Abstract
Manganese pollution in surface water has been a new concern in decentralized drinking water treatment. The dissolved manganese cannot be effectively removed by the traditional ultrafiltration (UF) process, but will cause severe membrane fouling. To address such issues, an innovative gravity-driven membrane (GDM) coupled with a dynamic manganese oxide (MnOx) film on the membrane surface was proposed, with hopes of enhancing manganese removal and alleviating membrane fouling. The results demonstrated that pre-coating a dynamic MnOx film on the membrane surface of a GDM system would effectively reduce start-up time for removing iron and manganese pollutants, without affecting the flux stabilization of the GDM. Effective manganese removal (~80%) primarily depended on the adsorption and auto-catalytic oxidation facilitated by the pre-coating of MnOx. Furthermore, the MnOx film notably enhanced organic pollutant removal efficiency. Additionally, the MnOx coated on the membrane surface acted as a skeleton, promoting the gradual formation of a biocake layer with a heterogeneous and porous structure, which benefited the flux stabilization of the GDM. In particular, the fine and homogeneous MnOx-M derived from the backflushing water of the mature manganese sand filter exhibited precise and uniform coating on the membrane surface, effectively mitigating the irreversible pore plugging caused by organic matter penetration and thereby enhancing stable flux by ~16.3% compared to the control. This study offered a novel strategy to enhance the purification efficiency of GDM system treating manganese pollution and was expected to contribute to the technological advancement of decentralized water supply scenarios.
1. Introduction
Recently, there has been increasingly focused on the seasonal outbreaks of manganese in surface water (such as lake water) which serves as a substantial water source for drinking water, especially in the case of rural areas [1,2,3,4]. An excessive concentration of manganese in the water would pose obvious challenges to water facilities and physical health [5,6,7]. Increasing manganese in the water supply networks would not only reduce water-carrying capacity but also facilitate microbial re-growth, along with resulting in issues of odor and discoloration to compromise water safety [8]. Moreover, a separate study even indicated that the long-term intake of manganese-excessive water showed a much higher risk of catching neurological and other related diseases [9]. Therefore, it is of great significance to remove manganese from surface water.
Compared to the cases of manganese pollution in groundwater sources, the manganese outbreak on surface water has been more difficult to diminish by conventional treatment strategies, due to the strong influence of the turbidity, particulate matter, and organic compounds in surface water. Thus, there is an urgent need for the development of new technologies [10,11]. Typically, manganese is present in water as a bivalent ion. Currently, common methods for manganese removal include automatic contact oxidation, biological oxidation, and chemical oxidation, which primarily involve oxidizing the dissolved Mn(II) ions into high-value precipitates [12,13]. The simple, cost-effective, and chemical-free approach is automatic contact oxidation; however, the presence of organics, colloids, and particles reduces the efficiency of manganese removal [14,15,16]. In most real cases, pre-treatment has been employed to pre-remove the organics, colloids, and particles, followed by the use of an automatic contact oxidation filter to remove residual manganese, which has required a long hybrid treatment process with more complex operation maintenance and energy consumption [17,18].
Ultrafiltration (UF) process can efficiently remove colloids, particles, and microorganisms to improve biosecurity, and are increasingly employed in drinking water treatment, especially for water supply purposes of rural areas [19]. However, the traditional UF technology has been ineffective in removing manganese and has instead led to severe membrane fouling [20]. In order to enhance manganese removal, pre-oxidation has been adopted in conjunction with the UF process. Potassium permanganate, a typical and popular oxidant, has been introduced for pre-oxidation before UF treatment, achieving highly effective manganese removal with effluent manganese levels as low as 0.02 mg/L [21,22]. Another study reported that using sodium hypochlorite oxidation prior to the UF process could enhance the simultaneous removal of iron and manganese [18]. However, in conventional UF processes, the involvement of pre-oxidation would cause extra chemical consumption and auxiliary equipment requirements, as well as risks of residual chemicals.
Gravity-driven membrane (GDM) technology has been extensively characterized for both its tolerance of biocake layer formation on the surface of the membrane and its distinctive operational modes which have no physical or chemical cleaning under ultra-low gravitational pressure during long-term filtration, giving it the merits of simple operation, less maintenance and less energy depletion [23,24,25]. Due to the essential role of the biocake layer, the flux of GDM system has been able to reach a stable state during prolonged filtration [26,27]. Additionally, the formation of the biocake layer can effectively promote the removal of viruses, trace organic substances (sulfamethoxazole, SMZ), ammonia, and low-molecular-weight organic compounds (assimilable organic carbon, AOC) [28,29]. Furthermore, some studies suggested that the biocake layer played a beneficial role in reducing concentrations of iron and manganese during groundwater treatment, demonstrating the potential of utilizing the GDM process for treating surface water containing iron and manganese [30,31]. However, previous studies indicated that the natural formation of biocake layer on the membrane surface of the GDM system required a reasonably long periods to achieve a steadily high removal efficiency for manganese [32,33]. Consequently, shortening the starting time for manganese removal via the GDM process holds significant importance.
Therefore, this study tried to coat activated manganese autocatalytic material on the membrane surface of the GDM system, aiming to shorten the start-up period and enhance the performance of manganese removal. The effects of different manganese autocatalytic materials on the removal of manganese and the changes in the flux of the GDM system were investigated. The structural and compositional characteristics of the biocake layer attached to the membrane surface of GDM system were evaluated. Furthermore, the mechanism behind flux stabilization and enhanced manganese removal was further explored. This study is expected to develop novel strategies using GDM technology for the removal of manganese to address manganese pollution in decentralized scenarios.
