google Scholar] [CrossRef]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef]
- Patiño, R.; Christensen, V.G.; Graham, J.L.; Rogosch, J.S.; Rosen, B.H. Toxic Algae in Inland Waters of the Conterminous United States—A Review and Synthesis. Water 2023, 15, 2808. [Google Scholar] [CrossRef]
- Manganelli, M. Blooms of toxic microorganisms in aquatic environments: Marine microalgae and freshwater cyanobacteria. A brief review with a particular focus on the Italian situation: Diffusion and health effects of toxic marine microalgae and freshwater cyanobacteria in Italy. Rendiconti Lincei 2016, 27, 135–143. [Google Scholar] [CrossRef]
- Falconer, I.R. Toxic cyanobacterial bloom problems in Australian waters: Risks and impacts on human health. Phycologia 2001, 40, 228–233. [Google Scholar] [CrossRef]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I.; et al. Towards the review of the European Union Water Framework Directive: Recommendations for more efficient assessment and management of chemical contamination in European surface water resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Yu, Q.; Eaglesham, G.; Connell, D.W.; McBroom, J.; Costanzo, S.; Shaw, G.R. Occurrence and seasonal variations of algal toxins in water, phytoplankton and shellfish from North Stradbroke Island, Queensland, Australia. Mar. Environ. Res. 2007, 64, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Burke, J.M.; Mosindy, T.; Fedorak, P.M.; Prepas, E.E. Cyanobacteria and microcystin-LR in a complex lake system representing a range in trophic status: Lake of the Woods, Ontario, Canada. J. Plankton Res. 2009, 31, 993–1008. [Google Scholar] [CrossRef]
- Oliveira, E.D.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef]
- Zhang, S.; Du, X.; Liu, H.; Losiewic, M.D.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Shi, L.; Guo, H.; et al. The latest advances in the reproductive toxicity of microcystin-LR. Environ. Res. 2021, 192, 110254. [Google Scholar] [CrossRef]
- Lone, Y.; Koiri, R.K.; Bhide, M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol. Rep. 2015, 2, 289–296. [Google Scholar] [CrossRef]
- Mbukwa, E.A.; Msagati, T.A.; Mamba, B.B. Quantitative Variations of Intracellular Microcystin-LR, -RR and -YR in Samples Collected from Four Locations in Hartbeespoort Dam in North West Province (South Africa) During the 2010/2011 Summer Season. Int. J. Environ. Res. Public Health 2012, 9, 3484–3505. [Google Scholar] [CrossRef]
- Dong, H.; Aziz, T.; Richardson, S.D. Transformation of Algal Toxins during the Oxidation/Disinfection Processes of Drinking Water: From Structure to Toxicity. Environ. Sci. Technol. 2023, 57, 12944–12957. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived intensification in harmful algal blooms is a wave of cumulative threat to the aquatic ecosystems. Biology 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Summers, E.J.; Ryder, J.L. A critical review of operational strategies for the management of harmful algal blooms (HABs) in inland reservoirs. J. Environ. Manag. 2023, 330, 117141. [Google Scholar] [CrossRef]
- Oduor, N.A.; Munga, C.N.; Ong’Anda, H.O.; Botwe, P.K.; Moosdorf, N. Nutrients and harmful algal blooms in Kenya’s coastal and marine waters: A review. Ocean Coast. Manag. 2023, 233, 106454. [Google Scholar] [CrossRef]
- Paerl, H.W.; Joyner, J.J.; Joyner, A.R.; Arthur, K.; Paul, V.; O’Neil, J.M.; Heil, C.A. Co-occurrence of dinoflagellate and cyanobacterial harmful algal blooms in southwest Florida coastal waters: Dual nutrient (N and P) input controls. Mar. Ecol. Prog. Ser. 2008, 371, 143–153. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Bouwman, A.F.; Brown, C.W.; Flynn, K.J.; Lewitus, A.J.; Madden, C.J. Modeling of HABs and eutrophication: Status, advances, challenges. J. Mar. Syst. 2010, 83, 262–275. [Google Scholar] [CrossRef]
- Yan, T.; Li, X.-D.; Tan, Z.-J.; Yu, R.-C.; Zou, J.-Z. Toxic effects, mechanisms, and ecological impacts of harmful algal blooms in China. Harmful Algae 2022, 111, 102148. [Google Scholar] [CrossRef]
- Šulčius, S.; Montvydienė, D.; Mazur-Marzec, H.; Kasperovičienė, J.; Rulevičius, R.; Cibulskaitė, Ž. The profound effect of harmful cyanobacterial blooms: From food-web and management perspectives. Sci. Total Environ. 2017, 609, 1443–1450. [Google Scholar] [CrossRef]
- Glibert, P.M.; Al-Azri, A.; Allen, J.I.; Bouwman, A.F.; Beusen, A.H.W.; Burford, M.A.; Harrison, P.J.; Zhou, M. Key Questions and Recent Research Advances on Harmful Algal Blooms in Relation to Nutrients and Eutrophication. In Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kudela, R.; Seeyave, S.; Cochlan, W. The role of nutrients in regulation and promotion of harmful algal blooms in upwelling systems. Prog. Oceanogr. 2010, 85, 122–135. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, T.; Chen, X.; Guo, M.; Meng, X.; Wang, X.; Bai, S. Exploring the resilience of constructed wetlands to harmful algal blooms disturbances: A study on microbial response mechanisms. Bioresour. Technol. 2023, 383, 129251. [Google Scholar] [CrossRef]
- Garcés, E.; Camp, J. Habitat changes in the Mediterranean Sea and the consequences for harmful algal blooms formation. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 519–541. [Google Scholar]
- Tao, F. Air–water CO2 flux in an algae bloom year for Lake Hongfeng, Southwest China: Implications for the carbon cycle of global inland waters. Acta Geochim. 2017, 36, 658–666. [Google Scholar] [CrossRef]
- McGowan, S.; Anderson, N.J.; Edwards, M.E.; Langdon, P.G.; Jones, V.J.; Turner, S.; van Hardenbroek, M.; Whiteford, E.; Wiik, E. Long-term perspectives on terrestrial and aquatic carbon cycling from palaeolimnology. WIREs Water 2015, 3, 211–234. [Google Scholar] [CrossRef]
- Legendre, L. The significance of microalgal blooms for fisheries and for the export of particulate organic carbon in oceans. J. Plankton Res. 1990, 12, 681–699. [Google Scholar] [CrossRef]
- Piatka, D.R.; Frank, A.H.; Köhler, I.; Castiglione, K.; van Geldern, R.; Barth, J.A. Balance of carbon species combined with stable isotope ratios show critical switch towards bicarbonate uptake during cyanobacteria blooms. Sci. Total Environ. 2022, 807, 151067. [Google Scholar] [CrossRef]
- Mei, Z.-P.; Legendre, L.; Tremblay, J.; Miller, L.; Gratton, Y.; Lovejoy, C.; Yager, P.; Gosselin, M. Carbon to nitrogen (C:N) stoichiometry of the spring–summer phytoplankton bloom in the North Water Polynya (NOW). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 2301–2314. [Google Scholar] [CrossRef]
- Humborg, C.; Conley, D.J.; Rahm, L.; Wulff, F.; Cociasu, A.; Ittekkot, V. Silicon Retention in River Basins: Far-reaching Effects on Biogeochemistry and Aquatic Food Webs in Coastal Marine Environments. AMBIO A J. Hum. Environ. 2000, 29, 45–50. [Google Scholar] [CrossRef]
- Liu, H.-C.; Nie, Z.-Y.; Long, X.-P.; Bouroubi, N.E.H.; Cao, S.-T.; Chen, Y.-X.; Zheng, X.-F.; Xia, J.-L. Biosynthesis and Detection of Domoic Acid from Diatom Pseudo-nitzschia: A Review. Curr. Pharm. Biotechnol. 2023, 24, 599–610. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, Y.; Liu, Q.; Yao, Y. Nutrient Compositions of Cultured Thalassiosira rotula and Skeletonema costatum from Jiaozhou Bay. In Studies of the Biogeochemistry of Typical Estuaries and Bays in China; Springer Earth System Sciences; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Pan, S.; Shen, Z.; Liu, W.; Han, X.; Miao, H.; Ma, H. Nutrient compositions of cultured Skeletonema costatum, Chaetoceros curvisetus, and Thalassiosira nordenskiöldii. Chin. J. Oceanol. Limnol. 2010, 28, 1131–1138. [Google Scholar] [CrossRef]
- Lipsewers, T.; Klais, R.; Camarena-Gómez, M.; Spilling, K. Effects of different plankton communities and spring bloom phases on seston C:N:P:Si:chl a ratios in the Baltic Sea. Mar. Ecol. Prog. Ser. 2020, 644, 15–31. [Google Scholar] [CrossRef]
- Liu, D.; Shen, X.; Di, B.; Shi, Y.; Keesing, J.K.; Wang, Y. Palaeoecological analysis of phytoplankton regime shifts in response to coastal eutrophication. Mar. Ecol. Prog. Ser. 2013, 475, 1–14. [Google Scholar] [CrossRef]
- Glibert, P.M.; Burkholder, J.M. Causes of harmful algal blooms. In Harmful Algal Bloom: A Compendium Desk Reference; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–38. [Google Scholar]
- Balode, M.; Purina, I.; Beéchemin, C.; Maestrini, S.Y. Effects of nutrient enrichment on the growth rates and community structure of summer phytoplankton from the Gulf of Riga, Baltic Sea. J. Plankton Res. 1998, 20, 2251–2272. [Google Scholar] [CrossRef]
- Ara, K.; Fukuyama, S.; Okutsu, T.; Nagasaka, S.; Shiomoto, A. Seasonal variability in phytoplankton carbon biomass and primary production, and their contribution to particulate carbon in the neritic area of Sagami Bay, Japan. Plankton Benthos Res. 2019, 14, 224–250. [Google Scholar] [CrossRef]
- Shroder, J.F.; Sivanpillai, R. Biological and Environmental Hazards, Risks, and Disasters; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Miyajima, T.; Nakanishi, M.; Nakano, S.-I.; Tezuka, Y. An autumnal bloom of the diatom Melosira granulata in a shallow eutrophic lake: Physical and chemical constraints on its population dynamics. Arch. Hydrobiol. 1994, 130, 143–162. [Google Scholar] [CrossRef]
- Kõiv, T.; Kangro, K. Resource Ratios and Phytoplankton Species Composition in a Strongly Stratified Lake. Hydrobiologia 2005, 547, 123–135. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes-Mella, J.; Flores-Leñero, A.; Yarimizu, K.; Godoy, M.; Artal, O.; Corredor-Acosta, A.; Marcus, L.; Cascales, E.; Espinoza, J.P.; et al. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian fjords: Insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2023, 210, 102921. [Google Scholar] [CrossRef]
- Davidson, K.; Gowen, R.J.; Harrison, P.J.; Fleming, L.E.; Hoagland, P.; Moschonas, G. Anthropogenic nutrients and harmful algae in coastal waters. J. Environ. Manag. 2014, 146, 206–216. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B. Harmful algal blooms in a changing ocean. In Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Berlin/Heidelberg, Germany, 2018; pp. 77–90. [Google Scholar]
- Wang, J.; Bouwman, A.F.; Liu, X.; Beusen, A.H.; Van Dingenen, R.; Dentener, F.; Yao, Y.; Glibert, P.M.; Ran, X.; Yao, Q.; et al. Harmful algal blooms in Chinese coastal waters will persist due to perturbed nutrient ratios. Environ. Sci. Technol. Lett. 2021, 8, 276–284. [Google Scholar] [CrossRef]
- Wang, H.; Bouwman, A.F.; Van Gils, J.; Vilmin, L.; Beusen, A.H.; Wang, J.; Liu, X.; Yu, Z.; Ran, X. Hindcasting harmful algal bloom risk due to land-based nutrient pollution in the Eastern Chinese coastal seas. Water Res. 2023, 231, 119669. [Google Scholar] [CrossRef]
- Shetye, S.; Pratihary, A.; Shenoy, D.; Kurian, S.; Gauns, M.; Uskaikar, H.; Naik, B.; Nandakumar, K.; Borker, S. Rice husk as a potential source of silicate to oceanic phytoplankton. Sci. Total Environ. 2023, 879, 162941. [Google Scholar] [CrossRef] [PubMed]
- Ragueneau, O.; Conley, D.J.; Leynaert, A.; Longphuirt, S.N.; Slomp, C.P. Role of diatoms in silicon cycling and coastal marine food webs. Silicon Cycle Hum. Human. Perturbations Impacts Aquat. Syst. 2006, 66, 163–195. [Google Scholar]
- Ragueneau, O.; Conley, D.J.; Leynaert, A.; Longphuirt, S.N.; Slomp, C.P.; Ittekkot, V. Responses of coastal ecosystems to an-thropogenic perturbations of silicon cycling. Silicon Cycle Hum. Human. Perturbations Impacts Aquat. Syst. 2006, 66, 197. [Google Scholar]
- Paerl, H.W. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol. Oceanogr. 1997, 42, 1154–1165. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Global nitrogen fertilizer demand and supply: Trend, current level and outlook. In Proceedings of the International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Available online: http://faostat.fao.org/ (accessed on 29 July 2021).
- Canton, H. The Europa Directory of International Organizations 2021; Routledge: Abingdon, UK, 2021; pp. 297–305. [Google Scholar]
- Randive, K.; Raut, T.; Jawadand, S. An overview of the global fertilizer trends and India’s position in 2020. Miner. Econ. 2021, 34, 1–14. [Google Scholar] [CrossRef]
- IFA. Fertilizer Outlook 2021–2025; IFA: Berlin, Germany, 2021. [Google Scholar]
- Peñuelas, J.; Sardans, J. The global nitrogen-phosphorus imbalance. Science 2022, 375, 266–267. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2020; FAO: Rome, Italy, 2017. [Google Scholar]
- Mayo, R.; Batello, C. World Fertilizer Trends and Outlook to 2018; FAO: Rome, Italy, 2015. [Google Scholar]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, S.X. Nitrate N loss by leaching and surface runoff in agricultural land: A global issue (a review). Adv. Agron. 2019, 156, 159–217. [Google Scholar] [CrossRef]
- Smith, D.; Owens, P.; Leytem, A.; Warnemuende, E.A. Nutrient losses from manure and fertilizer applications as impacted by time to first runoff event. Environ. Pollut. 2007, 147, 131–137. [Google Scholar] [CrossRef]
- Cirelli, A.F.; Arumí, J.L.; Rivera, D.; Boochs, P.W. Environmental Effects of irrigation in arid and semi-arid regions. Chil. J. Agric. Res. 2009, 69, 27–40. [Google Scholar] [CrossRef]
- Vogeler, I.; Rogasik, J.; Funder, U.; Panten, K.; Schnug, E. Effect of tillage systems and P-fertilization on soil physical and chemical properties, crop yield and nutrient uptake. Soil Tillage Res. 2009, 103, 137–143. [Google Scholar] [CrossRef]
- Kabir, Z. Tillage or no-tillage: Impact on mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Influence of Cover Crop, Tillage, and Crop Rotation Management on Soil Nutrients. Agriculture 2020, 10, 225. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, T.Y.; Pu, L.; Sun, J. Comparison of fertilizer use efficiency in grain production between developing countries and developed countries. J. Sci. Food Agric. 2021, 102, 2404–2412. [Google Scholar] [CrossRef]
- Kato, T.; Kuroda, H.; Nakasone, H. Runoff characteristics of nutrients from an agricultural watershed with intensive livestock production. J. Hydrol. 2009, 368, 79–87. [Google Scholar] [CrossRef]
- FAO. Global Dairy Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Egg Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Beef Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Poultry Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Pork Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Sheep and Goat Production Statistics; FAO: Rome, Italy, 2023. [Google Scholar]
- Wirasenjaya, F.; Dhar, A.R.; Oita, A.; Matsubae, K. Assessment of food-related nitrogen and phosphorus footprints in Indo-nesia. Sustain. Prod. Consum. 2023, 39, 30–41. [Google Scholar] [CrossRef]
- Russelle, M.P.; Entz, M.H.; Franzluebbers, A.J. Reconsidering Integrated Crop–Livestock Systems in North America. Agron. J. 2007, 99, 325–334. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Data Collection; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Contribution of Terrestrial Animal Source Food to Healthy Diets for Improved Nutrition and Health Outcomes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Peng, T.; Zhu, Z.; Du, J.; Liu, J. Effects of nutrient-rich submarine groundwater discharge on marine aquaculture: A case in Lianjiang, East China Sea. Sci. Total Environ. 2021, 786, 147388. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Boukhari, M.E.M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Bio/Technol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Loureiro, S.; Reñé, A.; Garcés, E.; Camp, J.; Vaqué, D. Harmful algal blooms (HABs), dissolved organic matter (DOM), and planktonic microbial community dynamics at a near-shore and a harbour station influenced by upwelling (SW Iberian Peninsula). J. Sea Res. 2011, 65, 401–413. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Gil, F.; Izydorczyk, G.; Mikula, K.; Gersz, A.; Hoppe, V.; Chojnacka, K.; Witek-Krowiak, A. Innovative bio-waste-based multilayer hydrogel fertilizers as a new solution for precision agriculture. J. Environ. Manag. 2022, 321, 116002. [Google Scholar] [CrossRef]
- Harary, D.C. Costs and Benefits of Nitrogen and Phosphate Fertilizer Use in the Lake Erie Basin. Cent. Dev. Strategy 2016, 2016. Available online: http://www.inquiriesjournal.com/articles/1531/costs-and-benefits-of-nitrogen-and-phosphate-fertilizer-use-in-the-lake-erie-basin (accessed on 28 August 2024).
