Degradation of Aniline and Antimony in Printing and Dyeing Wastewater by Micro-Oxygenated Hydrolytic Acidification and Their Removal Effects on Chemical Oxygen Demand and Ammonia Nitrogen
Abstract
1. Introduction
2. Material and Methods
2.1. Inoculation
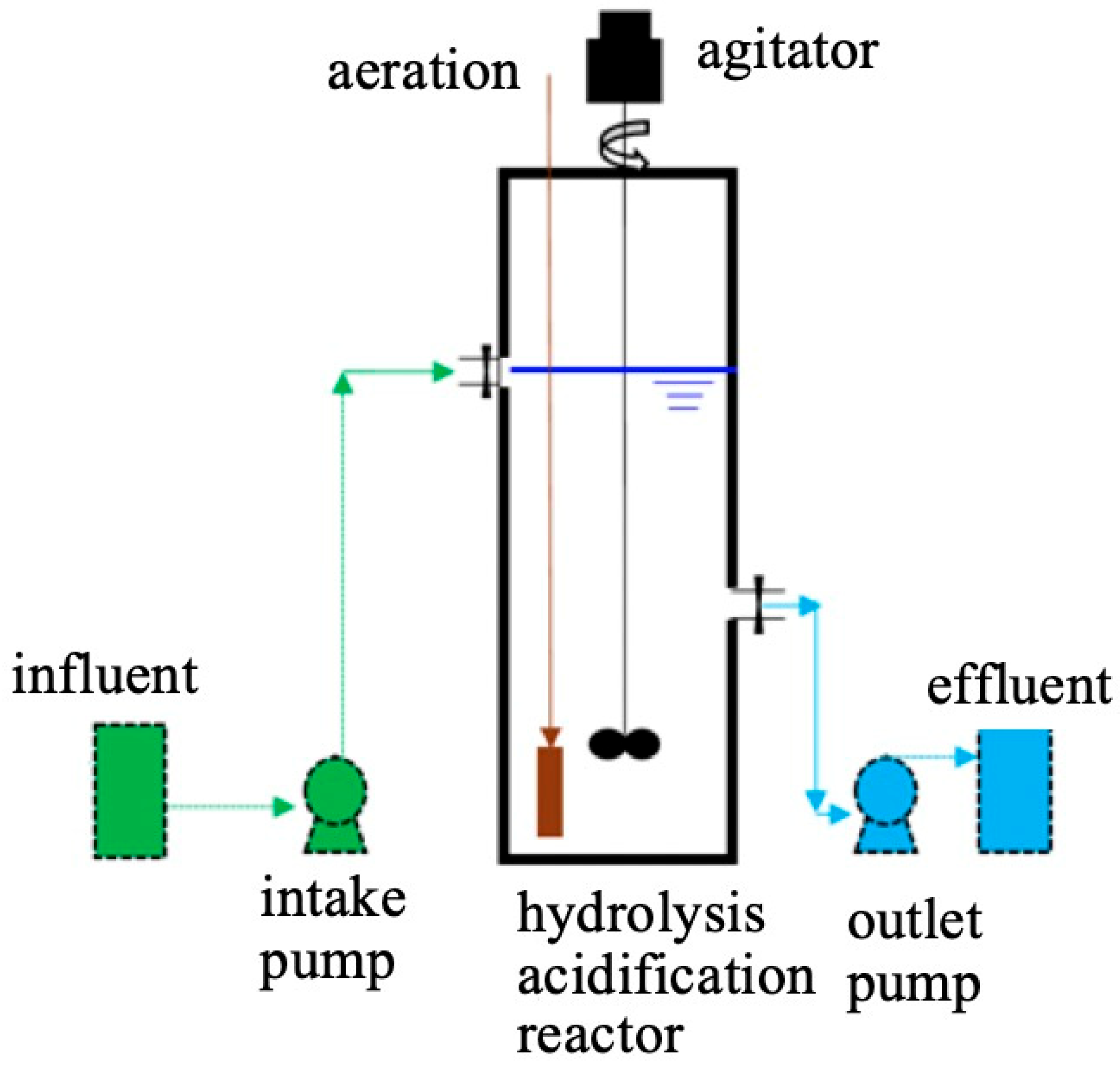
2.2. Experimental Setup
2.3. Experimental Method
2.4. Analytical Method
3. Results and Discussion
3.1. Factors Analysis of the Hydrolysis Acidification Process
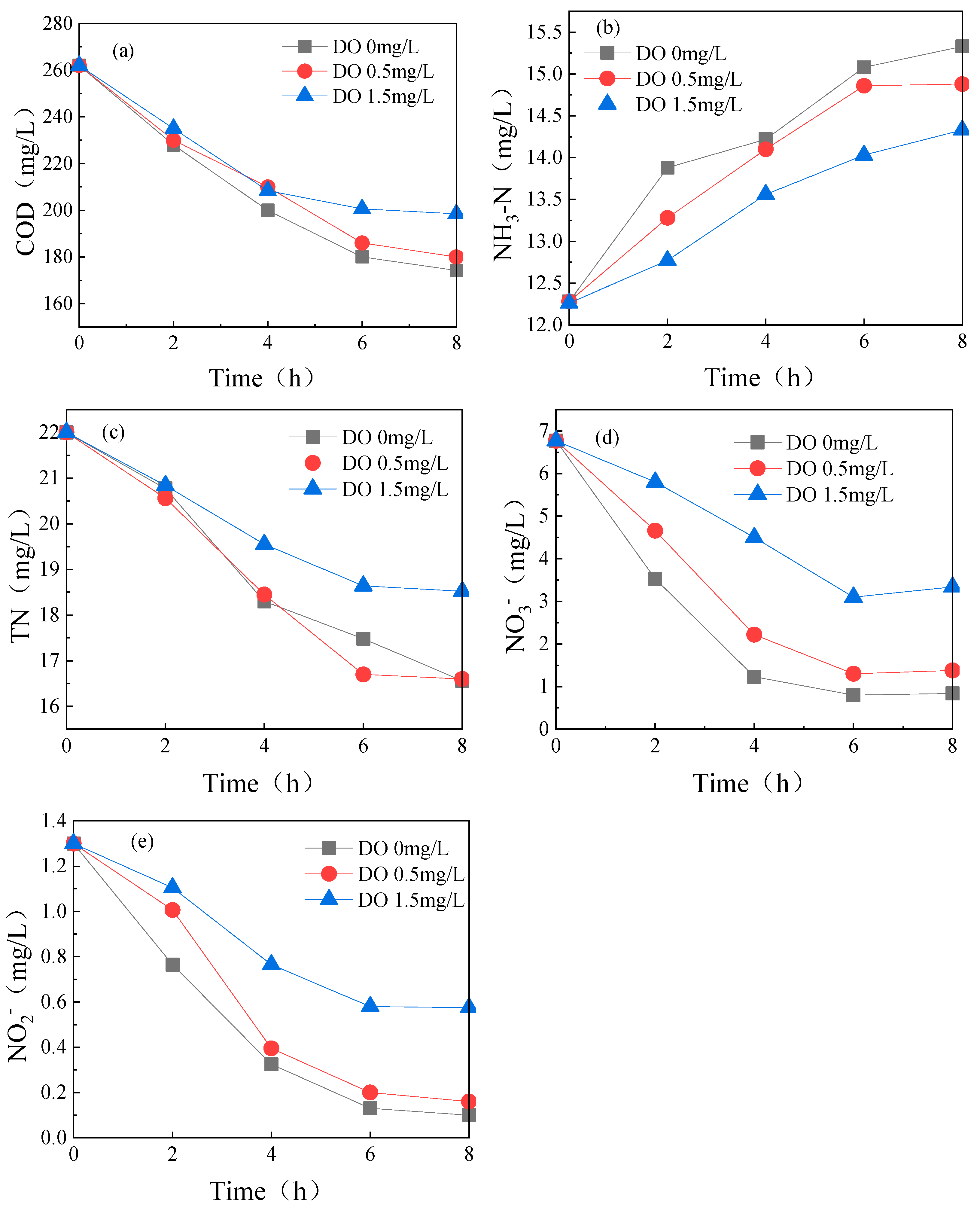
3.1.1. Dissolved Oxygen
3.1.2. pH Value
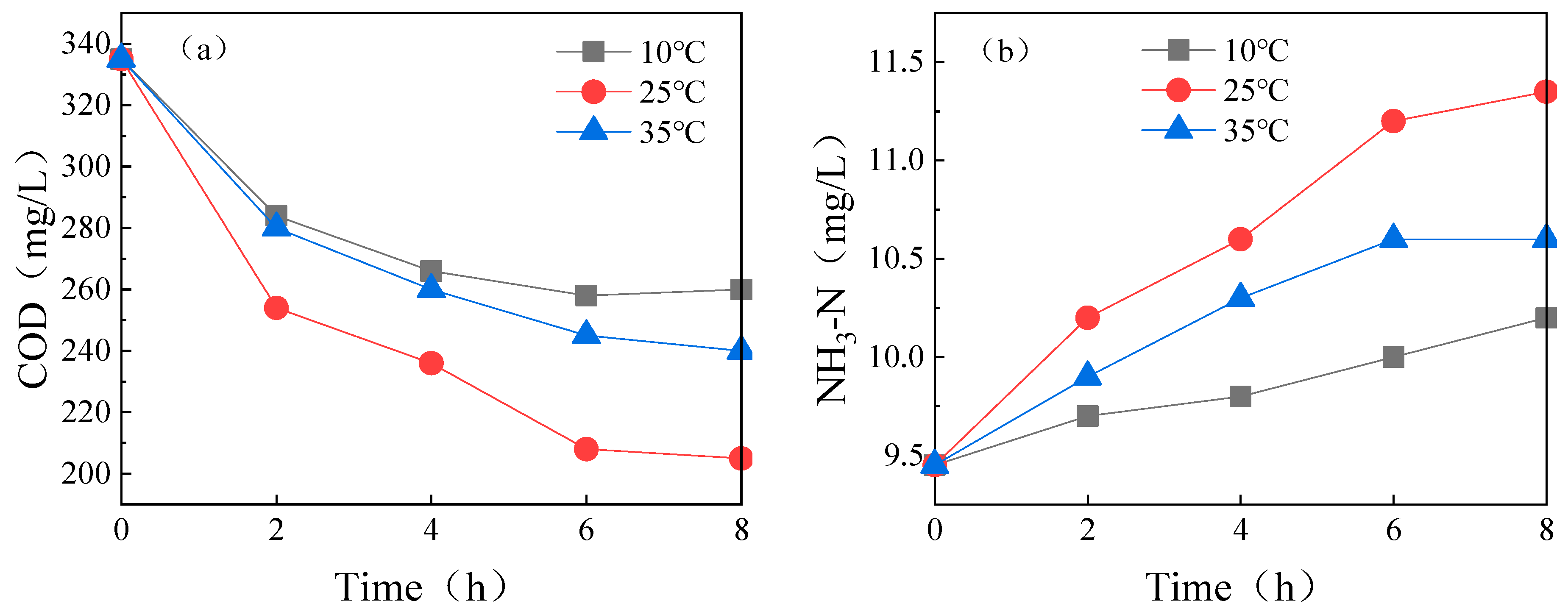
3.1.3. Temperature
3.1.4. Sludge Concentration
3.2. Effect of Aniline and Antimony on the Removal of COD and NH3-N
3.2.1. Effect of Aniline on COD Removal
3.2.2. Effect of Antimony on COD Removal
3.2.3. Effect of Aniline on NH3-N Removal
3.2.4. Effect of Antimony on NH3-N Removal
3.3. Effect of Aniline and Antimony on Sludge Penetration
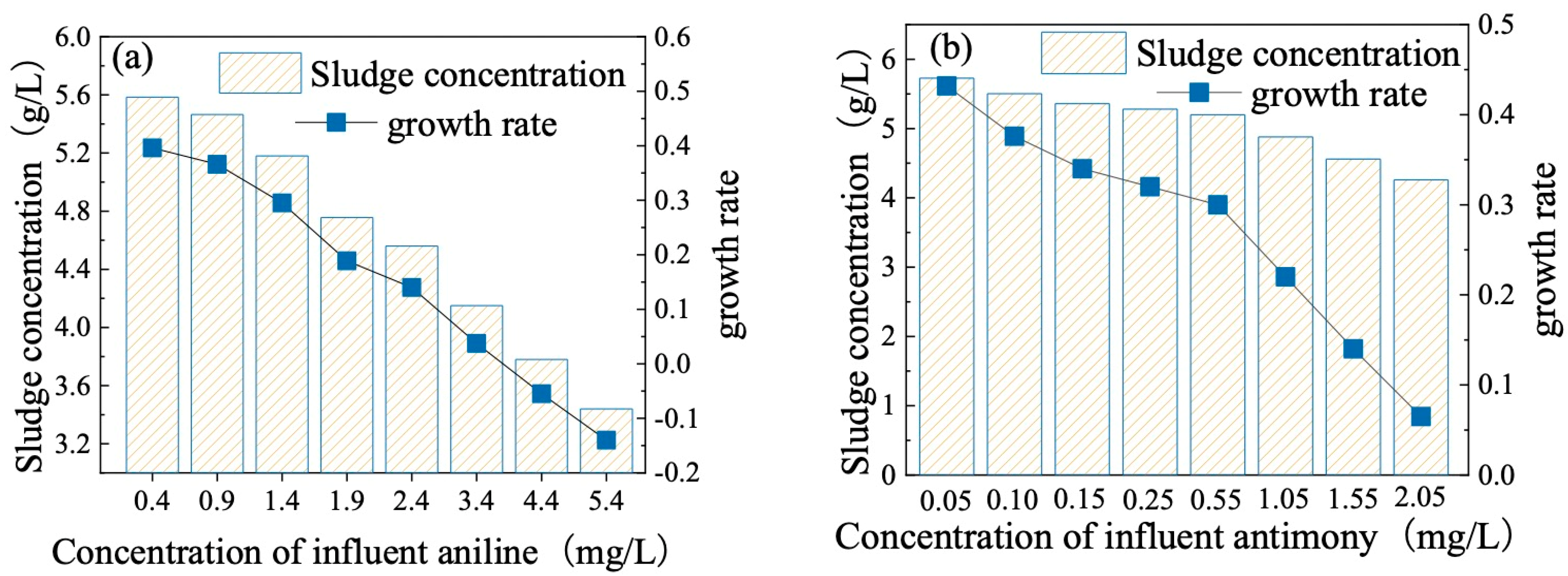
3.4. Effect of Aniline and Antimony on Sludge Concentration Change
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Li, F.; Xia, Q.; Gao, Y.; Cheng, Q.; Ding, L.; Yang, B.; Tian, Q.; Ma, C.; Sand, W.; Liu, Y.J.E.S.W.R.; et al. Anaerobic biodegradation and decolorization of a refractory acid dye by a forward osmosis membrane bioreactor. Water Res. Technol. 2018, 4, 272–280. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Mei, Y.; Pan, B. Treatment of reverse-osmosis concentrate of printing and dyeing wastewater by electro-oxidation process with controlled oxidation-reduction potential (ORP). Chemosphere 2018, 201, 621–626. