Characteristics and Nitrogen Removal Performance Optimization of Aerobic Denitrifying Bacteria Bacillus cereus J1 under Ammonium and Nitrate-Nitrogen Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Medium and the Isolation and Identification of Strain J1
2.2. Kinetics Experiments
2.3. Optimization of Nitrogen Removal Performance
2.4. Analytical Methods
3. Results
3.1. Isolation and Identification of Strain J1
3.2. Kinetics Experiments with Two Nitrogen Sources of Strain J1
3.2.1. Growth Characteristics of Strain J1
3.2.2. Characteristics of pH and Substrate Degradation for Two Nitrogen Sources
3.2.3. The Fit Curve between TIN and COD
3.3. Single-Factor Experiments for Two Nitrogen Sources with Strain J1
3.3.1. Shaking Speed
3.3.2. C/N Ratio
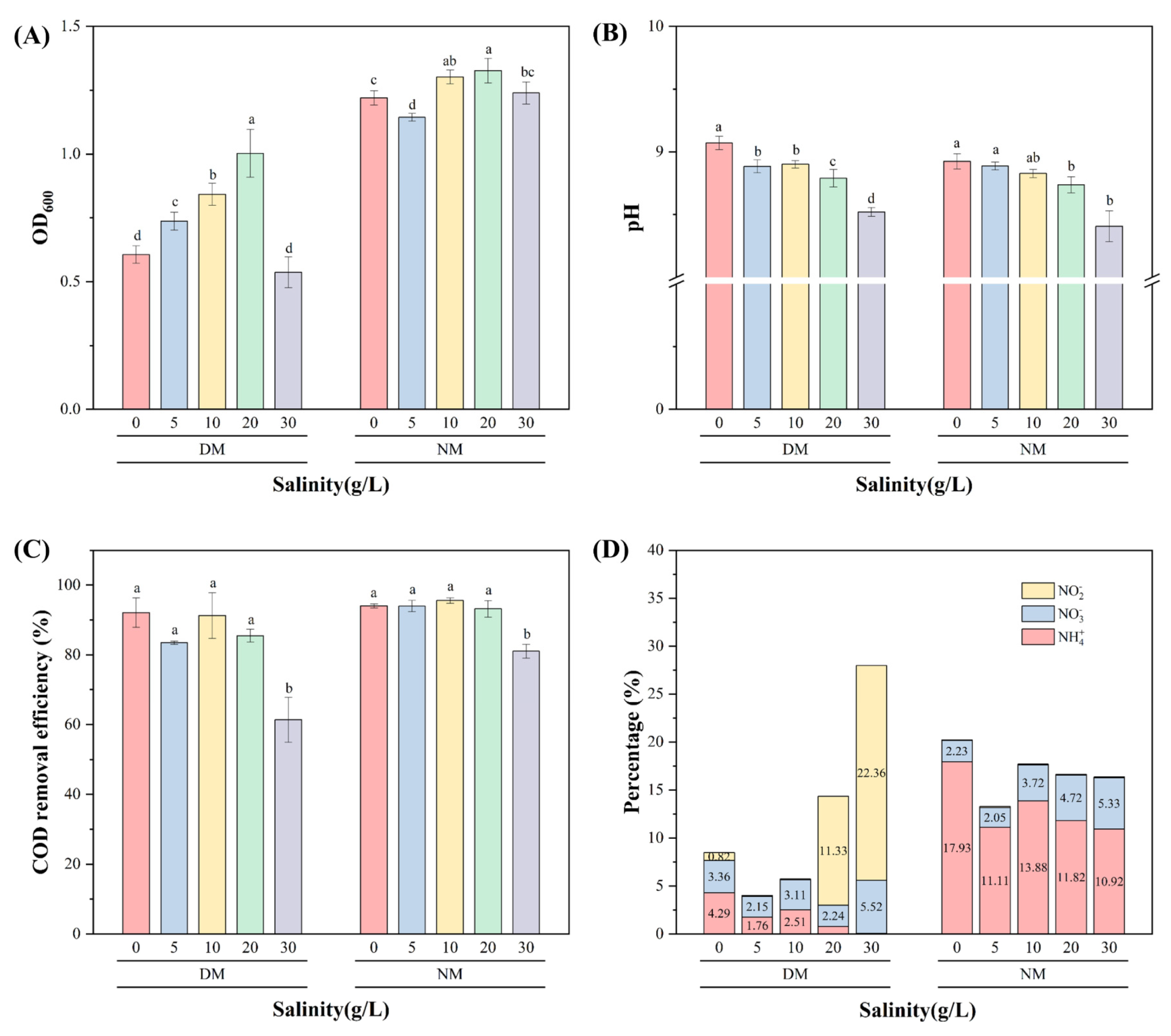
3.3.3. Salinity
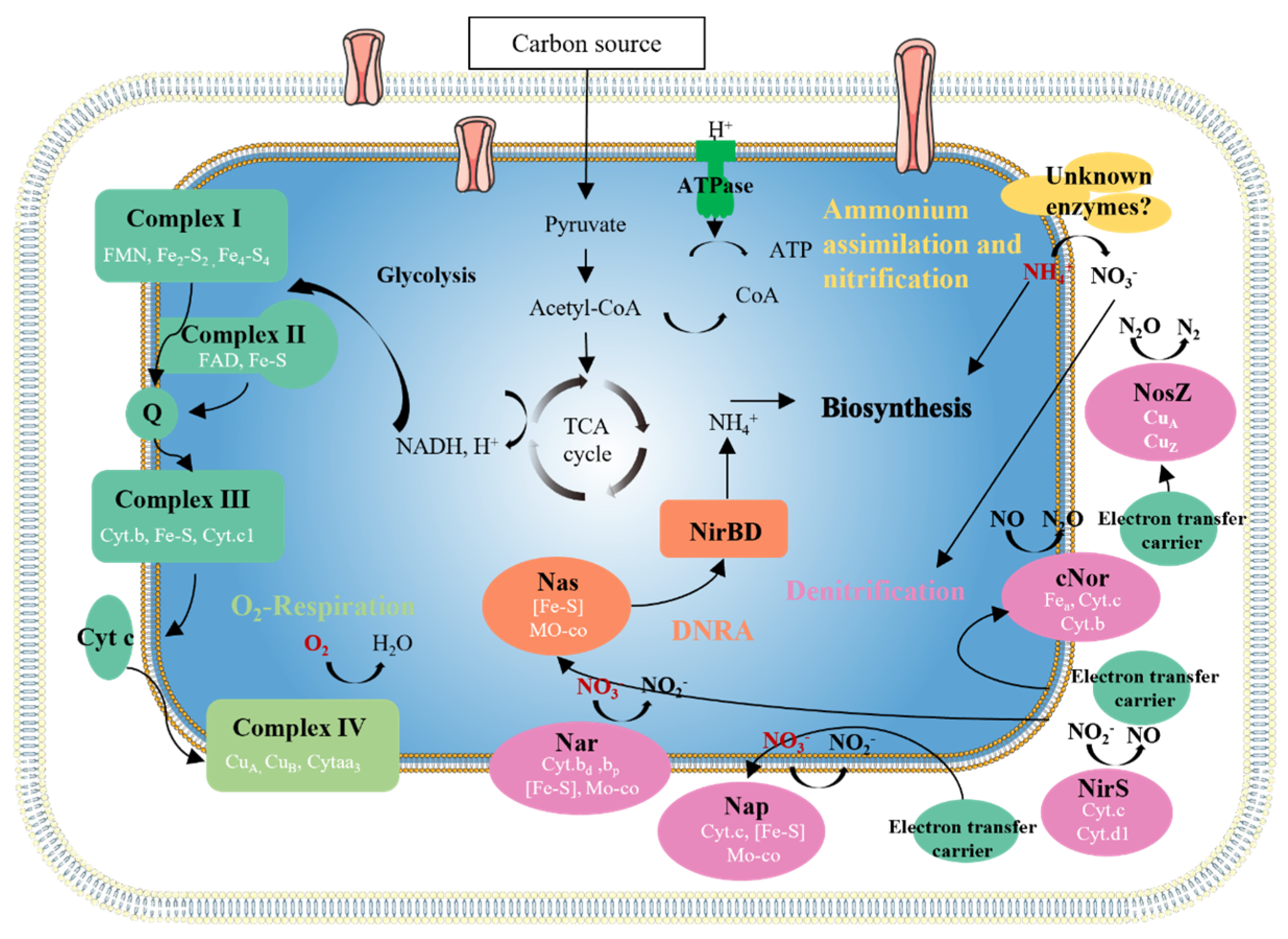
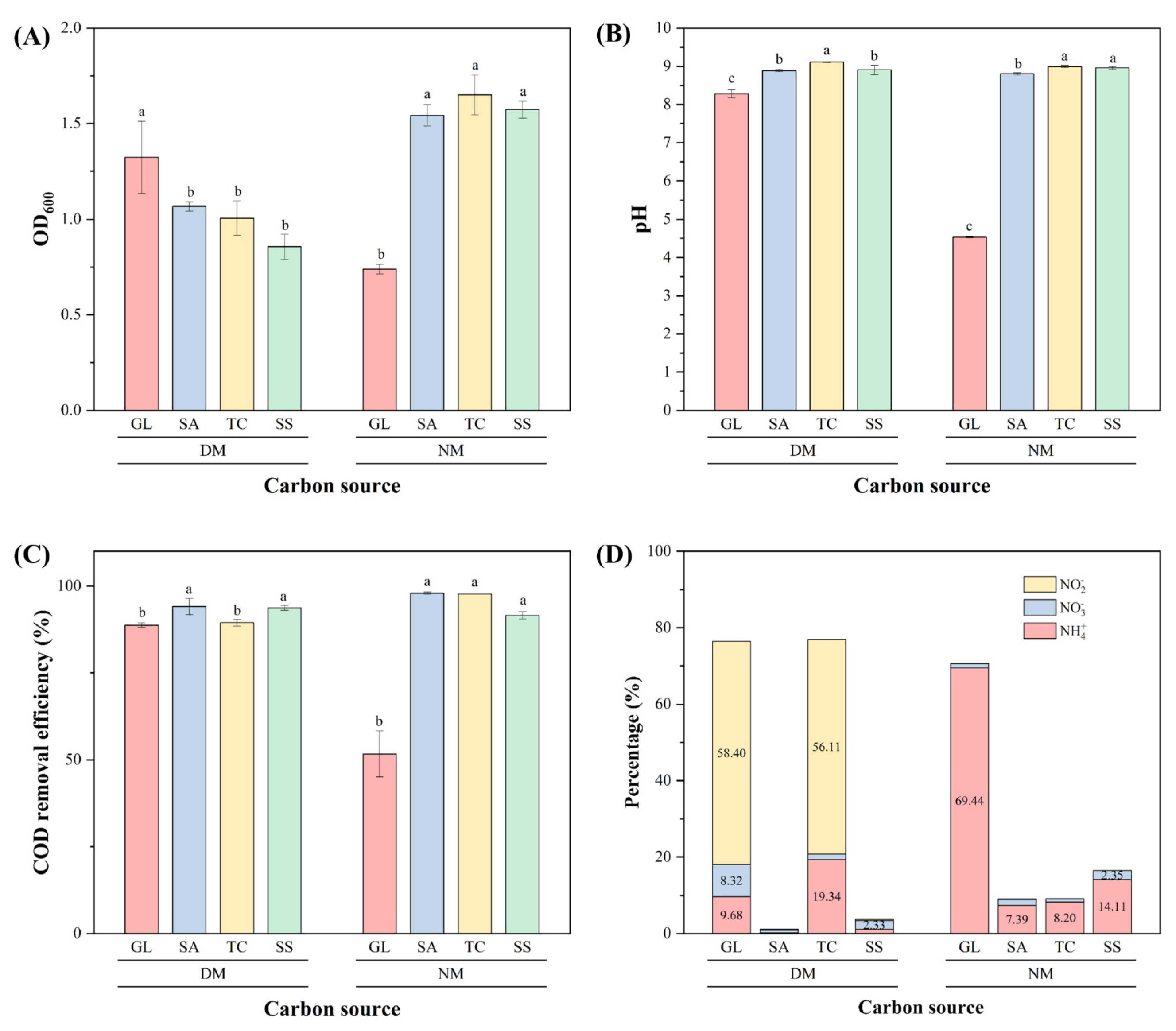
3.3.4. Carbon Source and Carbon Metabolism Genes
3.4. Box–Behnken Design for Nitrogen Removal Condition Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, R.; Huang, T.; Zhang, H.; Yang, S.; Li, N.; Huang, D. Aerobic Denitrification of Oligotrophic Source Water Driven by Reduced Metal Manganese. Chemosphere 2023, 317, 137764. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.K.; Tripathy, S.; Mohanty, S.; Maiti, N.K. Aerobic and Heterotrophic Nitrogen Removal by Enterobacter Cloacae CF-S27 with Efficient Utilization of Hydroxylamine. Bioresour. Technol. 2017, 232, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Wang, K.; Liu, X.; Wong, M.H.; Hu, Z.; Chen, H.; Yang, X.; Li, S. A study on the nitrogen removal efficacy of bacterium Acinetobacter tandoii MZ-5 from a contaminated river of Shenzhen, Guangdong Province, China. Bioresour. Technol. 2020, 315, 123888. [Google Scholar] [CrossRef]
- Robertson, L.A.; Cornelisse, R.; De Vos, P.; Hadioetomo, R.; Kuenen, J.G. Aerobic Denitrification in Various Heterotrophic Nitrifiers. Antonie Leeuwenhoek 1989, 56, 289–299. [Google Scholar] [CrossRef]