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Kay, A.N. An alginate-based fertilizer to reduce eutrophication of aquatic environments. Trans. Kans. Acad. Sci. 2004, 107, 155–164. [Google Scholar] [CrossRef]
- Mansouri, H.; Said, H.A.; Noukrati, H.; Oukarroum, A.; Ben Youcef, H.; Perreault, F. Advances in Controlled Release Fertilizers: Cost-Effective Coating Techniques and Smart Stimuli-Responsive Hydrogels. Adv. Sustain. Syst. 2023, 7, 2300149. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation—A review. Nutr. Cycl. Agroecosyst. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Singh, S.N.; Verma, A. Environmental Review: The Potential of Nitrification Inhibitors to Manage the Pollution Effect of Nitrogen Fertilizers in Agricultural and Other Soils: A Review. Environ. Pract. 2007, 9, 266–279. [Google Scholar] [CrossRef]
- Kumar, R.; Parmar, B.S.; Walia, S.; Saha, S. Nitrification Inhibitors: Classes and Its Use in Nitrification Management. In Nutrient Use Efficiency: From Basics to Advances; Springer: Amsterdam, The Netherlands, 2015; pp. 103–122. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.-J.; Schmidt, C.K.; Sacher, F. Occurrence and fate of nitrification and urease inhibitors in the aquatic environment. Environ. Sci. Process. Impacts 2016, 18, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’callaghan, T.F. Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Yin, J.; Chadwick, D.; Norse, D.; Lu, Y.; Liu, X.; Chen, X.; Zhang, F.; Powlson, D.; et al. Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Change Biol. 2018, 24, e511–e521. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2014, 95, 1131–1142. [Google Scholar] [CrossRef]
- Naik, M.R.; Kumar, B.K.; Manasa, K. Polymer coated fertilizers as advance technique in nutrient management. Asian J. SOIL Sci. 2017, 12, 228–232. [Google Scholar] [CrossRef]
- Chandra, M.S.; Lal, M.; Naresh, R.K.; Yadav, S.; Kumar, R.; Kumar, R.; Lavanya, N. Role of polymer coated fertilizers (PCFS) an advance technology for improving nutrient use efficiency and crop productivity: A review. Int. J. Chem. Stud. 2019, 7, 2667–2679. [Google Scholar]
- Vasseghian, Y.; Nadagouda, M.M.; Aminabhavi, T.M. Biochar-enhanced bioremediation of eutrophic waters impacted by algal blooms. J. Environ. Manag. 2024, 367, 122044. [Google Scholar] [CrossRef] [PubMed]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, J.; Xu, X.; Ma, M.; Li, Y.; Chen, Q.; Su, H. Full quantitative resource utilization of raw mustard waste through integrating a comprehensive approach for producing hydrogen and soil amendments. Microb. Cell Factories 2024, 23, 27. [Google Scholar] [CrossRef]
- Bougarne, L.; Ben Abbou, M.; El Haji, M.; Bouka, H. Consequences of surface water eutrophication: Remedy and environmental interest. Mater. Today Proc. 2019, 13, 654–662. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Bernas, J.; Mukosha, C.E.; Hoang, T.N. Environmental Assessment of Dryland and Irrigated Winter Wheat Cultivation under Compost Fertilization Strategies. Plants 2024, 13, 509. [Google Scholar] [CrossRef]
- Yu, P.; Baker, M.C.; Crump, A.R.; Vogler, M.; Strawn, D.G.; Möller, G. Biochar integrated reactive filtration of wastewater for P removal and recovery, micropollutant catalytic oxidation, and negative CO2e: Process operation and mechanism. Water Environ. Res. 2023, 95, e10926. [Google Scholar] [CrossRef]
- Alarefee, H.A.; Ishak, C.F.; Karam, D.S.; Othman, R. Efficiency of Rice Husk Biochar with Poultry Litter Co-Composts in Oxisols for Improving Soil Physico-Chemical Properties and Enhancing Maize Performance. Agronomy 2021, 11, 2409. [Google Scholar] [CrossRef]
- Choulot, M.; Le Guillard, C.; Bourgougnon, N.; Michalak, I. Seaweed-based fertilizing products. In Algae and Aquatic Macro-phytes in Cities; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–313. [Google Scholar]
- Dadson, R.B.; Javaid, I.; Hashem, F.M.; Joshi, J. Potential of fodder soybean genotypes for phosphorus removal in poultry manure-enriched soils. J. Plant Nutr. 2014, 38, 108–115. [Google Scholar] [CrossRef]
- Verdegem, M.C.J. Nutrient discharge from aquaculture operations in function of system design and production environment. Rev. Aquac. 2013, 5, 158–171. [Google Scholar] [CrossRef]
- Trottet, A.; George, C.; Drillet, G.; Lauro, F.M. Aquaculture in coastal urbanized areas: A comparative review of the challenges posed by Harmful Algal Blooms. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2888–2929. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Varela, D.; Pérez-Santos, I.; Díaz-Valdés, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Álvarez, G.; Uribe, E.; Rengel, J.A.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Centro Oceanográfico de A Coruña 2019. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.A. Water column stability and spring bloom dynamics in the Gulf of Alaska. J. Mar. Res. 2007, 65, 715–736. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justić, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Smith, J.; Caron, D.A.; Kudela, R.M.; Loftin, K.; Hayashi, K.; Fadness, R.; Fricke, S.; Kann, J.; Roethler, M.; et al. Integrative monitoring strategy for marine and freshwater harmful algal blooms and toxins across the freshwater-to-marine continuum. Integr. Environ. Assess. Manag. 2023, 19, 586–604. [Google Scholar] [CrossRef]
- Grogan, A.E.; Alves-De-Souza, C.; Cahoon, L.B.; Mallin, M.A. Harmful algal blooms: A prolific issue in urban stormwater ponds. Water 2023, 15, 2436. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Zhang, X.; Shen, Z. Transport process and source contribution of nitrogen in stormwater runoff from urban catchments. Environ. Pollut. 2021, 289, 117824. [Google Scholar] [CrossRef] [PubMed]
- Krimsky, L.S.; Lusk, M.G.; Abeels, H.; Seals, L. Sources and concentrations of nutrients in surface runoff from waterfront homes with different landscape practices. Sci. Total Environ. 2021, 750, 142320. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Y.; Zhang, X.; Shen, Z. Transport and sources of nitrogen in stormwater runoff at the urban catchment scale. Sci. Total Environ. 2022, 806, 150281. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, T. A comparison of sources and risk assessment of polycyclic aromatic hydrocarbons in urban stormwater runoff from ground and highway roads in Shanghai, China. Polycycl. Aromat. Compd. 2020, 40, 670–680. [Google Scholar] [CrossRef]
- Heil, C.A.; Muni-Morgan, A.L. Florida’s harmful algal bloom (HAB) problem: Escalating risks to human, environmental and economic health with climate change. Front. Ecol. Evol. 2021, 9, 646080. [Google Scholar] [CrossRef]
- Morée, A.L.; Beusen, A.H.W.; Bouwman, A.F.; Willems, W.J. Exploring global nitrogen and phosphorus flows in urban wastes during the twentieth century. Glob. Biogeochem. Cycles 2013, 27, 836–846. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Band, L.E.; Walsh, C.J.; Berke, P.E. Understanding, Managing, and Minimizing Urban Impacts on Surface Water Nitrogen Loading. Ann. N. Y. Acad. Sci. 2008, 1134, 61–96. [Google Scholar] [CrossRef]
- Salas, E.A.L.; Subburayalu, S.K. Analysis of phosphorus and nitrogen concentrations in the Great Miami and Little Miami basins in Ohio, USA from 2015 to 2017. River Res. Appl. 2020, 36, 1345–1352. [Google Scholar] [CrossRef]
- Sheibley, R.W.; Foreman, J.R.; Marshall, C.A.; Welch, W.B. Water and Nutrient Budgets for Vancouver Lake, Vancouver, Washington, October 2010–October 2012 (No. 2014-5201); US Geological Survey: Reston, VA, USA, 2014. [Google Scholar]
- Costa, M.L.R.; Henry, R. Phosphorus, nitrogen, and carbon contents of macrophytes in lakes lateral to a tropical river (Paranapanema River, São Paulo, Brazil). Acta Limnol. Bras. 2010, 22, 122–132. [Google Scholar] [CrossRef]
- Silva, M.A.L.; Calasans, C.F.; Ovalle, A.R.C.; Rezende, C.E. Dissolved Nitrogen and Phosphorus Dynamics in the Lower Portion of the Paraiba do Sul River, Campos dos Goytacazes, RJ, Brazil. Braz. Arch. Biol. Technol. 2001, 44, 365–371. [Google Scholar] [CrossRef]
- Torti, M.J.; Portela, S.I.; Andriulo, A.E. Phosphorus and nitrogen fractions during base flow conditions of a Pampean stream and their relationship with land use. Ecol. Austral 2020, 30, 331–343. [Google Scholar] [CrossRef]
- Rivas, Z.; de Medina, H.L.; Gutiérrez, J.; Gutiérrer, E. Nitrogen and phosphorus levels in sediments from tropical Catatumbo river (Venezuela). Water Air Soil Pollut. 2000, 117, 27–37. [Google Scholar] [CrossRef]
- Young, K.; Morse, G.; Scrimshaw, M.; Kinniburgh, J.; MacLeod, C.; Lester, J. The relation between phosphorus and eutrophication in the Thames catchment, UK. Sci. Total Environ. 1999, 228, 157–183. [Google Scholar] [CrossRef]
- Caille, F.; Riera, J.L.; Rosell-Melé, A. Modelling nitrogen and phosphorus loads in a Mediterranean river catchment (La Tordera, NE Spain). Hydrol. Earth Syst. Sci. 2012, 16, 2417–2435. [Google Scholar] [CrossRef]
- Wassen, M.J.; Venterink, H.O. Comparison of nitrogen and phosphorus fluxes in some European fens and floodplains. Appl. Veg. Sci. 2006, 9, 213–222. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M. Water quality assessment of River Nile from Idfo to Cairo. Egypt. J. Aquat. Res. 2005, 31, 200–223. [Google Scholar]
- Oladosu, N.O.; Abayomi, A.A.; Olayinka, K.O.; Alo, B.I. Wet nitrogen and phosphorus deposition in the eutrophication of the Lagos Lagoon, Nigeria. Environ. Sci. Pollut. Res. 2017, 24, 8645–8657. [Google Scholar] [CrossRef]
- Gizaw, E.; Legesse, W.; Haddis, A.; Deboch, B.; Birke, W. Assessment of Factors Contributing to Eutrophication of Aba Samuel Water Reservoir in Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2004, 14, 109–117. [Google Scholar]
- Kpannieu, D.E.; Kouadio, K.N.; M’Bra, I.C.; Hippolyte, C.N.; Coulibaly, L.; Ouffouet, S. Physico-Chemical Pollution and Trophic State of Biétry Bay (Ebrié Lagoon, Abidjan, Ivory Coast). J. Mater. Sci. Chem. Eng. 2022, 10, 87–99. [Google Scholar]
- Kubo, A.; Hashihama, F.; Kanda, J.; Horimoto-Miyazaki, N.; Ishimaru, T. Long-term variability of nutrient and dissolved organic matter concentrations in Tokyo Bay between 1989 and 2015. Limnol. Oceanogr. 2018, 64, S209–S222. [Google Scholar] [CrossRef]
- Liu, C.; Zou, C.; Wang, Q.; Hayashi, Y.; Yasunari, T. Impact assessment of human diet changes with rapid urbanization on regional nitrogen and phosphorus flows—A case study of the megacity Shanghai. Environ. Sci. Pollut. Res. 2013, 21, 1905–1914. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, Z.; Zhang, X.; Wang, X.; Sun, X.; Ballantine, D.J.; Wang, S. Nitrogen pollution and source identification of urban ecosystem surface water in Beijing. Front. Environ. Sci. Eng. 2013, 8, 106–116. [Google Scholar] [CrossRef]
- Yin, K.; Harrison, P.J.; Broom, M.; Chung, C. Ratio of nitrogen to phosphorus in the Pearl River and effects on the estuarine coastal waters: Nutrient management strategy in Hong Kong. Phys. Chem. Earth Parts A/B/C 2011, 36, 411–419. [Google Scholar] [CrossRef]
- Gin, K.Y.-H.; Ramaswamy, U.; Gopalakrishnan, A.P. Comparison of Nutrient Limitation in Freshwater and Estuarine Reservoirs in Tropical Urban Singapore. J. Environ. Eng. 2011, 137, 913–919. [Google Scholar] [CrossRef]
- Mathew, M.M.; Rao, N.S.; Mandla, V.R. Development of regression equation to study the Total Nitrogen, Total Phosphorus and Suspended Sediment using remote sensing data in Gujarat and Maharashtra coast of India. J. Coast. Conserv. 2017, 21, 917–927. [Google Scholar] [CrossRef]
- Harris, G.P. Biogeochemistry of nitrogen and phosphorus in Australian catchments, rivers and estuaries: Effects of land use and flow regulation and comparisons with global patterns. Mar. Freshw. Res. 2001, 52, 139–149. [Google Scholar] [CrossRef]
- Drewry, J.J.; Newham, L.T.H.; Greene, R.S.B.; Jakeman, A.J.; Croke, B.F.W. A review of nitrogen and phosphorus export to waterways: Context for catchment modelling. Mar. Freshw. Res. 2006, 57, 757–774. [Google Scholar] [CrossRef]
- Okus, E.; Ozturk, I.; Sur, H.I.; Yuksek, A.; Tas, S.; Aslan-Yilmaz, A.; Altiok, H.; Balkis, N.; Dogan, E.; Ovez, S.; et al. Critical evaluation of wastewater treatment and disposal strategies for Istanbul with regards to water quality monitoring study results. Desalination 2008, 226, 231–248. [Google Scholar] [CrossRef]
- van der Wulp, S.A.; Damar, A.; Ladwig, N.; Hesse, K.-J. Numerical simulations of river discharges, nutrient flux and nutrient dispersal in Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2016, 110, 675–685. [Google Scholar] [CrossRef]
- Sotto, L.P.A.; Beusen, A.H.W.; Villanoy, C.L.; Bouwman, L.F.; Jacinto, G.S. Nutrient load estimates for Manila Bay, Philippines using population data. Ocean Sci. J. 2015, 50, 467–474. [Google Scholar] [CrossRef]
- Tang, D.; Kawamura, H.; Oh, I.S.; Baker, J. Satellite evidence of harmful algal blooms and related oceanographic features in the Bohai Sea during autumn 1998. Adv. Space Res. 2006, 37, 681–689. [Google Scholar] [CrossRef]
- Niemi, A.; Michel, C.; Hille, K.; Poulin, M. Protist assemblages in winter sea ice: Setting the stage for the spring ice algal bloom. Polar Biol. 2011, 34, 1803–1817. [Google Scholar] [CrossRef]
- Katz, S.L.; Izmest’eva, L.R.; Hampton, S.E.; Ozersky, T.; Shchapov, K.; Moore, M.V.; Shimaraeva, S.V.; Silow, E.A. The “M elosira years” of Lake B aikal: Winter environmental conditions at ice onset predict under-ice algal blooms in spring. Limnol. Oceanogr. 2015, 60, 1950–1964. [Google Scholar] [CrossRef]
- Moncheva, S.; Gotsis-Skretas, O.; Pagou, K.; Krastev, A. Phytoplankton Blooms in Black Sea and Mediterranean Coastal Ecosystems Subjected to Anthropogenic Eutrophication: Similarities and Differences. Estuar. Coast. Shelf Sci. 2001, 53, 281–295. [Google Scholar] [CrossRef]
- Cokacar, T.; Oguz, T.; Kubilay, N. Satellite-detected early summer coccolithophore blooms and their interannual variability in the Black Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1017–1031. [Google Scholar] [CrossRef]
- Janssen, F.; Neumann, T.; Schmidt, M. Inter-annual variability in cyanobacteria blooms in the Baltic Sea controlled by wintertime hydrographic conditions. Mar. Ecol. Prog. Ser. 2004, 275, 59–68. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R.; Kaiser, J.; Wasmund, N.; Savchuk, O. Cyanobacterial blooms in the Baltic Sea: Correlations with environmental factors. Harmful Algae 2020, 92, 101739. [Google Scholar] [CrossRef]
- Love, R.C.; Loder, T.C.; Keafer, B.A. Nutrient conditions during Alexandrium fundyense blooms in the western Gulf of Maine, USA. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2450–2466. [Google Scholar] [CrossRef]
- Yan, D.; Nishioka, J.; Toyota, T.; Suzuki, K. Winter microalgal communities of the southern Sea of Okhotsk: A comparison of sea ice, coastal, and basinal seawater. Prog. Oceanogr. 2022, 204, 102806. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, H.; Song, J.; Li, X.; Duan, L. Hypoxia, acidification and nutrient accumulation in the Yellow Sea Cold Water of the South Yellow Sea. Sci. Total Environ. 2020, 745, 141050. [Google Scholar] [CrossRef]
- Peperzak, L. Climate change and harmful algal blooms in the North Sea. Acta Oecologica 2003, 24, S139–S144. [Google Scholar] [CrossRef]
- Friocourt, Y.; Skogen, M.; Stolte, W.; Albretsen, J. Marine downscaling of a future climate scenario in the North Sea and possible effects on dinoflagellate harmful algal blooms. Food Addit. Contam. Part A 2012, 29, 1630–1646. [Google Scholar] [CrossRef]
- Le Fouest, V.; Zakardjian, B.; Saucier, F.J.; Starr, M. Seasonal versus synoptic variability in planktonic production in a high-latitude marginal sea: The Gulf of St. Lawrence (Canada). J. Geophys. Res. Oceans 2005, 110. [Google Scholar] [CrossRef]
- Imai, I.; Yamaguchi, M.; Hori, Y. Eutrophication and occurrences of harmful algal blooms in the Seto Inland Sea, Japan. Plankton Benthos Res. 2006, 1, 71–84. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Global inequalities in CO2 emissions. Our World Data. 2023. Available online: https://ourworldindata.org/inequality-co2?ref=hackernoon.com (accessed on 28 August 2024).
- Nguyen, X.P.; Hoang, A.T.; Ölçer, A.I.; Huynh, T.T. Record decline in global CO2 emissions prompted by COVID-19 pandemic and its implications on future climate change policies. Energy Sources Part A Recover. Util. Environ. Eff. 2021, 1–4. [Google Scholar] [CrossRef]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Change 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Asefi, M.A.; Attaran-Fariman, G. An Overview of the Impact of Climate Change and Acidification of the Oceans on the Growth and Bloom of Marine Algae with Emphasis on Harmful Algae Blooms (HABs). J. Persian Gulf 2018, 9, 7–17. [Google Scholar]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Meng, R.; Du, X.; Ge, K.; Wu, C.; Zhang, Z.; Liang, X.; Yang, J.; Zhang, H. Does climate change increase the risk of marine toxins? Insights from changing seawater conditions. Arch. Toxicol. 2024, 98, 2743–2762. [Google Scholar] [CrossRef]
- Fricke, A.; Pey, A.; Gianni, F.; Lemée, R.; Mangialajo, L. Multiple stressors and benthic harmful algal blooms (BHABs): Potential effects of temperature rise and nutrient enrichment. Mar. Pollut. Bull. 2018, 131, 552–564. [Google Scholar] [CrossRef]
- Ahn, S.H.; Glibert, P.M.; Heil, C.A. In hot water: Interactions of temperature, nitrogen form and availability and photosynthetic and nitrogen uptake responses in natural Karenia brevis populations. Harmful Algae 2023, 129, 102519. [Google Scholar] [CrossRef]
- Ahn, S.H.; Glibert, P.M. Temperature-Dependent Mixotrophy in Natural Populations of the Toxic Dinoflagellate Karenia brevis. Water 2024, 16, 1555. [Google Scholar] [CrossRef]
- Errera, R.; Yvon-Lewis, S.; Kessler, J.; Campbell, L. Reponses of the dinoflagellate Karenia brevis to climate change: pCO2 and sea surface temperatures. Harmful Algae 2014, 37, 110–116. [Google Scholar] [CrossRef]
- Alonso-Rodríguez, R.; Pichardo-Velarde, J.G. Effects of temperature and nutrients on growth and toxicity of Alexandrium affine from southeastern Gulf of California. Mar. Pollut. Bull. 2024, 203, 116464. [Google Scholar] [CrossRef]
- Rial, P.; Sixto, M.; Vázquez, J.; Reguera, B.; Figueroa, R.; Riobó, P.; Rodríguez, F. Interaction between temperature and salinity stress on the physiology of Dinophysis spp. and Alexandrium minutum: Implications for niche range and blooming patterns. Aquat. Microb. Ecol. 2023, 89, 1–22. [Google Scholar] [CrossRef]
- Singh, A.; Hårding, K.; Reddy, H.; Godhe, A. An assessment of Dinophysis blooms in the coastal Arabian Sea. Harmful Algae 2014, 34, 29–35. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Kikuchi, T. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of temperature, light intensity and photoperiod. Harmful Algae 2008, 7, 163–173. [Google Scholar] [CrossRef]
- Meeson, B.W.; Sweeney, B.M. Adaptation of Ceratium furca and Gonyaulax polyedra (Dinophyceace) to Different Temperatures and Irradiances: Growth Rates and Cell Volumes. J. Phycol. 1982, 18, 241–245. [Google Scholar] [CrossRef]
- Lundholm, N.; Clarke, A.; Ellegaard, M. A 100-year record of changing Pseudo-nitzschia species in a sill-fjord in Denmark related to nitrogen loading and temperature. Harmful Algae 2010, 9, 449–457. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.M.; Court, M.; Seco, M.C.; Borges, F.O.; Vicente, B.; Lage, S.; Braga, A.C.; Duarte, B.; Santos, C.F.; Amorim, A.; et al. Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves. Toxins 2023, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J.; López-Cortés, D.J. Effect of temperature on growth and paralytic toxin profiles in isolates of Gymnodinium catenatum (Dinophyceae) from the Pacific coast of Mexico. Toxicon 2014, 90, 199–212. [Google Scholar] [CrossRef]
- Griffith, A.; Gobler, C. Temperature controls the toxicity of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides. Mar. Ecol. Prog. Ser. 2016, 545, 63–76. [Google Scholar] [CrossRef]
- Tyrell, A.; Jiang, H.; Fisher, N. Effects of seawater viscosity and temperature on the movement of the marine dinoflagellate Prorocentrum minimum. Aquat. Microb. Ecol. 2021, 86, 21–28. [Google Scholar] [CrossRef]
- Aquino-Cruz, A.; Purdie, D.A.; Morris, S. Effect of increasing sea water temperature on the growth and toxin production of the benthic dinoflagellate Prorocentrum lima. Hydrobiologia 2018, 813, 103–122. [Google Scholar] [CrossRef]
- Matsubara, T.; Nagasoe, S.; Yamasaki, Y.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. J. Exp. Mar. Biol. Ecol. 2007, 342, 226–230. [Google Scholar] [CrossRef]
- Ou, G.; Wang, H.; Si, R.; Guan, W. The dinoflagellate Akashiwo sanguinea will benefit from future climate change: The interactive effects of ocean acidification, warming and high irradiance on photophysiology and hemolytic activity. Harmful Algae 2017, 68, 118–127. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, M.; Liang, Y.; Lu, S. Effects of temperature and organic and inorganic nutrients on the growth of Chattonella marina (Raphidophyceae) from the Daya Bay, South China Sea. Acta Oceanol. Sin. 2011, 30, 124–131. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Mizushima, K.; Sakamoto, S.; Yamaguchi, M. Effects of temperature, salinity and irradiance on growth of the novel red tide flagellate Chattonella ovata (Raphidophyceae). Harmful Algae 2010, 9, 398–401. [Google Scholar] [CrossRef]
- Griffith, A.W.; Doherty, O.M.; Gobler, C.J. Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190340. [Google Scholar] [CrossRef] [PubMed]
- Terenko, G.; Krakhmalnyi, A. Red tide of the Lingulodinium polyedrum (Dinophyceae) in Odessa Bay (Black Sea). Turk. J. Fish. Aquat. Sci. 2021, 22, 4–10. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Shang, L.; Chai, Z.; Tang, Y.Z. Transcriptional Responses of the Heat Shock Protein 20 (Hsp20) and 40 (Hsp40) Genes to Temperature Stress and Alteration of Life Cycle Stages in the Harmful Alga Scrippsiella trochoidea (Dinophyceae). Biology 2020, 9, 408. [Google Scholar] [CrossRef]
- Tawong, W.; Yoshimatsu, T.; Yamaguchi, H.; Adachi, M. Effects of temperature, salinity and their interaction on growth of benthic dinoflagellates Ostreopsis spp. from Thailand. Harmful Algae 2015, 44, 37–45. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge 1. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Raven, J.A.; Gobler, C.J.; Hansen, P.J. Dynamic CO2 and pH levels in coastal, estuarine, and inland waters: Theoretical and observed effects on harmful algal blooms. Harmful Algae 2020, 91, 101594. [Google Scholar] [CrossRef]
- Lee, C.H.; Min, J.; Lee, H.-G.; Kim, K.Y. Thermal plasticity of growth and chain formation of the dinoflagellates Alexandrium affine and Alexandrium pacificum with respect to ocean acidification. Algae 2021, 36, 285–298. [Google Scholar] [CrossRef]
- Charrieau, L.M.; Nagai, Y.; Kimoto, K.; Dissard, D.; Below, B.; Fujita, K.; Toyofuku, T. The coral reef-dwelling Peneroplis spp. shows calcification recovery to ocean acidification conditions. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, G.; Brennan, G.; Wang, Z.; Jiang, T.; Sun, K.; Fan, X.; Bowler, C.; Zhang, X.; Zhang, Y.; et al. Plastic responses lead to increased neurotoxin production in the diatom Pseudo-nitzschia under ocean warming and acidification. ISME J. 2023, 17, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Ayache, N.; Lundholm, N.; Gai, F.; Hervé, F.; Amzil, Z.; Caruana, A. Impacts of ocean acidification on growth and toxin content of the marine diatoms Pseudo-nitzschia australis and P. fraudulenta. Mar. Environ. Res. 2021, 169, 105380. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Liu, Q.; Liu, S.; Zhu, Y.; Yao, J.; Wang, H.; Guan, W. The responses of harmful dinoflagellate Karenia mikimotoi to simulated ocean acidification at the transcriptional level. Harmful Algae 2022, 111, 102167. [Google Scholar] [CrossRef]
- Wang, H.; Niu, X.; Feng, X.; Gonçalves, R.J.; Guan, W. Effects of ocean acidification and phosphate limitation on physiology and toxicity of the dinoflagellate Karenia mikimotoi. Harmful Algae 2019, 87, 101621. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Darius, H.T.; Hess, P.; Chinain, M. Effects of pH and Nutrients (Nitrogen) on Growth and Toxin Profile of the Ciguatera-Causing Dinoflagellate Gambierdiscus polynesiensis (Dinophyceae). Toxins 2020, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Zepernick, B.N.; Gann, E.R.; Martin, R.M.; Pound, H.L.; Krausfeldt, L.E.; Chaffin, J.D.; Wilhelm, S.W. Elevated pH Conditions Associated with Microcystis spp. Blooms Decrease Viability of the Cultured Diatom Fragilaria crotonensis and Natural Diatoms in Lake Erie. Front. Microbiol. 2021, 12, 598736. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. Availability of Iron to the Marine Cyanobacterium Lyngbya Majuscula; UNSW Sydney: Kensington, Australia, 2005. [Google Scholar]
- Watkinson, A.; O’neil, J.; Dennison, W. Ecophysiology of the marine cyanobacterium, Lyngbya majuscula (Oscillatoriaceae) in Moreton Bay, Australia. Harmful Algae 2005, 4, 697–715. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Bedford, B.J.; Baumberger, R. Hurricanes Frances and Jeanne remove blooms of the invasive green algaCaulerpa brachypus formaparvifolia (Harvey) cribb from coral reefs off Northern Palm Beach County, Florida. Estuaries Coasts 2006, 29, 966–971. [Google Scholar] [CrossRef]
- Glibert, P.M.; Berdalet, E.; Burford, M.A.; Pitcher, G.C.; Zhou, M. Harmful Algal Blooms and the Importance of Understanding Their Ecology and Oceanography. In Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Rosa, M.; Holohan, B.; Shumway, S.; Bullard, S.; Wikfors, G.; Morton, S.; Getchis, T. Biofouling ascidians on aquaculture gear as potential vectors of harmful algal introductions. Harmful Algae 2013, 23, 1–7. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Waack, J.; Turner, A.D.; Lewis, A.M.; Lawton, L.A.; Edwards, C. Rapid uptake and slow depuration: Health risks following cyanotoxin accumulation in mussels? Environ. Pollut. 2021, 271, 116400. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Louw, D.C. Harmful algal blooms of the Benguela eastern boundary upwelling system. Harmful Algae 2021, 102, 101898. [Google Scholar] [CrossRef]
- Perez, M.S. Understanding Environmental and Anthropogenic Factors Affecting Dinoflagellate Communities in the Black and Caspian Seas. Ph.D. Thesis, University of Bristol, Bristol, UK, 2021. [Google Scholar]
- Benitt, C.; Young, C.S.; Sylvers, L.H.; Gobler, C.J. Inhibition of harmful algal blooms caused by Aureococcus anophagefferens (Pelagophyceae) using native (Gracilaria tikvahiae) and invasive (Dasysiphonia japonica) red seaweeds from North America. J. Appl. Phycol. 2022, 34, 965–983. [Google Scholar] [CrossRef]
- Wallentinus, I. Introduced marine algae and vascular plants in European aquatic environments. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Springer: Berlin/Heidelberg, Germany, 2002; pp. 27–52. [Google Scholar]
- Gauff, R. Diversity and Functioning of Harbor Communities. Influence on Non Indigenous Species in a Climate Change Driven Context. Ph.D. Thesis, Sorbonne Université, Paris, France, 2021. [Google Scholar]
- Neves, R.A.F.; Nascimento, S.M.; Santos, L.N. Harmful algal blooms and shellfish in the marine environment: An overview of the main molluscan responses, toxin dynamics, and risks for human health. Environ. Sci. Pollut. Res. 2021, 28, 55846–55868. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ge, Z.; Li, C.; Wan, F.; Xiao, X. Inhibition of harmful algae Phaeocystis globosa and Prorocentrum donghaiense by extracts of coastal invasive plant Spartina alterniflora. Sci. Total Environ. 2019, 696, 133930. [Google Scholar] [CrossRef]
- Soliño, L.; Widgy, S.; Pautonnier, A.; Turquet, J.; Loeffler, C.R.; Quintana, H.A.F.; Diogène, J. Prevalence of ciguatoxins in lionfish (Pterois spp.) from Guadeloupe, Saint Martin, and Saint Barthélmy Islands (Caribbean). Toxicon 2015, 102, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Straquadine, N.R.; Kudela, R.M.; Gobler, C.J. Hepatotoxic shellfish poisoning: Accumulation of microcystins in Eastern oysters (Crassostrea virginica) and Asian clams (Corbicula fluminea) exposed to wild and cultured populations of the harmful cyanobacteria, Microcystis. Harmful Algae 2022, 115, 102236. [Google Scholar] [CrossRef]
- Grima, M. How One of the World’s Worst Invasive Species, Carcinus maenas, Fares in Shinnecock Bay, New York. Master’s Thesis, State University of New York at Stony Brook, New York, NY, USA, 2022. [Google Scholar]
- Olenin, S.; Gollasch, S.; Lehtiniemi, M.; Sapota, M.; Zaiko, A. Biological Oceanography of the Baltic Sea; Springer Science & Business Media: Berlin, Germany, 2017; p. 193. [Google Scholar]
- Hiebenthal, C. Forschungskooperation: Konzept zum Monitoring der Entwicklung von Flachwas-ser-Hartbodengemeinschaften in der s.-h. Ostsee. [Aktenzeichen 0608.451722]-Endbericht-[Projektteile 1 und 2]. 2021. Available online: https://oceanrep.geomar.de/id/eprint/60405/1/Hiebenthal%202021_Hartbodenbiodiversit%C3%A4t_LLUR-Endbericht_2021%2011%2030.pdf (accessed on 28 August 2024).
