Sources, Transport, and Accumulation of Synthetic Microfiber Wastes in Aquatic and Terrestrial Environments
Abstract
1. Introduction
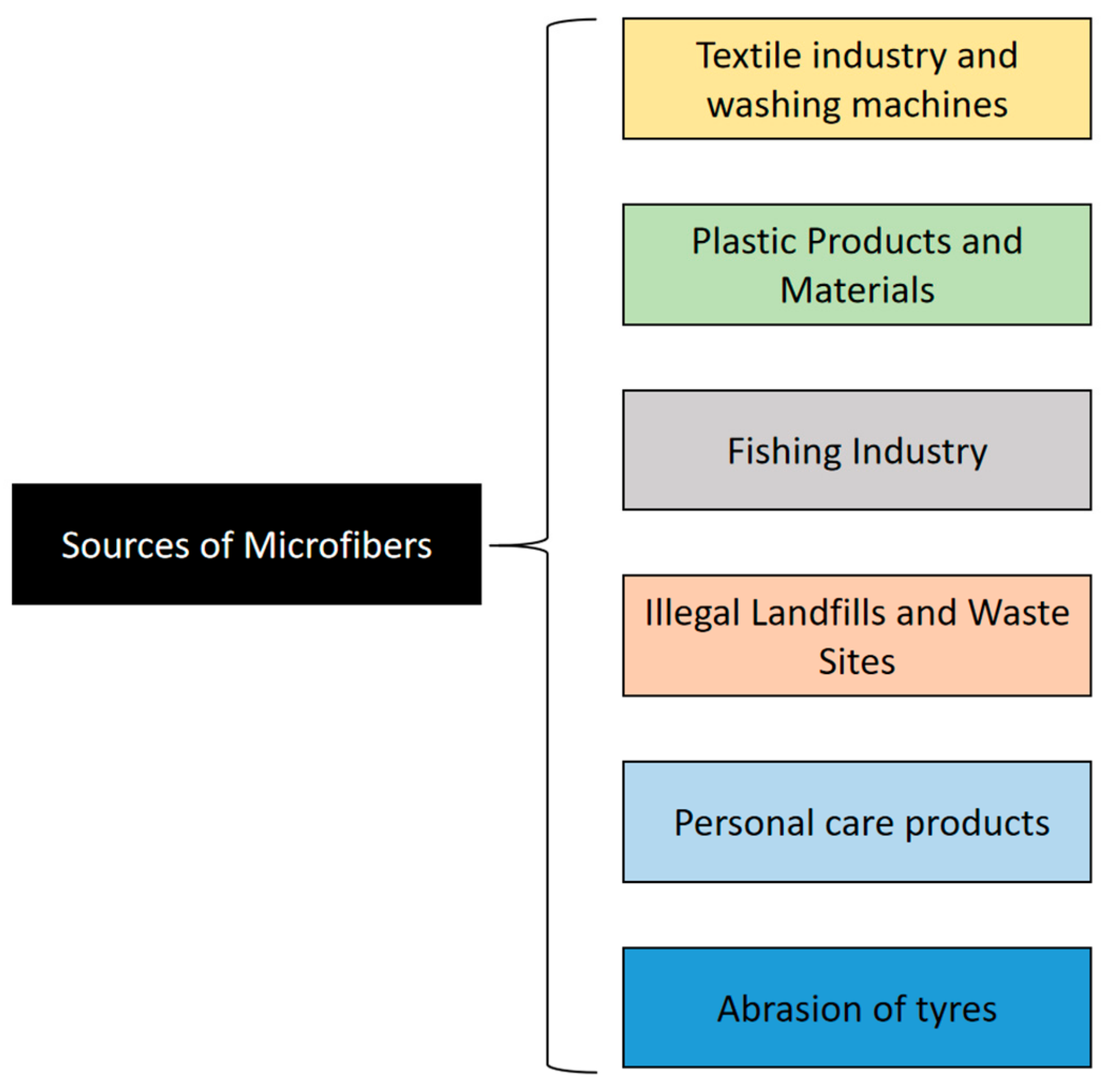
2. Sources of Synthetic Microfibers
2.1. The Textile Industry and Washing Machines
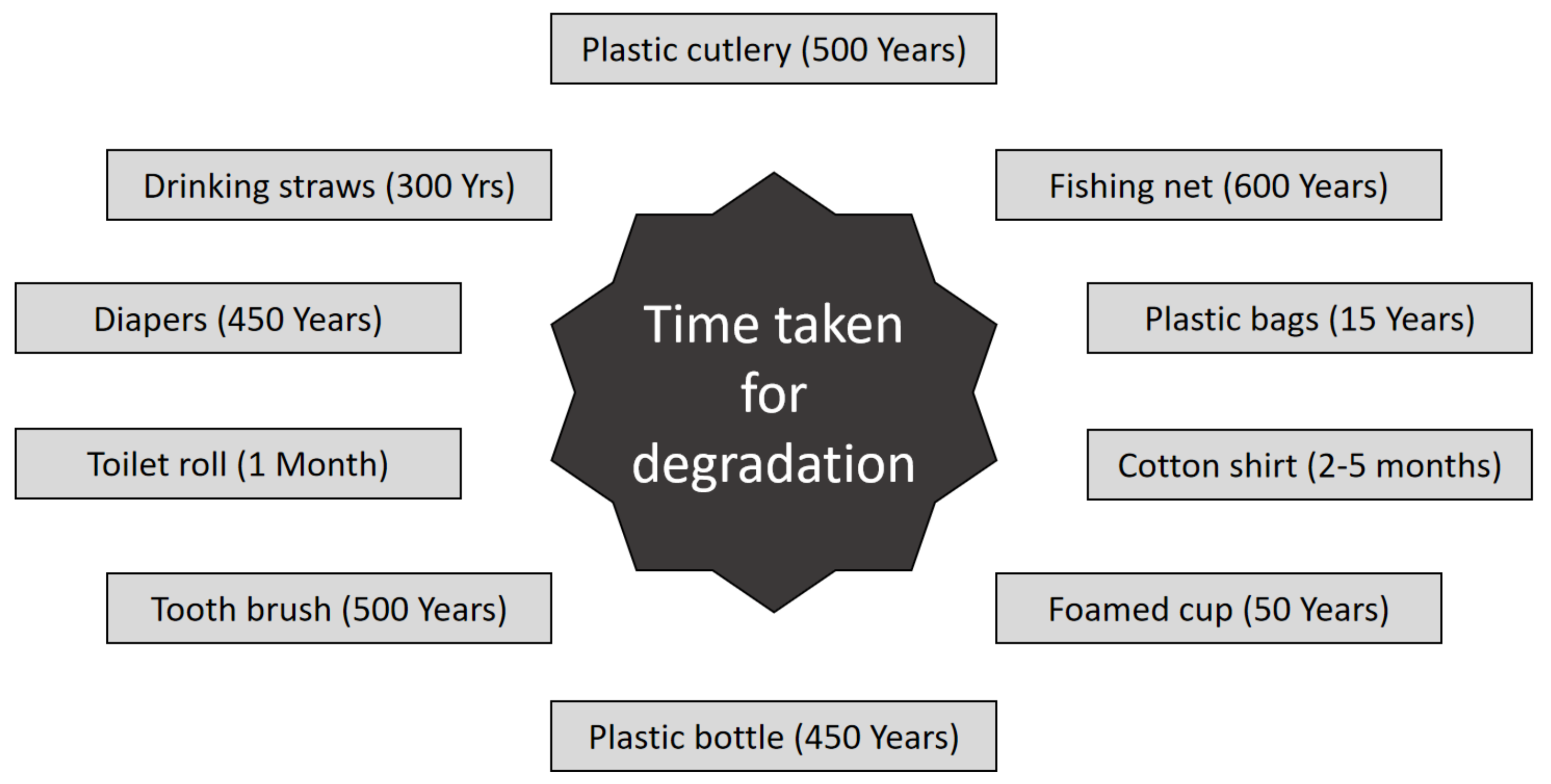
2.2. Plastic Products and Materials
2.3. The Fishing Industry
2.4. Landfills and Waste Sites
2.5. Personal Care Products
3. Transport Mechanisms
3.1. Waterborne Transport in Aquatic Environments
3.2. Transport in the Atmosphere
3.3. Terrestrial Transport
4. Accumulation in the Environment
4.1. Sediment Accumulation in Aquatic Environments
4.2. Bioaccumulation in Aquatic Food Webs
4.3. Resuspension and Re-Entry into the Water Column
5. Techniques for the Identification and Detection of Microplastics
6. Treatment Technologies
6.1. Wastewater Treatment Plants (WWTPs)
6.2. Electrochemical Oxidation
6.3. Photocatalytic Degradation
6.4. Magnetic Separation
6.5. Activated Carbon Filtration
6.6. Dissolved Air Flotation (DAF)
6.7. Electrocoagulation
6.8. Constructed Wetlands
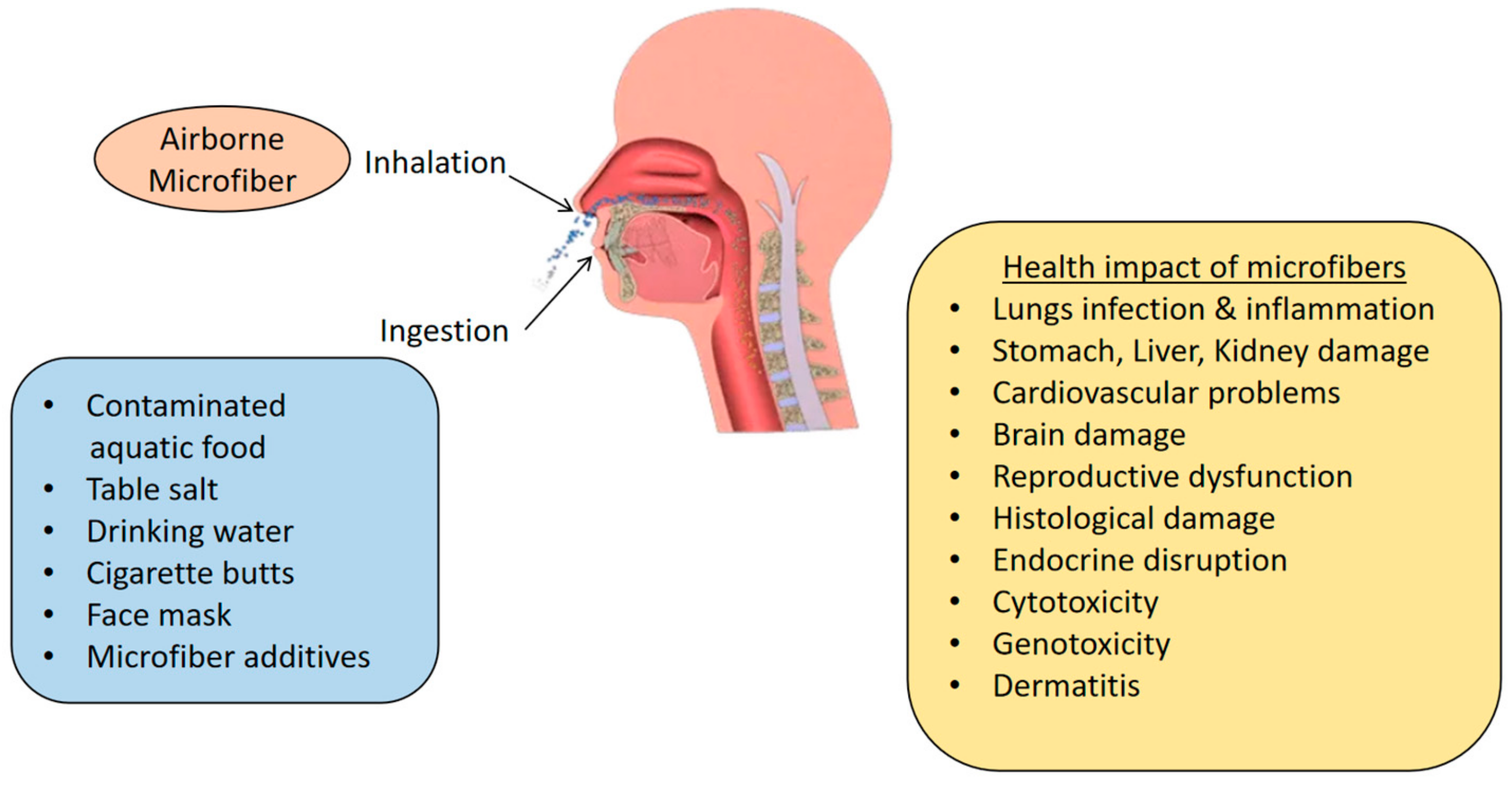
7. Environmental and Health Impacts
8. Mitigation and Solutions
