Abstract
Compared with other methods, the synthesis of metal nanoparticles by metal ion reduction using plant extracts as raw materials has the advantages of low cost, simple synthesis and environmental friendliness, and has garnered significant attention. To achieve this effect, in the form of green synthetic nano silver (AgNP), we mixed AgNO3 with attapulgite (ATP) and stirred it with clove plant extract at 80 °C. By changing the dosage of clove extract, a series of new samples were prepared by the same method. The shape and size of the synthesized silver nanoparticles on catalysts were visualized by transmission electron microscope (TEM) observations. The particle size of the optimally prepared nanoparticles ranges from 1 to 9 nm with spherical or roughly spherical forms. The inductively coupled plasma (ICP) results further demonstrated the reducing effect of clove extract on Ag. Increasing the amount of clove extract could promote the formation and loading of Ag on ATP. An outstanding catalytic performance of Ag/ATP under HCHO outperformed that synthesized without clove extract. With the addition of clove extract, the catalytic performance was enhanced by more than 40% compared to no addition. Among different nanoparticles, the catalytic oxidation activity of HCHO was best when the volume ratio of clove extract to Ag was 10:1. Therefore, the green synthesis of Ag/ATP catalysts using clove extracts can be considered an environmentally benign, superior approach.
1. Introduction
Due to the continuous release of volatile organic compounds in home decoration products, coatings and coatings, indoor air pollution has become more and more serious, and has become one of the primary factors affecting human life and health [1,2]. As an important chemical raw material, formaldehyde is widely found in various home improvement materials, and its release cycle is more than 20 years, making it one of the main pollutants in indoor air. Long-term exposure to formaldehyde can cause serious harm to human health, especially in closed environments such as submarines and space stations, and the continuous accumulation of formaldehyde will poison the human central system and respiratory system [3]. Therefore, purifying formaldehyde is essential for improving people’s quality of life and ensuring the sustainable development of society [4]. Thermal catalytic oxidation (TCO) is one of the most promising technologies in various formaldehyde emission reduction technologies [5,6,7]; other technologies include photocatalytic oxidation [7,8], physical [9] and chemical adsorption [10], and plasma catalytic oxidation [11]. Traditional formaldehyde is removed by activated carbon adsorption [12,13], but formaldehyde will be released again after activated carbon adsorption saturation, thus causing secondary pollution. The catalytic oxidation technology using oxygen as oxidant can deeply purify formaldehyde at room temperature and pressure [13,14,15,16]. On the surface of the catalytic material, formaldehyde undergoes the process of adsorption—decomposition—desorption, and finally forms water and carbon dioxide, which are harmless and free of secondary pollution. They do not require additional energy. Therefore, catalytic oxidation technology has become one of the ideal technologies to solve indoor formaldehyde pollution.
One of the keys to obtaining excellent catalytic oxidation activity is the selection of catalytic materials. At present, two major catalytic materials, noble metals (Pt, Pd, Au, Ag, etc.) [17,18,19,20] and non-noble metal oxides (MnO2, CuO, CeO2, etc.) [21,22,23], have been widely developed and utilized to degrade HCHO. In general, noble metal materials exhibit higher catalytic oxidation activity than non-noble metal oxide catalysts [24]. Noble metal catalysts have been the focus of attention because of their high activity, long life and good stability [25]. Ag, as the cheapest noble metal, has been shown to have effective HCHO catalytic oxidation properties [26,27]. In our previous work [28], we also found that Ag monometallic catalysts were able to achieve 100% HCHO conversion at relatively low temperatures (150 °C). Therefore, silver-based catalysts are potential candidates for catalytic oxidation of HCHO at low temperatures. Attapulgite is a kind of hydrated magnesium aluminosilicate crystal with a fibrous structure, widely distributed in nature. It costs very little, almost one-twentieth of a ton of activated carbon [29]. In fact, it is also a natural nanostructured material. The long and narrow attapulgite particles clump together to form bundles of fibers shaped like hay bundles. Because attapulgite has large surface area, high mechanical strength and high aspect ratio due to its unique structure, it is widely used as reinforcement filler, catalyst carrier and adsorbent for nanocomposites [30]. It has the advantages of strong acid and alkali corrosion resistance and high temperature resistance. Therefore, the catalyst uses attapulgite (ATP) as a carrier to support the precious metal Ag. Noble metal nanoparticles are common objects in the field of catalytic oxidation of HCHO [31,32,33,34], and have the advantages of good activity, stability and controllability, making them applicable in many aspects. The preparation methods of precious metal nanoparticles are usually categorized into physical [35], chemical [36,37,38] and biological methods [39,40,41]. The physical method is fast and easy, but there is the problem of large consumption of resources, which is accompanied by a great waste in the preparation of nanoparticles. While chemical methods produce less waste during preparation, due to the involvement of toxic and hazardous chemical reagents, they might have an adverse impact on the human body and the environment during the preparation process. Compared with the first two methods, the biosynthesis method has comprehensive advantages such as simple process, high economic efficiency, safety and environmental protection. Therefore, the preparation of noble metal nanoparticles with high activity by biomaterials has become a focus of the biosynthesis method.