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Song, X.; Sand, W. Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: A mini-review. World J. Microbiol. Biotechnol. 2018, 34, 165. [Google Scholar] [CrossRef]
- GB 4287-2012; Discharge Standards of Water Pollutants for Dyeing and Finishing of Textile Industry [including MODIFICATION 1]. Standardization Administration of China (SAC): Beijing, China, 2012. Available online: https://www.chinesestandard.net/PDF.aspx/GB4287-2012 (accessed on 28 July 2024).
- Chen, Q.; Yao, Y.; Zhao, Z.; Zhou, J.; Chen, Z. Long term catalytic activity of pyrite in Heterogeneous Fenton-like oxidation for the tertiary treatment of dyeing wastewater. J. Environ. Chem. Eng. 2021, 9, 105730. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Li, W.; Li, Y.; Wang, G.; Li, A.; Li, H. Efficient removal of acid dyes using permanent magnetic resin and its preliminary investigation for advanced treatment of dyeing effluents. J. Clean. Prod. 2020, 251, 119694. [Google Scholar] [CrossRef]
- Yao, H.-Y.; Guo, H.; Shen, F.; Li, T.; Show, D.-Y.; Ling, M.; Yan, Y.-G.; Show, K.-Y.; Lee, D.-J. Anaerobic-aerobic treatment of high-strength and recalcitrant textile dyeing effluents. Bioresour. Technol. 2023, 379, 129060. [Google Scholar] [CrossRef] [PubMed]