- Textile manufacturing innovations: One of the primary strategies for mitigating microfiber pollution is the development and use of sustainable textiles. Designing and producing clothing made from natural fibers (cotton, hemp, and wool) or less-shedding synthetic materials can significantly reduce the release of microfibers during washing and use [97,98,99]. Additionally, treatments such as surface coatings can make fabrics more resistant to abrasion. Sustainable textile manufacturing should prioritize the use of biodegradable or low-impact materials and incorporate innovative design and production techniques that minimize microfiber shedding.
- Wastewater treatment enhancements: Enhancing wastewater treatment technologies is crucial in reducing the release of microfibers into the environment [92]. Advanced filtration technologies such as membrane bioreactors, magnetic separation, and advanced oxidation processes can improve microfiber capture [77,79,81]. Implementing bio-based flocculants can also help in aggregating microfibers for easier removal. These systems can be integrated into existing treatment plants to improve their efficiency in removing microfibers.
- Microfiber filtration: At the household level, the installation of microfiber filtration devices (PlanetCare filter, XFiltra, Filtrol, etc.) in washing machines is an emerging solution. These devices, often referred to as microfiber filters or laundry filters, capture microfibers released from clothing during the wash cycle [100]. By preventing the release of microfibers into wastewater, they represent a proactive approach to reducing microfiber pollution at its source.
- Public awareness and education: Public awareness campaigns and consumer education are essential for combating microfiber pollution. Individuals can take steps to reduce their contribution by washing synthetic garments less frequently, using cooler water temperatures, and using laundry bags specifically designed to capture microfibers. Being informed about the environmental impacts of microfibers and making sustainable choices when purchasing clothing can also make a significant difference [100].
- Regulatory and policy measures: Governments and regulatory bodies can play a vital role in addressing microfiber pollution by implementing and enforcing regulations that require manufacturers to develop more sustainable products and textiles. Legislation can set standards for microfiber shedding and encourage the adoption of best practices for reducing pollution at the source [101,102].
