Trait Composition and Assemblage Structure Analyses of Lacustrine Fishes: Synthesizing a Proposal for Better Fishing Practices
Abstract
1. Introduction
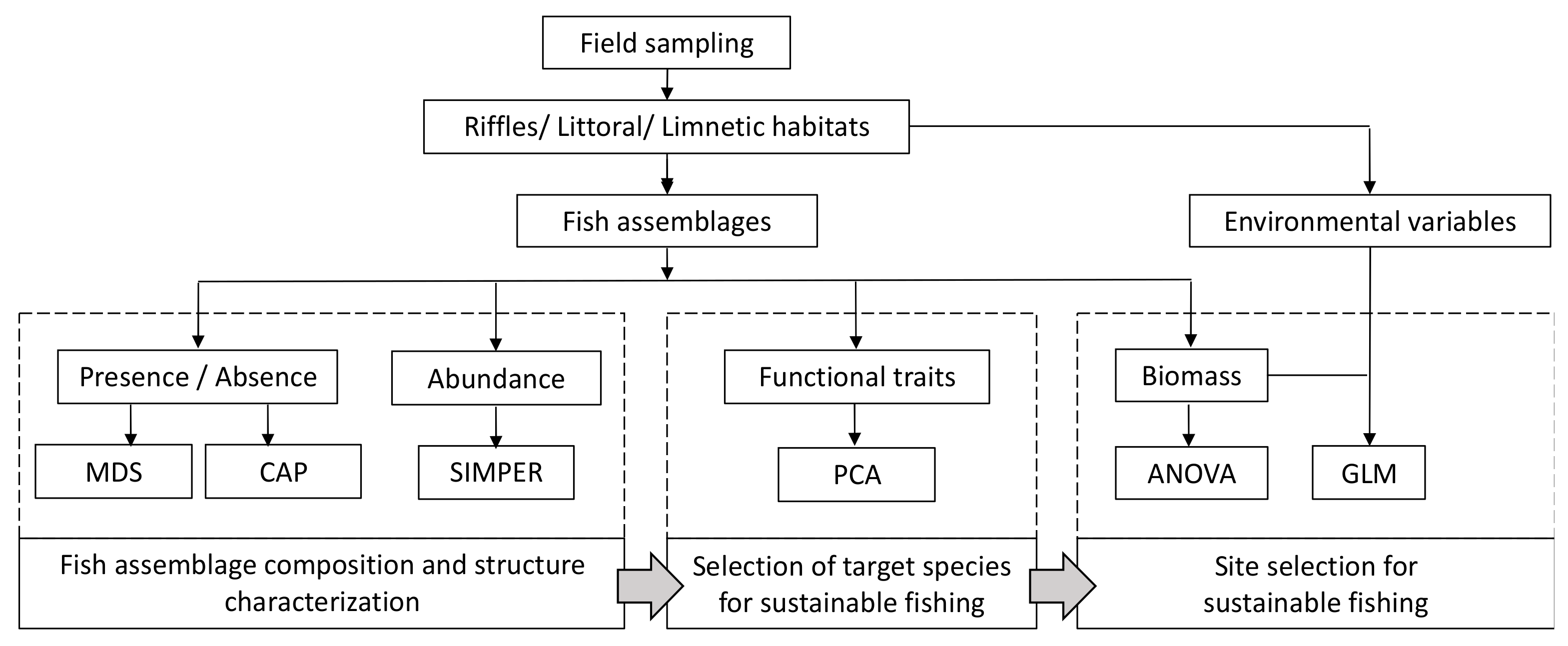
2. Materials and Methods
2.1. Study Area
2.2. Field Surveys
2.3. Statistical Analyses
3. Results
4. Discussion
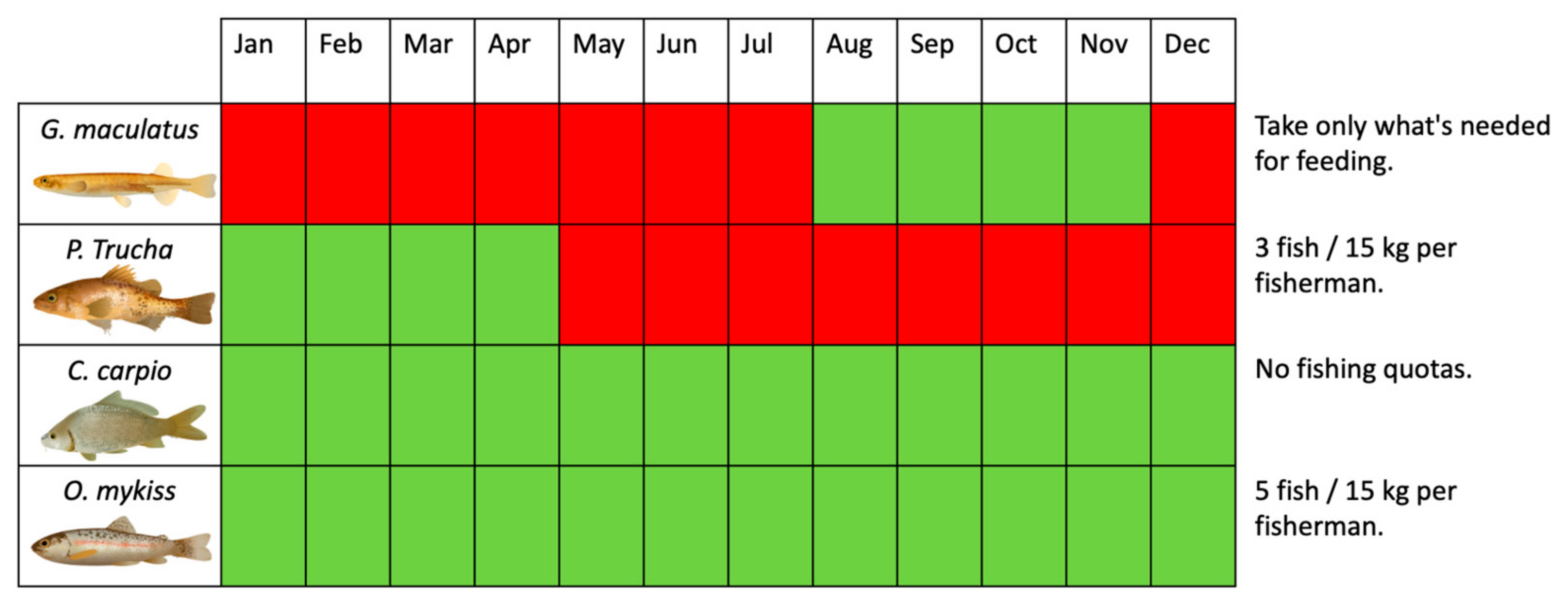
- Target species selection: We recommend specific species whose biology and ecology could support different fishing activities in the study area.