The biosynthesis method uses environmentally friendly biological materials instead of toxic and harmful chemical reagents to prepare and synthesize the nanoparticles we investigate, and its raw materials mainly come from microorganisms, algae and plants [42,43,44]. Compared with microorganisms and algae for the preparation of nanoparticles, plant materials have the advantages of easy availability and rapid synthesis. Components including terpenes, alkaloids, steroids, flavonoids, sugars and their derivative molecules in plant extracts act as reducing and stabilizing agents in the synthesis, effectively reducing or avoiding the need for surfactants and stabilizers in the synthesis of nanoparticles [45]. Ahmed et al. (2014) reported that biocomponents like flavonoids and terpenoids in laurel were significantly reduced after participating in the synthesis of silver nanoparticles, and the size and shape of silver nanoparticles prepared in this way were relatively stable [46]. Clove is a kind of Mulleinaceae plant, easily cultivated and without strict soil requirements. It has been cultivated in China for more than a thousand years, and its planting areas are mainly distributed in the southwest, northwest, north and northeast regions. Clove leaves are rich in flavonoids, terpenoids, polyphenols and a variety of other reducing substances, and thus can be used for the preparation of precious metal nanoparticles by bioreduction. In view of this, Lakhan et al., 2020 reported the ability to visually determine the formation of silver nanoparticles from the change in solution color during the preparation of silver nanoparticles using young clove extract. This method is simple, economical and environmentally friendly; in addition, the prepared silver nanoparticles demonstrate high activity in the experiment [47].
In this study, a series of Ag/ATP catalysts with different volume ratios of clove solution were prepared by hydrothermal synthesis. The structural composition and conformational relationship of the Ag/ATP catalysts were analyzed by characterization methods such as powder X-ray diffraction (XRD), ultraviolet–visible spectroscopy (UV–Vis), X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET) and inductively coupled plasma (ICP). We discuss the clove solution in the synthesis of Ag/ATP and its influence on the catalytic oxidation performance of HCHO.
2. Experimental Section
2.1. Chemicals and Materials
All the chemicals were analytical reagent grade and were used directly without any purification. Cloves were purchased from a local market in Jiangsu province, China. Attapulgite was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). AgNO3 was purchased from Beijing Chemical Engineering Co., Ltd. (Beijing, China).
2.2. Preparation of Catalysts
2.2.1. Preparation of Clove Extract
Clove leaves were dried and broken into powder form with a pulverizer.
Five grams of clove leaves was immersed in 250 mL of deionized water. The suspension was stirred at 80 °C for 2 h, followed by sonication for 0.5 h. After the sonication, the clove leaves were removed by filtration. Subsequently, the filtrate was collected and stored at 2 °C.
2.2.2. Synthesis of Ag/ATP Catalysts Using Clove Extracts
Ag/ATP catalysts were synthesized using the following conditions. The total Ag loading amount is 8 wt%. In a typical preparation, aqueous AgNO3 solution was added to clove extract. By changing the dosage of clove extract, the volume ratios of clove extract to Ag were 0:1, 1:1, 4:1, 10:1 and 20:1. A series of new samples were prepared in the same way and denoted as 0:1, 1:1, 4:1, 10:1 and 20:1, respectively. After the mixed solution was stirred continuously at 80 °C for 2 h, ATP was added to it and stirring continued for 2 h under the same conditions. The supernatant was discarded, and meanwhile, the resulting solids were washed three times with doubly distilled water. Subsequently, the obtained powder was calcined at 550 °C [28,48,49] for 2 h in a muffle furnace.
2.3. Characterization of Catalysts
ICP–AES was used to determine the mass percentage of the metallic element Ag in the samples using Optima 7300 DV inductively coupled plasma spectrometer (PerkinElmer, Shelton, CT, USA).
Powder X-ray diffraction (XRD) was performed at Rigaku D/max-γb (λ = 0.1542 nm), 40 kV and 200 mA. We used a position-sensitive detector with a step size of 0.02° to photograph the pattern in the 2θ range from 10° to 80°.
IR results were examined for the composition and structure of the samples using Cary 610/670 micro-infrared spectrometer (Varian, Las Vegas, NV, USA), with data collected in the scanning range of 400–4000 nm.