- Research and innovation: Continued research is essential for understanding the full extent of the problem and identifying effective mitigation strategies. Scientists and innovators are working to develop new materials and coatings that shed fewer microfibers and exploring the use of natural and biodegradable alternatives to synthetic textiles [103]. Innovations in textile recycling and circular economy models are also being explored to reduce waste and microfiber emissions.
- Circular economy and recycling: Promoting the recycling of textiles can reduce the need for virgin synthetic fibers and decrease microfiber pollution. Closed-loop recycling systems where textiles are collected, processed, and remade into new fabrics can help create a circular economy [104]. Encouraging manufacturers to establish take-back programs for old clothing can ensure textiles are recycled rather than discarded.
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathinamoorthy, R.; Raja Balasaraswathi, S. A review of the current status of microfiber pollution research in textiles. Int. J. Cloth. Sci. Technol. 2021, 33, 364–387. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, F.; Lei, K.; Li, H.; Kang, Y.; Cui, S.; An, L. Microfiber release from different fabrics during washing. Environ. Pollut. 2019, 249, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Woodward, J.C.; Rothwell, J.J. Ingestion of microplastics by freshwater tubifex worms. Environ. Sci. Technol. 2017, 51, 12844–12851. [Google Scholar] [CrossRef]
- Das, A.P.; Dutta, K.; Khatun, R.; Behera, I.D.; Singh, S.; Mishra, S. Microfiber pollution and its microbial mitigation: A review on current trends and future prospects. J. Tai. Inst. Chem. Eng. 2023, 105104. [Google Scholar] [CrossRef]
- Peng, G.; Xu, P.; Zhu, B.; Bai, M.; Li, D. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TrAC Trends Analyt. Chem. 2019, 111, 62–72. [Google Scholar]
- Liang, Y.; Lehmann, A.; Ballhausen, M.-B.; Muller, L.; Rillig, M.C. Increasing temperature and microplastic fibers jointly influence soil aggregation by saprobic fungi. Front. Microbiol. 2019, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Muniasamy, G.K.; Guevara, F.P.; Martínez, I.E.; Shruti, V.C. An overview of recent advances in micro/nano beads and microfibers research: Critical assessment and promoting the less known. Sci. Total Environ. 2020, 740, 139991. [Google Scholar] [CrossRef]
- Singh, R.P.; Mishra, S.; Das, A.P. Synthetic microfibers: Pollution toxicity and remediation. Chemosphere 2020, 257, 127199. [Google Scholar] [CrossRef]
- Samal, K.; Dash, R.R.; Bhunia, P. Treatment of wastewater by vermifiltration integrated with macrophyte filter: A review. J. Environ. Chem. Eng. 2017, 5, 2274–2289. [Google Scholar] [CrossRef]
- Habib, R.Z.; Abdoon, M.M.S.; Al Meqbaali, R.M.; Ghebremedhin, F.; Elkashlan, M.; Kittaneh, W.F.; Cherupurakal, N.; Mourad, A.H.I.; Thiemann, T.; Al Kindi, R. Analysis of microbeads in cosmetic products in the United Arab Emirates. Environ. Pollut. 2020, 258, 113831. [Google Scholar] [CrossRef]
- Ustabasi, G.S.; Baysal, A. Bacterial interactions of microplastics extracted from toothpaste under controlled conditions and the influence of seawater. Sci. Total Environ. 2020, 703, 135024. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, C.; Kumbur, H.; Gökdağ, K.; Kıdeyş, A.E.; Sanchez-Vidal, A. Microplastics composition and load from three wastewater treatment plants discharging into Mersin Bay, north eastern Mediterranean Sea. Mar. Pollut. Bull. 2020, 150, 110776. [Google Scholar] [CrossRef] [PubMed]
- Severini, M.D.F.; Villagran, D.M.; Buzzi, N.S.; Sartor, G.C. Microplastics in oysters (Crassostrea gigas) and water at the Bahía Blanca Estuary (Southwestern Atlantic): An emerging issue of global concern. Reg. Studies Mar. Sci. 2019, 32, 100829. [Google Scholar] [CrossRef]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Ding, J.; Zhang, G.; Zeng, X.; Yang, Q.; Zhu, B.; Gao, W. Microfiber pollution: An ongoing major environmental issue related to the sustainable development of textile and clothing industry. Environ. Dev. Sustain. 2021, 23, 11240–11256. [Google Scholar] [CrossRef]
- Measuring Cotton Consumption: Better Cotton Conversion Factors and Multipliers. Better Cotton Initiative: Geneva, Switzerland. 2022. 23p. Available online: https://ls.bettercotton.org/measuring-cotton-consumption/ (accessed on 10 June 2024).
- Johnson, J.; MacDonald, S.; Meyer, L.; Soley, G. The World and United States Cotton Outlook. 2021; pp. 1–21. Available online: https://econpapers.repec.org/paper/agsusao21/320767.htm (accessed on 10 June 2024).