- Ewuzie, U.; Saliu, O.D.; Dulta, K.; Ogunniyi, S.; Bajeh, A.O.; Iwuozor, K.O.; Ighalo, J.O. A review on treatment technologies for printing and dyeing wastewater (PDW). J. Water Process Eng. 2022, 50, 103273. [Google Scholar] [CrossRef]
- Guo, G.; Tian, F.; Zhang, L.; Ding, K.; Yang, F.; Hu, Z.; Liu, C.; Sun, Y.; Wang, S. Effect of salinity on removal performance in hydrolysis acidification reactors treating textile wastewater. Bioresour. Technol. 2020, 313, 123652. [Google Scholar] [CrossRef]
- Xie, X.; Qin, Y.; Yang, S.; Sun, Y.; Mo, H.; Zheng, H.; Liu, N.; Zhang, Q. Effect of Enhanced Hydrolytic Acidification Process on the Treatment of Azo Dye Wastewater. Molecules 2023, 28, 3930. [Google Scholar] [CrossRef]
- Liu, N.; Xie, X.; Yang, B.; Zhang, Q.; Yu, C.; Zheng, X.; Xu, L.; Li, R.; Liu, J. Performance and microbial community structures of hydrolysis acidification process treating azo and anthraquinone dyes in different stages. Environ. Sci. Pollut. Res. 2017, 24, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Yin, Q.; Wang, Z.; He, K.; Wu, G. Color and nitrogen removal from synthetic dye wastewater in an integrated mesophilic hydrolysis/acidification and multiple anoxic/aerobic process. Chemosphere 2018, 212, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, D.; Zhang, W. Newly Designed Hydrolysis Acidification Flat-Sheet Ceramic Membrane Bioreactor for Treating High-Strength Dyeing Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 777. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, X.; Yao, Z.; Li, H.; Wang, D.; Tian, M.; Xu, Z.; Wan, J. A novel inhibition mechanism of aniline on nitrification: Aniline degradation competes dissolved oxygen with nitrification. Sci. Total Environ. 2021, 770, 145205. [Google Scholar] [CrossRef]
- DB32/T 3410-2018; Interface Specification of Data Catalog Service for Food Safety Electronic Traceability. Jiangsu Provincial Market Supervision and Administration: Nanjin, China, 2018. Available online: https://www.codeofchina.com/standard/DB32T3410-2018.html (accessed on 28 July 2024).
- DB32/T 3761.20-2020; Technical Specifications of Novel Coronavirus Pneumonia Prevention and Control. Part 20: Medical Waste Disposal Institution. Jiangsu Provincial Market Supervision and Administration: Nanjin, China, 2020. Available online: https://www.codeofchina.com/standard/DB32T3761.20-2020.html (accessed on 28 July 2024).
- Tao, Y.; Su, H.; Li, H.; Zhu, Y.; Shi, D.; Wu, F.; Sun, F. Ecological and human health risk assessment of antimony (Sb) in surface and drinking water in China. J. Clean. Prod. 2021, 14, 128514. [Google Scholar] [CrossRef]
- Bai, J.; Xu, H.; Zhang, Y.; Peng, Z.; Xu, G. Combined industrial and domestic wastewater treatment by periodic allocating water hybrid hydrolysis acidification reactor followed by SBR. Biochem. Eng. J. 2013, 70, 115–119. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Development of partial nitrification as a first step of nitrite shunt process in a Sequential Batch Reactor (SBR) using Ammonium Oxidizing Bacteria (AOB) controlled by mixing regime. Bioresour. Technol. 