2. Materials and Methods
2.1. Characteristics of Feed Water
In this experiment, the feed water was simulated iron- and manganese-containing surface water, which was prepared by diluting domestic wastewater with ultrapure water at a ratio of 1:30, while maintaining the concentration of iron at 0.6 ± 0.3 mg/L and manganese at 0.4 ± 0.1 mg/L by adding FeCl2 and MnCl2, respectively. The other water quality was characterized by a 2.1–3.8 NTU, DOC content of 2.1–5.4 mg/L, and UV254 value of 0.035–0.08 cm−1.
2.2. Experimental Materials
The manganese oxide (MnOx) utilized for membrane surface coating was sourced from a manganese sand filtration-based full-scale drinking water treatment plant that has been operational for over a decade to treat groundwater containing iron and manganese. Four types of MnOx were used and prepared as follows: (i) and (ii): the MnOxs used were coarse manganese sand (CMS, 1~2 mm) and fine manganese sand (FMS, 0.5~1 mm) separated from the ordinary manganese sand through a screen mesh; (iii): the activated MnOx was scrapped from the surface of the manganese sand that was taken out from the operational manganese sand filter, and defined as MnOx-S; (iv): the activated MnOx was extracted from the backflushing water of a manganese sand filter using a filtration mesh, and defined as MnOx-M. All obtained activated MnOxs were rinsed with deionized water to remove any remaining solutes, then dried in a dryer at 80 °C for 24 h.
The static coating method was employed, with the flat membrane module positioned horizontally. Then, the MnOx turbidite solution was carefully poured onto the membrane surface. After gently shaking the membrane assembly, the turbid solution naturally and uniformly spread across the entire membrane surface, and subsequently settled for 60 min to ensure fixation.
2.3. Experimental Setup
The experimental GDM system and scheme are presented in Figure 1. The GDM system consisted of a feed tank, a constant tank, pipes, a pump, membrane modules, and collection bottles. The membrane module employed the Microdyn-Nadir PES UF membrane with a molecular weight cut-off (MWCO) of 150 kDa and an effective filtration area of 76.87 cm2. The GDM operated at an operating pressure of 7 kPa, which was provided by the difference in the distance (70 cm) of the liquid level between the feed water tank and the membrane reactor. A magnetic stirrer with a rotational speed of 100 r/min was set at the bottom of the constant water tank to ensure uniform water quality.

Figure 1.
Schematic diagram of the experimental GDM system.
In the GDM process of treating the iron- and manganese-containing surface water, the membrane reactor without the MnOx coating was established and defined as the GDM control. Moreover, four types of MnOx, namely CMS, FMS, MnOx-S, and MnOx-M, were pre-coated onto the UF membrane surface. Thus, the experimental systems corresponded to the designations of GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M, respectively. At least three GDM modules were arranged in parallel to minimize experimental errors. The experimental ambient temperature was maintained at 20 ± 1 °C.
2.4. Characterization and Analysis
The morphology and distribution of MnOx on the membrane surface were observed by the stereomicroscope (Olympus C-7070, Olympus, Tokyo, Japan). The cross-sectional structure and 3D characteristics of the MnOx-based biocake layer were captured using an optical coherence tomography system (Thorlabs GmbH, Dachau, Germany) with a central light source wavelength of 930 nm.
An inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 5300DV, PerkinElmer, Waltham, USA) was introduced to investigate the contents of Fe2+ and Mn2+ in both the raw water and GDM effluent. The detected solution was acidified using a sulfuric acid solution before performing the measurement. The dissolved organic carbon (DOC) in the water sample was ascertained using a total organic carbon analyzer (Multi N/C 2100 S, Jena, Germany), and the UV254 value was determined using a spectrophotometer (T6, Puxi, Beijing, China). The pH was recorded using a portable pH meter.
3. Results and Discussion
3.1. Removal Performance of Iron and Manganese
3.1.1. Iron Removal
Figure 2 illustrates the iron removal of each GDM system against filtration time. It can be seen that the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M exhibited an efficient removal capacity for the iron, with average removal rates of 95.46%, 94.0%, 95.63%, 94.44%, and 92.61%, respectively. Additionally, despite the fluctuation of iron concentration within the feed water ranging from 0.6 to 0.9 mg/L, the iron levels in all five GDM effluents decreased to a range of 0.055–0.087 mg/L, demonstrating the exceptional resilience of the GDM towards fluctuations in feed water quality. The extremely low iron content in the GDM effluent was consistent with the drinking water health standards. In general, the Fe2+ in the feed water could be easily oxidized due to its high reducibility, resulting in the formation of larger-sized Fe3+ precipitates that could be effectively rejected by the UF membrane. Furthermore, previous research indicated that ferrous ions could react with manganese oxides, usually following the following formula: 4H+ + MnO2 + 2Fe2+ = Mn2+ + 2Fe3+ + 2H2O. In addition, compared with the conventional GDM process seen in previous studies, the start-up time of iron removal was significantly shortened by pre-coating MnOx on the film surface, and higher iron removal occurred at the beginning of the experiment [34]. Thus, the membrane surface with the pre-coating of MnOx film was advantageous for improving iron removal and ensuring efficient stabilization duringlong-term operation.