- Robertson, L.A.; Kuenen, J.G. Aerobic Denitrification—Old Wine in New Bottles? Antonie Leeuwenhoek 1984, 50, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Yin, Q.; Wang, J.; Li, M.; Li, Y.; Li, B. Heterotrophic Nitrification-Aerobic Denitrification Performance of a Novel Strain, Pseudomonas Sp. B-1, Isolated from Membrane Aerated Biofilm Reactor. Environ. Res. 2023, 220, 115199. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Ma, M.; Ma, B.; Liu, H.; Niu, L.; Zhao, D.; Ni, T.; Yang, W.; Yang, Y. Nitrogen Reduction by Aerobic Denitrifying Fungi Isolated from Reservoirs Using Biodegradation Materials for Electron Donor: Capability and Adaptability in the Lower C/N Raw Water Treatment. Sci. Total Environ. 2023, 864, 161064. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of Heterotrophic Nitrifiers and Aerobic Denitrifiers in Simultaneous Nitrification and Denitrification Process: A Nonconventional Nitrogen Removal Pathway in Wastewater Treatment. Lett. Appl. Microbiol. 2022, 74, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, M.; Chen, Y.; Hu, Y.; Luo, J. Insight into Short-Cut of Simultaneous Nitrification and Denitrification Process in Moving Bed Biofilm Reactor: Effects of Carbon to Nitrogen Ratio. Chem. Eng. J. 2020, 400, 125905. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Q.; Gui, M.; Li, C.; Ni, J. Simultaneous Denitrification and Phosphorus Removal by Agrobacterium Sp. LAD9 under Varying Oxygen Concentration. Appl. Microbiol. Biotechnol. 2016, 100, 3337–3346. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, B.; An, Q.; Wang, X.; Zhang, Y.X. Kinetic Characteristics and Modelling of Growth and Substrate Removal by Alcaligenes faecalis Strain NR. Bioprocess. Biosyst. Eng. 2016, 39, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Lu, J.; Hai, C.; Liu, X.; Wu, P.; Sun, Y.; Yuan, C.; Zhao, J.; Hu, B. Exploring influence mechanism of small-molecule carbon source on heterotrophic nitrification-aerobic denitrification process from carbon metabolism, nitrogen metabolism and electron transport process. Bioresour. Technol. 2023, 387, 129681. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; He, T.; Wang, C.; Zhang, M.; Yang, L.; Yang, L. Key Enzymes, Functional Genes, and Metabolic Pathways of the Nitrogen Removal-Related Microorganisms. Crit. Rev. Environ. Sci. Technol. 2024, 1–20. [Google Scholar] [CrossRef]