- Schuhmann, P.W.; Irvine, J.; Oxenford, H.A.; Degia, A.K.; Valderrama, J.P. The Potential Economic Impacts of Sargassum in-Undations in the Caribbean, Part 1: Insights from the Literature; Final Draft; Centre for Resource Management and Environmental Studies, University of the West Indies: Cave Hill, Barbados, 2022; 57p. [Google Scholar]
- Mach, M.E. Research on Marine Coastal Impacts to Promote Ecosystem-Based Management: Nonnative Species in Northeast Pacific Estuaries. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2012. [Google Scholar]
- Murray, S.N.; Fernandez, L. Status, Environmental Threats, and Policy Considerations for Invasive Seaweeds for the Pacific Coast of North America. 2005. Available online: https://repository.library.noaa.gov/view/noaa/45831 (accessed on 28 August 2024).
- Xu, Z.L.; Shen, X.M.; Gao, Q. Marine Biology of the Changjiang (Yangtze River) Estuary and Adjacent East China Sea Shelf. In Ecological Continuum from the Changjiang (Yangtze River) Watersheds to the East China Sea Continental Margin; Estuaries of the World; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Whitfield, A.K.; James, N.C.; Lamberth, S.J.; Adams, J.B.; Perissinotto, R.; Rajkaran, A.; Bornman, T.G. The role of pioneers as indicators of biogeographic range expansion caused by global change in southern African coastal waters. Estuar. Coast. Shelf Sci. 2016, 172, 138–153. [Google Scholar] [CrossRef]
- Palinkas, C.; Mistri, M.; Staver, L.; Lipej, L.; Kružić, P.; Stevenson, J.C.; Tamburri, M.; Munari, C.; Orlando-Bonaca, M. Status of Critical Habitats and Invasive Species. Geophys. Monogr. 2020, 177–202. [Google Scholar] [CrossRef]
- Pederson, J.; Mieszkowska, N.; Carlton, J.T.; Gollasch, S.; Jelmert, A.; Minchin, D.; Occhipinti-Ambrogi, A.; Wallentinus, I. 11 Climate Change and Non-Native Species in the North Atlantic. 2011. Available online: https://livrepository.liverpool.ac.uk/id/eprint/3002502 (accessed on 28 August 2024).
- Inglis, G.; Gust, N.; Fitridge, I.; Floerl, O.; Woods, C.; Hayden, B.; Fenwick, G. Port of Wellington. In Baseline Survey for Non-indigenous Marine Species; Biosecurity New Zealand Technical Paper; 2006; Available online: https://webstatic.niwa.co.nz/static/marine-biosecurity/Inglis%20et%20al%202005_09%20PortOfWellington.pdf (accessed on 28 August 2024).
- Nakazwe, M. Comparing the Impact of Artificial Oyster Reefs and Oyster Farms on Wild Oyster Restoration, Aquatic Species Diversity, and Water Quality Enhancement in Rehoboth Bay, Delaware. Master’s Thesis, Delaware State University, Dover, DE, USA, 2021. [Google Scholar]
- Olenin, S.; Minchin, D.; Daunys, D. Assessment of biopollution in aquatic ecosystems. Mar. Pollut. Bull. 2007, 55, 379–394. [Google Scholar] [CrossRef]
- Rolton, A.; Rhodes, L.; Hutson, K.S.; Biessy, L.; Bui, T.; MacKenzie, L.; Symonds, J.E.; Smith, K.F. Effects of Harmful Algal Blooms on Fish and Shellfish Species: A Case Study of New Zealand in a Changing Environment. Toxins 2022, 14, 341. [Google Scholar] [CrossRef]
- Magaña, H.A.; Contreras, C.; Villareal, T.A. A historical assessment of Karenia brevis in the western Gulf of Mexico. Harmful Algae 2003, 2, 163–171. [Google Scholar] [CrossRef]
- Lu, D.; Goebel, J.; Qi, Y.; Zou, J.; Han, X.; Gao, Y.; Li, Y. Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species. Harmful Algae 2005, 4, 493–505. [Google Scholar] [CrossRef]
- Pechsiri, J.S.; Risén, E.; Malmström, M.E.; Brandt, N.; Gröndahl, F. Harvesting of Nodularia spumigena in the Baltic Sea: As-sessment of potentials and added benefits. J. Coast. Res. 2014, 30, 825–831. [Google Scholar] [CrossRef]
- Bravo, I.; Vila, M.; Masó, M.; Figueroa, R.I.; Ramilo, I. Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: Toward the identification of ecological niches. Harmful Algae 2008, 7, 515–522. [Google Scholar] [CrossRef]
- Davis, T.W.; Watson, S.B.; Rozmarynowycz, M.J.; Ciborowski, J.J.H.; McKay, R.M.; Bullerjahn, G.S. Phylogenies of Microcystin-Producing Cyanobacteria in the Lower Laurentian Great Lakes Suggest Extensive Genetic Connectivity. PLoS ONE 2014, 9, e106093. [Google Scholar] [CrossRef] [PubMed]
- Vershinin, A.O.; Moruchkov, A.A.; Leighfield, T.; Sukhanova, I.N.; Pan’kov, S.L.; Morton, S.L.; Ramsdell, J.S. Potentially toxic algae in the coastal phytoplankton of the northeast Black Sea in 2001–2002. Oceanology 2005, 45, 224–232. [Google Scholar]
- Wang, C.; Xu, Y.; Gu, H.; Luo, Z.; Luo, Z. Expansion risk of the toxic dinoflagellate Gymnodinium catenatum blooms in Chinese waters under climate change. Ecol. Inform. 2023, 75, 102042. [Google Scholar] [CrossRef]
- Tango, P.; Butler, W.; Lacouture, R.; Goshorn, D.; Magnien, R.; Michael, B.; Hall, S.; Browhawn, K.; Wittman, R.; Beatty, W. An unprecedented bloom of Dinophysis acuminata in Chesapeake Bay. Harmful Algae 2002, 358–360. [Google Scholar]
- Al-Azri, A.R.; Piontkovski, S.A.; Al-Hashmi, K.A.; Goes, J.I.; Gomes, H.D.R.; Glibert, P.M. Mesoscale and Nutrient Conditions Associated with the Massive 2008 Cochlodinium polykrikoides Bloom in the Sea of Oman/Arabian Gulf. Estuaries Coasts 2013, 37, 325–338. [Google Scholar] [CrossRef]
- Dela-Cruz, J.; Middleton, J.H.; Suthers, I.M. Population growth and transport of the red tide dinoflagellate, Noctiluca scintillans, in the coastal waters off Sydney Australia, using cell diameter as a tracer. Limnol. Oceanogr. 2003, 48, 656–674. [Google Scholar] [CrossRef]
- Kenitz, K.M.; Anderson, C.R.; Carter, M.L.; Eggleston, E.; Seech, K.; Shipe, R.; Smith, J.; Orenstein, E.C.; Franks, P.J.S.; Jaffe, J.S.; et al. Environmental and ecological drivers of harmful algal blooms revealed by automated underwater microscopy. Limnol. Oceanogr. 2023, 68, 598–615. [Google Scholar] [CrossRef]
- Medlin, L.K.; Orozco, J. Molecular Techniques for the Detection of Organisms in Aquatic Environments, with Emphasis on Harmful Algal Bloom Species. Sensors 2017, 17, 1184. [Google Scholar] [CrossRef]
- Toldrà, A.; O’Sullivan, C.K.; Diogène, J.; Campàs, M. Detecting harmful algal blooms with nucleic acid amplification-based biotechnological tools. Sci. Total Environ. 2020, 749, 141605. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.A.; D’Agostino, P.M.; Neilan, B.A. Recent developments in quantitative PCR for monitoring harmful marine microalgae. Harmful Algae 2021, 108, 102096. [Google Scholar] [CrossRef]
- Wang, K.; Mou, X.; Cao, H.; Struewing, I.; Allen, J.; Lu, J. Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environ. Pollut. 2021, 288, 117682. [Google Scholar] [CrossRef] [PubMed]
- Whale, A.S.; Huggett, J.F.; Cowen, S.; Speirs, V.; Shaw, J.; Ellison, S.; Foy, C.A.; Scott, D.J. Comparison of microfluidic digital PCR and conventional quantitative PCR for measuring copy number variation. Nucleic Acids Res. 2012, 40, e82. [Google Scholar] [CrossRef]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Zielinski, O.; Busch, J.A.; Cembella, A.D.; Daly, K.L.; Engelbrektsson, J.; Hannides, A.K.; Schmidt, H. Detecting marine hazardous substances and organisms: Sensors for pollutants, toxins, and pathogens. Ocean Sci. 2009, 5, 329–349. [Google Scholar] [CrossRef]
- Cooper, E.D.; Bentlage, B.; Gibbons, T.R.; Bachvaroff, T.R.; Delwiche, C.F. Metatranscriptome profiling of a harmful algal bloom. Harmful Algae 2014, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yancey, C.E.; Smith, D.J.; Uyl, P.A.D.; Mohamed, O.G.; Yu, F.; Ruberg, S.A.; Chaffin, J.D.; Goodwin, K.D.; Tripathi, A.; Sherman, D.H.; et al. Metagenomic and Metatranscriptomic Insights into Population Diversity of Microcystis Blooms: Spatial and Temporal Dynamics of mcy Genotypes, Including a Partial Operon That Can Be Abundant and Expressed. Appl. Environ. Microbiol. 2022, 88, e0246421. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Cai, Z.; Lao, Y.; Jin, H.; Yu, K.; Zhang, B.; Zhou, J. Profiles of quorum sensing (QS)-related sequences in phycospheric microorganisms during a marine dinoflagellate bloom, as determined by a metagenomic approach. Microbiol. Res. 2018, 217, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, F.M.; Lidie, K.B.; Monroe, E.A.; Bhattacharya, D.; Campbell, L.; Doucette, G.J.; Kamykowski, D. The Florida red tide dinoflagellate Karenia brevis: New insights into cellular and molecular processes underlying bloom dynamics. Harmful Algae 2009, 8, 562–572. [Google Scholar] [CrossRef]
- López-Legentil, S.; Song, B.; DeTure, M.; Baden, D.G. Characterization and Localization of a Hybrid Non-ribosomal Peptide Synthetase and Polyketide Synthase Gene from the Toxic Dinoflagellate Karenia brevis. Mar. Biotechnol. 2009, 12, 32–41. [Google Scholar] [CrossRef]
- Hwang, J.; Kang, H.W.; Moon, S.J.; Hyung, J.-H.; Lee, E.S.; Park, J. Metagenomic Analysis of the Species Composition and Seasonal Distribution of Marine Dinoflagellate Communities in Four Korean Coastal Regions. Microorganisms 2022, 10, 1459. [Google Scholar] [CrossRef]
- Shin, H.; Lee, E.; Shin, J.; Ko, S.-R.; Oh, H.-S.; Ahn, C.-Y.; Oh, H.-M.; Cho, B.-K.; Cho, S. Elucidation of the bacterial communities associated with the harmful microalgae Alexandrium tamarense and Cochlodinium polykrikoides using nanopore sequencing. Sci. Rep. 2018, 8, 5323. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Li, F.; Wang, Y.; Zhu, Y.; Kang, H.; Wang, S.; Qin, S. Metagenome of microorganisms associated with the toxic Cyanobacteria Microcystis aeruginosa analyzed using the 454 sequencing platform. Chin. J. Oceanol. Limnol. 2011, 29, 505–513. [Google Scholar] [CrossRef]
- Kenkel, C.D.; Smith, J.; Hubbard, K.A.; Chadwick, C.; Lorenzen, N.; Tatters, A.O.; Caron, D.A. Reduced representation sequencing accurately quantifies relative abundance and reveals population-level variation in Pseudo-nitzschia spp. Harmful Algae 2022, 118, 102314. [Google Scholar] [CrossRef] [PubMed]
- Rambo, I.M.; Dombrowski, N.; Constant, L.; Erdner, D.; Baker, B.J. Metabolic relationships of uncultured bacteria associated with the microalgae Gambierdiscus. Environ. Microbiol. 2019, 22, 1764–1783. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, J.S.; Jung, S.W.; Kim, H.; Joo, H.M.; Kang, D.; Seo, H.; Kim, S.; Jang, M.; Lee, K.; et al. Zooming on dynamics of marine microbial communities in the phycosphere of Akashiwo sanguinea (Dinophyta) blooms. Mol. Ecol. 2020, 30, 207–221. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.-B.; Wu, P.-F.; Zhang, S.-F.; Xie, Z.-X.; Li, D.-X.; Lin, L.; Chen, F.; Wang, D.-Z. Functional Differences in the Blooming Phytoplankton Heterosigma akashiwo and Prorocentrum donghaiense Revealed by Comparative Metaproteomics. Appl. Environ. Microbiol. 2019, 85, e01425-19. [Google Scholar] [CrossRef]
- Shang, L.; Hu, Z.; Deng, Y.; Liu, Y.; Zhai, X.; Chai, Z.; Liu, X.; Zhan, Z.; Dobbs, F.C.; Tang, Y.Z. Metagenomic Sequencing Identifies Highly Diverse Assemblages of Dinoflagellate Cysts in Sediments from Ships’ Ballast Tanks. Microorganisms 2019, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xu, H.; Guo, X. Satellite Remote Sensing of Harmful Algal Blooms (HABs) and a Potential Synthesized Framework. Sensors 2012, 12, 7778–7803. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.; Salehi, B.; Mahdianpari, M.; Mohammadimanesh, F.; Mountrakis, G.; Quackenbush, L.J. A Meta-Analysis on Harmful Algal Bloom (HAB) Detection and Monitoring: A Remote Sensing Perspective. Remote Sens. 2021, 13, 4347. [Google Scholar] [CrossRef]
- Liu, S.; Glamore, W.; Tamburic, B.; Morrow, A.; Johnson, F. Remote sensing to detect harmful algal blooms in inland waterbodies. Sci. Total Environ. 2022, 851, 158096. [Google Scholar] [CrossRef]
- Tomlinson, M.; Wynne, T.; Stumpf, R. An evaluation of remote sensing techniques for enhanced detection of the toxic dinoflagellate, Karenia brevis. Remote Sens. Environ. 2009, 113, 598–609. [Google Scholar] [CrossRef]
- Brando, V.E.; Sammartino, M.; Colella, S.; Bracaglia, M.; Di Cicco, A.; D’alimonte, D.; Kajiyama, T.; Kaitala, S.; Attila, J. Phytoplankton Bloom Dynamics in the Baltic Sea Using a Consistently Reprocessed Time Series of Multi-Sensor Reflectance and Novel Chlorophyll-a Retrievals. Remote Sens. 2021, 13, 3071. [Google Scholar] [CrossRef]
- Torres Palenzuela, J.M.; González Vilas, L.; Bellas, F.M.; Garet, E.; González-Fernández, Á.; Spyrakos, E. Pseudo-nitzschia Blooms in a Coastal Upwelling System: Remote Sensing Detection, Toxicity and Environmental Variables. Water 2019, 11, 1954. [Google Scholar] [CrossRef]
- Cannizzaro, J.P.; Carder, K.L.; Chen, F.R.; Heil, C.A.; Vargo, G.A. A novel technique for detection of the toxic dinoflagellate, Karenia brevis, in the Gulf of Mexico from remotely sensed ocean color data. Cont. Shelf Res. 2008, 28, 137–158. [Google Scholar] [CrossRef]
- Sayers, M.; Fahnenstiel, G.L.; Shuchman, R.A.; Whitley, M. Cyanobacteria blooms in three eutrophic basins of the Great Lakes: A comparative analysis using satellite remote sensing. Int. J. Remote Sens. 2016, 37, 4148–4171. [Google Scholar] [CrossRef]
- Shen, F.; Tang, R.; Sun, X.; Liu, D. Simple methods for satellite identification of algal blooms and species using 10-year time series data from the East China Sea. Remote Sens. Environ. 2019, 235, 111484. [Google Scholar] [CrossRef]
- Wolny, J.L.; Tomlinson, M.C.; Uz, S.S.; Egerton, T.A.; McKay, J.R.; Meredith, A.; Reece, K.S.; Scott, G.P.; Stumpf, R.P. Current and Future Remote Sensing of Harmful Algal Blooms in the Chesapeake Bay to Support the Shellfish Industry. Front. Mar. Sci. 2020, 7, 337. [Google Scholar] [CrossRef]
- El Hourany, R.; Saab, M.A.; Faour, G.; Mejia, C.; Crépon, M.; Thiria, S. Phytoplankton Diversity in the Mediterranean Sea From Satellite Data Using Self-Organizing Maps. J. Geophys. Res. Oceans 2019, 124, 5827–5843. [Google Scholar] [CrossRef]
- Polikarpov, I.; Al-Yamani, F.; Petrov, P.; Saburova, M.; Mihalkov, V.; Al-Enezi, A. Phytoplankton bloom detection during the COVID-19 lockdown with remote sensing data: Using Copernicus Sentinel-3 for north-western Arabian/Persian Gulf case study. Mar. Pollut. Bull. 2021, 171, 112734. [Google Scholar] [CrossRef] [PubMed]
- Kahru, M.; Elmgren, R. Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences 2014, 11, 3619–3633. [Google Scholar] [CrossRef]
- Jordan, C.; Cusack, C.; Tomlinson, M.C.; Meredith, A.; McGeady, R.; Salas, R.; Gregory, C.; Croot, P.L. Using the Red Band Difference Algorithm to Detect and Monitor a Karenia spp. Bloom Off the South Coast of Ireland, June 2019. Front. Mar. Sci. 2021, 8, 638889. [Google Scholar] [CrossRef]
- Thyssen, M.; Alvain, S.; Lefèbvre, A.; Dessailly, D.; Rijkeboer, M.; Guiselin, N.; Creach, V.; Artigas, L.-F. High-resolution analysis of a North Sea phytoplankton community structure based on in situ flow cytometry observations and potential implication for remote sensing. Biogeosciences 2015, 12, 4051–4066. [Google Scholar] [CrossRef]
- Bucci, A.F. Remotely Sensed Assessment of the Preferred Habitat of Alexandrium catenella in the Gulf of Maine and the Bay of Fundy; The University of Maine: Orono, ME, USA, 2022. [Google Scholar]
- Kopelevich, O.V.; Burenkov, V.I.; Sheberstov, S.V. Case studies of optical remote sensing in the Barents Sea, Black Sea and Caspian Sea. In Remote Sensing of the European Seas; Springer: Berlin/Heidelberg, Germany, 2008; pp. 53–66. [Google Scholar]
- Hill, P.R.; Kumar, A.; Temimi, M.; Bull, D.R. HABNet: Machine Learning, Remote Sensing-Based Detection of Harmful Algal Blooms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 3229–3239. [Google Scholar] [CrossRef]
- Gernez, P.; Zoffoli, M.L.; Lacour, T.; Fariñas, T.H.; Navarro, G.; Caballero, I.; Harmel, T. The many shades of red tides: Sentinel-2 optical types of highly-concentrated harmful algal blooms. Remote Sens. Environ. 2023, 287, 113486. [Google Scholar] [CrossRef]
- Johansen, R.A.; Reif, M.K.; Saltus, C.L.; Pokrzywinski, K.L. A Review of Empirical Algorithms for the Detection and Quantification of Harmful Algal Blooms Using Satellite-Borne Remote Sensing. 2022. Available online: https://erdc-library.erdc.dren.mil/bitstream/11681/44523/1/ERDC-EL%20SR-22-2.pdf (accessed on 28 August 2024).