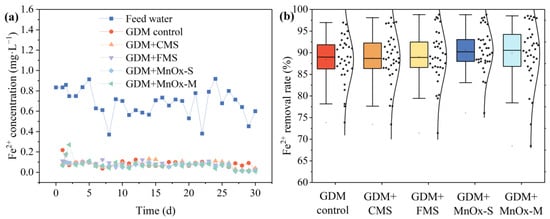
Figure 2.
(a) Fe2+ content in the feed water and GDM effluent; (b) averaged Fe2+ removal efficiency.
3.1.2. Manganese Removal
The manganese removal using the five GDM systems is depicted in Figure 3. Obviously, the GDM control demonstrated a relatively limited efficacy in removing the manganese, with only 15.09% of manganese removed during the whole experimental filtration period. It is well known that Mn2+, due to its weakened reducibility, is difficult to be oxidized by dissolved oxygen, necessitating the use of oxidants for its effective removal [35]. Specifically, the removal of manganese using the GDM process mainly relied on the adsorption, microbial degradation, and catalytic oxidation provided by the biocake layer, while the development of a mature biocake layer formed on the membrane surface typically required a long duration of ~20 to 40 days [36]. Thus, the GDM control showed relatively low and slow manganese removal, which was ascribed to the challenge in the self-establishment of a biocake layer with efficient manganese removal in a short time. Previous studies have also shown similar results, taking a longer time to effectively remove manganese [20]. Significantly, the four GDMs with the pre-coating of MnOx exhibited rapid and efficient manganese removal, with an average removal rate surpassing 60% within 2 days. In particular, the manganese concentration in the effluent of GDM + MnOx-S and GDM + FMS remained below 0.1 mg/L over a period of 2–12 days post-filtration. The results suggested that the pre-coating of MnOx on the membrane surface endowed GDM with a significant enhancement in manganese removal, attributable to its auto-catalytic oxidation function [37]. Additionally, the improvements in the removal capacity of manganese by MnOx for the GDMs were ranked in the following order: GDM + MnOx-M > GDM + FMS > GDM + MnOx-S > GDM + CMS, which should be related to the differences in the inherent active components and coating properties of the different manganese oxides.
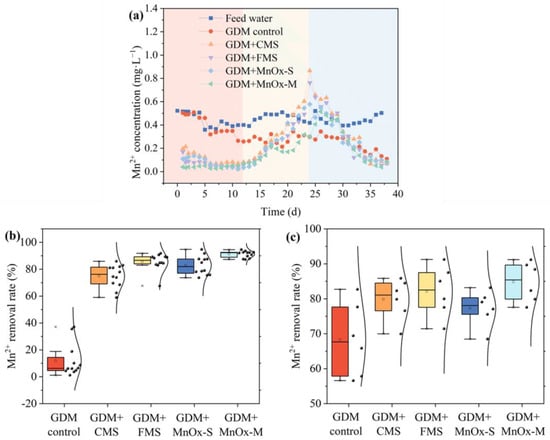
Figure 3.
(a) Mn2+ content in the feed water and GDM effluent; averaged Mn2+ removal efficiency over (b) 2–12 days and (c) 34–39 days.
Combined with the contents of Section 3.4, the conjecture was confirmed by the fact that the coverage of the membrane surface increased with the decrease in the particle size of the pre-coated MnOx. The fine and uniform MnOx-M formed a precise and uniform manganese oxide coating on the surface of the membrane, demonstrated the highest autocatalytic oxidation efficiency, and effectively improved the ability to remove manganese.
However, following a 12-day operation of the GDM system, there was a noted decrease in manganese removal, which was attributed to the adjustment of pH in the feed water to an acidic level due to a problem with the acid pump. The presence of acidic conditions in the feed water can impede the auto-catalytic oxidation of manganese, thereby diminishing its removal efficiency through the GDM process. Moreover, under the acidic conditions, the pre-coating of MnOx and accumulated MnOx products were prone to being dissolved through acidolysis reactions, resulting in the release of dissolved manganese ions [38]. As a result, the concentration of manganese in the effluent of GDM with the pre-coating of MnOx gradually increased and eventually exceeded that of the feed water throughout the operational period of 12–24 days. Until day 23, the pH of the feed water was maintained within a neutral range, resulting in a gradual decrease in manganese concentration in the GDM effluent. Subsequently, the manganese removal in the MnOx pre-coating-based GDM systems recovered, accounting for the outstanding average removal rate of 81.3% on days 34–39. The pH levels of the feed water were found to be instrumental in manganese removal, necessitating careful consideration for its promising application. Overall, the application of a pre-coated MnOx film to the membrane surface within a GDM system can significantly expedite the start-up period for manganese removal, thereby proving highly significant in practical applications.