- Sadeghi, P.; Sadeghi, B.; Marfavi, Y.; Kowsari, E.; Ramakrishna, S.; Chinnappan, A. Addressing the challenge of microfiber plastics as the marine pollution crisis using circular economy methods: A review. Mater. Circ. Econ. 2021, 3, 27. [Google Scholar] [CrossRef]
- Mishra, S.; Rout, P.K.; Das, A.P. Emerging microfiber pollution and its remediation. Environ. Pollut. Remediat. 2021, 247–266. [Google Scholar] [CrossRef]
- Samal, K.; Trivedi, S. A statistical and kinetic approach to develop an Ecological Floating Bed for the treatment of wastewater. J. Environ. Chem. Eng. 2020, 8, 104102. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, R.P.; Rath, C.C.; Das, A.P. Synthetic microfibers: Source, transport and their remediation. J. Wat. Proces. Eng. 2020, 38, 101612. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Krzan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chernick, M.; Lewis, A.M.; Ferguson, P.L.; Hinton, D.E. Chronic microfiber exposure in adult japanese medaka (Oryzias latipes). PLoS ONE 2020, 15, e0229962. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; charan Rath, C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; An, L.; Wang, M.; Yang, Q.; Zhu, B.; Ding, J.; Ye, C.; Xu, Y. Microfiber pollution in the earth system. Rev. Environ. Contam. Toxicol. 2022, 260, 13. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef]
- Samal, K.; Moulick, S.; Mohapatra, B.G.; Samanta, S.; Sasidharan, S.; Prakash, B.; Sarangi, S. Design of faecal sludge treatment plant (FSTP) and availability of its treatment technologies. Energy Nexus 2022, 7, 100091. [Google Scholar] [CrossRef]
- Li, L.; Su, L.; Cai, H.; Rochman, C.M.; Li, Q.; Kolandhasamy, P.; Peng, J.; Shi, H. The uptake of microfibers by freshwater asian clams (Corbicula fluminea) varies based upon physicochemical properties. Chemosphere 2019, 221, 107–114. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Kunej, U.; Skalar, T. Screening study of four environmentally relevant microplastic pollutants: Uptake and effects on Daphnia magna and Artemia franciscana. Chemosphere 2018, 208, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Zhang, L.; Sun, L.; Lin, C.; Wang, Q.; Yang, H. Microplastic fibers transfer from the water to the internal fluid of the sea cucumber Apostichopus japonicus. Environ. Pollut. 2020, 257, 113606. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.N.; Hong, T.J.; Baughman, D.; Andrews, G.; Fields, D.M.; Matrai, P.A. Accumulation and effects of microplastic fibers in american lobster larvae (Homarus americanus). Mar. Pollut. Bull. 2020, 157, 111280. [Google Scholar] [CrossRef]
- Leads, R.R.; Burnett, K.G.; Weinstein, J.E. The effect of microplastic ingestion on survival of the grass shrimp Palaemonetes pugio (Holthuis, 1949) challenged with Vibrio campbellii. Environ. Toxicol. Chem. 2019, 38, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Hankins, C.; Duffy, A.; Drisco, K. Scleractinian coral microplastic ingestion: Potential calcification effects, size limits, and retention. Mar. Pollut. Bull. 2018, 135, 587–593. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.A.; Granek, E.F.; Steele, C.L. Effects of environmentally relevant concentrations of microplastic fibers on Pacific mole crab (Emerita analoga) mortality and reproduction. Limnol. Oceanogr. Lett. 2020, 5, 74–83. [Google Scholar] [CrossRef]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.L.; Galloway, T.S.; Cole, M.; Fileman, E.S.; Queirós, A.M.; Lindeque, P.K. Microplastics alter feeding selectivity and faecal density in the copepod, Calanus helgolandicus. Sci. Total Environ. 2019, 687, 780–789. [Google Scholar] [CrossRef]
- Kolandhasamy, P.; Su, L.; Li, J.; Qu, X.; Jabeen, K.; Shi, H. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Sci. Total Environ. 2018, 610, 635–640. [Google Scholar] [CrossRef]
- Straub, S.; Hirsch, P.E.; Burkhardt-Holm, P. Biodegradable and petroleum-based microplastics do not differ in their ingestion and excretion but in their biological effects in the freshwater invertebrate Gammarus fossarum. Int. J. Environ. Res. Public Health 2017, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Dash, R.R. Modelling of pollutants removal in Integrated Vermifilter (IVmF) using response surface methodology. Clean. Eng. Technol. 2021, 2, 100060. [Google Scholar] [CrossRef]