- Zhao, Y.; Liu, D.; Wei, X. Monitoring cyanobacterial harmful algal blooms at high spatiotemporal resolution by fusing Landsat and MODIS imagery. Environ. Adv. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Wijata, A.M.; Foulon, M.-F.; Bobichon, Y.; Vitulli, R.; Celesti, M.; Camarero, R.; Di Cosimo, G.; Gascon, F.; Longépé, N.; Nieke, J.; et al. Taking Artificial Intelligence Into Space Through Objective Selection of Hyperspectral Earth Observation Applications: To bring the “brain” close to the “eyes” of satellite missions. IEEE Geosci. Remote Sens. Mag. 2023, 11, 10–39. [Google Scholar] [CrossRef]
- Cheng, K.; Chan, S.; Lee, J.H. Remote sensing of coastal algal blooms using unmanned aerial vehicles (UAVs). Mar. Pollut. Bull. 2020, 152, 110889. [Google Scholar] [CrossRef]
- Olivetti, D.; Cicerelli, R.; Martinez, J.-M.; Almeida, T.; Casari, R.; Borges, H.; Roig, H. Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming. Drones 2023, 7, 410. [Google Scholar] [CrossRef]
- Glibert, P.M.; Pitcher, G.C.; Bernard, S.; Li, M. Advancements and Continuing Challenges of Emerging Technologies and Tools for Detecting Harmful Algal Blooms, Their Antecedent Conditions and Toxins, and Applications in Predictive Models. In Global Ecology and Oceanography of Harmful Algal Blooms; Ecological Studies; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Roesler, C.; Uitz, J.; Claustre, H.; Boss, E.; Xing, X.; Organelli, E.; Briggs, N.; Bricaud, A.; Schmechtig, C.; Poteau, A.; et al. Recommendations for obtaining unbiased chlorophyll estimates from in situ chlorophyll fluorometers: A global analysis of WET Labs ECO sensors. Limnol. Oceanogr. Methods 2017, 15, 572–585. [Google Scholar] [CrossRef]
- Ove Moller, K.; Petersen, W.; Nair, R. Report on Best Practice in the Utilization of Sensors Used for Measuring Nutrients, Biology Related Optical Properties, Variables of the Marine Carbonate System, and for Coastal Profiling; IFREMER: Brest, France, 2019. [Google Scholar]
- Yoshida, M.; Shimasaki, Y.; Inokuchi, D.; Nakazato, A.; Otake, S.; Qiu, X.; Mukai, K.; Foloni-Neto, H.; Kato, H.; Honda, S.; et al. A New Fluorometer to Detect Harmful Algal Bloom Species and its Application as a Long–Term HABs Monitoring Tool. J. Fac. Agric. Kyushu Univ. 2021, 66, 37–43. [Google Scholar] [CrossRef]
- Glibert, P.M.; Kelly, V.; Alexander, J.; Codispoti, L.A.; Boicourt, W.C.; Trice, T.M.; Michael, B. In situ nutrient monitoring: A tool for capturing nutrient variability and the antecedent conditions that support algal blooms. Harmful Algae 2008, 8, 175–181. [Google Scholar] [CrossRef]
- Burton, L.; Jayachandran, K.; Bhansali, S. The “Real-Time” revolution for in situ soil nutrient sensing. J. Electrochem. Soc. 2020, 167, 037569. [Google Scholar] [CrossRef]
- Shapiro, J.; Dixon, L.K.; Schofield, O.M.; Kirkpatrick, B.; Kirkpatrick, G.J. New sensors for ocean observing: The optical phy-toplankton discriminator. In Coastal Ocean Observing Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 326–350. [Google Scholar]
- Duffy, G.; Regan, F. Recent developments in sensing methods for eutrophying nutrients with a focus on automation for environmental applications. Anal. 2017, 142, 4355–4372. [Google Scholar] [CrossRef]
- A McPartlin, D.; Loftus, J.H.; Crawley, A.S.; Silke, J.; Murphy, C.S.; O’kennedy, R.J. Biosensors for the monitoring of harmful algal blooms. Curr. Opin. Biotechnol. 2017, 45, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.K.; Mickett, J.B.; Doucette, G.J.; Adams, N.G.; Mikulski, C.M.; Birch, J.M.; Roman, B.; Michel-Hart, N.; Newton, J.A. An Autonomous Platform for Near Real-Time Surveillance of Harmful Algae and Their Toxins in Dynamic Coastal Shelf Environments. J. Mar. Sci. Eng. 2021, 9, 336. [Google Scholar] [CrossRef]
- Yamahara, K.; Demir-Hilton, E.; Preston, C.; Marin, R.; Pargett, D.; Roman, B.; Jensen, S.; Birch, J.; Boehm, A.; Scholin, C. Simultaneous monitoring of faecal indicators and harmful algae using an in-situ autonomous sensor. Lett. Appl. Microbiol. 2015, 61, 130–138. [Google Scholar] [CrossRef]
- Ralston, D.K.; Moore, S.K. Modeling harmful algal blooms in a changing climate. Harmful Algae 2020, 91, 101729. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Cusack, C.; Ruiz-Villarreal, M.; Silke, J.; McElligott, D.; Davidson, K. Applied simulations and integrated modelling for the understanding of toxic and harmful algal blooms (ASIMUTH): Integrated HAB forecast systems for Europe’s Atlantic Arc. Harmful Algae 2016, 53, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Wu, H.; Liu, F.; Peng, W.; Zhang, X.; Chang, F.; Xie, P.; Zhang, H. A Review and Perspective of eDNA Application to Eutrophication and HAB Control in Freshwater and Marine Ecosystems. Microorganisms 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Bao, M.; Lou, X.; Zhou, Z.; Yin, K. Monitoring, modeling and projection of harmful algal blooms in China. Harmful Algae 2022, 111, 102164. [Google Scholar] [CrossRef]
- Zahir, M.; Su, Y.; Shahzad, M.I.; Ayub, G.; Rahman, S.U.; Ijaz, J. A review on monitoring, forecasting, and early warning of harmful algal bloom. Aquaculture 2024, 593, 741351. [Google Scholar] [CrossRef]
- Wen, J.; Yang, J.; Li, Y.; Gao, L. Harmful algal bloom warning based on machine learning in maritime site monitoring. Knowledge-Based Syst. 2022, 245, 108569. [Google Scholar] [CrossRef]
- Flynn, K.J.; McGillicuddy, D.J. Modeling marine harmful algal blooms: Current status and future prospects. Harmful Algal Bloom. A Compend. Desk Ref. 2018, 115–134. [Google Scholar] [CrossRef]
- Pinto, L.; Mateus, M.; Silva, A. Modeling the transport pathways of harmful algal blooms in the Iberian coast. Harmful Algae 2016, 53, 8–16. [Google Scholar] [CrossRef]
- Yan, Z.; Kamanmalek, S.; Alamdari, N.; Nikoo, M.R. Comprehensive Insights into Harmful Algal Blooms: A Review of Chemical, Physical, Biological, and Climatological Influencers with Predictive Modeling Approaches. J. Environ. Eng. 2024, 150, 03124002. [Google Scholar] [CrossRef]
- Franks, P.J. Recent Advances in Modelling of Harmful Algal Blooms. Global Ecology and Oceanography of Harmful Algal Blooms, Ecological Studies; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lin, J.; Miller, P.I.; Jönsson, B.F.; Bedington, M. Early Warning of Harmful Algal Bloom Risk Using Satellite Ocean Color and Lagrangian Particle Trajectories. Front. Mar. Sci. 2021, 8, 736262. [Google Scholar] [CrossRef]
- El-Habashi, A.; Ioannou, I.; Tomlinson, M.C.; Stumpf, R.P.; Ahmed, S. Satellite Retrievals of Karenia brevis Harmful Algal Blooms in the West Florida Shelf Using Neural Networks and Comparisons with Other Techniques. Remote Sens. 2016, 8, 377. [Google Scholar] [CrossRef]
- Ahn, J.M.; Kim, J.; Kim, K. Ensemble Machine Learning of Gradient Boosting (XGBoost, LightGBM, CatBoost) and Attention-Based CNN-LSTM for Harmful Algal Blooms Forecasting. Toxins 2023, 15, 608. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lyubchich, V.; Glibert, P.M. Time series models of decadal trends in the harmful algal species Karlodinium veneficum in Chesapeake Bay. Harmful Algae 2018, 73, 110–118. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, C.; Zhang, Z.; Hu, H.; Tan, A.; Xing, Z. Temporal and spatial characteristics of harmful algal blooms in the offshore waters, China during 1990 to 2019. J. Appl. Remote Sens. 2022, 16, 012004. [Google Scholar] [CrossRef]
- Song, W.; Dolan, J.M.; Cline, D.; Xiong, G. Learning-Based Algal Bloom Event Recognition for Oceanographic Decision Support System Using Remote Sensing Data. Remote Sens. 2015, 7, 13564–13585. [Google Scholar] [CrossRef]
- Seoane, S.; Garmendia, M.; Revilla, M.; Borja, A.; Franco, J.; Orive, E.; Valencia, V. Phytoplankton pigments and epifluorescence microscopy as tools for ecological status assessment in coastal and estuarine waters, within the Water Framework Directive. Mar. Pollut. Bull. 2011, 62, 1484–1497. [Google Scholar] [CrossRef]
- Medlin, L. Molecular tools for monitoring harmful algal blooms. Environ. Sci. Pollut. Res. 2013, 20, 6683–6685. [Google Scholar] [CrossRef]
- Clark, D.; Pilditch, C.; Pearman, J.; Ellis, J.; Zaiko, A. Environmental DNA metabarcoding reveals estuarine benthic community response to nutrient enrichment—Evidence from an in-situ experiment. Environ. Pollut. 2020, 267, 115472. [Google Scholar] [CrossRef]
- Ininbergs, K.; Bergman, B.; Larsson, J.; Ekman, M. Microbial metagenomics in the Baltic Sea: Recent advancements and prospects for environmental monitoring. AMBIO 2015, 44, 439–450. [Google Scholar] [CrossRef]
- Vos, R.; Hakvoort, J.; Jordans, R.; Ibelings, B. Multiplatform optical monitoring of eutrophication in temporally and spatially variable lakes. Sci. Total Environ. 2003, 312, 221–243. [Google Scholar] [CrossRef]
- Chang, N.-B.; Imen, S.; Vannah, B. Remote Sensing for Monitoring Surface Water Quality Status and Ecosystem State in Relation to the Nutrient Cycle: A 40-Year Perspective. Crit. Rev. Environ. Sci. Technol. 2014, 45, 101–166. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Klinger, P.; Heege, T.; Oppelt, N. Multi-sensor satellite and in situ monitoring of phytoplankton development in a eutrophic-mesotrophic lake. Sci. Total Environ. 2018, 612, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Ménesguen, A.; Lacroix, G. Modelling the marine eutrophication: A review. Sci. Total Environ. 2018, 636, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and Solutions. In Eutrophication: Causes, Consequences and Control; Springer: Singapore, 2014; pp. 1–15. [Google Scholar]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Jiang, S.; Wu, R.; Ma, R.; Xue, Z. Adsorption of phosphorus from eutrophic seawater using microbial modified attapulgite-cleaner production, remove behavior, mechanism and cost-benefit analysis. Chem. Eng. J. 2023, 458, 141404. [Google Scholar] [CrossRef]
- Lenzi, M.; Persiano, M.L.; Ciarapica, M. Quality Improvement of Eutrophic Environments Degraded by Organic Matter, in Experiences Conducted in Sea-Water Microcosms. Eur. J. Biol. Biotechnol. 2021, 2, 29–35. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Mediterranean Action, P. In Eutrophication in the Mediterranean Sea: Receiving Capacity and Monitoring of Long Term Effects; UNEP: Nairobi, Kenya, 1988. [Google Scholar]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Wu, R. Research progress of phosphorus adsorption by attapulgite and its prospect as a filler of constructed wetlands to enhance phosphorus removal from mariculture wastewater. J. Environ. Chem. Eng. 2022, 10, 108748. [Google Scholar] [CrossRef]
- Mittal, Y.; Srivastava, P.; Kumar, N.; Tripathy, B.C.; Martinez, F.; Yadav, A.K. Nutrient removal in floating and vertical flow constructed wetlands using aluminium dross: An innovative approach to mitigate eutrophication. Bioresour. Technol. 2024, 410, 131205. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Zhou, Y.; Mao, X.-Z. Eutrophication control strategies for highly anthropogenic influenced coastal waters. Sci. Total Environ. 2020, 705, 135760. [Google Scholar] [CrossRef]
- Boyd, J. The Development and Evaluation of New Technologies for the Study of Eutrophication in Coastal Waters. Ph.D. Thesis, University of Southampton, Hampshire, UK, 2004. [Google Scholar]
- Sighicelli, M.; Iocola, I.; Pittalis, D.; Luglié, A.; Padedda, B.M.; Pulina, S.; Iannetta, M.; Menicucci, I.; Fiorani, L.; Palucci, A. An Innovative and High-Speed Technology for Seawater Monitoring of Asinara Gulf (Sardinia-Italy). Open J. Mar. Sci. 2014, 4, 42525. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.-L. Chemicals used forin situimmobilization to reduce the internal phosphorus loading from lake sediments for eutrophication control. Crit. Rev. Environ. Sci. Technol. 2016, 46, 947–997. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Wu, R. Adsorption and immobilization of phosphorus from eutrophic seawater and sediment using attapulgite—Behavior and mechanism. Chemosphere 2023, 313, 137390. [Google Scholar] [CrossRef]
- Zangarini, S.; Sciarria, T.P.; Tambone, F.; Adani, F. Phosphorus removal from livestock effluents: Recent technologies and new perspectives on low-cost strategies. Environ. Sci. Pollut. Res. 2020, 27, 5730–5743. [Google Scholar] [CrossRef]
- Boniardi, G.; Turolla, A.; Fiameni, L.; Gelmi, E.; Malpei, F.; Bontempi, E.; Canziani, R. Assessment of a simple and replicable procedure for selective phosphorus recovery from sewage sludge ashes by wet chemical extraction and precipitation. Chemosphere 2021, 285, 131476. [Google Scholar] [CrossRef]
- Lind, C.B. Precipitation of Phosphorus in Wastewater, Lakes, and Animal Wastes. 2013. Available online: https://elibrary.asabe.org/abstract.asp?aid=15544 (accessed on 28 August 2024).
- An, J.-S.; Back, Y.-J.; Kim, K.-C.; Cha, R.; Jeong, T.-Y.; Chung, H.-K. Optimization for the removal of orthophosphate from aqueous solution by chemical precipitation using ferrous chloride. Environ. Technol. 2014, 35, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, P.; Pesonen, J.; Romar, H.; Hu, T.; Tynjälä, P.; Lassi, U. The Use of Ca- and Mg-Rich Fly Ash as a Chemical Precipitant in the Simultaneous Removal of Nitrogen and Phosphorus—Recycling and Reuse. Recycling 2019, 4, 14. [Google Scholar] [CrossRef]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The effect of temperature on the properties of hydroxyapatite precipitated from calcium hydroxide and phosphoric acid. Thermochim. Acta 2001, 374, 13–22. [Google Scholar] [CrossRef]
- Neweshy, W.; Planas, D.; Tellier, E.; Demers, M.; Marsac, R.; Couture, R.M. Response of sediment phosphorus partitioning to lanthanum-modified clay amendment and porewater chemistry in a small eutrophic lake. Environ. Sci. Process. Impacts 2022, 24, 1494–1507. [Google Scholar] [CrossRef]
- Zhan, Y.; Qiu, B.; Lin, J. Effect of common ions aging treatment on adsorption of phosphate onto and control of phosphorus release from sediment by lanthanum-modified bentonite. J. Environ. Manag. 2023, 341, 118109. [Google Scholar] [CrossRef]
- Wu, G.; Liu, G.; Li, X.; Peng, Z.; Zhou, Q.; Qi, T. Enhanced phosphate removal with fine activated alumina synthesized from a sodium aluminate solution: Performance and mechanism. RSC Adv. 2022, 12, 4562–4571. [Google Scholar] [CrossRef]
- Van Truong, T.; Kim, D.-J. Synthesis of high quality boehmite and γ-alumina for phosphorus removal from water works sludge by extraction and hydrothermal treatment. Environ. Res. 2022, 212, 113448. [Google Scholar] [CrossRef]
- Wan, C.; Ding, S.; Zhang, C.; Tan, X.; Zou, W.; Liu, X.; Yang, X. Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Sep. Purif. Technol. 2017, 180, 1–12. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.-J.; van Loosdrecht, M.C.M. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-F.; Wang, J.-P.; Duan, E.-G.; Hu, W.-H.; Dong, Y.-B.; Zhang, G.-Q. Phosphorus removal by adsorbent based on poly-aluminum chloride sludge. Water Sci. Eng. 2020, 13, 193–201. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Kowalkowska, A.; Filipkowska, U.; Struk-Sokołowska, J.; Bolozan, L.; Gache, L.; Ilie, M. Recovery of phosphorus as soluble phosphates from aqueous solutions using chitosan hydrogel sorbents. Sci. Rep. 2021, 11, 16766. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Katuwal, S.; Zheng, W.; Sharma, B.; Cooke, R. Capture and recover dissolved phosphorous from aqueous solutions by a designer biochar: Mechanism and performance insights. Chemosphere 2021, 274, 129717. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, S.; Zheng, Q.; Huang, B.; Zhang, J.; Fu, H.; Gao, H. Removal and recovery of phosphorus from solution by bifunctional biochar. Inorg. Chem. Commun. 2022, 139, 109341. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Marti, E.J. A review of the application of cerium and lanthanum in phosphorus removal during wastewater treatment: Characteristics, mechanism, and recovery. Chemosphere 2022, 309, 136462. [Google Scholar] [CrossRef]
- Makita, Y.; Sonoda, A.; Sugiura, Y.; Ogata, A.; Suh, C.; Lee, J.-H.; Ooi, K. Phosphorus removal from model wastewater using lanthanum hydroxide microcapsules with poly(vinyl chloride) shells. Sep. Purif. Technol. 2020, 241, 116707. [Google Scholar] [CrossRef]
- Caravelli, A.H.; De Gregorio, C.; Zaritzky, N.E. Effect of operating conditions on the chemical phosphorus removal using ferric chloride by evaluating orthophosphate precipitation and sedimentation of formed precipitates in batch and continuous systems. Chem. Eng. J. 2012, 209, 469–477. [Google Scholar] [CrossRef]
- Sun, W.; Ma, G.; Sun, Y.; Liu, Y.; Song, N.; Xu, Y.; Zheng, H. Effective treatment of high phosphorus pharmaceutical wastewater by chemical precipitation. Can. J. Chem. Eng. 2017, 95, 1585–1593. [Google Scholar] [CrossRef]
- Liu, G.-R.; Ye, C.-S.; He, J.-H.; Qian, Q.; Jiang, H. Lake sediment treatment with aluminum, iron, calcium and nitrate additives to reduce phosphorus release. J. Zhejiang Univ.-Sci. A 2009, 10, 1367–1373. [Google Scholar] [CrossRef]