3.2. Removal of Organic Pollutants
During the drinking water treatment process, organic matter in surface water serves as another pollutant. The removal performance of GDM for organic contaminants was evaluated based on the analysis of the removal of DOC and UV254, as shown in Figure 4. The DOC concentrations in the effluents of the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M decreased to 4.05–2.00 mg/L, 3.37–1.85 mg/L, 3.07–1.64 mg/L, 3.18–1.71 mg/L, and 3.12–1.12 mg/L, with average removal rates of 31.27%, 36.37%, 38.07%, 36.57%, and 41.19%, respectively. Compared with the previous study using the conventional GDM to treat the iron- and manganese-containing water, pre-coating active MnOx on the membrane surface of GDM conferred an effective improvement in the removal effect of the above two organic pollutants [5]. Although the DOC concentration in the feed water fluctuated between 2.13 mg/L and 5.34 mg/L, the GDM was able to steadily remove the DOC, which was mainly attributable to the microbiological degradation function provided by the gradually formed biocake layer along with long-term filtration. In particular, the late stage of the GDM process exhibited a higher DOC removal efficiency compared to the early stage, suggesting that the enhanced biodegradation capacity in the biocake layer mainly resulted from the gradually matured microbial community through growth and reproduction.
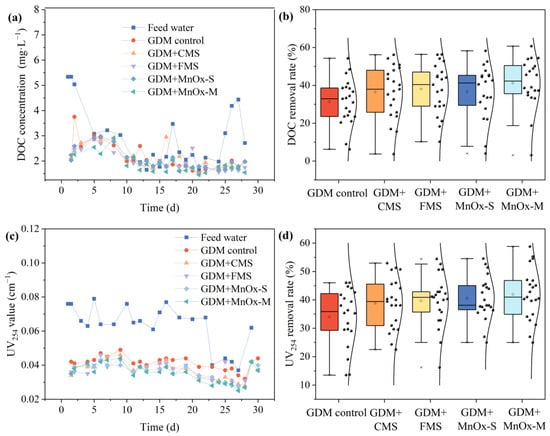
Figure 4.
(a,c) DOC and UV254 content in the feed water and GDM effluent; (b,d) removal of DOC and UV254 with GDM systems.
The value of UV254 in the feed water ranged from 0.037 to 0.079 cm−1. After filtration by the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M, the UV254 values decreased to 0.049–0.032 cm−1, 0.046–0.028 cm−1, 0.046–0.027 cm−1, 0.043–0.027 cm−1, and 0.044–0.025 cm−1, respectively, corresponding to average removal rates of 34%, 38.77%, 39.65%, 40.58%, and 41.94% (as shown in Figure 4). It can be observed that the GDM system demonstrated superior removal efficiency for UV254 compared to DOC. Apart from organic degradation by microorganisms, the biocake layer formed on the membrane surface created an enhanced rejection effect for the organics through their intermolecular adsorption or exclusion due to their increased size [27,39]. In general, the humic and aromatic organic compounds were mainly detected by UV254, and these organic matters, with a larger molecular size and functional groups, were more prone to be adsorbed and rejected by the MnOx pre-coating biocake layer, thereby resulting in higher UV254 removal. Furthermore, pre-coated MnOx on the membrane surface demonstrated an enhanced removal of organic pollutants of ~14–23% compared to the GDM control without MnOx assistance. The primary reason may be attributed to how the MnOx provided a stronger adsorption capacity and more growth sites for microorganisms, owing to its irregular surface and active components. Interestingly, the MnOx-S, MnOx-M, and FMS demonstrated superior efficacy in removing organics from the GDM compared to the CMS, which was attributable to the larger specific surface area and stronger adsorption function of the MnOx, with its smaller particle size. In short, these results indicated that the pre-coated MnOx film on the membrane surface not only exerted no detrimental influence on the effluent quality, but also showed effectiveness in organic matter removal.
3.3. Flux Development of GDM Process
Figure 5a illustrates the progression of flux across the five GDM systems. It can be seen that the development of the flux of the GDM showed a similar variation pattern and can be divided into stages I–IV over filtration time. Analogous developmental patterns have been identified in prior studies [5,40]. During phase I (1–3 days), the GDM flux linearly decreased from 43.16 to 8.58 L·m−2·h−1, attributable to the accumulation of a large amount of pollutants, such as inorganic ions, organics, and microbial components, on the membrane surface. In stage II (4–10 days), the flux of GDM gradually reached a stable stage, characterized by a slight decline and followed by a leveling off. Until stage III (11–25 days), a change in the development of the flux was observed in the five GDM systems, and showed an improvement to a certain degree. Nevertheless, as the operation of the GDM entered stage IV (26–39 days), the flux of the GDM gradually trended towards a stable stage, similar to stage II. The stabilization of flux in the GDM is crucial for the long-term operation of the GDM process without any membrane cleaning and maintenance. It can be seen that the pre-coated MnOx on the membrane surface did not cause a continuous flux decrease, while noting the stable development of the GDM flux. Previous studies indicated the heterogeneous and porous structure of the biocake layer was crucial for maintaining a stable flux [41]. In this study, pre-coated MnOx, due to its irregular shape and appropriate size, was intended to construct a heterogeneous biocake layer structure through its function as a skeleton. Supporting this, the biocake layer in the GDM exhibited a significantly heterogeneous and loose structure owing to the pre-coating of MnOx (see Figure 6 and Figure 7), which was beneficial in offsetting the increased water transport resistance caused by the coating film and in maintaining the stable development of the flux of the GDM.

Figure 5.
Flux variation in the whole experiment (a) and five groups’ stable flux (b).