2016, 221, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.J.; Bashir, M.J.K.; Ng, C.A.; Sethupathi, S.; Lim, J.-W. A sequential treatment of intermediate tropical landfill leachate using a sequencing batch reactor (SBR) and coagulation. J. Environ. Manag. 2018, 205, 244–252. [Google Scholar] [CrossRef]
- GB/T 11903-1989; Water Quality. Determination of Colority. Administration of China (SAC): Beijing, China, 1989. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT11903-1989 (accessed on 28 July 2024).
- HJ 535-2009; Water Quality. Determination of Ammonia Nitrogen. Nesslerreagent Spectrophotometry. Administration of China (SAC): Beijing, China, 2009. Available online: https://www.chinesestandard.net/PDF.aspx/HJ535-2009 (accessed on 28 July 2024).
- HJ/T 346-2007; Water Quality. Determination of Nitrate-Nitrogen. Ultraviolet Spectrophotometry. Standardization Administration of China (SAC): Beijing, China, 2007. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJT346-2007 (accessed on 28 July 2024).
- GB/T 11893-1989; Water Quality. Determination of Total Phosphorus. Ammonium Molybdate Spectrophotometric Method. Administration of China (SAC): Beijing, China, 1989. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT11893-1989#:~:text=This%20standard%20specifies%20the%20potassium,the%20determination%20of%20total%20phosphorus (accessed on 28 July 2024).
