Identification of Anthropogenic and Natural Inputs of Sulfate into River System of Carbonate Zn-Pb Mining Area in Southwest China: Evidence from Hydrochemical Composition, δ34SSO4 and δ18OSO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Data Analysis
- is the isotope value of the mixture (in this study = δ34SSO4 and δ18OSO4).
- is the source value of isotope , normally distributed with mean and standard deviation .
- is the contribution of source , estimated by the SIMMR model. Five sulfate sources were included in this study ( = 5).
- is the fractionation factor for isotope of source , normally distributed with a mean value and standard deviation . Fractionation factors were neglected due to the slow exchange of δ34SSO4 and δ18OSO4 after sulfate enters water [30].
- is the residual error, representing additional unquantified variation between individual mixtures, normally distributed with mean 0 and standard deviation . In this study, the residual error was not considered.
3. Results
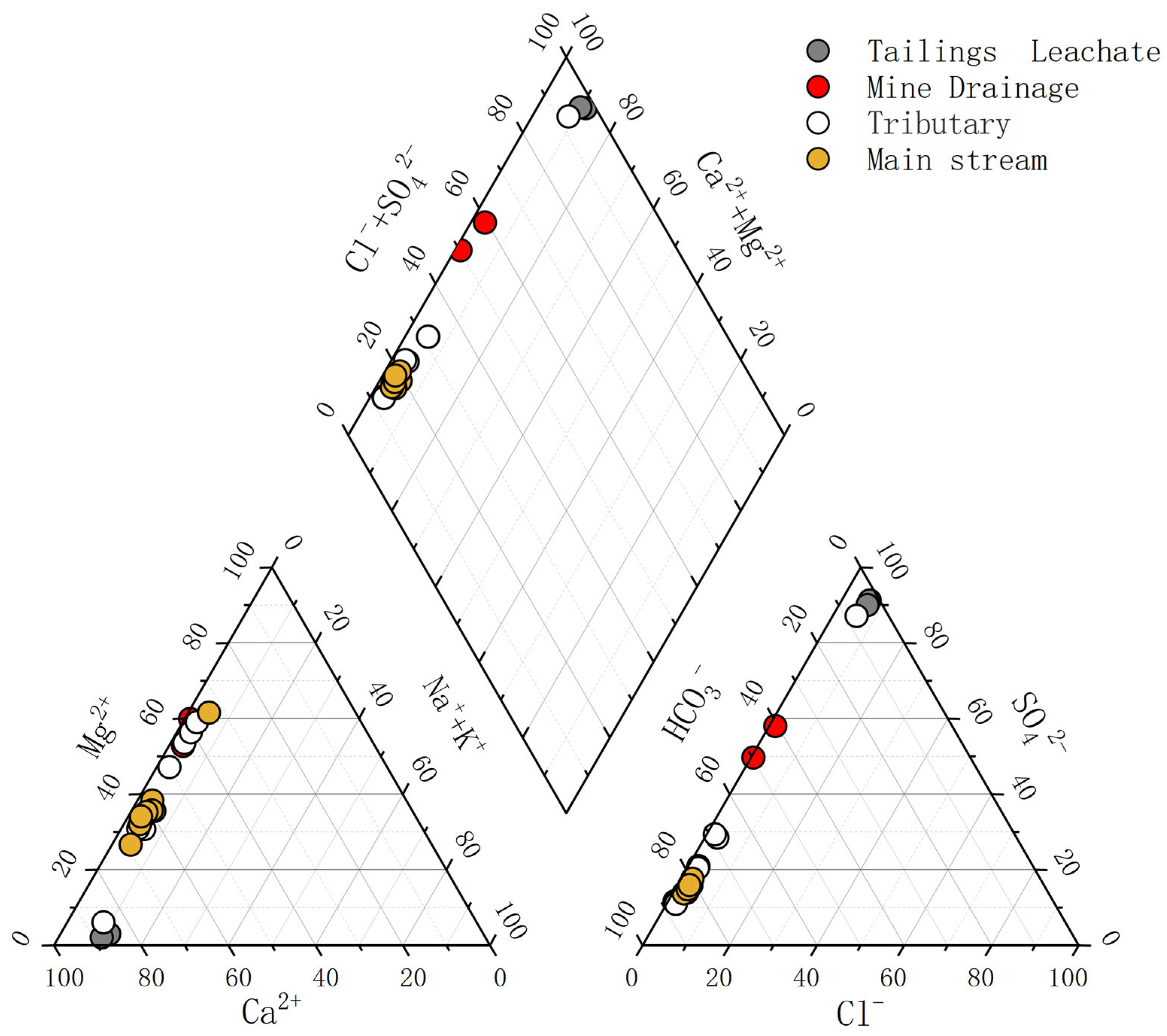
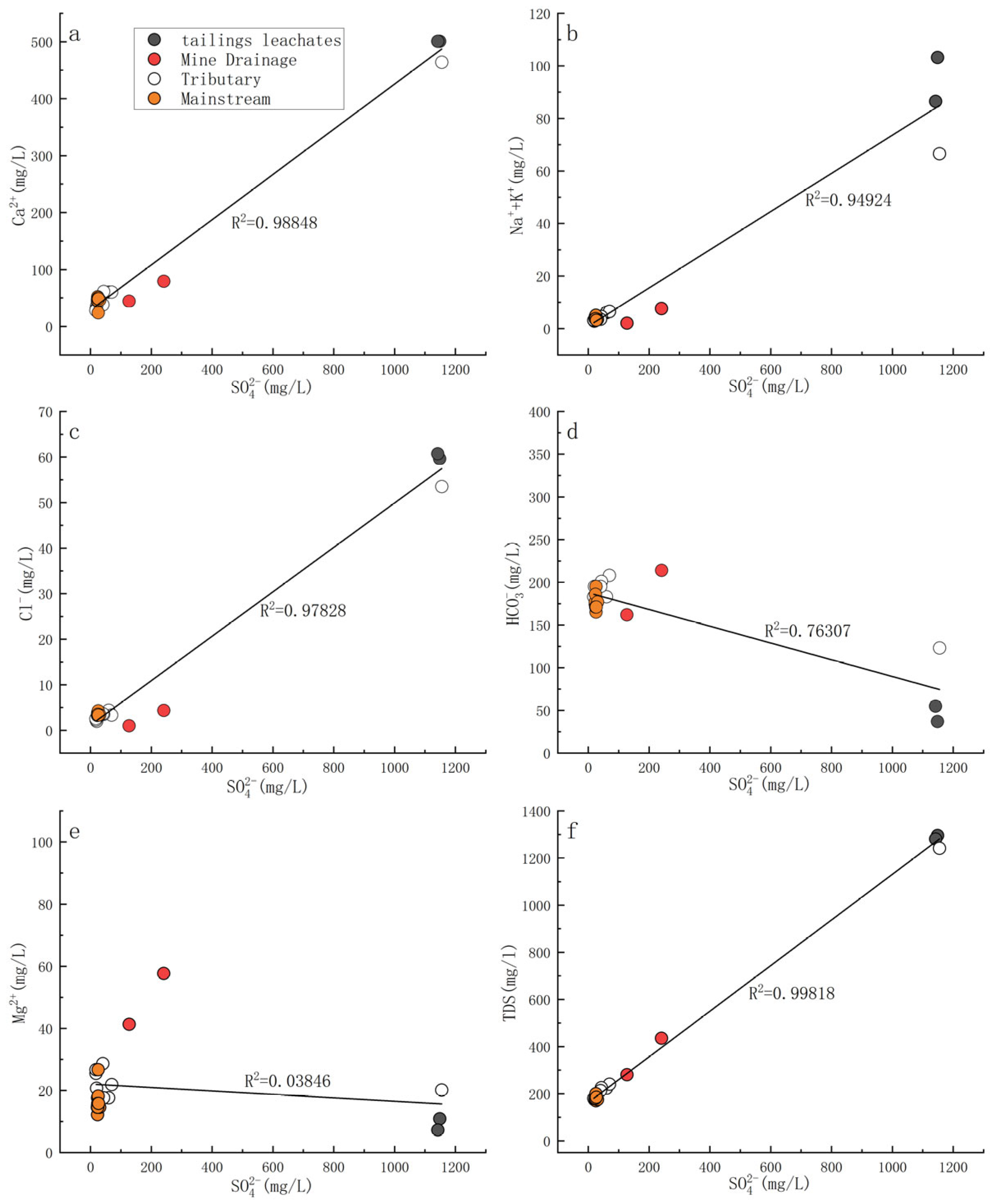
3.1. Hydrochemical Characteristics
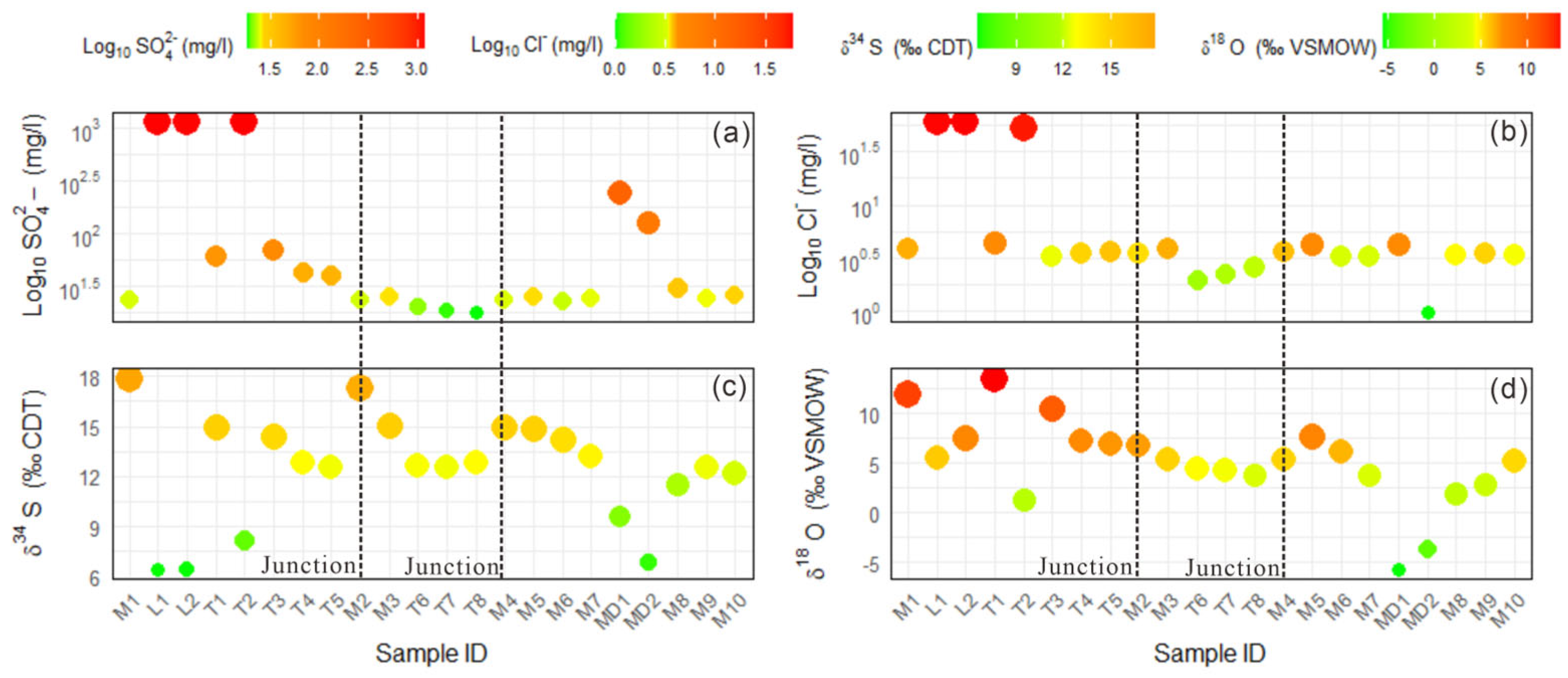
3.2. Sulfur and Oxygen Isotope Values of Sulfate
4. Discussion
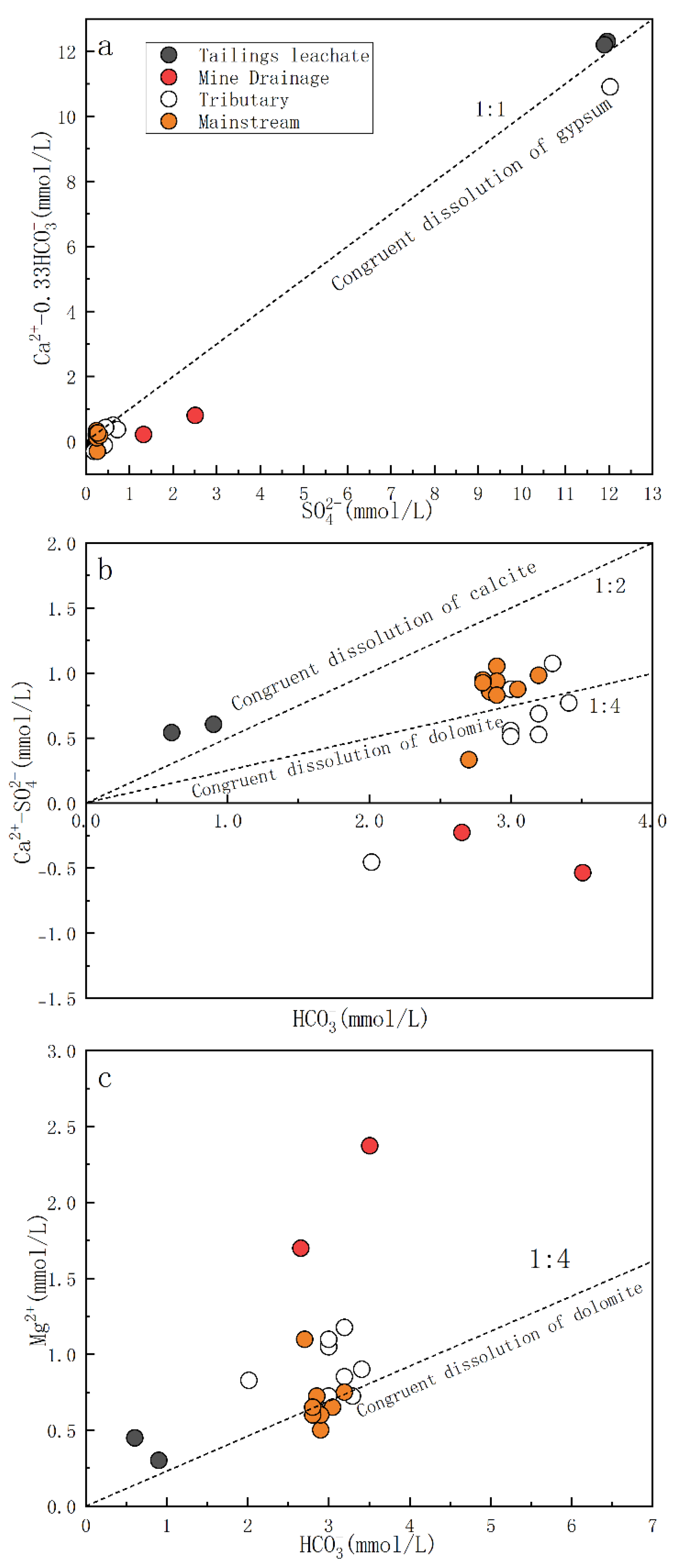
4.1. Impact of Water–Rock Interaction Processes on the Hydrochemical Compositions
4.2. Sulfate Sources and Mixing in the Daqiao River