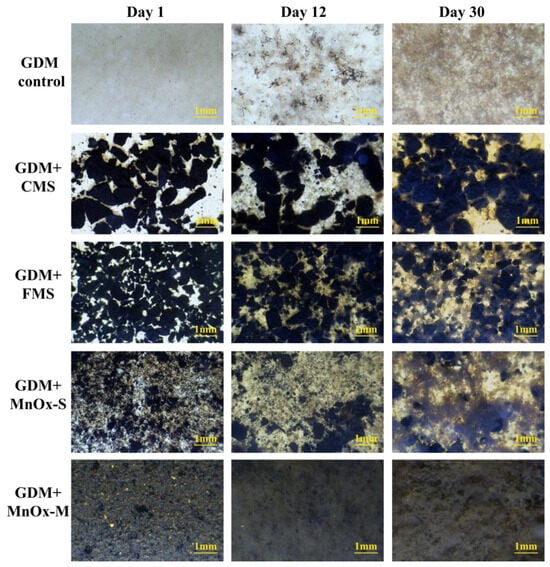
Figure 6.
The surface morphologies of the biocake layer in the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M.
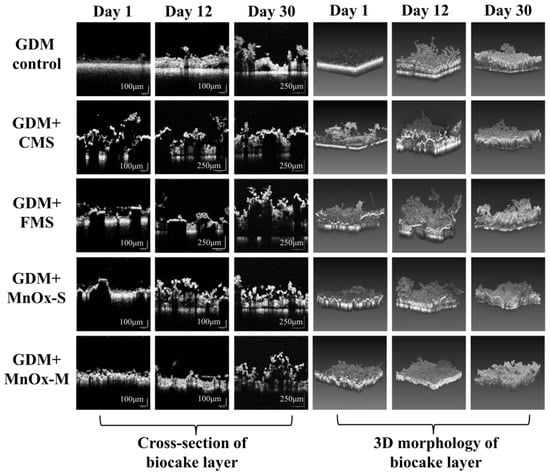
Figure 7.
The internal structure and stereo imaging morphology of the biocake layer in the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M.
As displayed in Figure 5b, the average stable flux (stage II and IV) of the GDM control, GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M were 6.37 L·m−2·h−1, 5.93 L·m−2·h−1, 6.08 L·m−2·h−1, 5.96 L·m−2·h−1, and 7.41 L·m−2·h−1, respectively. It was observed that the stable flux of GDM with MnOx-M improved by ~16.3% in comparison to the GDM control, while the stable flux of GDM pre-coated with CMS, FMS and MnOx-S decreased slightly. One explanation stated that the numerous exposed membrane areas caused by the large size of the MnOx, including CMS, FMS, and MnOx-S, contributed to the immersion of contaminants in the membrane pores, and combined with the coating resistance, thereby causing a larger water transport resistance and a relatively lower flux than the control. MnOx-M, characterized by its fine and homogeneous nature despite exceeding the pore size, facilitated the formation of a precise and uniform coverage layer on the membrane surface. Thus, the MnOx-M coated layer had a precise, uniform, and loose structure, which was beneficial for alleviating the pollution caused by the blockage of the membrane pore and to increasing the stable flux during long-term operation. Furthermore, it was important to highlight the significant increase in flux observed in the five GDM systems during Stage III. This result was mainly attributable to the decreased pH in the feed water, which affected the biocake layer, making it prone to form a loose structure due to acid humification. In particular, a segment of the biocake layer might become detached from the membrane surface during operation, leading to an increase in the permeate flux of the GDM. In summary, the pre-coating of MnOx on the membrane surface exerted a positive influence in optimizing the structure of the biocake layer to facilitate the flux stabilization of the GDM; as such, the MnOx-M demonstrated enhanced and stable flux.
3.4. Structure of GDM Biocake Layer
In this section, the membrane biocake layer was visualized using stereo microscopy and OCT at different filtration periods, which reflected its structural component, morphology, and distribution, and is displayed in Figure 6 and Figure 7. In the GDM control, it can be seen that the pollutants gradually accumulated on the membrane surface, and the formed biocake layer showed a brown and homogeneous structure. Nevertheless, a certain quantity of black particles was observed on the surface of the membrane, indicating the spontaneous formation of manganese oxides, these serving as the primary mechanism for manganese removal through MnOx catalytic oxidation in the GDM control. Previous research posited that the formation of manganese oxides on a GDM effectively contributed to manganese removal [42]. However, the development and maturity of the efficient manganese oxides were hindered by a prolonged filtration period. Thus, the relatively low and slow manganese removal in the GDM control could be attributed to the inadequate auto-catalytic oxidation provided by the small amount of MnOx.
In the GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M systems, the membrane surface was occupied by the activated MnOx, and a heterogeneous and rough biocake layer with continuous filtration was formed (Figure 6). Compared with conventional GDM studies without MnOx coating [5], the formed biocake layer under the influence of MnOx exhibits deeper coloration and increased component complexity, including the greater abundance of natural organic compounds, microorganism communities, and extracellular polymeric substances (EPS). This result was attributed to the greater accumulation of organics and microbial propagation owing to the pre-coating of MnOx creating more adsorption sites. Meanwhile, the improved organic removal mainly relied on the enhanced adsorption and microbial degradation functions supported by the MnOx. Moreover, with the reduction in the particle size of the pre-coating of MnOx, the coverage of the membrane surface increased (Figure 6). In other words, MnOx with a larger size exposed more of the membrane surface, and vice versa. Consequently, it could be reasonably inferred that the exposed membrane pores were susceptible to organic invasion and irreversible blockage fouling, thereby compromising the flux of GDM. Nevertheless, the MnOx-M materials, due to their small size, provided a precise and uniform coat for the membrane surface. This effectively prevented contaminants from deeply penetrating the membrane pores to mitigate the irreversible fouling, thereby improving the stable flux.