- Mpongwana, N.; Ntwampe, S.K.O.; Razanamahandry, L.C.; Chidi, B.S.; Omodanisi, E.I. Predictive Capability of Response Surface Methodology and Cybernetic Models for Cyanogenic Simultaneous Nitrification and Aerobic Denitrification Facilitated by Cyanide-Resistant Bacteria. Environ. Eng. Res. 2021, 26, 200346. [Google Scholar] [CrossRef]
- Bouchez, T.; Patureau, D.; Dabert, P.; Wagner, M.; Delgenès, J.P.; Moletta, R. Successful and Unsuccessful Bioaugmentation Experiments Monitored by Fluorescent in Situ Hybridization. Water Sci. Technol. 2000, 41, 61–68. [Google Scholar] [CrossRef]
- Patureau, D.; Helloin, E.; Rustrian, E.; Bouchez, T.; Delgenes, J.P.; Moletta, R. Combined Phosphate and Nitrogen Removal in a Sequencing Batch Reactor Using the Aerobic Denitrifier, Microvirgula aerodenitrificans. Water Res. 2001, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of Heterotrophic Aerobic Denitrifying Bacteria in Nitrate Removal from Wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic Denitrification: A Review of Important Advances of the Last 30 Years. Biotechnol. Bioprocess. Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Ren, Y.-X.; Yang, L.; Liang, X. The Characteristics of a Novel Heterotrophic Nitrifying and Aerobic Denitrifying Bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, R.; Chen, Y.; Li, Y.; Jin, P.; Zheng, Z. Enhanced Nitrogen Removal from Low-Temperature Wastewater by an Iterative Screening of Cold-Tolerant Denitrifying Bacteria. Bioprocess. Biosyst. Eng. 2022, 45, 381–390. [Google Scholar] [CrossRef]
- Huang, T.; Guo, L.; Zhang, H.; Su, J.; Wen, G.; Zhang, K. Nitrogen-Removal Efficiency of a Novel Aerobic Denitrifying Bacterium, Pseudomonas stutzeri Strain ZF31, Isolated from a Drinking-Water Reservoir. Bioresour. Technol. 2015, 196, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ni, J. Ammonium Removal by Agrobacterium Sp. LAD9 Capable of Heterotrophic Nitrification–Aerobic Denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, C.; Shen, J.; Wei, R.; Gao, Y.; Miao, A.; Xiao, L.; Yang, L. Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. Int. J. Environ. Res. Public Health 2019, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, X.; Liu, Y.; Zhao, K.; Li, Y.; Luo, K.; Wang, B.; Dong, S. Nitrogen removal capability and mechanism of a novel heterotrophic nitrifying–aerobic denitrifying strain H1 as a potential candidate in mariculture wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 106366–106377. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, Y.; Xin, Y.; Yang, T.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Insight into the Cold Adaptation Mechanism of an Aerobic Denitrifying Bacterium: Bacillus simplex H-b. Appl. Environ. Microbiol. 2023, 89, e01928-22. [Google Scholar] [CrossRef]
- Asamoto, C.K.; Rempfert, K.R.; Luu, V.H.; Younkin, A.D.; Kopf, S.H. Enzyme-Specific Coupling of Oxygen and Nitrogen Isotope Fractionation of the Nap and Nar Nitrate Reductases. Environ. Sci. Technol. 2021, 55, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Strous, M.; Tegetmeyer, H.E. Microbial Nitrate Respiration—Genes, Enzymes and Environmental Distribution. J. Biotechnol. 2011, 155, 104–117. [Google Scholar] [CrossRef]
- Hao, Z.-L.; Ali, A.; Ren, Y.; Su, J.-F.; Wang, Z. A Mechanistic Review on Aerobic Denitrification for Nitrogen Removal in Water Treatment. Sci. Total Environ. 2022, 847, 157452. [Google Scholar] [CrossRef]
- Loi, V.V.; Busche, T.; Kuropka, B.; Müller, S.; Methling, K.; Lalk, M.; Kalinowski, J.; Antelmann, H. Staphylococcus aureus Adapts to the Immunometabolite Itaconic Acid by Inducing Acid and Oxidative Stress Responses Including S-Bacillithiolations and S-Itaconations. Free Radic. Biol. Med. 2023, 208, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; de Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Harper, C.J.; Hayward, D.; Kidd, M.; Wiid, I.; van Helden, P. Glutamate Dehydrogenase and Glutamine Synthetase Are Regulated in Response to Nitrogen Availability in Myocbacterium smegmatis. BMC Microbiol. 2010, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Yutthanasirikul, R.; Kurdrid, P.; Saree, S.; Senachak, J.; Saelee, M.; Hongsthong, A. Increasing Activity of the GS-GOGAT Cycle Highlights the Compensation of N-Assimilation in the Absence of Nitrogen and Its Metabolic Effects in Cyanobacteria. Algal Res. 2024, 79, 103490. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell Biology and Molecular Basis of Denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Li, L.; Zhang, X.; Chen, F.; He, J. Heterotrophic Nitrification and Aerobic Denitrification Characteristics of the Psychrotolerant Pseudomonas peli NR-5 at Low Temperatures. Bioprocess. Biosyst. Eng. 2023, 46, 693–706. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, D.; Li, W.; Qin, W.; Huang, X.; Lv, L. Substrates Removal and Growth Kinetic Characteristics of a Heterotrophic Nitrifying-Aerobic Denitrifying Bacterium, Acinetobacter harbinensis HITLi7T at 2 °C. Bioresour. Technol. 2018, 259, 286–293. [Google Scholar] [CrossRef]
- Lang, X.; Li, Q.; Ji, M.; Yan, G.; Guo, S. Isolation and Niche Characteristics in Simultaneous Nitrification and Denitrification Application of an Aerobic Denitrifier, Acinetobacter Sp. YS2. Bioresour. Technol. 2020, 302, 122799. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, M.; Chen, Y.; Luo, J.; Hu, Y. Carbon Selection for Nitrogen Degradation Pathway by Stenotrophomonas maltophilia: Based on the Balances of Nitrogen, Carbon and Electron. Bioresour. Technol. 2019, 294, 122114. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Zhou, J.; Chen, M.; Wang, X. Biological Removal of Nitrate and Ammonium under Aerobic Atmosphere by Paracoccus versutus LYM. Bioresour. Technol. 2013, 148, 144–148. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A Review of Research Progress of Heterotrophic Nitrification and Aerobic Denitrification Microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Chen, F.; Xia, Q.; Ju, L.-K. A Recently Evolved Diflavin-Containing Monomeric Nitrate Reductase Is Responsible for Highly Efficient Bacterial Nitrate Assimilation. Appl. Environ. Microbiol. 2003, 69, 6715–6722. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Ma, T.; Wang, M.; Ni, J. Genomic Insights into Metabolic Potentials of Two Simultaneous Aerobic Denitrification and Phosphorus Removal Bacteria, Achromobacter Sp. GAD3 and Agrobacterium Sp. LAD9. FEMS Microbiol. Ecol. 2018, 94, fiy020. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Effect of ferric iron (Fe(Ш)) on heterotrophic solid-phase denitrification: Denitrification performance and metabolic pathway. Bioresour. Technol. 2023, 369, 128401. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Chen, M.; Ding, C.; Wu, Q.; Zhang, M. Hypothermia Pseudomonas taiwanensis J488 Exhibited Strong Tolerance Capacity to High Dosages of Divalent Metal Ions during Nitrogen Removal Process. Bioresour. Technol. 