- Lynn, A.K.; Bonfield, W. A Novel Method for the Simultaneous, Titrant-Free Control of pH and Calcium Phosphate Mass Yield. Accounts Chem. Res. 2005, 38, 202–207. [Google Scholar] [CrossRef]
- Shiba, N.C.; Ntuli, F. Extraction and precipitation of phosphorus from sewage sludge. Waste Manag. 2017, 60, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Zhang, Y.; Bao, S.; Huang, J. Precipitation of vanadium using ammonium salt in alkaline and acidic media and the effect of sodium and phosphorus. Hydrometallurgy 2018, 180, 113–120. [Google Scholar] [CrossRef]
- Meyer-Reil, L.-A.; Köster, M. Eutrophication of Marine Waters: Effects on Benthic Microbial Communities. Mar. Pollut. Bull. 2000, 41, 255–263. [Google Scholar] [CrossRef]
- Horta, P.A.; Rörig, L.R.; Costa, G.B.; Baruffi, J.B.; Bastos, E.; Rocha, L.S.; Destri, G.; Fonseca, A.L. Marine eutrophication: Overview from now to the future. In Anthropogenic Pollution of Aquatic Ecosystems; Springer: Cham, Switzerland, 2021; pp. 157–180. [Google Scholar]
- Jessen, C.; Bednarz, V.N.; Rix, L.; Teichberg, M.; Wild, C. Marine eutrophication. In Environmental Indicators; Springer: Dordrecht, The Netherlands, 2015; pp. 177–203. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a restoration tool to combat eutrophication: Recent advances and future challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar]
- Tribedi, P.; Goswami, M.; Chakraborty, P.; Mukherjee, K.; Mitra, G.; Bhattacharyya, P.; Dey, S. Bioaugmentation and biostimulation: A potential strategy for environmental remediation. J. Microbiol. Exp. 2018, 6, 62–65. [Google Scholar] [CrossRef]
- Anh, H.T.H.; Shahsavari, E.; Bott, N.J.; Ball, A.S. The application of Marinobacter hydrocarbonoclasticus as a bioaugmentation agent for the enhanced treatment of non-sterile fish wastewater. J. Environ. Manag. 2021, 291, 112658. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Gill, S.S.; Khan, F.A.; Naeem, M. Phytoremediation systems for the recovery of nutrients from eutrophic waters. In Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2014; pp. 239–248. [Google Scholar]
- Yamamoto, T.; Goto, I.; Kawaguchi, O.; Minagawa, K.; Ariyoshi, E.; Matsuda, O. Phytoremediation of shallow organically enriched marine sediments using benthic microalgae. Mar. Pollut. Bull. 2008, 57, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Zhou, X.; Xie, B.; Huang, C.; Uddin, M.M.; Chen, X.; Huang, L. Ecosystem stability and water quality improvement in a eutrophic shallow lake via long-term integrated biomanipulation in Southeast China. Ecol. Eng. 2021, 159, 106119. [Google Scholar] [CrossRef]
- Lindegren, M.; Möllmann, C.; Hansson, L.-A. Biomanipulation: A tool in marine ecosystem management and restoration? Ecol. Appl. 2010, 20, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Fennel, W.; Neumann, T. Introduction to the Modelling of Marine Ecosystems; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Spieles, D.J. The role of biomanipulation in aquatic ecosystem restoration. In Progress in Aquatic Ecosystem Research; Burk, A.R., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2005; pp. 59–82. [Google Scholar]
- Ren, L.; Wang, G.; Huang, Y.; Guo, J.; Li, C.; Jia, Y.; Chen, S.; Zhou, J.L.; Hu, H. Phthalic acid esters degradation by a novel marine bacterial strain Mycolicibacterium phocaicum RL-HY01: Characterization, metabolic pathway and bioaugmentation. Sci. Total Environ. 2021, 791, 148303. [Google Scholar] [CrossRef] [PubMed]
- Stallwood, B.; Shears, J.; Williams, P.; Hughes, K. Low temperature bioremediation of oil-contaminated soil using biostimulation and bioaugmentation with a Pseudomonas sp. from maritime Antarctica. J. Appl. Microbiol. 2005, 99, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Juhanson, J.; Truu, J.; Heinaru, E.; Heinaru, A. Survival and catabolic performance of introduced Pseudomonas strains during phytoremediation and bioaugmentation field experiment. FEMS Microbiol. Ecol. 2009, 70, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Laothamteep, N.; Naloka, K.; Pinyakong, O. Bioaugmentation with zeolite-immobilized bacterial consortium OPK results in a bacterial community shift and enhances the bioremediation of crude oil-polluted marine sandy soil microcosms. Environ. Pollut. 2022, 292, 118309. [Google Scholar] [CrossRef]
- Muangchinda, C.; Srisuwankarn, P.; Boubpha, S.; Chavanich, S.; Pinyakong, O. The effect of bioaugmentation with Exiguobacterium sp. AO-11 on crude oil removal and the bacterial community in sediment microcosms, and the development of a liquid ready-to-use inoculum. Chemosphere 2020, 250, 126303. [Google Scholar] [CrossRef]
- Keuter, S.; Beth, S.; Quantz, G.; Schulz, C.; Spieck, E. Longterm Monitoring of Nitrification and Nitrifying Communities during Biofilter Activation of Two Marine Recirculation Aquaculture Systems (RAS). Int. J. Aquac. Fish. Sci. 2017, 3, 051–061. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Wu, J.; Pang, W.; Hu, Z. Bioaugmented constructed wetlands for efficient saline wastewater treatment with multiple denitrification pathways. Bioresour. Technol. 2021, 335, 125236. [Google Scholar] [CrossRef]
- Stenström, F.; Jes la Cour, J. Impact on nitrifiers of full-scale bioaugmentation. Water Sci. Technol. 2017, 76, 3079–3085. [Google Scholar] [CrossRef]
- Kumar, V.R.; Sukumaran, V.; Achuthan, C.; Joseph, V.; Philip, R.; Singh, I.B. Molecular characterization of the nitrifying bacterial consortia employed for the activation of bioreactors used in brackish and marine aquaculture systems. Int. Biodeterior. Biodegradation 2013, 78, 74–81. [Google Scholar] [CrossRef]
- Wang, Y.; Tam, N.F. Microbial Remediation of organic pollutants. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–303. [Google Scholar]
- Yuan, Q.-B.; Shen, Y.; Huang, Y.-M.; Hu, N. A comparative study of aeration, biostimulation and bioaugmentation in contaminated urban river purification. Environ. Technol. Innov. 2018, 11, 276–285. [Google Scholar] [CrossRef]
- Amran, R.; Jamal, M.T.; Pugazhendi, A.; Al-Harbi, M.; Ghandourah, M.; Al-Otaibi, A.; Haque, F. Biodegradation and Bioremediation of Petroleum Hydrocarbons in Marine Ecosystems by Microorganisms: A Review. Nat. Environ. Pollut. Technol. 2022, 21, 1149–1157. [Google Scholar] [CrossRef]
- Sordes, F.; Pellequer, E.; Sahli, S.; Sarzynski, T.; Denes, M.; Techer, I. Phytoremediation of chloride from marine dredged sediments: A new model based on a natural vegetation recolonization. J. Environ. Manag. 2023, 344, 118508. [Google Scholar] [CrossRef] [PubMed]
- Masciandaro, G.; Di Biase, A.; Macci, C.; Peruzzi, E.; Iannelli, R.; Doni, S. Phytoremediation of dredged marine sediment: Monitoring of chemical and biochemical processes contributing to sediment reclamation. J. Environ. Manag. 2014, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Negrin, V.L.; Gironés, L.; Serra, A.V. Eco-friendly strategies of remediation in the marine system: Bioremediation and phy-toremediation. In Coastal and Deep Ocean Pollution; CRC Press: Boca Raton, FL, USA, 2020; pp. 184–214. [Google Scholar]
- Wang, R.; Xiao, H.; Zhang, P.; Qu, L.; Cai, H.; Tang, X. Allelopathic effects of Ulva pertusa, Corallina pilulifera and Sargassum thunbergii on the growth of the dinoflagellates Heterosigma akashiwo and Alexandrium tamarense. J. Appl. Phycol. 2007, 19, 109–121. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Tang, X.; Wang, J.; Dong, S. Allelopathic growth inhibition of Heterosigma akashiwo by the three Ulva spcieces (Ulva Pertusa, Ulva Linza, Enteromorpha intestinalis) under laboratory conditions. Acta Oceanol. Sin. 2012, 31, 138–144. [Google Scholar] [CrossRef]
- Laabir, M.; Grignon-Dubois, M.; Masseret, E.; Rezzonico, B.; Soteras, G.; Rouquette, M.; Rieuvilleneuve, F.; Cecchi, P. Algicidal effects of Zostera marina L. and Zostera noltii Hornem. extracts on the neuro-toxic bloom-forming dinoflagellate Alexandrium catenella. Aquat. Bot. 2013, 111, 16–25. [Google Scholar] [CrossRef]
- Onishi, Y.; Mohri, Y.; Tuji, A.; Ohgi, K.; Yamaguchi, A.; Imai, I. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense. Fish. Sci. 2014, 80, 353–362. [Google Scholar] [CrossRef]
- Alexandre, A.; Santos, R. Competition for nitrogen between the seaweed Caulerpa prolifera and the seagrass Cymodocea nodosa. Mar. Ecol. Prog. Ser. 2020, 648, 125–134. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Z.; Zhang, X.; Fan, X.; Wang, Y.; Li, D.; Wang, W.; Zhuang, Z.; Ye, N. Allelopathic Interactions between the Opportunistic Species Ulva prolifera and the Native Macroalga Gracilaria lichvoides. PLoS ONE 2012, 7, e33648. [Google Scholar] [CrossRef]
- Friedlander, M.; Gonen, Y.; Kashman, Y.; Beer, S. Gracilaria conferta and its epiphytes: 3. Allelopathic inhibition of the red seaweed by Ulva cf. lactuca. J. Appl. Phycol. 1996, 8, 21–25. [Google Scholar] [CrossRef]
- Raniello, R.; Mollo, E.; Lorenti, M.; Gavagnin, M.; Buia, M.C. Phytotoxic activity of caulerpenyne from the Mediterranean invasive variety of Caulerpa racemosa: A potential allelochemical. Biol. Invasions 2007, 9, 361–368. [Google Scholar] [CrossRef]
- Pergent, G.; Boudouresque, C.-F.; Dumay, O.; Pergent-Martini, C.; Wyllie-Echeverria, S. Competition between the invasive macrophyte Caulerpa taxifolia and the seagrass Posidonia oceanica: Contrasting strategies. BMC Ecol. 2008, 8, 20. [Google Scholar] [CrossRef]
- Lee, G.; Suonan, Z.; Kim, S.H.; Hwang, D.-W.; Lee, K.-S. Heavy metal accumulation and phytoremediation potential by transplants of the seagrass Zostera marina in the polluted bay systems. Mar. Pollut. Bull. 2019, 149, 110509. [Google Scholar] [CrossRef] [PubMed]
- Afifudin, A.F.M.; Irawanto, R.; Purwitasari, N. Selection of potential plants as phytoremediation for heavy metals in estuarine eco-system: A systematic review. In Proceedings of the 4th International Conference on Life Sciences and Biotechnology (ICOLIB 2021), Jember, Indonesia, 15–16 November 2021; Atlantis Press: Paris, France, 2022; pp. 420–434. [Google Scholar]
- Czakó, M.; Feng, X.; He, Y.; Liang, D.; Márton, L. Transgenic Spartina alterniflora for phytoremediation. Environ. Geochem. Health 2006, 28, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, J.; Zhu, Q.; Wang, Y.; Yang, Y. Accumulation and Output of Heavy Metals by the Invasive Plant Spartina alterniflora in a Coastal Salt Marsh. Pedosphere 2018, 28, 884–894. [Google Scholar] [CrossRef]
- Bastos, E.; Schneider, M.; de Quadros, D.P.C.; Welz, B.; Batista, M.B.; Horta, P.A.; Rörig, L.R.; Barufi, J.B. Phytoremediation potential of Ulva ohnoi (Chlorophyta): Influence of temperature and salinity on the uptake efficiency and toxicity of cadmium. Ecotoxicol. Environ. Saf. 2019, 174, 334–343. [Google Scholar] [CrossRef]
- Rahhou, A.; Layachi, M.; Akodad, M.; El Ouamari, N.; Rezzoum, N.E.; Skalli, A.; Oudra, B.; El Bakali, M.; Kolar, M.; Imperl, J.; et al. The Bioremediation Potential of Ulva lactuca (Chlorophyta) Causing Green Tide in Marchica Lagoon (NE Morocco, Mediterranean Sea): Biomass, Heavy Metals, and Health Risk Assessment. Water 2023, 15, 1310. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. NUTRIENT SEQUESTRATION, BIOMASS PRODUCTION BY MICROALGAE AND PHYTOREMEDIATION OF SEWAGE WATER. Int. J. Phytoremediation 2013, 15, 789–800. [Google Scholar] [CrossRef]
- Kumar, D.; Santhanam, S.P.; Jayalakshmi, T.; Nandakumar, R.; Ananth, S.; Devi, A.S.; Prasath, B.B. Excessive nutrients and heavy metals removal from diverse wastewaters using marine microalga Chlorella marina (Butcher). Indian J. Geo-Mar. Sci. 2015, 44, 97–103. [Google Scholar]
- Gillard, J.T.F.; Hernandez, A.L.; Contreras, J.A.; Francis, I.M.; Cabrales, L. Potential for Biomass Production and Remediation by Cultivation of the Marine Model Diatom Phaeodactylum tricornutum in Oil Field Produced Wastewater Media. Water 2021, 13, 2700. [Google Scholar] [CrossRef]
- Abd El-Hameed, M.M.; Abuarab, M.; Al-Ansari, N.; Mottaleb, S.A.; Bakeer, G.A.; Gyasi-Agyei, Y.; Mokhtar, A. Phytoremediation of Contaminated Water by Cadmium (Cd) Using Two Cyanobacteria Species (Anabaena Variabilis and Nostoc Muscorum). Res. Sq. 2021, 33, 135–146. [Google Scholar] [CrossRef]
- Tel-Or, E.; Forni, C. Phytoremediation of hazardous toxic metals and organics by photosynthetic aquatic systems. Plant Biosyst. 2011, 145, 224–235. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Olu-Owolabi, B.I.; Adebowale, K.O. Phytoremediation potential of Eichornia crassipes in metal-contaminated coastal water. Bioresour. Technol. 2009, 100, 4521–4526. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Olajire, T.; Afolabi, N.O.; Akinwumiju, A.S.; Akinbobola, E.; Hassan, U.O. Phytoremediation potentials of Eichhornia crassipes for nutrients and organic pollutants from textile wastewater. Int. J. Phytoremediation 2021, 23, 1333–1341. [Google Scholar] [CrossRef]
- Anacleto, P.; Heuvel, F.H.v.D.; Oliveira, C.; Rasmussen, R.R.; Fernandes, J.O.; Sloth, J.J.; Barbosa, V.; Alves, R.N.; Marques, A.; Cunha, S.C. Exploration of the phycoremediation potential of Laminaria digitata towards diflubenzuron, lindane, copper and cadmium in a multitrophic pilot-scale experiment. Food Chem. Toxicol. 2017, 104, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Omezzine, F.; Haouala, R.; El Ayeb, A.; Boughanmi, N. Allelopathic and antifungal potentialities of Padina pavonica (L.) extract. J. Plant Breed. Crop Sci. 2009, 1, 194–203. [Google Scholar]
- Budzałek, G.; Śliwińska-Wilczewska, S. Allelopathic effect of macroalgae Fucus vesiculosus (Ochrophyta) and Coccotylus brodiei (Rhodophyta) on the growth and photosynthesis performance of Baltic cyanobacteria. Ann. Univ. Paedagog. Cracoviensis Stud. Nat. 2021, 6, 81–94. [Google Scholar] [CrossRef]
- Creed, J.C.; Norton, T.; Kain Jones, J. Are neighbours harmful or helpful in Fucus vesiculosus populations? Mar. Ecol. Prog. Ser. 1996, 133, 191–201. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Li, H. Isolation and identification of a novel allelochemical from Ruppia maritima extract against the cyanobacteria Microcystis aeruginosa. Environ. Technol. Innov. 2021, 21, 101301. [Google Scholar] [CrossRef]
- Gross, E.M. Allelopathy of Aquatic Autotrophs. Crit. Rev. Plant Sci. 2003, 22, 313–339. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Antialgal ent-labdane diterpenes from Ruppia maritima. Phytochemistry 2000, 55, 909–913. [Google Scholar] [CrossRef]
- Iyapparaj, P.; Revathi, P.; Ramasubburayan, R.; Prakash, S.; Palavesam, A.; Immanuel, G.; Anantharaman, P.; Sautreau, A.; Hellio, C. Antifouling and toxic properties of the bioactive metabolites from the seagrasses Syringodium isoetifolium and Cymodocea serrulata. Ecotoxicol. Environ. Saf. 2014, 103, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Iyapparaj, P.; Revathi, P.; Ramasubburayan, R.; Prakash, S.; Anantharaman, P.; Immanuel, G.; Palavesam, A. Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicol. Environ. Saf. 2013, 89, 231–238. [Google Scholar] [CrossRef]
- Sun, X.; Xia, Y.M.; Xu, N.J. Allelopathic effects of Enteromorpha intestinalis (Ulvaceae, Chlorophyta) on Prorocentrum micans (Proro-centraceae, Dinophyta). Allelopath. J. 2012, 30, 299–310. [Google Scholar]
- Suzuki, Y.; Takabayashi, T.; Kawaguchi, T.; Matsunaga, K. Isolation of an allelopathic substance from the crustose coralline algae, Lithophyllum spp., and its effect on the brown alga, Laminaria religiosa Miyabe (Phaeophyta). J. Exp. Mar. Biol. Ecol. 1998, 225, 69–77. [Google Scholar] [CrossRef]
- Harlin, M.M.; Rice, E.L. Allelochemistry in marine macroalgae. Crit. Rev. Plant Sci. 1987, 5, 237–249. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Challenges 2023, 12, 100733. [Google Scholar] [CrossRef]
- Dhaka, A.; Chattopadhyay, P. A review on physical remediation techniques for treatment of marine oil spills. J. Environ. Manag. 2021, 288, 112428. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.; Lewis, R.R.R. Environmental impacts of dredging on seagrasses: A review. Mar. Pollut. Bull. 2006, 52, 1553–1572. [Google Scholar] [CrossRef]
- Harris, L.; Hodgkins, C.; Day, M.; Austin, D.; Testa, J.; Boynton, W.; Van Der Tak, L.; Chen, N. Optimizing recovery of eutrophic estuaries: Impact of destratification and re-aeration on nutrient and dissolved oxygen dynamics. Ecol. Eng. 2015, 75, 470–483. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, P.; Zhao, S.; Kang, S.; Wang, P.; Zhou, M.; Lyu, J. Control and remediation methods for eutrophic lakes in the past 30 years. Water Sci. Technol. 2020, 81, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research Progress and Prospects of Marine Oily Wastewater Treatment: A Review. Water 2019, 11, 2517. [Google Scholar] [CrossRef]
- Cooper, K.; Ware, S.; Vanstaen, K.; Barry, J. Gravel seeding—A suitable technique for restoring the seabed following marine aggregate dredging? Estuarine, Coast. Shelf Sci. 2011, 91, 121–132. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Trevathan-Tackett, S.M.; Nielsen, D.A.; Connolly, R.M.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I. Oxygen Consumption and Sulfate Reduction in Vegetated Coastal Habitats: Effects of Physical Disturbance. Front. Mar. Sci. 2019, 6, 14. [Google Scholar] [CrossRef]