- Fishing seasons: Timing matters! We propose well-defined fishing seasons to ensure minimal impact on fish populations during critical life stages.
- Best fishing areas: Based on our analysis, we highlight areas with abundant fish biomass and ecological significance.
- Fishing quotas: Our proposal suggests appropriate catch restrictions to prevent overexploitation.
4.1. Characterization of Fish Assemblages
- (1)
- Riverine (tributaries) habitats: Streams dominated by native catfish (Trichomycterus areolatus) and rainbow trout (Oncorhynchus mykiss), as occurs in Andean and other coastal rivers [34,72,73]. Notably, the abundance and biomass of rainbow trout in tributaries suggest its competitive success over native fish in terms of food and shelter [74] (see Supplementary Materials, Table S3).
- (2)
- Lacustrine (littoral and pelagic) habitats: While rainbow trout presented a lower abundance, they were represented by larger-bodied sizes (Supplementary Materials, Table S3). This coincides with findings from other Chilean coastal lakes but diverges from Andean oligotrophic lakes [75,76]. Mesotrophic coastal lakes are predominantly dominated by the native Creole perch (Percichthys trucha) and common carp (Cyprinus carpio), consistent with our results [20]. Interestingly, only large adult specimens of Creole perch were captured in pelagic habitats. This suggests that the species is as equally abundant and successful as in southern Andean lakes, as documented by Macchi et al. and Ortiz et al. [77,78]. Furthermore, the absence of P. trucha in rivers likely reflects their habitat preferences; juvenile P. trucha inhabit shallow habitats in larger rivers during summer [44,48]. Consequently, while they may be present in the outlet river of Lanalhue Lake (Paicaví River), their absence in small tributaries is consistent with their habitat choices.
4.2. Target Species for Subsistence and Recreational Fishing
4.3. Environmental Conditions That Explain Species Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Dasgupta, P.; Dietz, T.; Duraiappah, A.K.; Hassan, R.; et al. Ecosystems and Human Well-Being-Synthesis: A Report of the Millennium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005; ISBN 978-1-59726-040-4. [Google Scholar]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Alberti, M.; Folke, C.; Moran, E.; Pell, A.N.; Deadman, P.; Kratz, T.; Lubchenco, J.; et al. Complexity of Coupled Human and Natural Systems. Science 2007, 317, 1513–1516. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem Services Generated by Fish Populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Farber, S.C.; Costanza, R.; Wilson, M.A. Economic and Ecological Concepts for Valuing Ecosystem Services. Ecol. Econ. 2002, 41, 375–392. [Google Scholar] [CrossRef]
- Steinman, A.D.; Cardinale, B.J.; Munns, W.R.; Ogdahl, M.E.; Allan, J.D.; Angadi, T.; Bartlett, S.; Brauman, K.; Byappanahalli, M.; Doss, M.; et al. Ecosystem Services in the Great Lakes. J. Great Lakes Res. 2017, 43, 161–168. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de la Pesca y la Acuicultura. Hacia la Transformación Azul; FAO: Roma, Italy, 2022; ISSN 2663-8649. [Google Scholar] [CrossRef]
- Dugan, P.J.; Barlow, C.; Agostinho, A.A.; Baran, E.; Cada, G.F.; Chen, D.; Cowx, I.G.; Ferguson, J.W.; Jutagate, T.; Mallen-Cooper, M.; et al. Fish Migration, Dams, and Loss of Ecosystem Services in the Mekong Basin. Ambio 2010, 39, 344–348. [Google Scholar] [CrossRef]
- Ziv, G.; Baran, E.; Nam, S.; Rodríguez-Iturbe, I.; Levin, S.A. Trading-Off Fish Biodiversity, Food Security, and Hydropower in the Mekong River Basin. Proc. Natl. Acad. Sci. USA 2012, 109, 5609–5614. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.K.; Smith, L.M.; Case, J.L.; Linthurst, R.A. A Review of the Elements of Human Well-Being with an Emphasis on the Contribution of Ecosystem Services. Ambio 2012, 41, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Arismendi, I.; González, J.; Sanzana, J.; Jara, F.; Jara, C.; Guzman, E.; Lara, A. Southern Chile, Trout and Salmon Country: Invasion Patterns and Threats for Native Species. Rev. Chil. Hist. Nat. 2006, 79, 97–117. [Google Scholar] [CrossRef]