- Behera, S.; Samal, K. Sustainable approach to manage solid waste through biochar assisted composting. Energy Nexus. 2022, 7, 100121. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Skalar, T.; Parramon Dolcet, L.; Hurley, R.; van Gestel, C.A.M. Exploring the impacts of plastics in soil—the effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- Kim, S.W.; Waldman, W.R.; Kim, T.-Y.; Rillig, M.C. Effects of different microplastics on nematodes in the soil environment: Tracking the extractable additives using an ecotoxicological approach. Environ. Sci. Technol. 2020, 54, 13868–13878. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-derived microfibre impacts on the soil-dwelling earthworm lumbricus terrestris. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, C.; Qiu, R.; Hu, J.; Liu, M.; Lu, S.; Shi, H.; Raley-Susman, K.M.; He, D. Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 2019, 250, 447–455. [Google Scholar] [CrossRef]
- Kwak, J.I.; Liu, H.; Wang, D.; Lee, Y.H.; Lee, J.S.; Joo, Y.-A. Critical review of environmental impacts of microfibers in different environmental matrices. Compar. Biochem. Physiol. Part C 2022, 251, 109196. [Google Scholar] [CrossRef]
- Joos, L.; De Tender, C. Soil under stress: The importance of soil life and how it is influenced by (micro) plastic pollution. Comput. Struct. Biotechnol. J. 2022, 20, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in ecosystems: Their implications and mitigation pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, B.; An, L.; Ding, J.; Xu, Y. Atmospheric microfibers dominated by natural and regenerated cellulosic fibers: Explanations from the textile engineering perspective. Environ. Pollut. 2023, 317, 120771. [Google Scholar] [CrossRef]
- Peller, J.R.; Eberhardt, L.; Clark, R.; Nelson, C.; Kostelnik, E.; Iceman, C. Tracking the distribution of microfiber pollution in a southern Lake Michigan watershed through the analysis of water, sediment and air. Environ. Sci. Process. Impacts 2019, 21, 1549–1559. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Fortin, S.; Song, B.; Burbage, C. Quantifying and identifying microplastics in the effluent of advanced wastewater treatment systems using Raman microspectroscopy. Mar. Pollut. Bull. 2019, 149, 110579. [Google Scholar] [CrossRef] [PubMed]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ouarda, Y.; Tiwari, B.; Azaïs, A.; Vaudreuil, M.A.; Ndiaye, S.D.; Drogui, P.; Tyagi, R.D.; Sauvé, S.; Desrosiers, M.; Buelna, G.; et al. Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment. Chemosphere 2018, 193, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kiendrebeogo, M.; Estahbanati, M.K.; Mostafazadeh, A.K.; Drogui, P.; Tyagi, R.D. Treatment of microplastics in water by anodic oxidation: A case study for polystyrene. Environ. Pollut. 2021, 269, 116168. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.; Ribeiro-Claro, P.J. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Demir-Yilmaz, I.; Yakovenko, N.; Roux, C.; Guiraud, P.; Collin, F.; Coudret, C.; ter Halle, A.; Formosa-Dague, C. The role of microplastics in microalgae cells aggregation: A study at the molecular scale using atomic force microscopy. Sci. Total Environ. 2022, 832, 155036. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, P.; Ni, F.; Cizdziel, J.; Wu, D.; Zhao, Q.; Zhou, Y. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Mar. Pollut. Bull. 2019, 145, 153–160. [Google Scholar] [CrossRef]
- Lou, F.; Wang, J.; Sun, C.; Song, J.; Wang, W.; Pan, Y.; Huang, Q.; Yan, J. Influence of interaction on accuracy of quantification of mixed microplastics using Py-GC/MS. J. Environ. Chem. Eng. 2022, 10, 108012. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Kanyathare, B.; Roussey, M.; Peiponen, K.E. A prototype of a portable optical sensor for the detection of transparent and translucent microplastics in freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef]
- Yu, S.; Dai, J.; Liao, R.; Chen, L.; Zhong, W.; Wang, H.; Jiang, Y.; Li, J.; Ma, H. Probing the nanoplastics adsorbed by microalgae in water using polarized light scattering. Optik 2021, 231, 166407. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Sundbæk, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J. Appl. Phycol. 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-based biopolymer for nano-and microplastic removal: A possible biosolution for wastewater treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Zeng, H.; Lin, H.; Li, H.; Chai, Y.Y.; Choong, M.K.; Mohamed, A.R. Green synthesis of Fe-ZnO nanoparticles with improved sunlight photocatalytic performance for polyethylene film deterioration and bacterial inactivation. Mater. Sci. Semicond. Process. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 62, 68–72. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Eiband, M.M.S.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Sotiropoulou, M.; Stefanatou, A.; Schiza, S.; Petousi, I.; Stasinakis, A.S.; Fountoulakis, M.S. Removal of microfiber in vertical flow constructed wetlands treating greywater. Sci. Total Environ. 2023, 858, 159723. [Google Scholar] [CrossRef]