4.2.1. Potential Sulfate Sources and Their Isotope Compositions
4.2.2. Sulfate Mixing in the Daqiao River
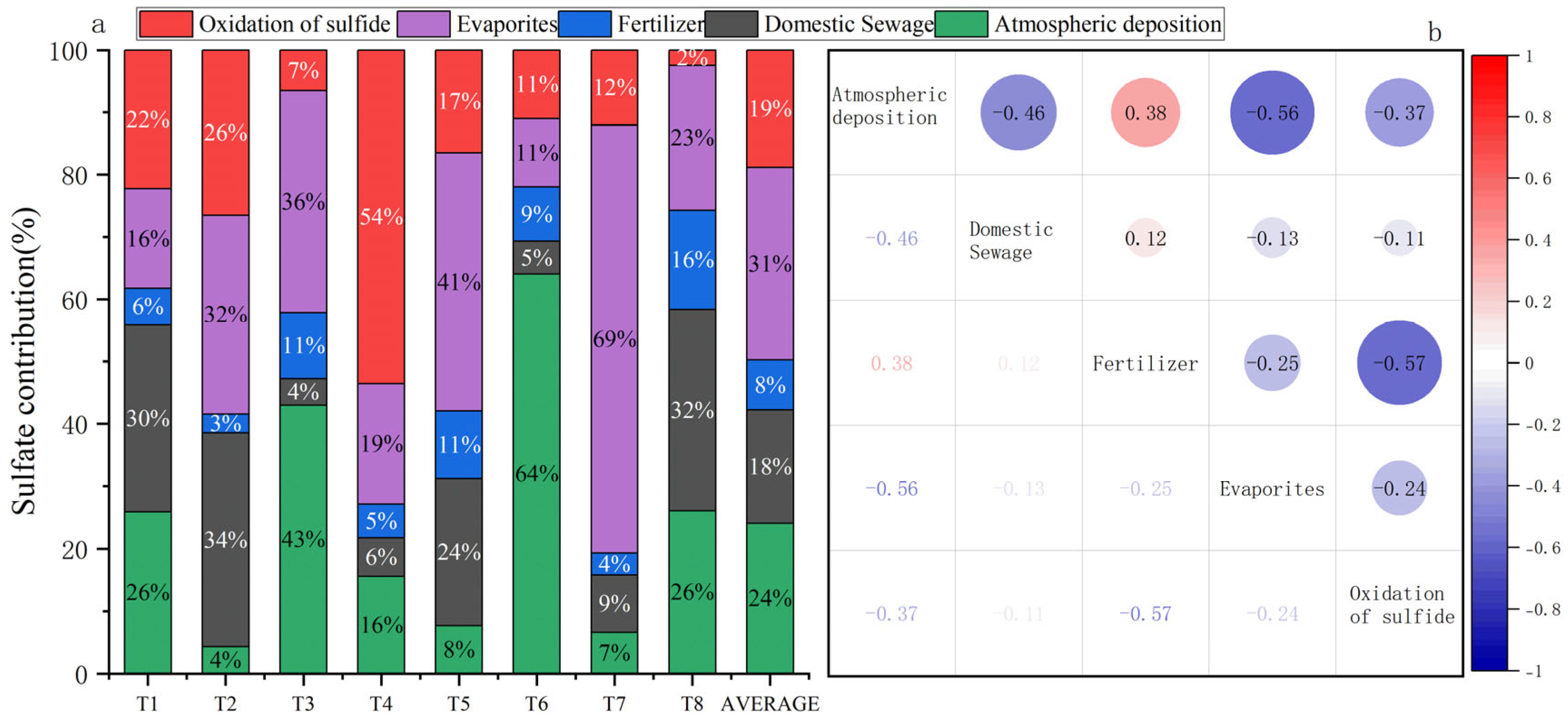
4.3. Contributions of Potential Sources to Riverine Sulfate
4.3.1. Sulfate Contributions to the Tributary Water
4.3.2. Sulfate Contributions to the Mainstream Water
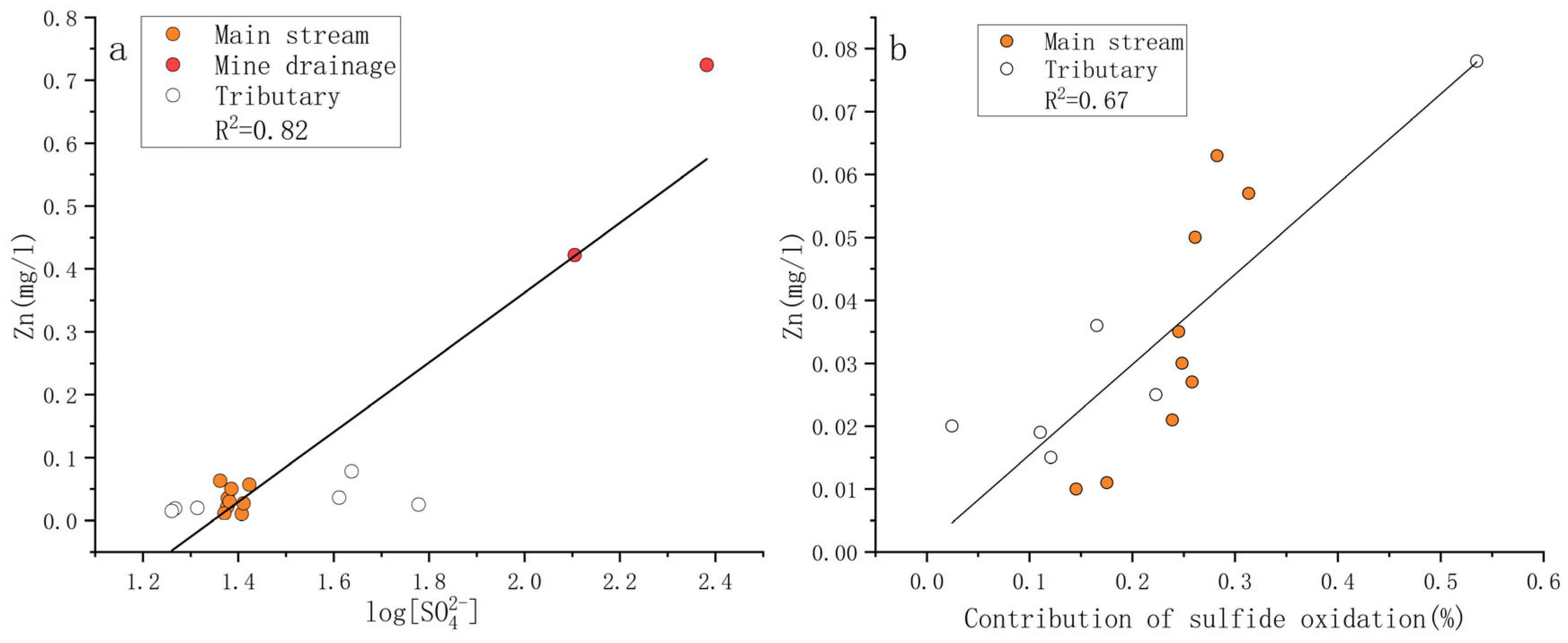
4.4. Sulfate Sources to Trace Zinc
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024.