- Valbo-Jørgensen, J.; Soto, D.; Gumy, A. La Pesca Continental en América Latina: Su Contribución Económica y Social e Instrumentos Normativos Asociados. COPESCAL Documento Ocasional No. 11; FAO: Roma, Italy, 2008; ISBN 978-92-5-305998-0. [Google Scholar]
- FAO/FishCode. Seminar on Responsible Fisheries Management in Large Rivers and Reservoirs of Latin America. FAO/FishCode Review. No. 5; FAO: Roma, Italy, 2004; ISSN 1728-4392. [Google Scholar]
- Aigo, J.; Ladio, A. Traditional Mapuche Ecological Knowledge in Patagonia, Argentina: Fishes and Other Living Beings Inhabiting Continental Waters, as a Reflection of Processes of Change. J. Ethnobiol. Ethnomed. 2016, 12, 56. [Google Scholar] [CrossRef]
- Ruiz, V.H.; Marchant, M. Ictiofauna de Aguas Continentales Chilenas; Universidad de Concepción: Concepción, Chile, 2004; 356p. [Google Scholar]
- Habit, E.; Dyer, B.; Vila, I. Estado de Conocimiento de los Peces Dulceacuícolas de Chile. Gayana 2006, 70, 100–113. [Google Scholar] [CrossRef]
- Penaluna, B.E.; Arismendi, I.; Soto, D. Evidence of Interactive Segregation between Introduced Trout and Native Fishes in Northern Patagonian Rivers, Chile. Trans. Am. Fish. Soc. 2009, 138, 839–845. [Google Scholar] [CrossRef]
- Habit, E.; González, J.; Ortiz-Sandoval, J.; Elgueta, A.; Sobenes, C. Effects of salmonid invasion in rivers and lakes of Chile. ECOS 2014, 24, 43–51. [Google Scholar] [CrossRef]
- Cooke, S.J.; Allison, E.H.; Beard, T.D.; Arlinghaus, R.; Arthington, A.H.; Bartley, D.M.; Cowx, I.G.; Fuentevilla, C.; Leonard, N.J.; Lorenzen, K.; et al. On the Sustainability of Inland Fisheries: Finding a Future for the Forgotten. Ambio 2016, 45, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Nyboer, E.; Bennett, A.; Lynch, A.J.; Infante, D.M.; Cowx, I.G.; Beard, T.D.; Bartley, D.; Paukert, C.P.; Reid, A.J.; et al. The Ten Steps to Responsible Inland Fisheries in Practice: Reflections from Diverse Regional Case Studies around the Globe. Rev. Fish Biol. Fish. 2021, 31, 843–877. [Google Scholar] [CrossRef]
- Parra, O. Caracterización y tendencias tróficas de cinco lagos costeros de Chile Central. Limnetica 2003, 22, 51–83. [Google Scholar] [CrossRef]
- Smith-Ramírez, C. The Chilean Coastal Range: A Vanishing Center of Biodiversity and Endemism in South American Temperate Rainforests. Biodivers. Conserv. 2004, 13, 373–393. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid Deforestation and Fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Huber, A.; Iroumé, A.; Mohr, C.; Frêne, C. Efecto de Plantaciones de Pinus Radiata y Eucalyptus Globulus Sobre el Recurso Agua en la Cordillera de la Costa de la Región del Biobío, Chile. Bosque 2010, 31, 219–230. [Google Scholar] [CrossRef]
- Subsecretaría de Desarrollo Regional. Guía Identificación de Áreas Potenciales para ser Declaradas “Áreas Preferenciales de Pesca Recreativa” Elementos Para el Ordenamiento Territorial. 2016. Available online: https://www.descentralizachile.cl/wp-content/uploads/2020/02/002-Gu%C3%ADa-Identificaci%C3%B3n-%C3%81reas-Potenciales-para-ser-declaradas-%E2%80%9C%C3%81reas-Preferenciales-de-Pesca-Recreativa%E2%80%9D-SUBDERE-2016.pdf (accessed on 10 May 2024).
- Aguayo, C.; Eames, C. Promoting Community Socio-Ecological Sustainability through Technology: A Case Study from Chile. Int. Rev. Educ. 2017, 63, 871–895. [Google Scholar] [CrossRef]
- Municipalidad de Contulmo, Actualización PLADECO, Plan de Desarrollo Comunal. 2019. Available online: https://contulmo.cl/Transparencia/Documentos/Pladeco_2019_2025.pdf (accessed on 24 March 2024).
- Biblioteca del Congreso Nacional, Reportes Comunales, Comuna Contulmo. 2021. Available online: https://www.bcn.cl/siit/reportescomunales/comunas_v.html?anno=2021&idcom=8204 (accessed on 24 March 2024).