- GB 11889-89; Water Quality—Determination of Amiline Compounds—Spectrophotometric Method with N-(1-naphthyl)ethylenediamine. Standardization Administration of China (SAC): Beijing, China, 1990. Available online: https://www.mee.gov.cn/image20010518/3758.pdf (accessed on 28 July 2024).
- HJ 776-2015; Water Quality—Determination of 32 Elements-Inductively Coupled Plasma Optical Emission Spectrometry. Administration of China (SAC): Beijing, China, 2015. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJ776-2015 (accessed on 28 July 2024).
- Huang, C.; Xiang, Z.; Huang, J.; Liu, G.; Yu, X.; Liu, W.; Cao, H. Efficient adsorptive removal of Sb(III) and Sb(V) from printing and dyeing wastewater by TiCl4. Chem. Eng. J. 2024, 493, 152858. [Google Scholar] [CrossRef]
- Feng, G.; Bai, T.; Ma, H.; Hu, Z.; Guo, Y.; Tan, W. Establishment of the Permeability Model for Soft Solid Sludge Conditioned with Flocculants. ACS Omega 2019, 4, 18574–18581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Y.; Xi, H.; Zhou, Y. Review of micro-aeration hydrolysis acidification for the pretreatment of toxic and refractory organic wastewater. J. Clean. Prod. 2021, 317, 128343. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, J.; Xie, J.; Wu, H.; Wei, C.; Li, Z.; Qiu, G.; Wei, C.; Zhu, S. Microbial community composition and function prediction involved in the hydrolytic bioreactor of coking wastewater treatment process. Arch. Microbiol. 2022, 204, 426. [Google Scholar] [CrossRef]
- An, Y.; Wang, M.; Zhou, Z.; Sun, X.; Cheng, C.; Zhu, S.; Wang, L.; Wu, Z. Enhancing biodegradability of industrial park wastewater by packing carriers and limited aeration in the hydrolysis process. J. Clean. Prod. 2020, 264, 121638. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Liu, H.; Liu, H.; Fu, B. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: Alkaline or neutral pH? Waste Manag. 2016, 48, 397–403. [Google Scholar] [CrossRef]
- Zhan, W.; Li, L.; Tian, Y.; Lei, Y.; Zuo, W.; Zhang, J.; Jin, Y.; Xie, A.; Zhang, X.; Wang, P.; et al. Insight into the roles of ferric chloride on short-chain fatty acids production in anaerobic fermentation of waste activated sludge: Performance and mechanism. Chem. Eng. J. 2021, 420, 129809. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, B.; Gong, X.; Liu, Y.; Li, G. Effects of pH buffering agents on the anaerobic hydrolysis acidification stage of kitchen waste. Waste Manag. 2017, 68, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Shi, S.; Lin, H.; Xia, Y.; He, X.; Zhou, J. The performance and microbial response of zero valent iron alleviating the thermal-alkaline stress and enhancing hydrolysis-acidification of primary sludge. J. Environ. Manag. 2023, 347, 119134. [Google Scholar] [CrossRef]
- Lin, Q.; De Vrieze, J.; Li, C.; Li, J.; Li, J.; Yao, M.; Hedenec, P.; Li, H.; Li, T.; Rui, J.; et al. Temperature regulates deterministic processes and the succession of microbial interactions in anaerobic digestion process. Water Res. 2017, 123, 134–143. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Fang, X.; Bai, Y.; Su, B.; Huang, H. Comparative study on the performance of hydrolytic acidification immobilized filler and sludge in the pretreatment of municipal wastewater. Environ. Technol. Innov. 2021, 24, 101885. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Muhmood, A.; Zhang, Q.; Wang, J.; Ruan, R.; Wang, Y.; Cui, X. Effect of time and temperature of pretreatment and anaerobic co-digestion of rice straw and swine wastewater by domesticated paddy soil microbes. J. Environ. Manag. 2022, 323, 116218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, L.; Liu, Y.; Zhang, Q.; Ruan, R.; Luo, X. Effect of acclimatized paddy soil microorganisms using swine wastewater on degradation of rice straw. Bioresour. Technol. 2021, 332, 125039. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, Y.; Sheng, Y.; Lv, X.; Yin, J. Enhancing biological denitrification with adding sludge liquor of hydrolytic acidification pretreated by high-pressure homogenization. Int. Biodeterior. Biodegrad. 2016, 113, 222–227. [Google Scholar] [CrossRef]