- Calero, S.; Segura, M.; Rojo, C.; Rodrigo, M.A. Shifts in plankton assemblages promoted by free water surface constructed wetlands and their implications in eutrophication remediation. Ecol. Eng. 2015, 74, 385–393. [Google Scholar] [CrossRef]
- Martín, M.; Oliver, N.; Hernández-Crespo, C.; Gargallo, S.; Regidor, M. The use of free water surface constructed wetland to treat the eutrophicated waters of lake L’Albufera de Valencia (Spain). Ecol. Eng. 2013, 50, 52–61. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; de Bettignies, T. Bubble Curtains: Herbivore Exclusion Devices for Ecology and Restoration of Marine Ecosystems? Front. Mar. Sci. 2017, 4, 302. [Google Scholar] [CrossRef]
- Sunaryani, A.; Soewondo, P.; Santoso, A.B. Sediment capping technology for eutrophication control and its potential for application in Indonesian lakes: A review. Limnotek Perair. Darat Trop. Indones. 2023, 29, 5–17. [Google Scholar] [CrossRef]
- Berg, U.; Neumann, T.; Donnert, D.; Nüesch, R.; Stüben, D. Sediment capping in eutrophic lakes—Efficiency of undisturbed calcite barriers to immobilize phosphorus. Appl. Geochem. 2004, 19, 1759–1771. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Ni, J.; Li, Z. Evaluation of laboratory-scale in situ capping sediments with purple parent rock to control the eutrophication. Environ. Sci. Pollut. Res. 2017, 24, 7114–7123. [Google Scholar] [CrossRef]
- Kamilya, T.; Majumder, A.; Yadav, M.K.; Ayoob, S.; Tripathy, S.; Gupta, A.K. Nutrient pollution and its remediation using constructed wetlands: Insights into removal and recovery mechanisms, modifications and sustainable aspects. J. Environ. Chem. Eng. 2022, 10, 107444. [Google Scholar] [CrossRef]
- Lemley, D.A.; Lakane, C.P.; Taljaard, S.; Adams, J.B. Inorganic nutrient removal efficiency of a constructed wetland before discharging into an urban eutrophic estuary. Mar. Pollut. Bull. 2022, 179, 113727. [Google Scholar] [CrossRef]
- Cheng, R.; Zhu, H.; Shutes, B.; Yan, B. Treatment of microcystin (MC-LR) and nutrients in eutrophic water by constructed wetlands: Performance and microbial community. Chemosphere 2021, 263, 128139. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Z.; Li, B.; Bai, G.; Yang, L.; Chi, Y.; Wang, M.; Ren, Y. Root vertical spatial stress: A method for enhancing rhizosphere effect of plants in subsurface flow constructed wetland. Environ. Res. 2023, 231, 116083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, D.; Bai, G.; Cao, T.; Liu, W.; Hu, Z.; Chen, D.; Qiu, D.; Wu, Z. Changes of microbial community structure during the initial stage of biological clogging in horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2021, 337, 125405. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yue, J.; Yao, D.; Zhang, X.; Li, Y.; Hu, Z.; Liang, S.; Wu, H.; Xie, H.; Zhang, J. Plant development alters the nitrogen cycle in subsurface flow constructed wetlands: Implications to the strategies for intensified treatment performance. Water Res. 2023, 246, 120750. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R. Comparison of free water and horizontal subsurface treatment wetlands. Ecol. Eng. 2009, 35, 159–174. [Google Scholar] [CrossRef]
- Wiessner, A.; Kappelmeyer, U.; Kaestner, M.; Schultze-Nobre, L.; Kuschk, P. Response of ammonium removal to growth and transpiration of Juncus effusus during the treatment of artificial sewage in laboratory-scale wetlands. Water Res. 2013, 47, 4265–4273. [Google Scholar] [CrossRef]
- Vymazal, J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: A review of a recent development. Water Res. 2013, 47, 4795–4811. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Kröpfelová, L. Multistage hybrid constructed wetland for enhanced removal of nitrogen. Ecol. Eng. 2015, 84, 202–208. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A.; Gersberg, R.M. Nutrient removal efficiency and resource economics of vertical flow and horizontal flow constructed wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Stefanakis, A.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment; Elsevier Science: Newnes, Australia, 2014. [Google Scholar]
- Jadhav, R.S.; Buchberger, S.G. Effects of vegetation on flow through free water surface wetlands. Ecol. Eng. 1995, 5, 481–496. [Google Scholar] [CrossRef]
- Alvarado, S.; Guédez, M.; Lué-Merú, M.P.; Nelson, G.; Alvaro, A.; Jesús, A.C.; Gyula, Z. Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour. Technol. 2008, 99, 8436–8440. [Google Scholar] [CrossRef]
- Wu, Y.; Chung, A.; Tam, N.; Pi, N.; Wong, M. Constructed mangrove wetland as secondary treatment system for municipal wastewater. Ecol. Eng. 2008, 34, 137–146. [Google Scholar] [CrossRef]
- Yang, Q.; Tam, N.; Wong, Y.; Luan, T.; Su, W.; Lan, C.; Shin, P.; Cheung, S. Potential use of mangroves as constructed wetland for municipal sewage treatment in Futian, Shenzhen, China. Mar. Pollut. Bull. 2008, 57, 735–743. [Google Scholar] [CrossRef]
- Kenworthy, J.W.; Thayer, G.W. Production and decomposition of the roots and rhizomes of seagrasses, Zostera marina and Thalassia testudinum, in temperate and subtropical marine ecosystems. Bull. Mar. Sci. 1984, 35, 364–379. [Google Scholar]
- El-Sheekh, M.; Abdel-Daim, M.M.; Okba, M.; Gharib, S.; Soliman, A.; El-Kassas, H. Green technology for bioremediation of the eutrophication phenomenon in aquatic ecosystems: A review. Afr. J. Aquat. Sci. 2021, 46, 274–292. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Yu, P.; Yang, X.; Zhang, L.; Geng, Z.; He, K. Oxygenation and synchronous control of nitrogen and phosphorus release at the sediment-water interface using oxygen nano-bubble modified material. Sci. Total Environ. 2020, 725, 138258. [Google Scholar] [CrossRef]
- Pandit, S.; Yadav, N.; Sharma, P.; Prakash, A.; Kuila, A. Life cycle assessment and techno-economic analysis of nanotech-nology-based wastewater treatment: Status, challenges and future prospectives. J. Taiwan Inst. Chem. Eng. 2024, 105567. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of electro-coagulation–flocculation for harvesting marine and freshwater microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Visconcin, K.C.L.; Meneghel, B.L.; Silva, A.J.d.; Dias, C.T.d.S. Nitrogen and phosphorus removal from synthetic aquaculture water through electrocoagulation. Rev. Ambiente Água 2024, 19, e2977. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, Y.; Chen, P.; He, Y.; Yi, C.; Feng, C. Electrocoagulation coupled with electrooxidation for the simultaneous treatment of multiple pollutants in contaminated sediments. J. Environ. Sci. 2023, 124, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Peng, Y.; Zhang, Z.; Zhang, M.; Zhou, Y.; Duan, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter. Ultrason. Sonochem. 2019, 56, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process. Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Edmundson, S.J.; Duncan, J.G. Indigenous algae for local bioresource production: Phycoprospecting. Energy Sustain. Dev. 2011, 15, 365–371. [Google Scholar] [CrossRef]
- Corcoran, A.A.; Hunt, R.W. Capitalizing on harmful algal blooms: From problems to products. Algal Res. 2021, 55, 102265. [Google Scholar] [CrossRef]
- Page, M.; MacAllister, B.; Urban, A.; Veinotte, C.; MacAllister, I.; Pokrzywinski, K.; Riley, J.; Martinez-Guerra, E.; White, C.; Grasso, C.; et al. Harmful Algal Bloom Interception, Treatment, and Transformation System, HABITATS; ERDC TR-20-1; US Army Corps of Engineers Engineer Research and Development Center: Vicksburg, MS, USA, 2020. [Google Scholar]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing solar energy using phototrophic microorganisms: A sustainable pathway to bioenergy, biomaterials, and environmental solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Lim, J.; Kim, J.; Hong, S.; Al-Amoudi, A.S.; Park, B. Fouling control in SWRO desalination during harmful algal blooms: A historical review and future developments. Desalination 2022, 543, 116094. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Chia, W.Y.; Cheah, W.Y.; Munawaroh, H.S.H.; Ong, W.-J. Abatement of hazardous materials and biomass waste via pyrolysis and co-pyrolysis for environmental sustainability and circular economy. Environ. Pollut. 2021, 278, 116836. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, W.-J.; Ma, L.-L.; Yu, H.-Q. Sustainable Conversion of Harmful Algae Biomass into a CO2 Reduction Electrocatalyst for Two-Fold Carbon Utilization. Environ. Sci. Technol. 2023, 57, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.D.; Chaudhuri, A. Valorization of algal blooms and their residues into renewable energy. In Biodegradable Waste Processing for Sustainable Developments; CPR Press: Boca Raton, FL, USA, 2024; p. 257. [Google Scholar]
- Ferreira, J.G.; Andersen, J.H.; Borja, A.; Bricker, S.B.; Camp, J.; da Silva, M.C.; Garcés, E.; Heiskanen, A.-S.; Humborg, C.; Ignatiades, L.; et al. Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar. Coast. Shelf Sci. 2011, 93, 117–131. [Google Scholar] [CrossRef]
- Friedland, R.; Macias, D.; Cossarini, G.; Daewel, U.; Estournel, C.; Garcia-Gorriz, E.; Grizzetti, B.; Grégoire, M.; Gustafson, B.; Kalaroni, S.; et al. Effects of Nutrient Management Scenarios on Marine Eutrophication Indicators: A Pan-European, Multi-Model Assessment in Support of the Marine Strategy Framework Directive. Front. Mar. Sci. 2021, 8, 596126. [Google Scholar] [CrossRef]
- Lintern, A.; McPhillips, L.; Winfrey, B.; Duncan, J.; Grady, C. Best Management Practices for Diffuse Nutrient Pollution: Wicked Problems Across Urban and Agricultural Watersheds. Environ. Sci. Technol. 2020, 54, 9159–9174. [Google Scholar] [CrossRef]
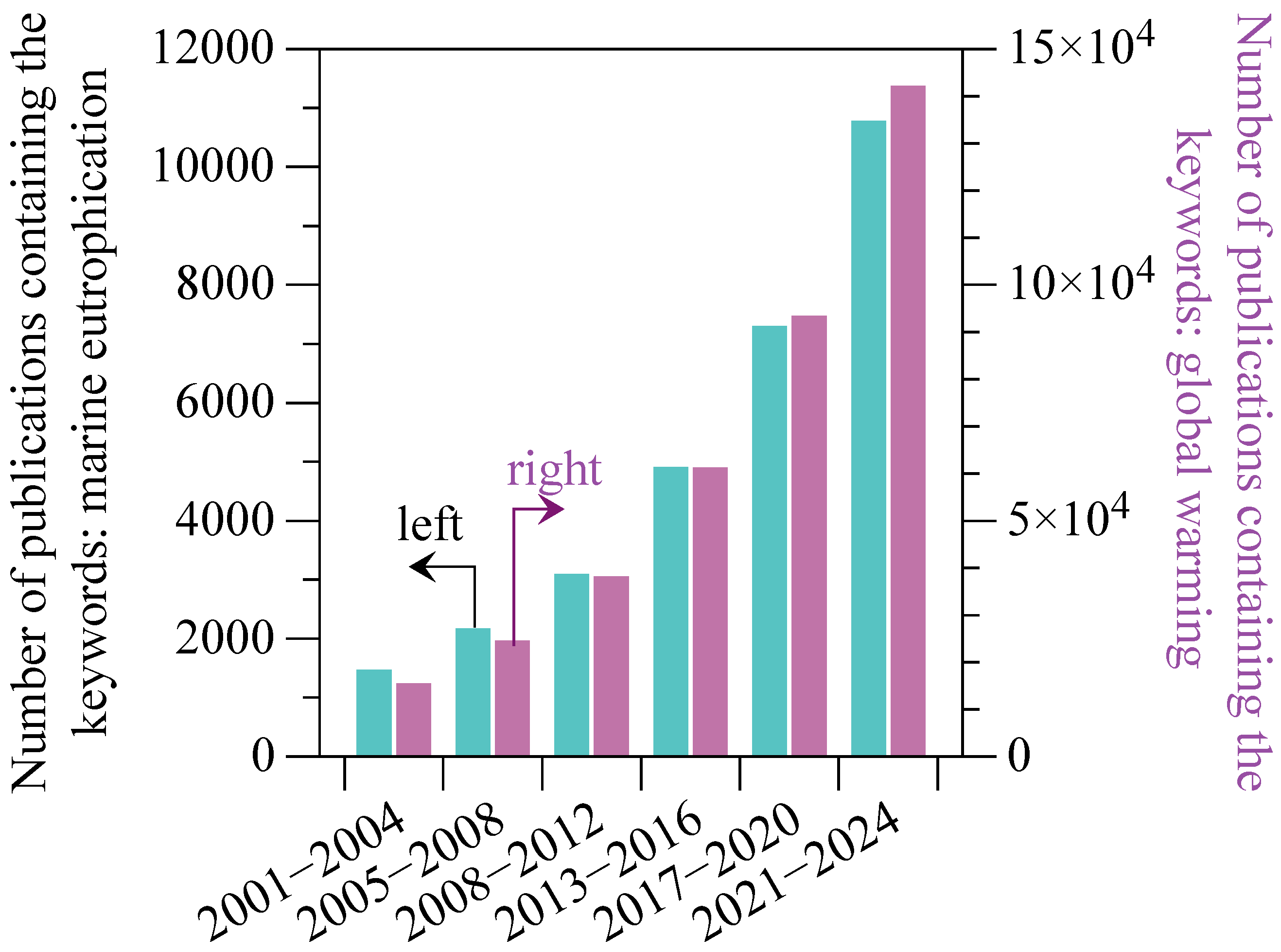


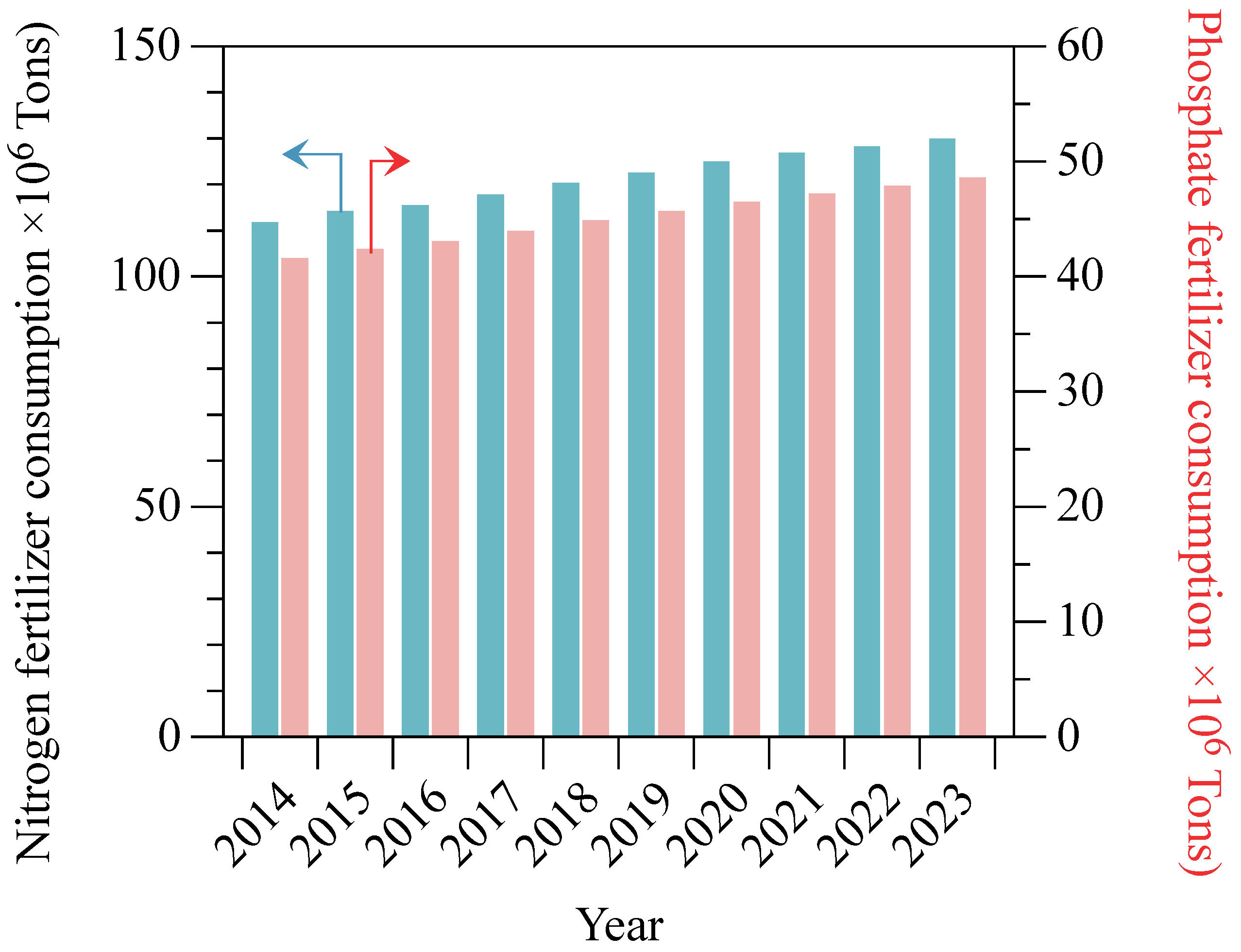
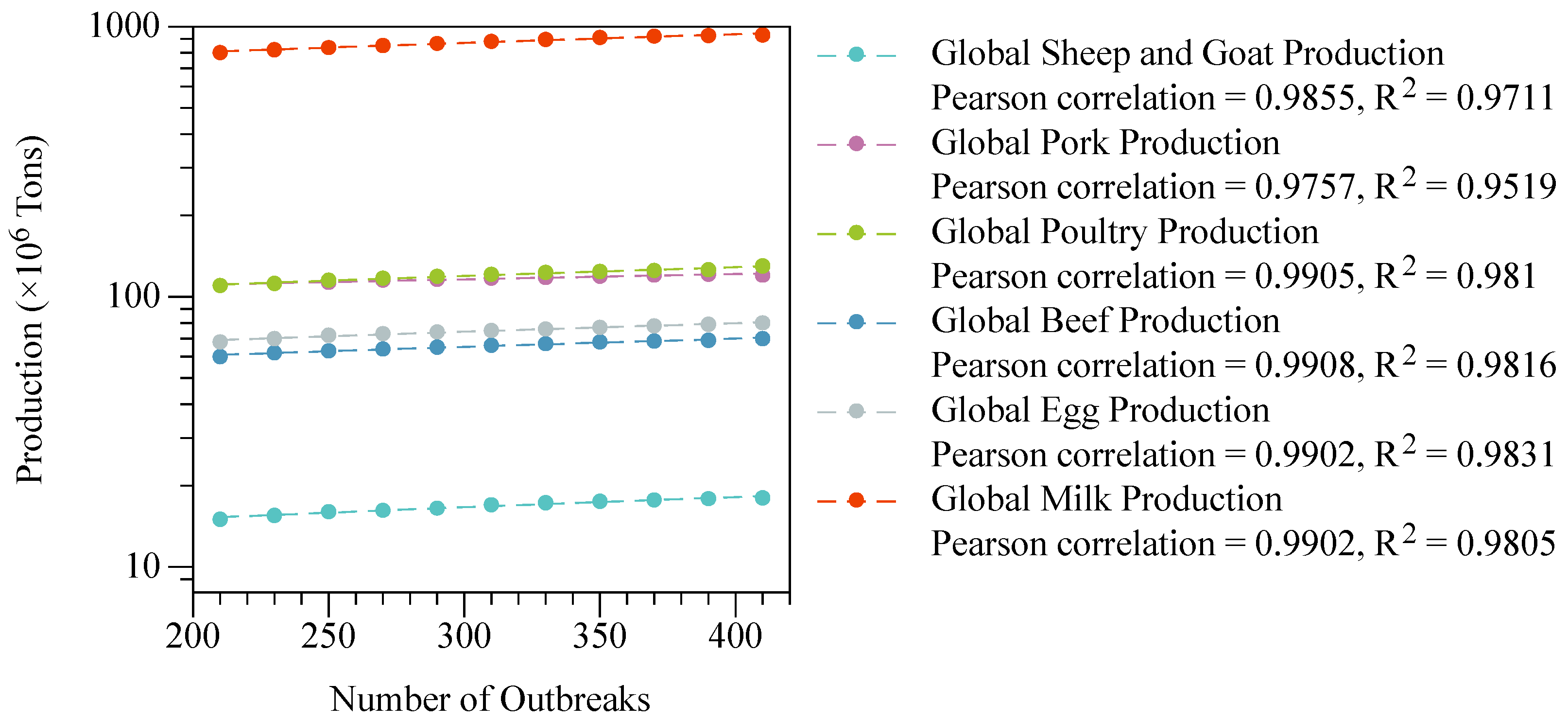
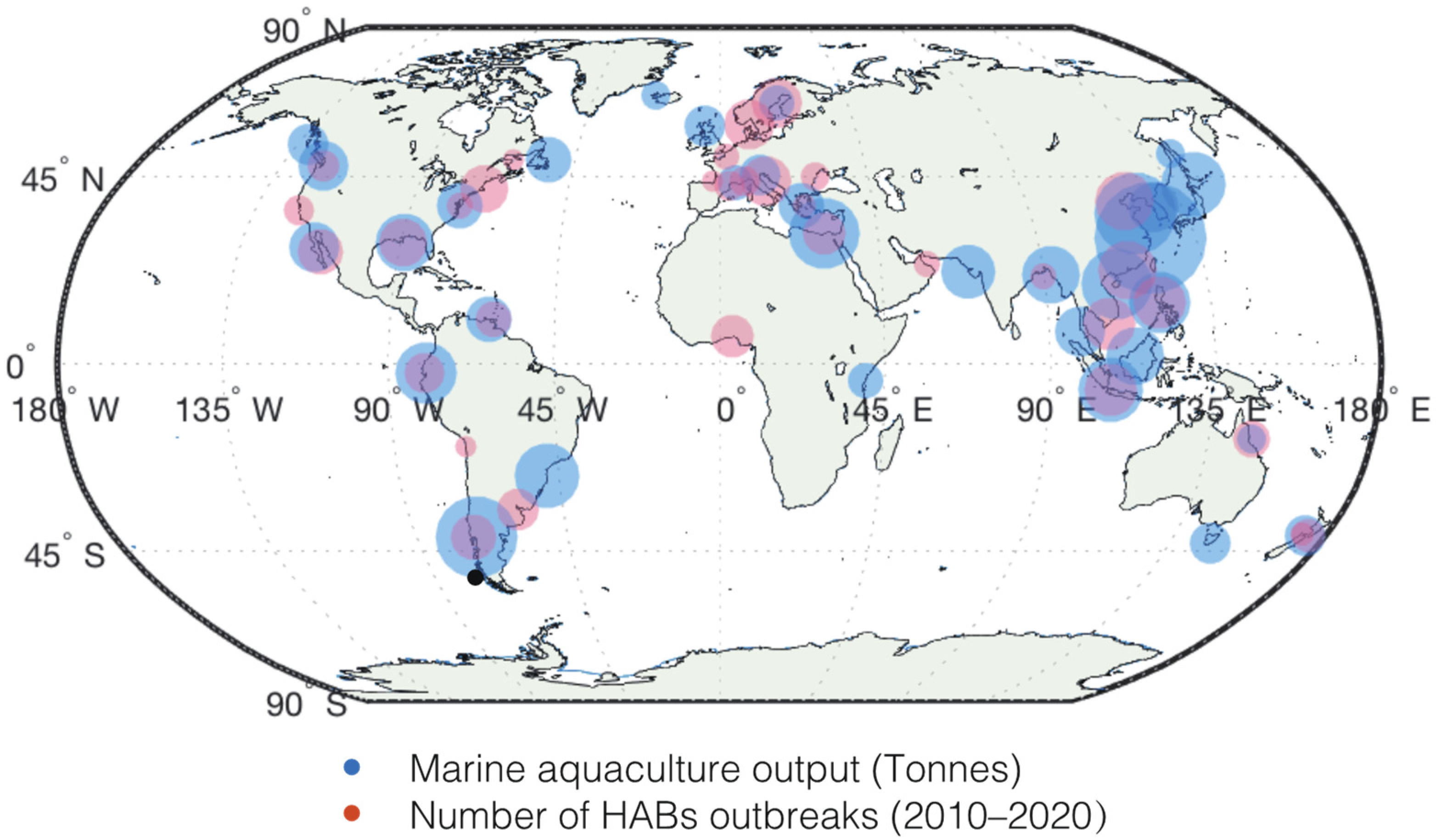
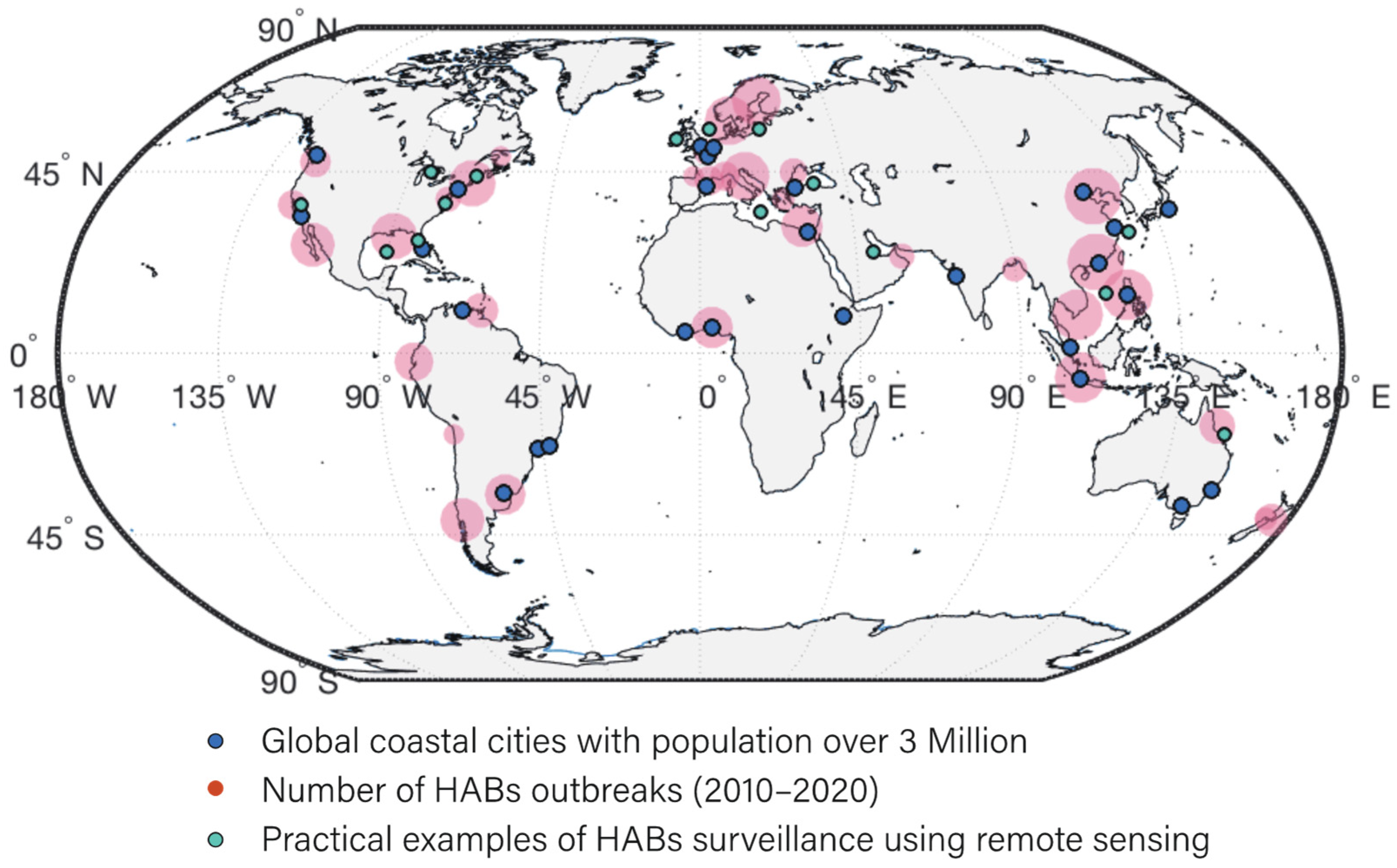

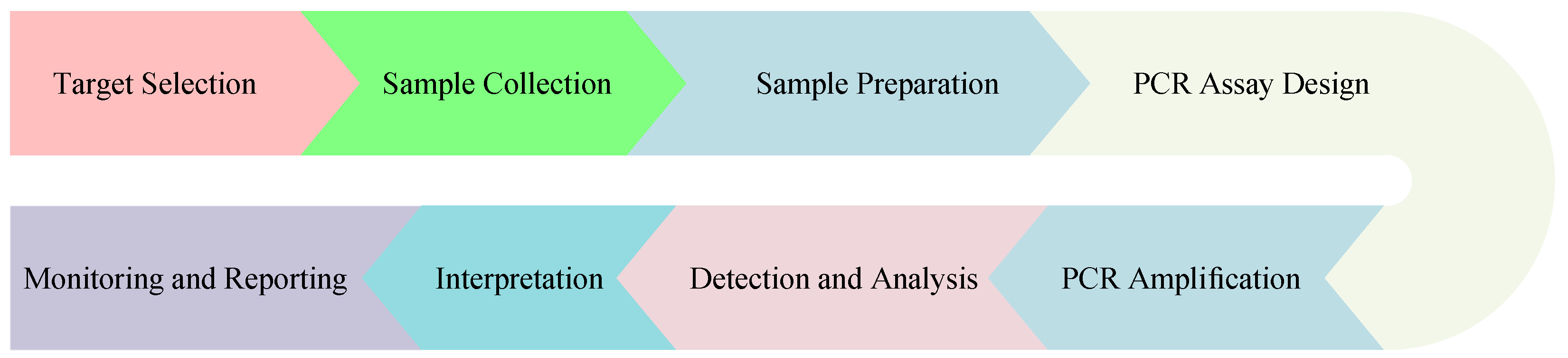
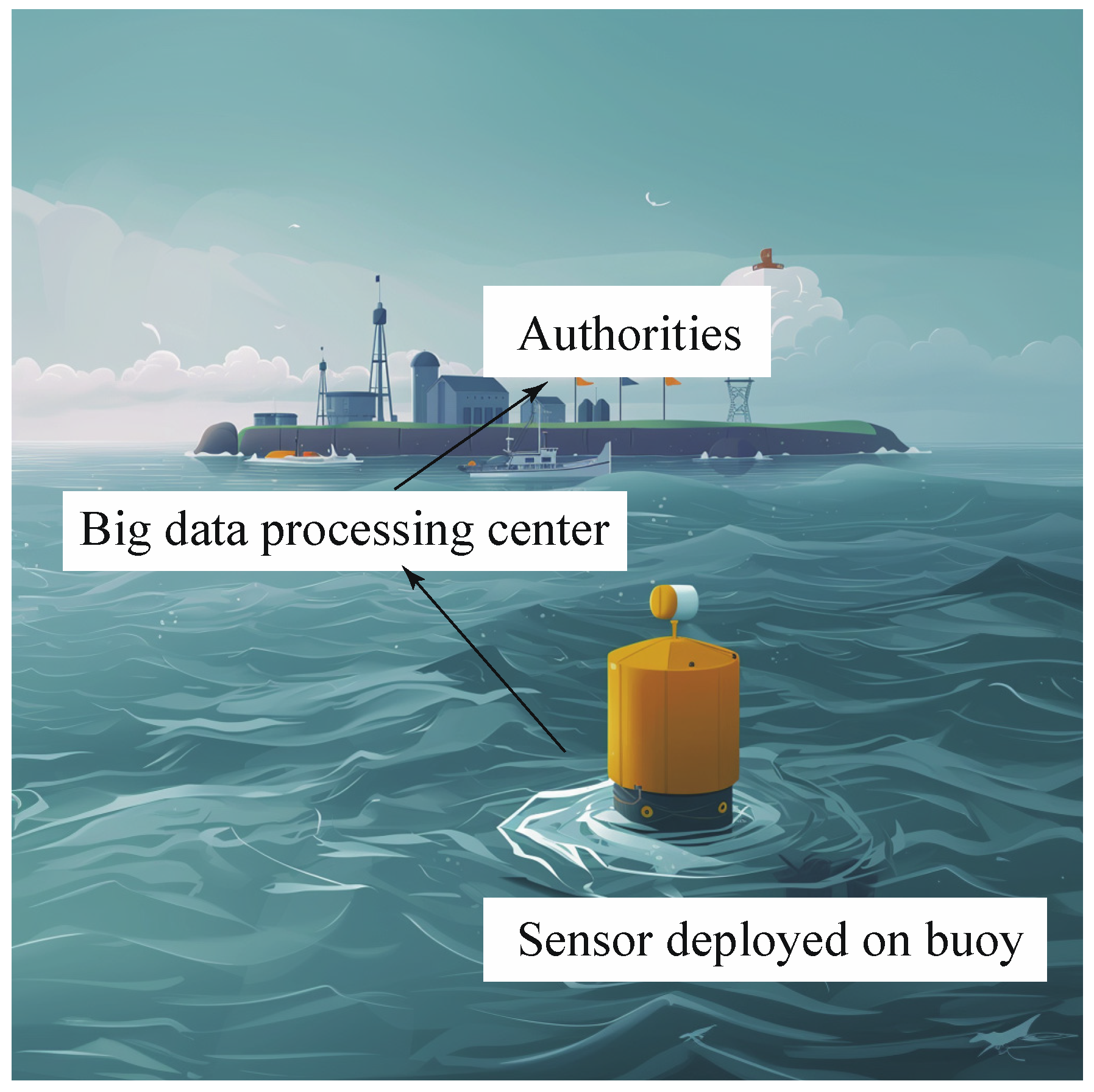
| Criteria | Eutrophication | HABs |
|---|---|---|
| Definition | Nutrient enrichment in water bodies, leading to excessive algal growth | Rapid and excessive growth of algae |
| Key Nutrients | Nitrogen, Phosphorus | Nitrogen, Phosphorus |
| Chlorophyll-a Concentration | >20 µg/L | >30 µg/L |
| Cell Density | N/A | >100,000 cells/mL [45] |
| Dissolved Oxygen | Hypoxia (DO < 2 mg/L) in bottom waters [46] | Significant diurnal fluctuation (supersaturation during day, hypoxia at night) [47] |
| Odor | N/A | Unpleasant odors due to volatile organic compounds (VOCs) [48] |
| Toxin Production | N/A | Presence of harmful algal toxins [49] |
| HAB Type | Dominant Species | Characteristics | Conditions for Dominance | Refs. |
|---|---|---|---|---|
| Cyanobacterial Blooms (Blue-Green Algae) | Microcystis, Anabaena, Nodularia | Produces microcystins, nodularins; toxic to humans and animals; common in freshwater and brackish environments | High nutrient levels (especially phosphorus); warm, stagnant waters; low water flow | [51] |
| Dinoflagellate Blooms | Alexandrium, Karenia, Gymnodinium | Known for “red tides”; produces neurotoxins (e.g., saxitoxins, brevetoxins) causing shellfish poisoning | Nutrient-rich environments; presence of specific minerals (e.g., silica for diatoms); stratified water column | [52,53] |
| Diatom Blooms | Pseudo-nitzschia, Chaetoceros | Produces domoic acid leading to amnesic shellfish poisoning; common in colder waters | Presence of silica; low predation; cold, nutrient-rich waters | [54,55] |
| Haptophyte Blooms | Phaeocystis, Chrysochromulina | Forms gelatinous masses disrupting food webs; produces dimethyl sulfide influencing climate | High nutrient availability; low predation; stable, stratified waters | [56,57] |
| Raphidophyte Blooms | Heterosigma, Chattonella | Produces reactive oxygen species; associated with fish kills | High nutrient and iron levels; warm temperatures; low grazing pressure | [58,59] |
| Green Algae Blooms | Ulva, Enteromorpha | Forms thick surface mats leading to hypoxia; disrupts marine habitats | High light availability; shallow, nutrient-rich waters; limited grazing | [60,61] |
| Type of Microorganism | Species Name | Method of Decomposing Algal Biomass | Products after Decomposition | Refs. |
|---|---|---|---|---|
| Bacteria | Pseudomonas putida | Oxidizes organic matter in the presence of oxygen | CO2, H2O | [71,72] |
| Bacteria | Bacillus subtilis | Aerobic decomposition of proteins and polysaccharides | CO2, H2O | [73,74] |
| Bacteria | Desulfovibrio vulgaris | Anaerobic sulfate reduction, using sulfate as an electron acceptor | H2S, CO2 | [75] |
| Archaea | Methanosarcina barkeri | Anaerobic decomposition of organic acids and alcohols | CH₄, CO2 | [76] |
| Fungi | Aspergillus terreus | Breaks down complex polysaccharides (cellulose, hemicellulose) | Simpler organic molecules, CO2 | [77,78] |
| Bacteria | Clostridium butyricum | Fermentation of organic matter under anaerobic conditions | Butyric acid, H2, CO2 | [79] |
| Fungi | Penicillium chrysogenum | Decomposes polysaccharides and proteins, especially in low-oxygen environments | Organic acids, CO2 | [80] |
| Country/Region | Algal Toxin | Standard (µg/L) | Water Type | Remarks | Ref. |
|---|---|---|---|---|---|
| United States | Microcystin-LR | 1.6 | Drinking Water | EPA’s Health Advisory level for infants and young children. | [135] |
| United States | Microcystin-LR | 8 | Drinking Water | EPA’s Health Advisory level for adults. | [136] |
| United States | Cylindrospermopsin | 0.7 | Drinking Water | EPA’s Health Advisory level for infants and young children. | [137] |
| United States | Cylindrospermopsin | 3 | Drinking Water | EPA’s Health Advisory level for adults. | [138] |
| United States | Anatoxin-a | 20 | Recreational Water | No federal standard for drinking water. | [139] |
| European Union | Microcystin-LR | 1 | Drinking Water | Set by the European Commission for safe drinking water. | [140] |
| Australia | Microcystin-LR | 1.3 | Drinking Water | Based on WHO guidelines for drinking water safety. | [141] |
| Canada | Microcystin-LR | 1.5 | Drinking Water | Guidelines set by Health Canada. | [142] |
| Brazil | Microcystin-LR | 1 | Drinking Water | Aligned with WHO recommendations. | [143] |
| New Zealand | Microcystin-LR | 1 | Drinking Water | Based on WHO guidelines for safe drinking water. | [144] |
| China | Microcystin-LR | 1 | Drinking Water | National standard for safe drinking water. | [145] |
| Japan | Microcystin-LR | 1 | Drinking Water | National guidelines for safe drinking water. | [146] |
| South Africa | Microcystin-LR | 1 | Drinking Water | Set by the Department of Water Affairs and Forestry. | [147] |
| WHO | Microcystin-LR | 1 | Drinking Water | International guideline value for safe drinking water. | [148] |
| Pathway | Contribution | Details | Refs. |
|---|---|---|---|
| Oxygen Depletion | High | Major driver of habitat degradation; leads to hypoxia or anoxia, causing widespread die-offs and loss of marine organisms. | [154,155] |
| Toxicity | High | Severe impact through poisoning of marine life and humans; disrupts health of various species and ecosystem balance. | [156] |
| Disruption of Food Webs | Moderate to High | Alters food web dynamics by outcompeting primary producers and reducing food availability for herbivores. | [157] |
| Light Reduction | Moderate | Reduces light penetration, affecting photosynthesis of submerged vegetation and causing habitat decline. | [149] |
| Physical Damage | Moderate | Causes direct harm to benthic habitats through smothering by mucilaginous or gelatinous substances. | [158] |
| Nutrient Imbalance | Moderate | Leads to persistent algal blooms by disrupting nutrient levels, exacerbating other degradation pathways. | [159,160] |
| Altered Community Structure | Variable | Shifts species composition, affecting biodiversity and ecosystem resilience; impact depends on specific species changes. | [161] |
| Algal Group/Species | Key Elemental Requirements | Optimal N:P:Si Ratio | Comments | Refs. |
|---|---|---|---|---|
| Pseudonitzschia spp. (Diatom) | High Si, Moderate N, Moderate P | 15:01:16 | Produces domoic acid, a neurotoxin causing amnesic shellfish poisoning (ASP). | [168] |
| Skeletonema costatum (Diatom) | High Si, Moderate N, Moderate P | 14:01:16 | Common in temperate regions; requires silicon for robust growth. | [169,170] |
| Alexandrium spp. (Dinoflagellate) | High N, Moderate P, Low Si | 16:01:00 | Thrives in nitrogen-rich environments; does not require silicon. | [171,172] |
| Karenia brevis (Dinoflagellate) | High N, Moderate P, Low Si | 20:01:00 | Produces brevetoxins, linked to “red tides” in the Gulf of Mexico. | [173] |
| Dinophysis spp. (Dinoflagellate) | High N, Moderate P, Low Si | 18:01:00 | Produces okadaic acid, causing diarrhetic shellfish poisoning (DSP). | [174] |
| Noctiluca scintillans (Dinoflagellate) | High N, Moderate P, Low Si | 10:01:00 | Non-toxic but can cause hypoxia; benefits from high N inputs. | [175] |