- Cowx, I.G.; Portocarrero Aya, M. Paradigm Shifts in Fish Conservation: Moving to the Ecosystem Services Concept. J. Fish Biol. 2011, 79, 1663–1680. [Google Scholar] [CrossRef]
- Johnston, F.D.; Arlinghaus, R.; Dieckmann, U. Fish Life History, Angler Behaviour and Optimal Management of Recreational Fisheries. Fish Fish. 2013, 14, 554–579. [Google Scholar] [CrossRef]
- Muñoz, M.D.; Azocar, G.; Cornejo, S.; Aguayo, M.; Figueroa, R. Diversidad en la Cuenca del Lago Lanalhue: Comprendiendo la Relación Entre su Patrimonio Natural y Cultura; Centro de Ciencias Ambientales EULA-Chile, Universidad de Concepción: Concepción, Chile, 2019. [Google Scholar]
- Muñoz-Pedreros, A.; Merino, C. Diversity of Aquatic Bird Species in a Wetland Complex in Southern Chile. J. Nat. Hist. 2014, 48, 1453–1465. [Google Scholar] [CrossRef]
- Campos, H. Sistemática del Género Cheirodon (Pisces: Characidae) en Chile Con Descriptión de una Nueva Especie. Análisis de Multivarianza. Stud. Neotrop. Fauna Environ. 1982, 17, 129–162. [Google Scholar] [CrossRef]
- Dyer, B.S. Systematic review and biogeography of the freshwater fishes of Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Habit, E.; Victoriano, P. Peces de agua dulce de la Cordillera de la Costa. In Historia, Biodiversidad y Ecología de los Bosques Costeros de Chile; Smith-Ramírez, C., Armesto, J., Valdovinos, C., Eds.; Editorial Universitaria: Santiago, Chile, 2005; pp. 392–406. ISBN 956-11-1777-0. [Google Scholar]
- Dyer, B.S. Systematic review of the silverside fishes of Chile (Teleostei, Atheriniformes). Estud. Oceanol. 2000, 19, 99–127. [Google Scholar]
- Salas, D.; Véliz, D.; Scott, S. Diferenciación Morfológica en Especies del Género Cheirodon (Ostariophysi: Characidae) Mediante Morfometría Tradicional y Geométrica. Gayana 2012, 76, 142–152. [Google Scholar] [CrossRef]
- Fenichel, E.P.; Gentner, B.; Arlinghaus, R. Normative Considerations for Recreational Fishery Management: A Bioeconomic Framework for Linking Positive Science and Normative Fisheries Policy Decisions. Fish. Manag. Eco 2013, 20, 223–233. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2001, 2.6-6.1. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 25 June 2024).
- Anderson, M.J.; Robinson, J. Generalized Discriminant Analysis Based on Distances. Aust. New Zealand J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Arratia, G. Preferencias de Habitat de Peces Siluriformes de Aguas Continentales de Chile (Fam. Diplomystidae y Trichomycteridae). Stud. Neotrop. Fauna Environ. 1983, 18, 217–237. [Google Scholar] [CrossRef]
- Chiang, G.; Munkittrick, K.R.; Saavedra, M.F.; Tucca, F.; McMaster, M.E.; Urrutia, R.; Tetreault, G.; Barra, R. Seasonal Changes in Reproductive Endpoints in Trichomycterus areolatus (Siluriformes: Trichomycteridae) and Percilia gillissi (Perciformes, Perciliidae), and the Consequences for Environmental Monitoring. Stud. Neotrop. Fauna Environ. 2011, 46, 185–196. [Google Scholar] [CrossRef]
- Cifuentes, R.; González, J.; Montoya, G.; Jara, A.; Ortíz, N.; Piedra, P.; Habit, E. Relación Longitud-Peso y Factor de Condición de Los Peces Nativos Del Río San Pedro (Cuenca Del Río Valdivia, Chile). Gayana 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Fernández, M.V.; Rechencq, M.; Lallement, M.E.; Zattara, E.E.; Juárez, S.M.; Sosnovsky, A.; Lippolt, G.E.; Alonso, M.F.; Vigliano, P.H.; Milan, D.; et al. Seasonal and reproductive migrations in the Creole perch (Actinopterygii: Percichthydae) promote both intra-lake and inter-lake habitat connectivity. Iran. J. Ichthyol. 2019, 6, 226–239. [Google Scholar]
- Ferriz, R.A.; Bentos, C.A.; Fernández, E.M.; López, G.R. Reproducción y Dinámica Poblacional de Cheirodon interruptus (Ostariophysi: Characidae) En El Arroyo El Portugués, Alta Cuenca Del Río Samborombón, Argentina. Lat. Am. J. Aquat. Res. 2011, 39, 151–160. [Google Scholar] [CrossRef]
- Habit, E.; Belk, M.C. Threatened Fishes of the World: Percilia irwini (Eigenmann 1927) (Perciliidae). Environ. Biol. Fish 2007, 78, 213–214. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Brown, D.; Castiñeira, L.; Renzi, M.; Luizon, C.; Urbanski, J.; Cussac, V. Reproduction of Landlocked Aplochiton zebra Jenyns (Pisces, Galaxiidae). Ecol. Freshw. Fish 2008, 17, 394–405. [Google Scholar] [CrossRef]
- Lopez Cazorla, A.; Sidorkewicj, N. Age, Growth and Reproduction in Creole Perch (Percichthys trucha) in the Negro River, Argentinean Patagonia. J. Appl. Ichthyol. 2011, 27, 30–38. [Google Scholar] [CrossRef]