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Singh, N.; Li, J.H. Environmental Impacts of Lead Ore Mining and Smelting. Adv. Mater. Res. 2014, 878, 338–347. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef]
- Gammons, C.H.; Brown, A.; Poulson, S.R.; Henderson, T.H. Using stable isotopes (S, O) of sulfate to track local contamination of the Madison karst aquifer, Montana, from abandoned coal mine drainage. Appl. Geochem. 2013, 31, 228–238. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C. Prediction of Acid Mine Drainage (AMD) and Metal Release Sources at the Kure Copper Mine Site, Kastamonu, NW Turkey. Mine Water Environ. 2018, 37, 56–74. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Bio-Technol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Qin, W.; Han, D.; Song, X.; Liu, S. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef] [PubMed]
- Komarek, M.; Ettler, V.; Chrastny, V.; Mihaljevic, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Mao, J.W.; Yan, X.H.; Wu, Y.; Zhang, F.; Zhao, L.L. Sources of metallogenic materials and metallogenic mechanism of Daliangzi Ore Field in Sichuan Province: Constraints from geochemistry of S, C, H, O, Sr isotope and trace element in sphalerite. Acta Petrol. Sin. 2014, 30, 209–220. [Google Scholar]
- García-Lorenzo, M.L.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; Agudo, I.; Recio, C. Isotope geochemistry of waters affected by mining activities in Sierra Minera and Portman Bay (SE, Spain). Appl. Geochem. 2014, 51, 139–147. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Dogramaci, S.; McLean, L.; Skrzypek, G. Hydrochemical and stable isotope indicators of pyrite oxidation in carbonate-rich environment; the Hamersley Basin, Western Australia. J. Hydrol. 2017, 545, 288–298. [Google Scholar] [CrossRef]
- Cao, X.; Wu, P.; Zhou, S.; Sun, J.; Han, Z. Tracing the origin and geochemical processes of dissolved sulphate in a karst-dominated wetland catchment using stable isotope indicators. J. Hydrol. 2018, 562, 210–222. [Google Scholar] [CrossRef]
- Guan, H.; Xiao, H.-Y.; Xiao, H.-W.; Xu, Y. The oxygen and sulfur isotopic compositions of soluble sulfate in the needles of Pinus massoniana Lamb.: Source discrimination and contribution estimation. J. Geochem. Explor. 2020, 208, 106402. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Lee, J.S. δ34S and δ18O of sulfates and Zn/Cd ratios reveal the cause of soil and groundwater contamination in metalliferous mining areas. J. Geochem. Explor. 2020, 209, 106437. [Google Scholar] [CrossRef]
- Kim, D.-M.; Lim, W.-L.; Im, D.-G.; Seo, E.-Y. Source and pathway determination of mine seepages using sulfate and Pb isotopes at the Daema and Okdong mines, South Korea. Appl. Geochem. 2020, 118, 104642. [Google Scholar] [CrossRef]
- Sanci, R.; Panarello, H.O.; Gozalvez, M.R. Environmental isotopes as tracers of mining activities and natural processes: A case study of San Antonio de los Cobres River Basin, Puna Argentina. J. Geochem. Explor. 2020, 213, 106517. [Google Scholar] [CrossRef]
- He, N.; Li, S.; Li, X.; Tang, Y.; Yang, J.; Zhou, J. Abiotic aerobic oxidation pathways of stibnite revealed by oxygen and sulfur isotope systematics of sulfate. J. Environ. Sci. 2023, 147, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, G.; Zhou, A.; Fang, L.; He, N. Stable sulfur and oxygen isotopes of sulfate as tracers of antimony and arsenic pollution sources related to antimony mine activities in an impacted river. Appl. Geochem. 2022, 142, 105351. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.; Dong, X.; Jiang, C.; Wei, X. Sources and mixing of sulfate contamination in the water environment of a typical coal mining city, China: Evidence from stable isotope characteristics. Environ. Geochem. Health 2020, 42, 2865–2879. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, M.; Cao, M.; Huang, X.; Zhang, Z.; Zhang, L. Sources and behaviors of dissolved sulfate in the Jinan karst spring catchment in northern China identified by using environmental stable isotopes and a Bayesian isotope-mixing model. Appl. Geochem. 2021, 134, 105109. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Lu, C. Tracing sulfate origin and transformation in an area with multiple sources of pollution in northern China by using environmental isotopes and Bayesian isotope mixing model. Environ. Pollut. 2020, 265, 115105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xue, T.; Xiao, J.; Chai, N.; Gong, S.-G. Significant influence of water diversion and anthropogenic input on riverine sulfate based on sulfur and oxygen isotopes. J. Hazard. Mater. 2024, 461, 132622. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, N.; Dragisic, V.; Stojkovic, J.; Papic, P.; Zivanovic, V. Hydrochemical characteristics of mine waters from abandoned mining sites in Serbia and their impact on surface water quality. Environ. Sci. Pollut. Res. Int. 2013, 20, 7615–7626. [Google Scholar] [CrossRef]
- Lloyd, R.M. Oxygen isotope behavior in the Sulfate-Water System. J. Geophys. Res. 1968, 73, 6099–6110. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, S.M.; Wang, Y.J.; Feng, Q.C.; Li, J.L.; Cui, C.F. The Progress of Mineral Processing Technology of Lead-zinc Mine in China. Appl. Mech. Mater. 2015, 737, 874–877. [Google Scholar] [CrossRef]
- Kalhor, K.; Ghasemizadeh, R.; Rajic, L.; Alshawabkeh, A. Assessment of groundwater quality and remediation in karst aquifers: A review. Groundw. Sustain. Dev. 2019, 8, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qu, S.; Nel, W.; Ji, J. The impact of natural weathering and mining on heavy metal accumulation in the karst areas of the Pearl River Basin, China. Sci. Total Environ. 2020, 734, 139480. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.G.; Chen, X.; Dong, X.L.; Wei, X.P.; Jiang, C.L.; Tang, Q. Using δ34S-SO4 and δ18O-SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Drever, J.I.; Hall, P. (Eds.) The Geochemistry of Natural Waters; Prentice Hall: Englewood Cliffs, NJ, USA, 1988; ISBN 0-13-272790-0. [Google Scholar]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1970.