2021, 341, 125785. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Q.; Cui, Y.-W.; Yang, H.-J.; Xu, M.-J.; Cui, Y.; Chen, Z. A Halophilic Aerobic-Heterotrophic Strain Halomonas venusta SND-01: Nitrogen Removal by Ammonium Assimilation and Heterotrophic Nitrification-Aerobic Denitrification. Bioresour. Technol. 2023, 374, 128758. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Li, M.; Wang, N.; Sun, Z.; Li, X.; Li, B. Effects of Sulfamethoxazole on Nitrogen Transformation and Antibiotic Resistance Genes in Short-Cut Nitrification and Denitrification Process Treating Mariculture Wastewater. Chem. Eng. J. 2023, 454. [Google Scholar] [CrossRef]
- Maier, R.M. Biogeochemical Cycling. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 339–373. ISBN 978-0-12-394626-3. [Google Scholar]
- Yan, L.; Jiang, J.; Liu, S.; Yin, M.; Yang, M.; Zhang, X. Performance and Mechanism of Nitrate Removal by the Aerobic Denitrifying Bacterium JI-2 with a Strong Autoaggregation Capacity. Bioresour. Technol. 2022, 365, 128111. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Halotolerance, Stress Mechanisms, and Circadian Clock of Salt-Tolerant Cyanobacteria. Appl. Microbiol. Biotechnol. 2023, 107, 1129–1141. [Google Scholar] [CrossRef]
- Oren, A. Bioenergetic Aspects of Halophilism. Microbiol. Mol. Biol. Rev. 1999, 63, 334–348. [Google Scholar] [CrossRef]
- Gui, M.; Chen, Q.; Ni, J. Effect of NaCl on Aerobic Denitrification by Strain Achromobacter Sp. GAD-3. Appl. Microbiol. Biotechnol. 2017, 101, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Takano, T.; Liu, S. The Role of Ammonium Transporter (AMT) against Salt Stress in Plants. Plant Signal Behav. 2019, 14, 1625696. [Google Scholar] [CrossRef]
- Oide, S.; Inui, M. Trehalose Acts as a Uridine 5′-Diphosphoglucose-Competitive Inhibitor of Trehalose 6-Phosphate Synthase in Corynebacterium glutamicum. FEBS J. 2017, 284, 4298–4313. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; An, L.; Fan, X.; Ju, F.; Zhang, B.; Sun, H.; Xiao, J.; Hu, W.; Qu, T.; Guan, L.; et al. A Trehalose Biosynthetic Enzyme Doubles as an Osmotic Stress Sensor to Regulate Bacterial Morphogenesis. PLoS Genet. 2017, 13, e1007062. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; El-Din, M.G. Heterotrophic Nitrification and Aerobic Denitrification Process: Promising but a Long Way to Go in the Wastewater Treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Lai, Y.; Wang, L.; Liu, W.; Liu, J.; Liu, D.; Li, K.; Cao, Q.; Wei, K.; Lan, H. Treatment of Nitrate−Nitrogen-Containing Wastewater via Aerobic Denitrifying Bacteria Using Different Carbon Sources. Bioresources 2022, 17, 1972–1987. [Google Scholar] [CrossRef]
- Chen, J.; Gu, S.; Hao, H.; Chen, J. Characteristics and Metabolic Pathway of Alcaligenes Sp. TB for Simultaneous Heterotrophic Nitrification-Aerob. Denitrification. Appl. Microbiol. Biotechnol. 2016, 100, 9787–9794. [Google Scholar] [CrossRef]
- Huang, Q.; Alengebawy, A.; Zhu, X.; Raza, A.F.; Chen, L.; Chen, W.; Guo, J.; Ai, P.; Li, D. Performance of Paracoccus pantotrophus MA3 in Heterotrophic Nitrification–Anaerobic Denitrification Using Formic Acid as a Carbon Source. Bioprocess. Biosyst. Eng. 2022, 45, 1661–1672. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Jin, Y.; Lu, Y.; Wang, Y.; Zhao, T.; Chen, P.; Huang, S.; Zhang, Y. Characteristics and Nitrogen Removal Performance Optimization of Aerobic Denitrifying Bacteria Bacillus cereus J1 under Ammonium and Nitrate-Nitrogen Conditions. Water 2024, 16, 2231. https://doi.org/10.3390/w16162231
Cao Y, Jin Y, Lu Y, Wang Y, Zhao T, Chen P, Huang S, Zhang Y. Characteristics and Nitrogen Removal Performance Optimization of Aerobic Denitrifying Bacteria Bacillus cereus J1 under Ammonium and Nitrate-Nitrogen Conditions. Water. 2024; 16(16):2231. https://doi.org/10.3390/w16162231
Chicago/Turabian StyleCao, Ying, Yi Jin, Yao Lu, Yanling Wang, Tianyu Zhao, Pengfei Chen, Shaobin Huang, and Yongqing Zhang. 2024. "Characteristics and Nitrogen Removal Performance Optimization of Aerobic Denitrifying Bacteria Bacillus cereus J1 under Ammonium and Nitrate-Nitrogen Conditions" Water 16, no. 16: 2231. https://doi.org/10.3390/w16162231
APA StyleCao, Y., Jin, Y., Lu, Y., Wang, Y., Zhao, T., Chen, P., Huang, S., & Zhang, Y. (2024). Characteristics and Nitrogen Removal Performance Optimization of Aerobic Denitrifying Bacteria Bacillus cereus J1 under Ammonium and Nitrate-Nitrogen Conditions. Water, 16(16), 2231. https://doi.org/10.3390/w16162231