- Manriquez, A.; Huaqúin, L.; Arellano, M.; Arratia, G. Aspectos Reproductivos de Trichomycterus areolatus Valenciennes, 1846 (Pisces: Teleostei: Siluriformes) En Rio Angostura, Chile. Stud. Neotrop. Fauna Environ. 1988, 23, 89–102. [Google Scholar] [CrossRef]
- McDowall, R.M. The Galaxiid Fishes of South America. Zool. J. Linn. Soc. 1971, 50, 33–73. [Google Scholar] [CrossRef]
- Montoya, G.; Jara, A.; Solis-Lufí, K.; Colin, N.; Habit, E. Primeros Estadios Del Ciclo de Vida de Peces Nativos Del Río San Pedro (Cuenca Del Rio Valdivia, Chile). Gayana 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Vila, I.; Soto, D. Atherinidae (Pisces) of Rapel Reservoir, Chile. Verh. Int. Ver. Theor. Angew. Limnol. 1981, 21, 1334–1338. [Google Scholar] [CrossRef]
- Jimenez, É.A.; Gonzalez, J.G.; Amaral, M.T.; Lucena Frédou, F. Sustainability Indicators for the Integrated Assessment of Coastal Small-Scale Fisheries in the Brazilian Amazon. Ecol. Econ. 2021, 181, 106910. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Hoffman, J.C. Coastal Wetland Support of Great Lakes Fisheries: Progress from Concept to Quantification. Trans. Am. Fish. Soc. 2015, 144, 352–372. [Google Scholar] [CrossRef]
- Ricker, W.E. Big Effects from Small Causes: Two Examples from Fish Population Dynamics. J. Fish. Res. Bd. Can. 1963, 20, 257–264. [Google Scholar] [CrossRef]
- Dabrowksa, K.; Hunt, L.M.; Haider, W. Understanding How Angler Characteristics and Context Influence Angler Preferences for Fishing Sites. N. Am. J. Fish Manag. 2017, 37, 1350–1361. [Google Scholar] [CrossRef]
- Golden, A.S.; Free, C.M.; Jensen, O.P. Angler Preferences and Satisfaction in a High-Threshold Bucket-List Recreational Fishery. Fish. Res. 2019, 220, 105364. [Google Scholar] [CrossRef]
- Trippel, E.A. Relations of Fecundity, Maturation, and Body Size of Lake Trout, and Implications for Management in Northwestern Ontario Lakes. North Am. J. Fish. Manag. 1993, 13, 64–72. [Google Scholar] [CrossRef]
- Winemiller, K.O. Life History Strategies, Population Regulation, and Implications for Fisheries Management. Can. J. Fish. Aquat. Sci. 2005, 62, 872–885. [Google Scholar] [CrossRef]
- Froese, R. Keep It Simple: Three Indicators to Deal with Overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Lorenzen, K.; Cowx, I.G.; Entsua-Mensah, R.E.M.; Lester, N.P.; Koehn, J.D.; Randall, R.G.; So, N.; Bonar, S.A.; Bunnell, D.B.; Venturelli, P.; et al. Stock Assessment in Inland Fisheries: A Foundation for Sustainable Use and Conservation. Rev. Fish Biol. Fish. 2016, 26, 405–440. [Google Scholar] [CrossRef]
- García, A.; González, J.; Habit, E. Caracterización del Hábitat de Peces Nativos en el Río San Pedro (Cuenca del Rio Valdivia, Chile). Gayana 2012, 76, 36–44. [Google Scholar] [CrossRef]
- Vega, R.; Dantagnan, P.; Mardones, A.; Valdebenito, I.; Zamorano, J.; Encina, F. Bases Biológicas para el Cultivo del Puye Galaxias Maculatus (Jenyns, 1842): Una Revisión. Lat. Am. J. Aquat. Res. 2013, 41, 369–386. [Google Scholar] [CrossRef]
- Fierro, P.; Valdovinos, C.; Arismendi, I.; Díaz, G.; Jara-Flores, A.; Habit, E.; Vargas-Chacoff, L. Examining the Influence of Human Stressors on Benthic Algae, Macroinvertebrate, and Fish Assemblages in Mediterranean Streams of Chile. Sci. Total Environ. 2019, 686, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Cowx, I.G. Contrasting Recreational and Commercial Fishing: Searching for Common Issues to Promote Unified Conservation of Fisheries Resources and Aquatic Environments. Biol. Conserv. 2006, 128, 93–108. [Google Scholar] [CrossRef]
- R Core Team. R Project for Statistical Computing. 2019. Available online: https://www.r-project.org (accessed on 9 August 2020).
- Kindt, R. BiodiversityR: Package for Community Ecology and Suitability Analysis 2007, 2.16-1. Available online: https://cran.r-project.org/web/packages/BiodiversityR/BiodiversityR.pdf (accessed on 25 June 2024).
- Ramírez-Álvarez, R.; Contreras, S.; Vivancos, A.; Reid, M.; López-Rodríguez, R.; Górski, K. Unpacking the Complexity of Longitudinal Movement and Recruitment Patterns of Facultative Amphidromous Fish. Sci. Rep. 2022, 12, 3164. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J. Conservation, Ecology and Management of Migratory Galaxiids and the Whitebait Fishery: A Summary of Current Knowledge and Information Gaps; Department of Conservation (DoC): Wellington, New Zealand, 2018; ISBN 978-1-98-851463-5. [Google Scholar]
- Servicio Nacional de Pesca y Acuicultura, Medidas de Administración de Pesca Recreativa en Chile 2021–2022. 2021. Available online: http://www.sernapesca.cl/sites/default/files/medidas_de_administracion_de_pesca_recreativa_en_chile_2021-2022_v20220706.pdf (accessed on 24 March 2024).