- Li, X.; Gan, Y.; Zhou, A.; Liu, Y.; Wang, D. Hydrological controls on the sources of dissolved sulfate in the Heihe River, a large inland river in the arid northwestern China, inferred from S and O isotopes. Appl. Geochem. 2013, 35, 99–109. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118591488. [Google Scholar]
- Han, D.; Song, X.; Currell, M.J. Identification of anthropogenic and natural inputs of sulfate into a karstic coastal groundwater system in northeast China: Evidence from major ions, δ13CDIC and δ34SSO4. Hydrol. Earth Syst. Sci. 2016, 20, 1983–1999. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Q.; Su, C.; Ma, T. Strontium isotope characterization and major ion geochemistry of karst water flow, Shentou, northern China. J. Hydrol. 2006, 328, 592–603. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Wang, J.; Jia, D.; Shi, Y.; Chen, L.; Xu, Z. Sources of metallogenic materials and metallogenic mechanism of Tianbaoshan Pb-Zn deposit in Sichuan Province: Constraints from fluid inclusions and isotopic evidences. Acta Petrol. Sin. 2021, 37, 1830–1846. [Google Scholar] [CrossRef]
- Sharma, M.K.; Kumar, M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020, 192, 74. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, T.; Qin, Y.; Liu, Y.; Chen, H.; Gao, Z.; Huang, X.; Ma, B. Dissolved Ion Concentrations and Isotope Values in Agricultural Fertilizer Locally Applied in Henan Province. Environ. Sci. 2023, 44, 1040–1050. [Google Scholar]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef]
- Li, X.-D.; Masuda, H.; Kusakabe, M.; Yanagisawa, F.; Zeng, H.-A. Degradation of groundwater quality due to anthropogenic sulfur and nitrogen contamination in the Sichuan Basin, China. Geochem. J. 2006, 40, 309–332. [Google Scholar] [CrossRef]
- Li, X.; Bao, H.; Gan, Y.; Zhou, A.; Liu, Y. Multiple oxygen and sulfur isotope compositions of secondary atmospheric sulfate in a mega-city in central China. Atmos. Environ. 2013, 81, 591–599. [Google Scholar] [CrossRef]
- Krouse, H.R.; Mayer, B. Sulphur and Oxygen Isotopes in Sulphate. In Environmental Tracers in Subsurface Hydrology; Springer: Boston, MA, USA, 2000; pp. 195–231. ISBN 978-1-4613-7057-4. [Google Scholar]
- Edraki, M.; Golding, S.D.; Baublys, K.A.; Lawrence, M.G. Hydrochemistry, mineralogy and sulfur isotope geochemistry of acid mine drainage at the Mt. Morgan mine environment, Queensland, Australia. Appl. Geochem. 2005, 20, 789–805. [Google Scholar] [CrossRef]
- Haefner, R.J. A sulfur-isotope mixing model to trace leachate from pressurized fluidized bed combustion byproducts in an abandoned-coal-mine setting. Fuel 2001, 80, 829–836. [Google Scholar] [CrossRef]
- Budakoglu, M.; Pratt, L. Sulfur-isotope distribution and contamination related to the Balya Pb-Zn Mine in Turkey. Environ. Geol. 2005, 47, 773–781. [Google Scholar] [CrossRef]
- Moses, C.O.; Herman, J.S. Pyrite oxidation at circumneutral pH. Geochim. Cosmochim. Acta 1991, 55, 471–482. [Google Scholar] [CrossRef]
- Llyod, R.M. Oxygen-18 composition of oceanic sulfate. Science 1967, 156, 1228–1231. [Google Scholar] [CrossRef]
- McCarthy, M. Oxygen isotopic compositions of sulphate from coals: Implications for primary sulphate sources and secondary weathering processes. Fuel 1998, 77, 677–682. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, C.Q.; Liu, X.L.; Bao, L.R. Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J. Asian Earth Sci. 2011, 42, 370–380. [Google Scholar] [CrossRef]
- Liu, Z. General Study on the Stable Isotope Geochemistry of the Daliangzi Pb-Zn Deposit in Huidong, Sichuan. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2016. [Google Scholar]