The cross-sectional structure and three-dimensional imaging morphology of the biocake layer are presented in Figure 7. The thickness of the biocake layer in the GDM control escalated throughout continuous filtration, which was adverse to the flux improvement. Nevertheless, the structure of biocake layer became more porous and loose, which ensured the flux stabilization. In the GDM + CMS, GDM + FMS, GDM + MnOx-S, and GDM + MnOx-M systems, the pre-coating of MnOx resulted in a rough, porous, and heterogeneous structure of the biocake layer through the skeleton’s function, which significantly mitigated the increased mass transfer resistance caused by coating and ensured the flux stabilization during the long-term operation. The 3D imaging morphology of the biocake layer also confirmed that the developed biocake layer exhibited heterogeneity and porosity. Generally, the gradual development of a microorganism community is instrumental in the structural evolution of the biocake layer. The vital activities of these microorganisms, including organic degradation, predation, and movement, played a pivotal role in the formation of the porous biocake layer and in maintaining stable flux. However, the EPS, consisting of the proteins or polysaccharides secreted by microorganisms, usually caused severe membrane fouling and flux reduction. Thus, a reasonable explanation was that the irreversible membrane fouling caused by the EPS could be efficiently alleviated by coating with manganese oxides. In particular, the MnOx-M provided a uniform and precise coating on the UF membrane surface, demonstrating higher fouling alleviation efficiency than manganese oxides used, as well as an obvious improvement in stable flux. In short, the pre-coating of the MnOx promoted the formation of heterogeneous and porous biocake layers, thereby contributing to flux stabilization. Notably, MnOx-M exhibited a superior enhancement in stable flux, which held significant implications for its practical applications.
4. Conclusions
In summary, this study proposed an innovative hybrid process using MnOx coat and GDM for the direct treatment of surface water containing iron and manganese. Pre-coating MnOx on the membrane surface of GDM process could effectively reduce the ripening time for the manganese removal, as well as enhance its removal efficiency for manganese, owing to the auto-catalytic oxidation by the activated MnOx film. Furthermore, introducing activated MnOx film into the GDM system could simultaneously enhance the removal of organic contaminants, accounting for the average removal rates of DOC and UV254 of 31.27–41.19% and 34–41.94%, respectively. Importantly, compared to the GDM control, introducing a MnOx coat on the membrane surface contributed to improving the stable flux level of the hybrid GDM process by approximately 16%, since the coated MnOx particles with irregular shapes could function as a scaffold to promote the development of the membrane biocake layer with a heterogeneous, rough, and porous structure. Consequently, pre-coating the membrane with a MnOx film could both enhance contaminant removal and promote flux elevation during long-term GDM filtration. This was of significant importance for extending the application of UF technology in decentralized drinking water supply scenarios.
Author Contributions
J.L. designed and performed the experiment, data analysis, and the manuscript writing. Y.Z., H.C., C.L., Y.H., H.W. and Y.W. contributed to the experimental investigation, data collection, discussion, and subsequent revision of the manuscript. X.T. provided the administration, funding, supervision, conceptualization, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (52370007) and Excellent Youth Foundation of Heilongjiang Province of China (YQ2022E034), the China Postdoctoral Science Foundation ((2019M651290) and (2020T130153)), and the Natural Science Foundation of the Heilongjiang Province of China (Grant No. LH2021E007).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors assert that they possess no known competing financial interests or personal relationships that could potentially have influenced the research presented in this paper.