- Long, Y.; Zhou, Z.; Yin, L.; Wen, X.; Xiao, R.; Du, L.; Zhu, L.; Liu, R.; Xu, Q.; Li, H.; et al. Microplastics removal and characteristics of constructed wetlands WWTPs in rural area of Changsha, China: A different situation from urban WWTPs. Sci. Total Environ. 2022, 811, 152352. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Xiao, M.R.; Zhang, G.S. The persistent impacts of polyester microfibers on soil bio-physical properties following thermal treatment. J. Hazard. Mater. 2021, 420, 126671. [Google Scholar] [CrossRef]
- Sharma, U.; Sharma, S.; Rana, V.S.; Rana, N.; Kumar, V.; Sharma, S.; Qadri, H.; Kumar, V.; Bhat, S.A. Assessment of microplastics pollution on soil health and eco-toxicological risk in horticulture. Soil. Syst. 2023, 7, 7. [Google Scholar] [CrossRef]
- Mahmud, A.; Wasif, M.M.; Roy, H.; Mehnaz, F.; Ahmed, T.; Pervez, M.N.; Naddeo, V.; Islam, M.S. Aquatic microplastic pollution control strategies: Sustainable degradation techniques, resource recovery, and recommendations for Bangladesh. Water 2022, 14, 3968. [Google Scholar] [CrossRef]
- Mondal, I.; Ghosh, D.; Biswas, P.K. Cost-effective remedial to microfiber pollution from wash effluent in Kolkata and Ranaghat. Chemosphere 2023, 313, 137548. [Google Scholar] [CrossRef]
- Weis, J.S.; De Falco, F. Microfibers: Environmental problems and textile solutions. Microplastics 2022, 1, 626–639. [Google Scholar] [CrossRef]
- Ramasamy, R.; Subramanian, R.B. Synthetic textile and microfiber pollution: A review on mitigation strategies. Environ. Sci. Pollut. Res. 2021, 28, 41596–41611. [Google Scholar] [CrossRef] [PubMed]
- Erdle, L.M.; Nouri Parto, D.; Sweetnam, D.; Rochman, C.M. Washing machine filters reduce microfiber emissions: Evidence from a community-scale pilot in Parry Sound, Ontario. Front. Mar. Sci. 2021, 8, 777865. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Harkes, P.; Beriot, N.; Larreta, J.; Basurko, O.C. Microplastics: A review of policies and responses. Microplastics 2022, 2, 1–26. [Google Scholar] [CrossRef]
- Garcia-Vazquez, E.; Garcia-Ael, C. The invisible enemy. Public knowledge of microplastics is needed to face the current microplastics crisis. Sustain. Prod. Consum. 2021, 28, 1076–1089. [Google Scholar] [CrossRef]
- Wojciechowska, P. Fibres and textiles in the circular economy. In Fundamentals of Natural Fibres and Textiles; Woodhead Publishing: Sawston, UK, 2021; Volume 1611, pp. 691–717. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from synthetic textiles as a major source of microplastics in the environment: A review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]

| Destination | Microfiber Types | Size Range (μm) | Shape | References |
|---|---|---|---|---|
| Tubifex worms | PET and acrylic microfibers | 55–4100 | Fibrous | [4] |
| Sediment | PP | <400 | Spherical | [6] |
| Fish | PE, PP, and cellulose | 1000–5000 | Fibrous | [7] |
| Body scrubs | PE, WS, PAA, MC, silica, and pumice | 12.3–273.4 | Irregular, spherical, granular | [12] |
| Toothpaste | PE and CaCO3 | <20 | Irregular | [13] |
| Surface water | PP, PS, PVC, PET, and polyamide | 100–5000 | Fibrous | [14] |
| Facial scrubs | PE or LDPE, wax, Luwax, and PVC | 85–186 | Irregular, granular, spherical | [15] |
| Surface water | PE, PP, and PS | 200 | Fibrous | [16] |
| Wastewater treatment plant | PE, PP, acrylic, PS, and cellulose acetate | ≤2500 | Fibrous | [17] |
| Oyster | - | 240–1000 | Spherical | [18] |
| Shrimp P. australiensis | Polyamide, rayon, PP, and PE | - | Fibrous | [19] |
| Microfibers | Organism | Exposure Time | Concentration | Ecotoxicological Effects | References |
|---|---|---|---|---|---|
| PET | Daphnia magna | 48 h | 12–100 mg/L | Mortality rate increased | [27] |
| PES | Oryzias latipes | 21 days | 10,000 MFs/L | Embryo production increased | [28] |
| PET | Corbicula fluminea | 48 h | 100, 1000 MFs/L | Polyester fibers were taken up in the small size range | [33] |
| PET | Daphnia magna | 48 h | 100 mg/L | No acute effect on test organism | [34] |
| Polyester | Apostichopus japonicus | 72 h | 25, 40 MFs/mL | Coelomic fluid accumulation, lysozyme toxicity | [35] |
| PET | Homarus gammarus | 5 days | 1, 10, 25 MFs/mL | Survival rate decreased at 25 MFs/mL | [36] |
| PES | Palaemon pugio | 96 h | 45,000 MFs/L | No significant mortality rate | [37] |
| PET | Montastraea cavernosa | 48 h | 30 mg/L | MFs did not elicit a feeding response | [38] |