- González-Acebrón, L.; Barroso-Barcenilla, F.; Cambra-Moo, O.; Carenas, B.; Segura, M. Environmental significance of gypsum-bearing layers at the “Lo Hueco” paleontological site (Upper Cretaceous, Cuenca, Spain): Petrography, fluid inclusions, and isotopic relations. Facies 2014, 60, 755–771. [Google Scholar] [CrossRef]
- Antler, G.; Turchyn, A.V.; Rennie, V.; Herut, B.; Sivan, O. Coupled sulfur and oxygen isotope insight into bacterial sulfate reduction in the natural environment. Geochim. Cosmochim. Acta 2013, 118, 98–117. [Google Scholar] [CrossRef]
- Mizutani, Y.; Rafter, T.A. Isotopic behaviour of sulphate oxygen in the bacterial reduction of sulphate. Geochem. J. 1973, 6, 183–191. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Zhang, C.; Zhu, M.; Li, J. Sulfur isotope geochemistry indicating the source of dissolved sulfate in Gonghe geothermal waters, northwestern China. Procedia Earth Planet. Sci. 2017, 17, 157–160. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Y. Distribution characteristic and migration pathways of metals in subsidence zone in a coal mine, China. Bull. Environ. Contam. Toxicol. 2017, 98, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jiang, C.; Li, C.; Zheng, L. Tracing sulfate source and transformation in the groundwater of the linhuan coal mining area, huaibei coalfield, China. Int. J. Environ. Res. Public Health 2022, 19, 14434. [Google Scholar] [CrossRef]
- Taylor, B.E.; Wheeler, M.C. Sulfur- and Oxygen-Isotope Geochemistry of Acid Mine Drainage in the Western United States. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society: Washington, DC, USA, 1994; ISBN 0-8412-2772-1. [Google Scholar]
- Cloquet, C.; Carignan, J.; Lehmann, M.F.; Vanhaecke, F. Variation in the isotopic composition of zinc in the natural environment and the use of zinc isotopes in biogeosciences: A review. Anal. Bioanal. Chem. 2008, 390, 451–463. [Google Scholar] [CrossRef]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C. The geochemistry of acid mine. Environ. Geochem. 2005, 9, 149. [Google Scholar]
- Seal, R.R., II; Foley, N.K.; Wanty, R.B. Introduction to geoenvironmental models of mineral deposits. Prog. Geoenviron. Models Sel. Miner. Depos. Types 2002, 2, 1. [Google Scholar]



| Sample | pH | Ca | Mg | Na | K | HCO3− | SO42− | Cl− | Pb | Zn | TDS | δ34SSO4 | δ18OSO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 10.9 | 501 | 10.9 | 34.4 | 68.8 | 37 | 1148.81 | 59.6 | 3.04 | 0.103 | 1296 | 6.47 | 5.56 |
| L2 | 10.5 | 501 | 7.29 | 33.5 | 53 | 55 | 1142.54 | 60.7 | 4.11 | 1.28 | 1281 | 6.48 | 7.46 |
| MD1 | 8.1 | 79.2 | 57.7 | 5.2 | 2.4 | 214 | 241.22 | 4.34 | 0.23 | 0.724 | 435 | 9.55 | −5.66 |
| MD2 | 7.94 | 44.1 | 41.3 | 1 | 1.1 | 162 | 127.5 | 0.99 | 0.01 | 0.422 | 280 | 6.93 | −3.7 |
| S01 | 9.06 | 10 | 7.29 | 23.5 | 8.6 | 54.9 | 31.76 | 0.52 | 0.01 | 0.037 | 55 | 17.96 | 13.98 |
| T1 | 8.41 | 60.1 | 17.6 | 5.2 | 0.9 | 183 | 59.97 | 4.41 | 0.01 | 0.025 | 223 | 14.92 | 13.52 |
| T2 | 7.33 | 463.9 | 20.1 | 31.1 | 35.4 | 123 | 1155.41 | 53.5 | 0.12 | 0.302 | 1241 | 8.17 | 1.37 |
| T3 | 8.21 | 60.1 | 21.9 | 5.5 | 1 | 208 | 70.03 | 3.33 | 0.01 | 0.011 | 240 | 14.44 | 10.47 |
| T4 | 7.58 | 61.1 | 17.6 | 3.4 | 1.3 | 201 | 43.4 | 3.49 | 0.01 | 0.078 | 225 | 12.83 | 7.24 |
| T5 | 8.25 | 38.1 | 28.6 | 2.5 | 1.1 | 195 | 40.85 | 3.65 | 0.01 | 0.036 | 213 | 12.58 | 6.91 |
| T6 | 8.08 | 36.1 | 20.7 | 1.9 | 0.9 | 195 | 20.62 | 1.98 | 0.01 | 0.02 | 175 | 12.67 | 4.41 |
| T7 | 8.08 | 30.1 | 25.5 | 2.2 | 0.9 | 183 | 18.51 | 2.26 | 0.01 | 0.019 | 180 | 12.56 | 4.22 |