| Cylindrospermopsis raciborskii (Cyanobacterium) | High P, Moderate N, Low Si | 05:01:00 | Thrives in phosphorus-rich environments; produces cylindrospermopsin toxin. | [176] |
| Microcystis spp. (Cyanobacterium) | High N, High P, Low Si | 20:01:00 | Common in eutrophic freshwater; produces microcystins, toxic to humans and animals. | [177,178] |
| Heterosigma akashiwo (Raphidophyte) | High N, Moderate P, Low Si | 25:01:00 | Known for causing fish kills; thrives in high N, low Si environments. | [179] |
| Nutrient | Role in HABs | Ecosystem | Processes and Conditions | Dominant Algal Species | Refs. |
|---|---|---|---|---|---|
| N | Major driver of algal growth; often limiting nutrient | Coastal Waters | High inputs from agriculture, urban runoff | Dinoflagellates, Cyanobacteria | [152,159,180] |
| Open Ocean | Upwelling, atmospheric deposition | Diatoms, Dinoflagellates | |||
| Polar Regions | Seasonal nutrient release from ice melt | Diatoms, Phaeocystis | |||
| P | Key driver in phosphorus-limited systems | Estuaries and Coastal Lagoons | Runoff from agriculture, sewage discharge | Cyanobacteria, Dinoflagellates | [181,182,183] |
| Freshwater-Influenced Marine Systems | High phosphorus inputs | Cyanobacteria, Diatoms | |||
| Si | Essential for diatom growth | Upwelling Zones | Nutrient-rich upwelled waters | Diatoms | [184,185,186] |
| River-Influenced Systems | Abundant silicon from riverine inputs | Diatoms |
| Factor | Description | Impact on Nutrient Runoff | Refs. |
|---|---|---|---|
| Over-application of Fertilizers | Applying fertilizers in amounts exceeding crop needs. | High risk of nutrient runoff, especially during heavy rain. | [198] |
| Timing of Fertilizer Application | Fertilizer applied during non-growing seasons or prior to heavy rainfall. | Increased nutrient loss to runoff. | [199] |
| Irrigation Practices | Excessive or inefficient irrigation methods. | Increases leaching and runoff, particularly of N. | [200] |
| Tillage Practices | Conventional tillage increases soil disturbance and erosion. | Higher erosion and surface runoff leading to nutrient loss. | [201,202] |
| Crop Rotation and Cover Cropping | Rotating crops with different nutrient needs and using cover crops to absorb residual nutrients. | Reduces nutrient buildup and runoff. | [203] |
| Fertilizer Technology | Nutrients Provided | Mechanism | Suitable Crops | Refs. |
|---|---|---|---|---|
| Slow-Release Fertilizers (SRFs) | Nitrogen, Phosphorus, Potassium | Gradual nutrient release aligned with crop uptake | Cereals, horticultural crops, turfgrass | [221,222] |
| Controlled-Release Fertilizers (CRFs) | Nitrogen, Phosphorus, Potassium | Coating controls nutrient release over time | Vegetables, fruits, ornamental plants | [223,224] |
| Nitrification Inhibitors | Nitrogen | Inhibits nitrification, reducing nitrate leaching | Maize, wheat, rice | [225,226] |
| Urease Inhibitors | Nitrogen (Urea-based) | Prevents rapid urea conversion, reducing ammonia loss | Rice, cereals, pasture | [227,228] |
| Enhanced Efficiency Fertilizers (EEFs) | Nitrogen, Phosphorus | Combines slow and controlled release with inhibitors | Various crops including cereals, fruits, vegetables | [229,230] |
| Polymer-Coated Fertilizers | Nitrogen, Potassium | Encapsulated nutrients in a polymer for controlled release | High-value crops like fruits, vegetables, ornamentals | [231,232] |
| Biochar-Enhanced Fertilizers | Nitrogen, Phosphorus, Potassium, micronutrients | Uses biochar to retain nutrients and reduce leaching | Cereals, legumes, vegetables | [233] |
| Struvite Fertilizers | Phosphorus, Nitrogen, Magnesium | Mineral compound with slow nutrient release | Horticultural crops, cereals | [234] |
| Fertilizer Name | Nutrients Provided | Principle | Suitable Crops | Refs. |
|---|---|---|---|---|
| Humic Acid-Enriched Fertilizers | N, P, K, Micronutrients | Enhances nutrient absorption and soil structure with humic acid. | Cereals, vegetables, fruits, legumes | [235] |
| Compost-Based Mineral Fertilizers | N, P, K, Organic Matter | Integrates compost with minerals to improve soil organic content and nutrient retention. | Vegetables, fruits, horticultural crops | [236,237] |
| Biochar-Integrated Fertilizers | N, P, K, Carbon | Uses biochar to enhance nutrient retention and reduce leaching. | Row crops, perennials, reclamation projects | [238,239] |
| Seaweed Extract-Based Fertilizers | N, P, K, Trace Elements | Combines seaweed extracts for growth promotion with minerals for nutrient availability. | Vegetables, fruits, ornamental plants | [240] |
| Poultry Manure-Enriched Fertilizers | N, P, K, Calcium | Combines poultry manure with minerals for balanced nutrient supply. | Grain crops, pasture, vegetables | [241] |
| City | Population | Nitrogen Discharge (tons/Year) | Phosphorus Discharge (tons/Year) | Sources of Nitrogen and Phosphorus Discharge | Ref. |
|---|---|---|---|---|---|
| New York | 8,336,817 | 120,000 | 8500 | Domestic sewage, agricultural runoff, industrial waste | [259] |
| Los Angeles | 3,979,576 | 85,000 | 6000 | Domestic sewage, urban runoff, industrial effluents | [260] |
| Miami | 467,963 | 45,000 | 3200 | Domestic sewage, urban runoff, aquaculture, agricultural runoff | [261] |
| Vancouver | 631,486 | 55,000 | 4000 | Domestic sewage, urban runoff, aquaculture | [262] |
| Sao Paulo | 12,252,023 | 200,000 | 15,000 | Domestic sewage, industrial waste, agricultural runoff | [263] |
| Rio de Janeiro | 6,747,815 | 130,000 | 9500 | Domestic sewage, industrial waste, agricultural runoff | [264] |
| Buenos Aires | 15,153,729 | 180,000 | 12,500 | Domestic sewage, urban runoff, industrial effluents, agricultural runoff | [265] |
| Caracas | 2,939,000 | 75,000 | 5000 | Domestic sewage, urban runoff, industrial waste | [266] |
| London | 9,304,000 | 150,000 | 10,000 | Domestic sewage, urban runoff, industrial effluents | [267] |
| Paris | 11,017,000 | 160,000 | 11,000 | Domestic sewage, urban runoff, agricultural runoff | [259] |
| Barcelona | 5,575,000 | 85,000 | 6500 | Domestic sewage, urban runoff, aquaculture, industrial waste | [268] |
| Antwerp | 520,000 | 50,000 | 3000 | Domestic sewage, industrial effluents, port activities | [269] |
| Cairo | 20,900,000 | 300,000 | 22,000 | Domestic sewage, agricultural runoff, industrial effluents | [270] |
| Lagos | 15,388,000 | 270,000 | 20,000 | Domestic sewage, urban runoff, industrial waste, aquaculture | [271] |
| Addis Ababa | 5,228,000 | 90,000 | 7000 | Domestic sewage, urban runoff, agricultural runoff | [272] |
| Abidjan | 5,158,000 | 85,000 | 6500 | Domestic sewage, urban runoff, industrial waste | [273] |
| Tokyo | 14,043,000 | 320,000 | 25,000 | Domestic sewage, urban runoff, industrial effluents, agricultural runoff | [274] |
| Shanghai | 24,870,000 | 500,000 | 35,000 | Domestic sewage, industrial effluents, aquaculture, agricultural runoff | [275] |
| Beijing | 21,540,000 | 400,000 | 30,000 | Domestic sewage, urban runoff, industrial waste, agricultural runoff | [276] |
| Hong Kong | 7,474,200 | 150,000 | 10,000 | Domestic sewage, urban runoff, aquaculture | [277] |
| Singapore | 5,637,000 | 200,000 | 15,000 | Domestic sewage, urban runoff, aquaculture | [278] |
| Mumbai | 20,667,000 | 350,000 | 26,000 | Domestic sewage, urban runoff, industrial waste | [279] |
| Sydney | 5,367,000 | 90,000 | 7000 | Domestic sewage, urban runoff, aquaculture, agricultural runoff | [280] |
| Melbourne | 5,159,000 | 85,000 | 6500 | Domestic sewage, urban runoff, aquaculture | [281] |
| Istanbul | 15,519,000 | 250,000 | 18,000 | Domestic sewage, urban runoff, industrial effluents | [282] |
| Jakarta | 10,770,000 | 270,000 | 20,000 | Domestic sewage, urban runoff, industrial waste, aquaculture | [283] |
| Manila | 1,780,148 | 80,000 | 5500 | Domestic sewage, urban runoff, aquaculture | [284] |
| Sea Area Name | Outbreak Time | Outbreak Cause | Outbreak Frequency | Dominant Species | Refs. |
|---|---|---|---|---|---|
| Black Sea | December–March | Nutrient loading, temperature inversion | Annually | Pseudo-nitzschia spp. Emiliania huxleyi | [288,289] |
| Baltic Sea | November–February | Low temperature, high nutrient levels | Biennially | Aphanizomenon spp. | [290,291] |
| Gulf of Maine | January–March | Cold water upwelling, nutrient input | Annually | Alexandrium fundyense | [292] |
| Bering Sea | December–April | Ice melt, nutrient upwelling | Annually | Chaetoceros spp. | [31,32,33] |
| Sea of Okhotsk | November–March | Ice edge upwelling, nutrient inflow | Annually | Pseudo-nitzschia spp. | [293] |
| Yellow Sea | December–February | Cold water mixing, agricultural runoff | Annually | Skeletonema costatum | [294] |
| Barents Sea | February–April | Temperature drops, nutrient influx | Biennially | Chaetoceros spp. | [29,30] |
| North Sea | December–March | Cold currents, high nutrient load | Annually | Phaeocystis globosa | [295,296] |
| Gulf of St. Lawrence | December–February | Cold water upwelling, river runoff | Annually | Alexandrium tamarense | [297] |
| Sea of Japan | January–March | Cold currents, nutrient inputs | Annually | Dinophysis spp. | [298] |
| Algal Species | Toxin Produced | Effects | Refs. |
|---|---|---|---|
| Karenia brevis | Brevetoxins | Causes red tides, respiratory irritation, fish kills | [307,308,309] |
| Alexandrium fundyense | Saxitoxins | Affects human health through contaminated shellfish | [310] |
| Dinophysis spp. | Okadaic acid | Causes gastrointestinal issues in humans | [311,312] |
| Ceratium furca | None specific | Disrupts marine ecosystems, depletes oxygen | [313] |
| Pseudo-nitzschia spp. | Domoic acid | Neurological effects in humans and marine animals | [314,315] |
| Gymnodinium catenatum | Saxitoxins | Affects human health through contaminated shellfish | [316,317] |
| Cochlodinium polykrikoides | None specific | Causes fish kills, disrupts marine ecosystems | [10,318] |
| Prorocentrum minimum | Yessotoxins | Affects marine life, potential human health impacts | [319,320] |
| Akashiwo sanguinea | Hemolysins | Fish kills, disrupts marine ecosystems | [321,322] |
| Chattonella spp. | Brevetoxins | Causes fish kills, respiratory irritation | [323,324] |
| Heterosigma akashiwo | Unknown toxins | Fish kills, disrupts marine ecosystems | [315] |
| Margalefidinium polykrikoides | Unknown toxins | Causes fish kills, disrupts marine ecosystems | [325] |
| Lingulodinium polyedrum | Yessotoxins | Causes red tides, bioluminescence, disrupts ecosystems | [326] |
| Scrippsiella trochoidea | None specific | Disrupts marine ecosystems, depletes oxygen | [327] |
| Ostreopsis spp. | Palytoxin-like compounds | Respiratory issues, skin irritation in humans, marine life effects | [328] |
| Algal Species | Toxins | Distribution | Refs. |
|---|---|---|---|
| Alexandrium spp. | saxitoxins | Widely distributed in temperate and tropical coastal waters around the world. | [332,333] |
| Pseudo-nitzschia spp. | domoic acid | Found globally in both coastal and open ocean waters, with notable blooms along the west coast of North America (California and Alaska), the Gulf of Mexico, and parts of Europe (North Sea). | [334,335] |
| Dinophysis spp. | okadaic acid pectenotoxins | Found in coastal waters worldwide, including the North Atlantic, the Mediterranean Sea, and the coasts of South America and Asia. | [333] |
| Karenia brevis | brevetoxins | Primarily found in the Gulf of Mexico and along the southeastern coast of the United States, particularly in Florida. | [336,337] |
| Gambierdiscus spp. | ciguatoxins | Predominantly found in tropical and subtropical regions, including the Caribbean, the Pacific Islands, and the coasts of Australia and Southeast Asia. | [338] |
| Microcystis spp. | microcystins | Primarily found in freshwater, brackish, and marine environments, with occurrences in coastal regions worldwide, including the Baltic Sea and estuaries. | [339,340] |
| Lyngbya majuscula | lyngbyatoxin | Found in tropical and subtropical coastal waters, including the Caribbean, the Indian Ocean, and the Pacific Ocean, particularly around Australia and Southeast Asia. | [341,342] |
| Alien Species | Impact Mechanism | Location of Occurrence | Dominant Species during Algal Outbreaks | Ref. |
|---|---|---|---|---|
| Caulerpa taxifolia | Outcompetes native seagrasses, alters nutrient cycling | Mediterranean Sea | Alexandrium minutum | [343] |
| Mnemiopsis leidyi | Disrupts zooplankton populations, leading to increased algal biomass | Black Sea | Prymnesium parvum | [344] |
| Didemnum vexillum | Biofouling on structures, altering local nutrient dynamics | New Zealand Coast | Karenia brevis | [345] |
| Undaria pinnatifida | Outcompetes native kelp, changes habitat structure | North Atlantic Ocean | Phaeocystis globosa | [346] |
| Perna viridis | Alters nutrient cycles through biofiltration | Gulf of Mexico | Microcystis aeruginosa | [347] |
| Mytilus galloprovincialis | Outcompetes native mussels, changes nutrient dynamics | South African Coast | Alexandrium catenella | [348] |
| Ctenophora (comb jellies) | Predation on zooplankton, reducing grazing pressure on phytoplankton | Caspian Sea | Gonyaulax spinifera | [349] |
| Codium fragile | Competes with native algae, modifies nutrient availability | Japan Sea | Prorocentrum minimum | [98] |
| Gracilaria vermiculophylla | Creates dense mats, alters local hydrodynamics and nutrient flow | Western Baltic Sea | Dinophysis acuminata | [350] |
| Sargassum muticum | Forms large floating mats, modifies nutrient dynamics | English Channel | Pseudo-nitzschia spp. | [351] |
| Ciona intestinalis | Biofouling, alters nutrient recycling in coastal areas | Scandinavian Coasts | Chaetoceros spp. | [352] |
| Littorina littorea | Alters sediment structure, impacting nutrient availability | North American East Coast | Heterosigma akashiwo | [353] |
| Spartina alterniflora | Invades and modifies coastal wetlands, alters nutrient dynamics | Pacific Coast of the US | Coscinodiscus spp. | [354] |
| Styela clava | Biofouling and competition, altering habitat complexity | South Korean Coast | Skeletonema costatum | [345] |
| Pterois volitans (Lionfish) | Predation on herbivorous fish, leading to overgrowth of algae | Caribbean Sea | Karenia brevis | [355] |
| Corbicula fluminea | Alters sediment and nutrient dynamics | Southeast Asian Coast | Cylindrospermopsis raciborskii | [356] |
| Carcinus maenas (Green Crab) | Preys on native species, modifies ecosystem structure | US Atlantic Coast | Alexandrium fundyense | [357] |
| Eriocheir sinensis (Chinese Mitten Crab) | Burrowing activity alters sediment and nutrient flow | European Rivers | Microcystis aeruginosa | [358] |
| Lepidochitona cinerea | Grazes on native algae, shifts competitive balance | Mediterranean Coast | Noctiluca scintillans | [359] |
| Halophila stipulacea | Competes with native seagrasses, changes nutrient dynamics | Caribbean Sea | Pyrodinium bahamense | [360] |
| Batillaria attramentaria | Alters sediment composition, impacts nutrient cycles | West Coast of North America | Lingulodinium polyedra | [361] |
| Ascophyllum nodosum | Changes in nutrient uptake dynamics | North Atlantic Ocean | Aureococcus anophagefferens | [362] |
| Charybdis japonica | Predation and competition, altering nutrient dynamics | New Zealand Estuaries | Akashiwo sanguinea | [363] |
| Melanoides tuberculata | Modifies sediment and nutrient availability | Florida Wetlands | Karlodinium veneficum | [364] |
| Rapana venosa | Predation on bivalves, impacting nutrient recycling | Black Sea | Alexandrium tamarense | [365] |
| Tunicata (Sea Squirts) | Filter feeding, changes nutrient dynamics | Scottish Coast | Prymnesium parvum | [366] |
| Clytia hemisphaerica | Alters plankton community dynamics | Northern Adriatic Sea | Karenia mikimotoi | [367] |
| Chthamalus stellatus | Changes intertidal nutrient flows | Portuguese Coast | Dinophysis acuta | [368] |
| Tricellaria inopinata | Biofouling on marine structures, altering local nutrient dynamics | Italian Coast | Pseudochattonella verruculosa | [366] |
| Balanus improvisus | Displaces native species, modifies nutrient availability | Baltic Sea | Aphanizomenon flos-aquae | [369] |
| Crassostrea gigas (Pacific Oyster) | Alters local nutrient cycles through biofiltration | Normandy Coast, France | Chattonella marina | [370] |
| Location | Dominant Algal Species | Toxin | Geographical Distribution | Environmental Factors | Oceanographic Features | Mechanism of Influence | Ref. |
|---|---|---|---|---|---|---|---|
| Gulf of Mexico, USA | Karenia brevis | Brevetoxins | Subtropical, coastal waters | Warm temperatures, high nutrient runoff | Seasonal stratification, riverine inputs | Warm waters and high nutrient runoff from agriculture promote bloom frequency and intensity. | [371] |
| East China Sea, China | Prorocentrum donghaiense | Okadaic acid | Coastal, heavily industrialized | High nutrient input from agriculture and urbanization | Strong currents, upwelling zones | Nutrient pollution and strong ocean currents promote frequent blooms. | [372] |