References
- Earle, M.R.; Stoddart, A.K.; Gagnon, G.A. Raw water biofiltration for surface water manganese control. Sci. Rep. 2023, 13, 9020. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Singh, A.K. Risk assessment, statistical source identification and seasonal fluctuation of dissolved metals in the Subarnarekha River, India. J. Hazard. Mater. 2014, 265, 305–314. [Google Scholar] [CrossRef] [PubMed]
- McCormick, N.E.; Earle, M.; Ha, C.; Hakes, L.; Evans, A.; Anderson, L.; Stoddart, A.K.; Langille, M.G.I.; Gagnon, G.A. Biological and physico-chemical mechanisms accelerating the acclimation of Mn-removing biofilters. Water Res. 2021, 207, 117793. [Google Scholar] [CrossRef]
- Superville, P.-J.; Ivanovsky, A.; Bhurtun, P.; Prygiel, J.; Billon, G. Diel cycles of reduced manganese and their seasonal variability in the Marque River (northern France). Sci. Total Environ. 2018, 624, 918–925. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Zhang, H.; Yu, M.; Guo, Y.; Li, G.; Liang, H. Respective role of iron and manganese in direct ultrafiltration: From membrane fouling to flux improvements. Sep. Purif. Technol. 2021, 259, 118174. [Google Scholar] [CrossRef]
- Dion, L.-A.; Bouchard, M.F.; Sauve, S.; Barbeau, B.; Tucholka, A.; Major, P.; Gilbert, G.; Mergler, D.; Saint-Amour, D. MRI pallidal signal in children exposed to manganese in drinking water. Neurotoxicology 2016, 53, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Goher, M.E.; Khedr, A.I. Novel Combined Toxicity Indices (CTI) to assess the ecological risk of metals in sediments dependent on fractions and total metal content, application on Qarun Lake sediment, case study. Water Cycle 2024, 5, 59–75. [Google Scholar] [CrossRef]
- Hoyland, V.W.; Knocke, W.R.; Falkinham, J.O., III; Pruden, A.; Singh, G. Effect of drinking water treatment process parameters on biological removal of manganese from surface water. Water Res. 2014, 66, 31–39. [Google Scholar] [CrossRef]
- Li, G.; Ma, X.; Chen, R.; Yu, Y.; Tao, H.; Shi, B. Field studies of manganese deposition and release in drinking water distribution systems: Insight into deposit control. Water Res. 2019, 163, 114897. [Google Scholar] [CrossRef]
- Zheng, X.-w.; Fang, Y.-y.; Lin, J.-j.; Luo, J.-j.; Li, S.-j.; Aschner, M.; Jiang, Y.-m. Signal Transduction Associated with Mn-induced Neurological Dysfunction. Biol. Trace Elem. Res. 2023, 202, 4158–4169. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Huang, T.; Cheng, L.; Yao, X. Effects of coagulants on the catalytic properties of iron-manganese co-oxide filter films for ammonium and manganese removal from surface water. J. Clean. Prod. 2020, 242, 118494. [Google Scholar] [CrossRef]
- Cerrato, J.M.; Falkinham, J.O., III; Dietrich, A.M.; Knocke, W.R.; McKinney, C.W.; Pruden, A. Manganese-oxidizing and -reducing microorganisms isolated from biofilms in chlorinated drinking water systems. Water Res. 2010, 44, 3935–3945. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, X.; Wang, Y.; Wen, X.; Chang, H.; Wang, J.; Li, G.; Liang, H.; Tang, X. NaClO-based rapid sand filter in treating manganese-containing surface water: Fast ripening and mechanism. J. Environ. Chem. Eng. 2023, 11, 109082. [Google Scholar] [CrossRef]
- Bruins, J.H.; Petrusevski, B.; Siokar, Y.M.; Huysman, K.; Joris, K.; Kruithof, L.C.; Kennedy, M.D. Biological and physico-chemical formation of Birnessite during the ripening of manganese removal filters. Water Res. 2015, 69, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, R.; Huang, T.; Wen, G. The simultaneous removal of ammonium and manganese from surface water by MeOx: Side effect of ammonium presence on manganese removal. J. Environ. Sci. 2019, 77, 346–353. [Google Scholar] [CrossRef]
- Chang, H.; Sun, W.; Wang, Y.; Jiang, S.; Wang, J.; Liang, H.; Li, G.; Tang, X. Effects of organics concentration on the gravity-driven membrane (GDM) filtration in treating iron- and manganese-containing surface water. Water Res. 2022, 226, 119223. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Choo, K.H.; Lee, H.; Choi, S.J. Iron and manganese removal and membrane fouling during UF in conjunction with prechlorination for drinking water treatment. J. Membr. Sci. 2005, 267, 18–26. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Bai, Y.; Liu, R.; Chen, W.; Liu, H.; Qu, J. Enhanced alleviation of ultrafiltration membrane fouling by regulating cake layer thickness with pre-coagulation during drinking water treatment. J. Membr. Sci. 2020, 596, 117732. [Google Scholar] [CrossRef]
- Tang, X.; Qiao, J.; Wang, J.; Huang, K.; Guo, Y.; Xu, D.; Li, G.; Liang, H. Bio-cake layer based ultrafiltration in treating iron-and manganese-containing groundwater: Fast ripening and shock loading. Chemosphere 2021, 268, 128842. [Google Scholar] [CrossRef]
- Ke, Z.; Liang, H.; Sun, Y.; Wang, T.; Luo, J.; Tang, Y.; Li, G.; Tang, X.; Wang, J. Permanganate-assisted pilot-scale gravity-driven membrane (GDM) filtration in treating Mn(II)-containing groundwater: Fast startup and mechanism. J. Environ. Chem. Eng. 2024, 12, 112073. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Z.; Zhou, Z.; Ren, J.; Yang, Y.; Li, X.; Tan, X. Oxidants-assisted gravity-driven membrane (GDM) process for manganese removal from surface water: Rapid maturation and manganese oxide regulation. J. Water Process Eng. 2023, 56, 104452. [Google Scholar] [CrossRef]