| T8 | 8.21 | 28.1 | 26.7 | 2.2 | 1 | 183 | 18.21 | 2.59 | 0.01 | 0.015 | 180 | 12.84 | 3.85 |
| min | 7.33 | 28.1 | 17.6 | 1.9 | 0.9 | 123 | 18.21 | 1.98 | 0.01 | 0.011 | 175 | 8.17 | 1.37 |
| max | 8.41 | 463.9 | 28.6 | 31.1 | 35.4 | 208 | 1155.41 | 53.5 | 0.12 | 0.302 | 1241 | 14.92 | 13.52 |
| mean | 8.02 | 97.2 | 22.34 | 6.75 | 5.31 | 183.88 | 178.38 | 9.40 | 0.02 | 0.06 | 334.63 | 12.63 | 6.50 |
| M01 | 8.36 | 46.1 | 15.2 | 1.9 | 1.8 | 174 | 23.82 | 3.85 | 0.01 | 0.021 | 178 | 17.86 | 11.95 |
| M02 | 8.3 | 44.1 | 17.6 | 1.9 | 1.8 | 174 | 23.49 | 3.46 | 0.01 | 0.011 | 183 | 17.31 | 6.77 |
| M03 | 7.95 | 24 | 26.7 | 2.5 | 2.4 | 165 | 25.56 | 3.85 | 0.01 | 0.01 | 170 | 15.02 | 5.38 |
| M04 | 7.92 | 52.1 | 12.2 | 2.5 | 2.1 | 177 | 23.89 | 3.68 | 0.01 | 0.035 | 180 | 14.99 | 5.35 |
| M05 | 8.23 | 50.1 | 18.2 | 3.4 | 1.7 | 195 | 25.76 | 4.29 | 0.01 | 0.027 | 200 | 14.83 | 7.71 |
| M06 | 8.18 | 47.1 | 14.6 | 2.2 | 1.5 | 177 | 23.01 | 3.25 | 0.04 | 0.063 | 178 | 14.21 | 6.14 |
| M07 | 7.74 | 45.1 | 15.8 | 2.2 | 1.4 | 186 | 24.09 | 3.29 | 0.01 | 0.03 | 178 | 13.22 | 3.85 |
| M08 | 8.16 | 46.1 | 14.6 | 1.9 | 1.6 | 177 | 30.81 | 3.37 | 0.14 | 0.527 | 175 | 11.5 | 1.91 |
| M09 | 8.19 | 48.1 | 14.6 | 2.2 | 1.6 | 171 | 24.31 | 3.49 | 0.01 | 0.05 | 180 | 12.52 | 2.83 |
| M10 | 8.39 | 48.1 | 15.8 | 1.6 | 1.6 | 171 | 26.5 | 3.35 | 0.01 | 0.057 | 185 | 12.15 | 5.25 |
| min | 7.74 | 24 | 12.2 | 1.6 | 1.4 | 165 | 23.01 | 3.25 | 0.01 | 0.01 | 170 | 11.5 | 1.91 |
| max | 8.39 | 52.1 | 26.7 | 3.4 | 2.4 | 195 | 30.81 | 4.29 | 0.14 | 0.527 | 200 | 17.86 | 11.95 |
| mean | 8.142 | 45.09 | 16.53 | 2.23 | 1.75 | 176.7 | 25.124 | 3.588 | 0.026 | 0.0831 | 180.7 | 14.361 | 5.714 |
| ID | Sulfate Sources | δ34SSO4 (‰) | δ18OSO4 (‰) | Reference | ||
|---|---|---|---|---|---|---|
| Average Value | Standard Deviation | Average Value | Standard Deviation | |||
| 1 | Oxidation of sulfide | 8.24 | 1.85 | −4.68 | 1.39 | This study |
| 2 | Evaporite | 17.96 | 0 | 13.98 | 0 | This study |
| 3 | Chemical fertilizer | 5.8 | 5.5 | 10.7 | 2.7 | [44] |
| 4 | Domestic sewage | 5.98 | 1.98 | 6.86 | 1.6 | [45] |
| 5 | Atmospheric deposition | 3.9 | 0 | 11.65 | 1.54 | [46,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Shi, Z.; Ding, X.; Ge, L.; Xiong, M.; Zhang, Q.; Lai, W.; Ge, L. Identification of Anthropogenic and Natural Inputs of Sulfate into River System of Carbonate Zn-Pb Mining Area in Southwest China: Evidence from Hydrochemical Composition, δ34SSO4 and δ18OSO4. Water 2024, 16, 2311. https://doi.org/10.3390/w16162311
Zhang K, Shi Z, Ding X, Ge L, Xiong M, Zhang Q, Lai W, Ge L. Identification of Anthropogenic and Natural Inputs of Sulfate into River System of Carbonate Zn-Pb Mining Area in Southwest China: Evidence from Hydrochemical Composition, δ34SSO4 and δ18OSO4. Water. 2024; 16(16):2311. https://doi.org/10.3390/w16162311
Chicago/Turabian StyleZhang, Kailiang, Zeming Shi, Xiaoyan Ding, Liquan Ge, Maolin Xiong, Qingxian Zhang, Wanchang Lai, and Liangquan Ge. 2024. "Identification of Anthropogenic and Natural Inputs of Sulfate into River System of Carbonate Zn-Pb Mining Area in Southwest China: Evidence from Hydrochemical Composition, δ34SSO4 and δ18OSO4" Water 16, no. 16: 2311. https://doi.org/10.3390/w16162311
APA StyleZhang, K., Shi, Z., Ding, X., Ge, L., Xiong, M., Zhang, Q., Lai, W., & Ge, L. (2024). Identification of Anthropogenic and Natural Inputs of Sulfate into River System of Carbonate Zn-Pb Mining Area in Southwest China: Evidence from Hydrochemical Composition, δ34SSO4 and δ18OSO4. Water, 16(16), 2311. https://doi.org/10.3390/w16162311