- Welcomme, R.L.; Cowx, I.G.; Coates, D.; Béné, C.; Funge-Smith, S.; Halls, A.; Lorenzen, K. Inland Capture Fisheries. Phil. Trans. R. Soc. B 2010, 365, 2881–2896. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.H. Ictiofauna del río Andalién (Concepción, Chile). Gayana Zool. 1993, 57, 109–278. [Google Scholar]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P.; Link, O.; Habit, E. The Longest Fragment Drives Fish Beta Diversity in Fragmented River Networks: Implications for River Management and Conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.; Ruiz, V.H.; Berrios, P.; Palma, A.; Villegas, P.; Andreu-Soler, A. Trophic Ecology of Native and Introduced Fish Species from the Chillán River, South-Central Chile. J. Appl. Ichthyol. 2010, 26, 78–83. [Google Scholar] [CrossRef]
- Campos, H.; Ruiz, V.H.; Gavilán, J.F.; Alay, F. Peces del Río Bío-Bío; Serie Publicaciones de Divulgación EULA; Universidad de Concepción: Concepción, Chile, 1993. [Google Scholar]
- Scasso, F.; Campos, H. Pelagic Fish Communities and Eutrophication in Lakes of an Andean Basin of Central Chile. J. Freshw. Ecol. 2000, 15, 71–82. [Google Scholar] [CrossRef]
- Macchi, P.J.; Pascual, M.A.; Vigliano, P.H. Differential Piscivory of the Native Percichthys trucha and Exotic Salmonids upon the Native Forage Fish Galaxias maculatus in Patagonian Andean Lakes. Limnologica 2007, 37, 76–87. [Google Scholar] [CrossRef]
- Ortiz-Sandoval, J.J.; Górski, K.; González-Díaz, A.; Habit, E. Trophic Scaling of Percichthys trucha (Percichthyidae) in Monospecific and Multispecific Lakes in Western Patagonia. Limnologica 2015, 53, 50–59. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Mangi, S.C. Gear-Based Management of a Tropical Artisanal Fishery Based on Species Selectivity and Capture Size. Fish. Manag. Ecol. 2004, 11, 51–60. [Google Scholar] [CrossRef]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; Gonzalez, J.; Colin, N. Changes in the Distribution of Native Fishes in Response to Introduced Species and Other Anthropogenic Effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Habit, E.; González, S.; Victoriano, P. Alcances sobre el uso sustentable de la ictiofauna de sistemas fluviales. Theoria 2022, 11, 15–20. [Google Scholar]
- Ignatius, S.; Haapasaari, P. Justification Theory for the Analysis of the Socio-Cultural Value of Fish and Fisheries: The Case of Baltic Salmon. Mar. Policy 2018, 88, 167–173. [Google Scholar] [CrossRef]
- Liu, Y.; Bailey, J.L.; Davidsen, J.G. Social-Cultural Ecosystem Services of Sea Trout Recreational Fishing in Norway. Front. Mar. Sci. 2019, 6, 178. [Google Scholar] [CrossRef]
- Torres, P.; Cuevas, C.; Tang, M.; Barra, M.; Franjola, R.; Navarrete, N.; Montefusco, A.; Otth, L.; Wilson, G.; Puga, S.; et al. Introduced and Native Fishes as Infection Foci of Diphyllobothrium spp. in Humans and Dogs from Two Localities at Lake Panguipulli in Southern Chile. Copa 2004, 71, 111–117. [Google Scholar] [CrossRef]
- Torres, P.; Andrade, P.; Silva, R. On a New Species of Hysterothylacium (Nematoda: Anisakidae) from Cauque mauleanum (Pisces: Atherinidae) by Brightfield and Scanning Electron Microscopy. Mem. Inst. Oswaldo Cruz 1998, 93, 745–752. [Google Scholar] [CrossRef]
- Mardones, A.; Vega, R.; Encina, F. Cultivation of Whitebait (Galaxias maculatus) in Chile. Aquac. Res. 2008, 39, 731–737. [Google Scholar] [CrossRef]
- Iriarte, J.A.; Lobos, G.A.; Jaksic, F.M. Invasive Vertebrate Species in Chile and Their Control and Monitoring by Governmental Agencies. Rev. Chil. Hist. Nat. 2005, 78, 143–154. [Google Scholar] [CrossRef]
- Vega, R.; De los Ríos, P.; Encina, F.; Norambuena, J.A.; Barile, J.; Mardones, A. First Report of Inventory and Role of Macroinvertebrates and Fish in Cautín River (38° S, Araucania Region Chile). Braz. J. Biol. 2019, 80, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Fierro, P.; Valdovinos, C.; Vargas-Chacoff, L.; Bertrán, C.; Arismendi, I.; Fierro, P.; Valdovinos, C.; Vargas-Chacoff, L.; Bertrán, C.; Arismendi, I. Macroinvertebrates and Fishes as Bioindicators of Stream Water Pollution. In Water Quality; IntechOpen: London, UK, 2017; ISBN 978-953-51-2882-3. [Google Scholar]