| Baltic Sea, Europe | Nodularia spumigena | Nodularin | Enclosed sea, brackish water | Eutrophication, limited water exchange | Stratification, low salinity | Limited water exchange and nutrient accumulation contribute to persistent cyanobacterial blooms. | [373] |
| Mediterranean Sea, Europe | Alexandrium minutum | Saxitoxins (Paralytic Shellfish Toxins) | Semi-enclosed sea, temperate | Variable nutrient sources, urban runoff | Complex circulation patterns, semi-enclosed nature | High nutrient input and complex circulation patterns enhance HAB development. | [374] |
| Great Lakes, USA | Microcystis aeruginosa | Microcystins | Freshwater, temperate region | Agricultural runoff, warm summers | Stratification, freshwater system | Agricultural runoff and warm summers lead to frequent cyanobacterial blooms. | [375] |
| Black Sea, Europe | Alexandrium tamarense | Saxitoxins (Paralytic Shellfish Toxins) | Enclosed sea, nutrient-rich | Nutrient input from rivers, limited circulation | Low salinity, poor water circulation | Limited circulation and high nutrient input exacerbate toxic bloom events. | [376] |
| South China Sea, Asia | Gymnodinium catenatum | Saxitoxins (Paralytic Shellfish Toxins) | Tropical, coastal waters | Marine traffic, coastal development, nutrient loading | Monsoon-driven circulation, coastal upwelling | High nutrient loading and monsoon-driven currents promote frequent toxic blooms. | [377] |
| Chesapeake Bay, USA | Dinophysis acuminata | Okadaic acid, Dinophysistoxins | Estuarine, temperate region | High nutrient input from agriculture and urban runoff | Stratification, tidal mixing | Nutrient pollution and stratification enhance bloom occurrences. | [378] |
| Arabian Gulf, Middle East | Cochlodinium polykrikoides | No specific toxin identified, but associated with fish kills | Arid, warm coastal waters | Desalination effluents, high salinity | High salinity, weak circulation | High salinity and nutrient input from desalination create favorable conditions for HABs. | [379] |
| Australian Coastal Waters | Noctiluca scintillans | Ammonia (indirectly harmful, not a toxin in the traditional sense) | Coastal, temperate to tropical | Upwelling, nutrient-rich waters | Coastal upwelling, strong currents | Upwelling and nutrient enrichment lead to frequent red tide events. | [380] |
| Feature | Principle of Operation | Quantification Method | Sensitivity and Precision |
|---|---|---|---|
| Traditional PCR | Amplifies DNA for qualitative detection | Qualitative (presence/absence) | Less sensitive, suitable for detection only |
| qPCR | Quantifies DNA via fluorescence measurement | Relative quantification based on standard curves | Sensitive, but dependent on PCR efficiency and standard curves |
| dPCR | Partitions sample, performs PCR in each, and counts positive reactions | Absolute quantification using Poisson statistics | Highly sensitive and precise, minimal influence from PCR efficiency |
| Technology | Resolution | Typical Accuracy | Refs. |
|---|---|---|---|
| MODIS | 250–1000 m | Major blooms detected | [402] |
| Sentinel-1 | 10–20 m (SAR) | N/A | [402] |
| Sentinel-2 | 10–60 m | 70–80% | [419] |
| Sentinel-3 | 300 m (OLCI) | 70–80% | [420] |
| Landsat 8 | 30 m | 80–90% | [421,422] |
| Hyperspectral Camera (Drones/Aircraft) | 1–5 m | >90% | [423] |
| Multispectral Sensors (Drones) | 1–5 m | 80–90% | [424,425] |
| Sensor Type | Function | Deployment Examples | Applications | Refs. |
|---|---|---|---|---|
| Fluorometers | Measure chlorophyll fluorescence to estimate phytoplankton concentration. | Gulf of Maine | Monitoring Alexandrium spp. blooms for Paralytic Shellfish Poisoning prevention. | [428] |
| Spectrophotometers | Analyze water samples to detect specific pigments associated with harmful algae. | Chesapeake Bay, USA | Detecting and tracking Prorocentrum minimum blooms, aiding in shellfish safety management. | [429] |
| Nutrient Sensors | Monitor concentrations of nutrients like nitrate, phosphate, and silicate. | Great Lakes, USA | Monitoring Microcystis spp. blooms to protect drinking water sources. | [430] |
| Optical Sensors | Capture data on water clarity, turbidity, and light penetration. | Chesapeake Bay, USA | Detecting and tracking Prorocentrum minimum blooms, aiding in shellfish safety management. | [431,432] |
| DNA Biosensors | Detect and quantify specific genetic markers of harmful algae. | Great Lakes, USA | Monitoring Microcystis spp. blooms to protect drinking water sources. | [433] |
| Predictive Model | Practical Example | Description | Ref. |
|---|---|---|---|
| Numerical Models | Gulf of Mexico: Predicting Karenia brevis blooms | Utilizes physical, chemical, and biological data to simulate bloom dynamics and predict outbreaks | [438,439] |
| Statistical Models | Chesapeake Bay: Forecasting Prorocentrum minimum blooms | Uses historical data and statistical methods to predict bloom occurrences | [440] |
| Machine Learning Models | Great Lakes: Forecasting cyanobacterial blooms | Employs machine learning algorithms to analyze large datasets and predict HABs | [441] |
| Hydrodynamic Models | North Sea: Predicting Phaeocystis globosa blooms | Integrates oceanographic data to model water movement and predict bloom dispersion | [442] |
| Biogeochemical Models | Baltic Sea: Forecasting Nodularia spumigena blooms | Combines nutrient cycling and algal growth dynamics to forecast blooms | [443] |
| Coupled Physical-Biological Models | California Coast: Predicting Alexandrium catenella blooms | Integrates physical oceanography and biological processes to predict bloom dynamics | [444] |
| Remote Sensing Integrated Models | Florida Coast: Predicting Karenia brevis blooms using satellite data | Combines satellite remote sensing with modeling to enhance bloom prediction accuracy | [402] |
| Ecosystem Models | Puget Sound: Forecasting harmful algal bloom impacts on marine ecosystems | Models interactions between HABs and marine ecosystems to predict ecological impacts | [445] |
| Bayesian Network Models | New Zealand: Forecasting Alexandrium minutum blooms | Uses probabilistic methods to integrate various data sources for bloom prediction | [445] |
| Lagrangian Particle Tracking Models | East China Sea: Predicting Dinophysis acuminata blooms | Tracks the movement of individual water parcels to predict the transport and spread of blooms | [446] |
| Artificial Neural Networks | Mediterranean Sea: Predicting Dinoflagellate blooms | Uses neural network algorithms to learn and predict complex bloom patterns | [447] |
| Ensemble Models | Black Sea: Forecasting Cyanobacteria blooms | Combines multiple model outputs to improve prediction reliability and accuracy | [448] |
| Time Series Models | Hong Kong Waters: Predicting Cochlodinium polykrikoides blooms | Analyzes time series data to identify trends and predict future bloom events | [449] |
| Spatial–Temporal Models | South Korea: Forecasting Cochlodinium polykrikoides blooms | Integrates spatial and temporal data to predict the occurrence and spread of blooms | [450] |
| Decision Support Systems | Norway: Forecasting Karenia mikimotoi blooms | Provides integrated tools for real-time monitoring, prediction, and decision-making support | [451] |
| Technique | Advantages | Disadvantages | Cost | Sustainability | Refs. |
|---|---|---|---|---|---|
| Field Sampling and Microscopy | Direct observation, extensive historical data, low initial cost | Labor-intensive, time-consuming, requires skilled personnel | Moderate | Low | [452] |
| Molecular Techniques | High sensitivity and specificity, rapid results | Requires specialized equipment and personnel, high setup costs | High initial, moderate ongoing | Moderate | [453] |
| DNA/RNA Analysis | Detailed genetic information, multiple species identification | Advanced bioinformatics needed, high sequencing costs | High | Moderate to High | [454] |
| Metagenomics | Comprehensive view, identifies unknown species | High-throughput sequencing, complex data interpretation | Very High | High | [455] |
| Remote Sensing | Large-scale, real-time, non-invasive | Limited to surface, requires calibration, high setup cost | Moderate to High | High | [456,457] |
| Automated in situ Sensors | Continuous real-time monitoring, various parameter measurements | High setup and maintenance costs, limited to fixed locations | High initial, moderate to high maintenance | High | [458] |
| Modeling and Forecasting | Predictive, integrates multiple data sources, proactive management | Requires accurate data and robust models, high computational resources | High | High | [459] |
| Technology Type | Example Methods | Description | Refs. |
|---|---|---|---|
| Chemical Treatments | Nutrient Binding Agents | Uses chemicals to precipitate or bind nutrients | [460,461] |
| Biological Treatments | Bioremediation, Aquatic Plants | Utilizes microorganisms or plants to remove nutrients | [462,463] |
| Physical Treatments | Dredging, Aeration and Oxygenation, Flocculation | Involves physical processes to remove or reduce nutrients | [464] |
| Constructed Wetlands | Natural Filtration | Mimics natural wetlands for nutrient removal | [465,466] |
| Advanced Technologies | Membrane Filtration, Electrochemical Treatment, Photocatalysis | Uses advanced methods to filter or degrade nutrients | [467] |
| Innovative Technologies | Biochar Application, Microbial Fuel Cells | Employs emerging technologies for nutrient removal and management | [468] |
| Bacteria | Mechanism | Usage Scenarios | Refs. |
|---|---|---|---|
| Pseudomonas | Degrades organic matter and assimilates nutrients | Used in nutrient-rich water bodies to reduce nitrogen and phosphorus levels | [508,509] |
| Bacillus | Breaks down organic matter and assimilates nutrients | Applied in coastal areas to mitigate nutrient pollution | [510,511] |
| Nitrosomonas | Converts ammonia to nitrite (nitrification) | Used in aquaculture and wastewater treatment to manage ammonia levels | [512,513] |
| Nitrobacter | Converts nitrite to nitrate (nitrification) | Applied in marine environments to complete the nitrification process | [514,515] |
| Rhodobacter | Uptakes and removes phosphorus | Utilized in phosphorus-rich environments to reduce eutrophication | [516,517] |
| Alcaligenes | Degrades organic pollutants and nutrients | Applied in wastewater treatment and coastal waters | [518] |
| Treatment | Mechanism | Usage Scenarios | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|
| Dredging | Removes nutrient-rich sediments | Effective in areas with significant sediment buildup | Immediate reduction in internal nutrient loading | Expensive, disrupts benthic habitats | [558,562] |
| Aeration and Oxygenation | Introduces oxygen to enhance aerobic conditions and reduce nutrient release | Suitable for lakes, ponds, and enclosed coastal areas | Improves oxygen levels and water quality | Requires continuous operation and energy input | [559,563] |
| Flushing | Increases water circulation to dilute and remove nutrients | Effective in enclosed or semi-enclosed water bodies | Reduces nutrient concentrations and improves water movement | Dependent on tidal or external water sources | [560] |
| Constructed Wetlands | Filters and absorbs nutrients through plant uptake and sedimentation | Suitable for nutrient runoff areas | Provides habitats and improves water quality | Requires large areas and ongoing maintenance | [564,565] |
| Barriers and Curtains | Prevents spread of nutrients and algal blooms | Effective in localized bloom control | Immediate containment of algal blooms | Limited to small, contained areas | [566] |
| Sediment Capping | Covers nutrient-rich sediments with inert material to prevent nutrient release | Suitable for areas with high nutrient sediment loads | Prevents nutrient release and improves water quality | Can be expensive, may require repeated applications | [567,568] |
| Technology Used | Main Species Involved | Location | Refs. |
|---|---|---|---|
| Subsurface Flow Wetlands | Phragmites australis, Typha angustifolia | Coastal areas in China and the USA | [573,574,575,576] |
| Surface Flow Wetlands | Scirpus validus, Juncus effusus | Coastal regions in Europe, Australia | [577] |
| Hybrid Wetlands | Carex spp., Cyperus papyrus | Coastal regions in North America and Europe | [578,579] |
| Vertical Flow Wetlands | Canna indica, Phragmites australis | Coastal areas in Asia and the Mediterranean | [580,581] |
| Free Water Surface Wetlands | Eichhornia crassipes, Lemna minor | Tropical and subtropical regions worldwide | [582,583] |
| Constructed Mangrove Wetlands | Avicennia marina, Rhizophora stylosa | Southeast Asia and Northern Australia | [584,585] |
| Constructed Seagrass Beds | Zostera marina, Thalassia testudinum | Mediterranean and Caribbean coastal regions | [586] |
| Technology | Mechanism | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Nano-technology | Uses nanoparticles to target and adsorb nutrients | High precision in targeting specific contaminants | Potential ecological risks, high cost | [588,589] |
| Electrocoagulation | Uses electrical currents to coagulate and remove suspended particles | Effective in removing a wide range of contaminants | High energy consumption, maintenance requirements | [590,591,592] |
| Ultrasonic Treatment | Disrupts algal cells using high-frequency sound waves | Non-chemical, prevents algal bloom formation | Limited to small areas, high initial setup cost | [593,594] |
| Advanced Oxidation Processes | Uses strong oxidants to degrade organic pollutants and reduce nutrient levels | Effective in degrading a wide range of pollutants | High operational cost, potential formation of by-products | [595] |
| Method | Advantages | Disadvantages | Product after Treatment | Function of the Product | Refs. |
|---|---|---|---|---|---|
| Biological Treatment | Eco-friendly, can selectively target harmful species | Slow process, needs specific environmental conditions | Biogas, organic fertilizers | Renewable energy, nutrient recycling | [598,599] |
| Thermophilic Fermentation | High energy yield, effective pathogen destruction | Requires controlled conditions, infrastructure investment | Biogas, biochar | Renewable energy, soil enhancement | [600,601] |
| Dewatering and Drying | Simple technology, reduces volume of algae | Energy-intensive, potential loss of nutrients | Dried algae powder | Animal feed, fertilizer additive | [602] |
| Pyrolysis | Converts algae into energy-rich products, reduces biomass | High temperature requirement, complex setup | Bio-oil, biochar | Renewable energy, carbon sequestration | [603,604] |
| Anaerobic Digestion | Converts algae to biogas, reduces odor | Requires pretreatment, slow process | Biogas, digestate | Renewable energy, soil conditioner | [605] |
| Treatment | Advantages | Disadvantages | Cost | Sustainability |
|---|---|---|---|---|
| Physical Treatments | Immediate results, effective at removing algae and debris | High operational and maintenance costs, short-term solution | High | Low (temporary solution) |
| Nano-technology | High precision, effective at targeting specific contaminants | Potential ecological risks, high cost, limited large-scale application | Very High | Moderate (potential risks) |
| Electrocoagulation | Effective for a wide range of contaminants, relatively fast | High energy consumption, maintenance requirements, potential chemical by-products | High | Moderate |
| Advanced Oxidation | Effective in degrading various pollutants, non-selective | High operational cost, potential formation of harmful by-products | Very High | Moderate (risk of by-products) |
| Constructed Wetlands | Natural, sustainable, enhances biodiversity | Requires large land area, long establishment time | Moderate | High (long-term benefits) |
| Phytoremediation | Natural, cost-effective, enhances habitat | Limited to certain types of contaminants, slow process | Low to Moderate | High (natural process) |
| Bioaugmentation | Targets specific contaminants, enhances microbial diversity | Potential for non-native species introduction, monitoring required | Moderate | Moderate to High |
| Biomanipulation | Enhances ecosystem balance, uses natural predators | Complex to manage, slow process | Low to Moderate | High (natural balance) |
| Chemical Treatments | Fast-acting, effective for immediate nutrient reduction | Potential toxic effects, temporary solution, repeated applications needed | Low to Moderate | Low (temporary and potentially harmful) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. https://doi.org/10.3390/w16172525
Lan J, Liu P, Hu X, Zhu S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water. 2024; 16(17):2525. https://doi.org/10.3390/w16172525
Chicago/Turabian StyleLan, Jiaxin, Pengfei Liu, Xi Hu, and Shanshan Zhu. 2024. "Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment" Water 16, no. 17: 2525. https://doi.org/10.3390/w16172525
APA StyleLan, J., Liu, P., Hu, X., & Zhu, S. (2024). Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water, 16(17), 2525. https://doi.org/10.3390/w16172525