- Ye, X.; Nan, J.; Ge, Z.; Xiao, Q.; Liu, B.; Chen, M.; Wu, F. Unexpected iron-enhanced water flux and pollutant removal by low-pressure ultrafiltration. J. Membr. Sci. 2023, 679, 121708. [Google Scholar] [CrossRef]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.B.; Cheng, X.X.; Zhu, X.W.; Xie, B.H.; Guo, Y.Q.; Wang, J.L.; Ding, A.; Li, G.B.; Liang, H. Ultra-low pressure membrane-based bio-purification process for decentralized drinking water supply: Improved permeability and removal performance. Chemosphere 2018, 211, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, X.; Yang, Y.; Fan, X.; Zhou, Z.; Ren, J.; Tan, X.; Li, H. Insight into biofouling mechanism in biofiltration-facilitated gravity-driven membrane (GDM) system: Beneficial effects of pre-deposited adsorbents. J. Membr. Sci. 2022, 662, 121017. [Google Scholar] [CrossRef]
- Derlon, N.; Mimoso, J.; Klein, T.; Koetzsch, S.; Morgenroth, E. Presence of biofilms on ultrafiltration membrane surfaces increases the quality of permeate produced during ultra-low pressure gravity-driven membrane filtration. Water Res. 2014, 60, 164–173. [Google Scholar] [CrossRef]
- Chen, R.; Hu, L.; Zhang, H.; Lin, D.; Wang, J.; Xu, D.; Gong, W.; Liang, H. Toward emerging contaminants removal using acclimated activated sludge in the gravity-driven membrane filtration system. J. Hazard. Mater. 2022, 438, 129541. [Google Scholar] [CrossRef]
- Ding, A.; Song, R.; Cui, H.; Cao, H.; Ngo, H.H.; Chang, H.; Nan, J.; Li, G.; Ma, J. Presence of powdered activated carbon/zeolite layer on the performances of gravity-driven membrane (GDM) system for drinking water treatment: Ammonia removal and flux stabilization. Sci. Total Environ. 2021, 799, 149415. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, X.; Huang, K.; Wang, J.; Guo, Y.; Xie, B.; Li, G.; Liang, H. Can ultrafiltration singly treat the iron- and manganese-containing groundwater? J. Hazard. Mater. 2021, 409, 124983. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Shi, D.; Liang, J.; Shao, S. Mechanism of Mn(II) removal by the cake layer containing biogenic manganese oxides in a flow-through mode: Biological or chemical catalysis? Sep. Purif. Technol. 2024, 330, 125214. [Google Scholar] [CrossRef]
- Ye, X.; Nan, J.; Ge, Z.; Xiao, Q.; Liu, B.; Men, Y.; Liu, J. Simultaneous removal of iron, manganese, and ammonia enhanced by preloaded MnO2 on low-pressure ultrafiltration membrane. J. Membr. Sci. 2022, 656, 120641. [Google Scholar] [CrossRef]
- Feng, C.-L.; Liu, C.; Yu, M.-Y.; Chen, S.-Q.; Mehmood, T. Removal performance and mechanism of the dissolved manganese in groundwater using ultrafiltration coupled with HA complexation. J. Environ. Chem. Eng. 2022, 10, 108931. [Google Scholar] [CrossRef]
- Tang, X.B.; Xie, B.H.; Chen, R.; Wang, J.L.; Huang, K.J.; Zhu, X.W.; Li, G.B.; Liang, H. Gravity-driven membrane filtration treating manganese-contaminated surface water: Flux stabilization and removal performance. Chem. Eng. J. 2020, 397, 125248. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, T.; Liu, C.; Zhang, S. Effects of dissolved oxygen on the start-up of manganese oxides filter for catalytic oxidative removal of manganese from groundwater. Chem. Eng. J. 2019, 371, 88–95. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, T.; Cheng, L.; Sun, Y.; Zhu, L.; Li, Y. Structural characteristic and ammonium and manganese catalytic activity of two types of filter media in groundwater treatment. J. Environ. Sci. 2018, 72, 89–97. [Google Scholar] [CrossRef]
- Sun, S.; Song, S.; Yang, S.; He, Y.L.; Shi, Y.; Zhou, P.; Xiong, Z.K.; Liu, Y.; Zhang, H.; Du, Y.; et al. Manganese oxide and derivative-modified materials in advanced oxidation processes: A review of modification enhancement and activation mechanisms. Chin. Chem. Lett. 2024, 35, 109242. [Google Scholar] [CrossRef]
- Shao, S.; Feng, Y.; Yu, H.; Li, J.; Li, G.; Liang, H. Presence of an adsorbent cake layer improves the performance of gravity-driven membrane (GDM) filtration system. Water Res. 2017, 108, 240–249. [Google Scholar] [CrossRef]
- Chomiak, A.; Traber, J.; Morgenroth, E.; Derlon, N. Biofilm increases permeate quality by organic carbon degradation in low pressure ultrafiltration. Water Res. 2015, 85, 512–520. [Google Scholar] [CrossRef]
- Akhondi, E.; Wu, B.; Sun, S.; Marxer, B.; Lim, W.; Gu, J.; Liu, L.; Burkhardt, M.; McDougald, D.; Pronk, W.; et al. Gravity-driven membrane filtration as pretreatment for seawater reverse osmosis: Linking biofouling layer morphology with flux stabilization. Water Res. 2015, 70, 158–173. [Google Scholar] [CrossRef]
- Chomiak, A.; Mimoso, J.; Koetzsch, S.; Sinnet, B.; Pronk, W.; Derlon, N.; Morgenroth, E. Biofilm formation and permeate quality improvement in Gravity Driven Membrane ultrafiltration. Water Sci. Technol.-Water Supply 2014, 14, 274–282. [Google Scholar] [CrossRef]
- Li, K.; Xu, W.; Han, M.; Cheng, Y.; Wen, G.; Huang, T. Integration of iron-manganese co-oxide (FMO) with gravity-driven membrane (GDM) for efficient treatment of surface water containing manganese and ammonium. Sep. Purif. Technol. 2022, 282, 119977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).