- Shengquan, Y.; Hui, W.; Chaohua, Z.; Xiaoming, Q. Study of the hydrolytic acidification-SBR process in aquatic products processing wastewater treatment. Desalination 2008, 222, 318–322. [Google Scholar] [CrossRef]
- Song, G.; Yu, Y.; Liu, T.; Xi, H.; Zhou, Y. Performance of microaeration hydrolytic acidification process in the pretreatment of 2-butenal manufacture wastewater. J. Hazard. Mater. 2019, 369, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Geng, J.; Hu, H.; Shi, Y.; Gao, R.; Wang, X.; Ren, H. In-situ sludge reduction performance and mechanism in an anoxic/aerobic process coupled with alternating aerobic/anaerobic side-stream reactor. Sci. Total Environ. 2021, 777, 145856. [Google Scholar] [CrossRef]
- Xie, X.; Liu, N.; Yang, B.; Yu, C.; Zhang, Q.; Zheng, X.; Xu, L.; Li, R.; Liu, J. Comparison of microbial community in hydrolysis acidification reactor depending on different structure dyes by Illumina MiSeq sequencing. Int. Biodeterior. Biodegrad. 2016, 111, 14–21. [Google Scholar] [CrossRef]
- Arora, P.K. Bacterial degradation of monocyclic aromatic amines. Front. Microbiol. 2015, 6, 820. [Google Scholar] [CrossRef]
- Xie, X.; Spiteller, D.; Huhn, T.; Schink, B.; Müller, N. Desulfatiglans anilini Initiates Degradation of Aniline With the Production of Phenylphosphoamidate and 4-Aminobenzoate as Intermediates Through Synthases and Carboxylases From Different Gene Clusters. Front. Microbiol. 2020, 11, 2064. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Constantino, C.; Dotro, G.; Cartmell, E. Mercury and antimony in wastewater: Fate and treatment. Water Air Soil Pollut. 2016, 227, 89. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the Environment: A Review Focused on Natural Waters. Part 2. Relevant Solution Chemistry. ChemInform 2003, 34, 23. [Google Scholar] [CrossRef]







| Index | Influent | Discharge |
|---|---|---|
| CODCr (mg/L) | 180–400 | 100 (50 *) |
| NH3-N (mg/L) | 7–18 | 12 (5 *) |
| Chroma | 450–700 | 70 |
| Aniline (mg/L) | 0.4–1.2 | 1.0 |
| Antimony (μg/L) | 50–150 | 50 (20 *) |
| TN (mg/L) | 12–25 | 20 |
| Nitrate (mg/L) | 3–8 | / |
| Nitrite Nitrogen (mg/L) | 0.2–0.6 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ye, S.; Liu, H. Degradation of Aniline and Antimony in Printing and Dyeing Wastewater by Micro-Oxygenated Hydrolytic Acidification and Their Removal Effects on Chemical Oxygen Demand and Ammonia Nitrogen. Water 2024, 16, 2436. https://doi.org/10.3390/w16172436
Zhang K, Ye S, Liu H. Degradation of Aniline and Antimony in Printing and Dyeing Wastewater by Micro-Oxygenated Hydrolytic Acidification and Their Removal Effects on Chemical Oxygen Demand and Ammonia Nitrogen. Water. 2024; 16(17):2436. https://doi.org/10.3390/w16172436
Chicago/Turabian StyleZhang, Kun, Shiqing Ye, and Hong Liu. 2024. "Degradation of Aniline and Antimony in Printing and Dyeing Wastewater by Micro-Oxygenated Hydrolytic Acidification and Their Removal Effects on Chemical Oxygen Demand and Ammonia Nitrogen" Water 16, no. 17: 2436. https://doi.org/10.3390/w16172436
APA StyleZhang, K., Ye, S., & Liu, H. (2024). Degradation of Aniline and Antimony in Printing and Dyeing Wastewater by Micro-Oxygenated Hydrolytic Acidification and Their Removal Effects on Chemical Oxygen Demand and Ammonia Nitrogen. Water, 16(17), 2436. https://doi.org/10.3390/w16172436