- Lacy, S.; Ugalde, F.; Mao, L. Invasive Rainbow Trout (Oncorhynchus mykiss) Are Not Affected by Different Land Uses in a Multi-Use, Mediterranean Climate Landscape. Fishes 2018, 3, 37. [Google Scholar] [CrossRef]
- Rowe, D.K.; Dean, T.L. Effects of Turbidity on the Feeding Ability of the Juvenile Migrant Stage of Six New Zealand Freshwater Fish Species. New Zealand J. Mar. Freshw. Res. 1998, 32, 21–29. [Google Scholar] [CrossRef]
- Rojo, J.H.; Rodríguez, P.; Boy, C.C. Morphological Differentiation in the Widespread Fish Galaxias maculatus: Do Darker Environments Imply Bigger Eyes? Hydrobiologia 2020, 847, 2863–2872. [Google Scholar] [CrossRef]
- Encina-Montoya, F.; Vega-Aguayo, R.; Mardones-Lazcano, A.; Rueda, T.; Tello, A. Characterization of Whitebait (Galaxias maculatus) Respiratory Rates to Optimize Intensive Culture Carrying Capacities. Aquac. Res. 2011, 42, 835–843. [Google Scholar] [CrossRef]
- Milano, D.; Vigliano, P.; Beauchamp, D. Effect of Body Size and Temperature on Respiration of Galaxias maculatus (Pisces: Galaxiidae). New Zealand J. Mar. Freshw. Res. 2017, 51, 295–303. [Google Scholar] [CrossRef]
- Buria, L.; Walde, S.J.; Battini, M.; Macchi, P.J.; Alonso, M.; Ruzzante, D.E.; Cussac, V.E. Movement of a South American Perch Percichthys Trucha in a Mountain Patagonian Lake during Spawning and Prespawning Periods. J. Fish Biol. 2007, 70, 215–230. [Google Scholar] [CrossRef]
- Prochelle, O.; Campos, H. The Biology of the Introduced Carp Cyprinus carpio L., in the River Cayumapu, Valdivia, Chile. Stud. Neotrop. Fauna Environ. 1985, 20, 65–82. [Google Scholar] [CrossRef]
- Stoner, A.W. Effects of Environmental Variables on Fish Feeding Ecology: Implications for the Performance of Baited Fishing Gear and Stock Assessment. J. Fish Biol. 2004, 65, 1445–1471. [Google Scholar] [CrossRef]
- Roset, N.; Grenouillet, G.; Goffaux, D.; Pont, D.; Kestemont, P. A Review of Existing Fish Assemblage Indicators and Methodologies. Fish. Manag. Eco 2007, 14, 393–405. [Google Scholar] [CrossRef]






| Numeral Categories | Type of Variable | Argument or Biological Feature | ||
|---|---|---|---|---|
| Life history traits | Maximum total length record | Numerical | Maximum length of each fish species recorded in the literature | |
| Maximum weight observed | Numerical | Maximum weight of each fish species registered during fieldwork | ||
| Fecundity | Categorical | 1 (Low) | Less than 100 eggs for each fish per spawn event | |
| 2 (Medium) | Between 100 and 999 eggs for each fish per spawn event | |||
| 3 (High) | Between 1000 and 9999 eggs for each fish per spawn event | |||
| 4 (Very high) | More than 100,000 eggs for each fish per spawn event | |||
| Habitat use | Categorical | 1 (Benthic) | Associated with substrate (boulders, pebbles, gravel, or sand). | |
| 2 (Benthic–pelagic) | Associated both with substrate and water column | |||
| 3 (Pelagic) | Uses only the water column | |||
| Shoaling behavior | Categorical | 0 (No shoaling behavior) | No shoaling behavior | |
| 1 (Exhibit shoaling behavior) | Fish form a part of shoals | |||
| Sustainability indicators | Mean total length observed | Numerical | Mean total length of each fish species registered during fieldwork | |
| Percentage of mature fish (ratio) | Numerical | Proportion of adults for each fish species |
| Species | Riverine | Lacustrine | Conservation Status | Records of Prior Catches | ||
|---|---|---|---|---|---|---|
| Family | Scientific name (abbreviation) | Tributaries | Littoral | Pelagic | ||
| Native | ||||||
| Geotriidae | Geotria australis (Ga) | x | Vulnerable | Subsistence fishing | ||
| Characidae | Cheirodon galusdae (Cg) | x | x | x | Vulnerable | No |
| Trichomycteridae | Trichomycterus areolatus (Ta) | x | Vulnerable | No | ||
| Galaxiidae | Galaxias maculatus (Gm) | x | x | Less concern | Subsistence fishing | |
| Brachygalaxias bullocki (Bb) | x | Vulnerable | No | |||
| Aplochiton zebra (Az) | x | Endangered | Subsistence and recreational fishing | |||
| Atheriniidae | Basilichthys microlepidotus (Bm) | x | Near-threatened | Subsistence and recreational fishing | ||