| PP | Danio rerio | 24 h | 20 mg/L | Intestine damaged by ingestion of microfibers | [39] |
| PP | Emerita analoga | 71 days | 3 MFs/L | Retention of egg clutches, embryonic development increased | [40] |
| PP | Nephrops norvegicus | 8 months | 5 MFs/per feeding | Reductions in blood protein and lipids, body mass reduced | [41] |
| Fibers, PET | Calanus helgolandicus | 24 h | 100 MFs/mL | Feeding reduction | [42] |
| Fibers | Mytilus edulis | 48 h | 2000 MFs/mL | Microfibers taken up in multiple organs | [43] |
| Fibers; PA | Gammarus fossarum | 24 h | 10–100,000 microbeads/ individual | Reduction in assimilation and wet weight gain | [44] |
| Microfibers | Organism | Exposure Time | Concentration | Ecotoxicological Effects | References |
|---|---|---|---|---|---|
| Polyacrylic | Mucor fragilis, Fusarium sp. | 42 days | 0.4% | Water-stable aggregates were decreased with four specific strains | [8] |
| Acrylic and nylon mixture | Acrasis rosea, Lolium perenne | 30 days | 10 mg/kg (0.001%) | No negative impact on plant and earthworm biomass or worm mortality; germination rate decreased and chlorophyll a/b ratio altered | [47] |
| PES blanket | Enchytraeus crypticus | 3 weeks | 0.02, 0.06, 0.17, 0.5, 1.5% (various) | Soil exposure to long fibers significantly affected reproduction | [48] |
| Polyacrylic nitrile | Caenorhabditis elegans | 24 h | 0.001, 0.01, 0.1% | Toxicity of MFs on the organism increased after long-term wet–dry cycles in soil | [49] |
| PE cushion | Lumbricus terrestris | 35 days | 0.1, 1% | No mortality and no bioaccumulation, but alteration of mt and hsp70 | [50] |
| PET | Achatina fulica | 28 days | 0.014, 0.14, 0.71 g/kg | No mortality and no growth inhibition | [51] |
| Identification Methods | Benefits | Limitations | References |
|---|---|---|---|
| FTIR |
|
| [67] |
| Raman spectroscopy |
|
| [68] |
| Atomic force microscopy |
|
| [69] |
| Thermal analysis |
|
| [70] |
| Py-GC-MS |
|
| [71] |
| Optical sensing |
|
| [72] |
| Polarized light scattering |
|
| [73] |
| Methods | MP Particle Removed | Efficiency (%) | Advantages | Challenges | References |
|---|---|---|---|---|---|
| Electrocoagulation | - | 90 (pH 3–10) 99.24 (pH 7.5) | Does not rely on chemicals or microorganisms, energy-efficient | Operation time needs to be reduced | [74] |
| Al and Fe salt | <0.5 mm | 45.34 | Simple process, does not require additional set-up | Low efficiency | [75] |
| Wastewater treatment plant | 100 μm | 99 | Conventional process, no additional cost | Not possible to remove MPs of size < 100 μm | [76] |
| Filtration with granular activated carbon | 1–5 μm | 56.8–60.9 | Efficient to remove plastic particles in the nanoscale size range | Frequent clogging, more regeneration time | [77] |
| Algal masses | 20 μm | 94.5 | No chemical, electrical, or mechanical operations | Efficiency will vary owing to physiological and environmental conditions | [78] |
| Membrane bioreactor (MBR) | 250 μm | 99.3 | MBR process helped to retain moremicroplastics compared to the conventional activated sludge process | Not possible to remove MPs of size < 250 μm | [79] |
| Bioremediation (Ideonellasakaiensis 201-F6) | PET | - | PET could be degraded within 6 weeks | Identification of other microorganisms of the same type that could be more effective for these bioremediation processes is required | [80] |
| Bioremediation (EPS by Cyanothece sp.) | <300 μm | - | Hetero-aggregation capability at 1 and 10 mg/L of both nanoplastics and microplastics | - | [81] |
| Air flotation and nano-ferrofluid processes | PE 75 μm, PVC 150 μm, PES 300 μm | PE 85%, PVC 82%, PES 69% | - | Nanofluid particles were ineffective to remove NPs/MPs | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, K.; Samal, S.R.; Mishra, S.; Nayak, J.K. Sources, Transport, and Accumulation of Synthetic Microfiber Wastes in Aquatic and Terrestrial Environments. Water 2024, 16, 2238. https://doi.org/10.3390/w16162238
Samal K, Samal SR, Mishra S, Nayak JK. Sources, Transport, and Accumulation of Synthetic Microfiber Wastes in Aquatic and Terrestrial Environments. Water. 2024; 16(16):2238. https://doi.org/10.3390/w16162238
Chicago/Turabian StyleSamal, Kundan, Satya Ranjan Samal, Saurabh Mishra, and Jagdeep Kumar Nayak. 2024. "Sources, Transport, and Accumulation of Synthetic Microfiber Wastes in Aquatic and Terrestrial Environments" Water 16, no. 16: 2238. https://doi.org/10.3390/w16162238
APA StyleSamal, K., Samal, S. R., Mishra, S., & Nayak, J. K. (2024). Sources, Transport, and Accumulation of Synthetic Microfiber Wastes in Aquatic and Terrestrial Environments. Water, 16(16), 2238. https://doi.org/10.3390/w16162238