| Odontesthes mauleanum (Oma) | x | x | Vulnerable | Subsistence and recreational fishing | ||
| Percychthidae | Percichthys trucha (Pt) | x | Less concern | Subsistence and recreational fishing | ||
| Perciliidae | Percilia gillissi (Pg) | x | x | Endangered | No | |
| Non-native | ||||||
| Salmonidae | Oncorhynchus mykiss (Omy) | x | x | x | - | Subsistence and recreational fishing |
| Cyprinidae | Cyprinus carpio (Cc) | x | - | Subsistence and recreational fishing | ||
| Species | Av. Abundance | Contribution % | ||
|---|---|---|---|---|
| Riverine | Tributaries | Oncorhynchus mykiss | 0.71 | 65.91 |
| Trichomycterus areolatus | 0.50 | 18.62 | ||
| Geotria australis | 0.29 | 10.30 | ||
| Lacustrine | Littoral | Galaxias maculatus | 0.92 | 56.91 |
| Basilichthys microlepidotus | 0.63 | 42.18 | ||
| Pelagic | Percichthys trucha | 0.12 | 59.23 | |
| Cyprinus carpio | 0.07 | 20.04 | ||
| Odonthestes mauleanum | 0.07 | 14.33 | ||
| Species | Riverine Habitat | Lacustrine Habitats | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tributaries | Littoral | Pelagic | |||||||||||||||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | L1 | L2 | L3 | L4 | L5 | P1 | P2 | P3 | P4 | P5 | ||
| O. mykiss | Total abundance | 65 | 11 | 2 | 23 | 13 | 38 | 6 | 12 | 38 | 1 | 1 | 1 | ||||||||
| Maximum length | 17.7 | 25.9 | 6.6 | 16.8 | 5.9 | 25.4 | 21 | 18.2 | 13.3 | 6.5 | 40.5 | 24.7 | |||||||||
| Mean length | 9.0 | 7.7 | 6.2 | 6.1 | 4.1 | 7.8 | 10.1 | 9.5 | 4.7 | 6.5 | 40.5 | 24.7 | |||||||||
| G. maculatus | Total abundance | 1 | 1 | 60 | 77 | 37 | 109 | 131 | |||||||||||||
| Maximum length | 6.1 | 5.7 | 5.4 | 5.5 | 5.7 | 5.3 | 5.3 | ||||||||||||||
| Mean length | 6.1 | 5.7 | 4.1 | 4.5 | 4.8 | 4.4 | 4.6 | ||||||||||||||
| P. trucha | Total abundance | 9 | 20 | 11 | 24 | 2 | |||||||||||||||
| Maximum length | 29.5 | 33.2 | 33.3 | 33.8 | 28.0 | ||||||||||||||||
| Mean length | 25.9 | 26.6 | 30.8 | 27.8 | 26.7 | ||||||||||||||||
| C. carpio | Total abundance | 17 | 1 | 1 | 4 | ||||||||||||||||
| Maximum length | 44.4 | 42.0 | 40.0 | 41.5 | |||||||||||||||||
| Mean length | 33.1 | 42.0 | 40.0 | 34.7 | |||||||||||||||||
| Species | Environmental Variables in the Model | Estimate | Std. Error | z | p | |
|---|---|---|---|---|---|---|
| Riverine | O. mykiss | Depth | −15.353 | 0.747 | −20.553 | <0.001 |
| Tributaries | Temperature | −0.227 | 0.025 | −9.039 | <0.001 | |
| Dissolved Oxygen | −0.340 | 0.071 | −4.785 | <0.001 | ||
| Oxygen saturation | 0.034 | 0.005 | 6.066 | <0.001 | ||
| Turbidity | −0.255 | 0.012 | −20.535 | <0.001 | ||
| Total P | 35.926 | 2.609 | 13.766 | <0.001 | ||
| Total N | −8.505 | 0.699 | −12.155 | <0.001 | ||
| pH | 1.192 | 0.063 | 18.812 | <0.001 | ||
| Lacustrine | G. maculatus | Depth | −7.209 | 2.159 | −3.339 | <0.001 |
| Littoral | Dissolved Oxygen | −0.678 | 0.280 | −2.419 | 0.015 | |
| Oxygen saturation | −0.070 | 0.030 | −2.309 | 0.020 | ||
| Turbidity | −0.437 | 0.069 | −6.325 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, G.; Habit, E.; Urrutia, R.; Manosalva, A.; Barra, R.O.; Figueroa, R. Trait Composition and Assemblage Structure Analyses of Lacustrine Fishes: Synthesizing a Proposal for Better Fishing Practices. Water 2024, 16, 2333. https://doi.org/10.3390/w16162333
Díaz G, Habit E, Urrutia R, Manosalva A, Barra RO, Figueroa R. Trait Composition and Assemblage Structure Analyses of Lacustrine Fishes: Synthesizing a Proposal for Better Fishing Practices. Water. 2024; 16(16):2333. https://doi.org/10.3390/w16162333
Chicago/Turabian StyleDíaz, Gustavo, Evelyn Habit, Roberto Urrutia, Aliro Manosalva, Ricardo O. Barra, and Ricardo Figueroa. 2024. "Trait Composition and Assemblage Structure Analyses of Lacustrine Fishes: Synthesizing a Proposal for Better Fishing Practices" Water 16, no. 16: 2333. https://doi.org/10.3390/w16162333
APA StyleDíaz, G., Habit, E., Urrutia, R., Manosalva, A., Barra, R. O., & Figueroa, R. (2024). Trait Composition and Assemblage Structure Analyses of Lacustrine Fishes: Synthesizing a Proposal for Better Fishing Practices. Water, 16(16), 2333. https://doi.org/10.3390/w16162333