The TEM pictures were taken on Tecnai 12 (Philips, Amsterdam, The Netherlands) at 120 kV to study the morphology of the metal catalyst. Prior to measurement, the sample was suspended in ethanol. The samples were then supported on the copper wire for TEM analysis.
UV–Vis results were detected by Cary 5000 (Varian, Las Vegas, NV, USA) to determine the state of the metal on the catalyst samples over a scan range from 200 to 800 nm.
The Brunauer–Emmett–Teller (BET) method was used to determine the specific area, pore volume and pore size distribution on Quantachrome NOVA4200e. Before measurement, the sample was degassed at 300 °C for 2 h.
The XPS experiments were performed on the ESCALAB250Xi high-performance electron spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using monochromatic Al Kα radiation (1486.6 eV). All binding energies (BE) were calibrated with an uncertain carbon C1s peak of 284.6 eV to compensate for the charging effect of the sample.
The H2-TPR was performed on a Quantachrome automated chemisorption analyzer. The sample was exposed to an H2/Ar (10 vol% H2) mixture with a flow rate of 30 mL/min. The temperature was set to a constant heating rate of 10 °C/min.
2.4. Catalytic Activity Test
The oxidation activity of HCHO was measured under atmospheric pressure and 0.2 g catalyst in a fixed-bed flow reactor. Prior to the catalytic test, all samples required pretreatment using 30% O2 in nitrogen (total flow rate 30 mL/min), prepared at 500 °C (10 °C min−1 heated slope, held for 1 h). The feed stream consisted of a mixture of 500 ppm HCHO, 20 vol% oxygen and balanced N2. In all experiments, the flow rate through the reactor was controlled at 50 mL/min and mass flowmeters were used. The reactor effluent was analyzed using an in-line gas chromatograph (GC 7890II, Techcomp, Shanghai, China) equipped with an FID detector. To determine the exact concentration of carbon dioxide produced, a nickel catalyst converter was placed before the FID detector, which was used to quantitatively convert carbon dioxide to methane in the presence of hydrogen. In a typical run, reaction data are obtained after forming HCHO oxidation for 1 h to achieve steady-state conditions. Other than carbon dioxide, no other carbon-containing compounds were detected in any of the catalyst products tested. Therefore, the HCHO conversion is calculated as follows:
where [HCHO] is the concentration of HCHO in the effluent, and [CO2] is the concentration of CO2 produced at different reaction temperatures.
HCHO conversion (%) = [CO2]/[HCHO] × 100%
3. Results and Discussion
3.1. HCHO Catalytic Oxidation Reaction Activity
Given that the catalytic HCHO oxidation over various silver monometallic catalysts draws great attention, we focused on the Ag/ATP catalysts prepared with different ratios of clove extract to investigate the catalytic behavior of metallic silver nanoparticles in the HCHO oxidation reaction. As shown in Figure 1, the effects of reaction temperature of different Ag/ATP catalysts on HCHO transformation, catalyst stability and repeatability were investigated. Figure 1a shows that the Ag/ATP catalyst had the worst reactivity at a volume ratio of 20:1 of clove extract, with less than 10% HCHO conversion efficiency under the reaction condition of 110 °C, which was less than that of the catalyst prepared without the addition of clove extract (more than 60%).
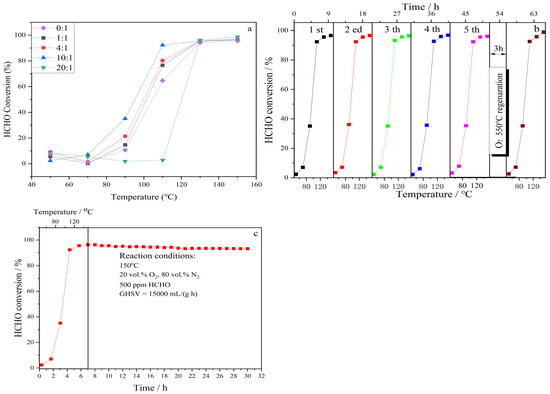
Figure 1.
(a) Catalytic oxidation activity of HCHO on Ag/ATP catalyst samples prepared with different amounts of clove addition, (b) repeatability experiment of Ag/ATP 10:1 catalyst activity, (c) stability experiment of Ag/ATP 10:1 catalyst activity.
The lower HCHO conversion efficiency of the Ag/ATP catalyst with a 20:1 volume ratio of clove extract might be explained by the excessive amount of bioreductive substances that entered the carrier structure, blocking its pores. This reduced the interaction between silver and the carrier, leading to a lower silver particle loading rate. Alternatively, the large amount of bioreductive substances that surrounded the silver nanoparticles made them difficult to load onto ATP, resulting in fewer metal active sites for the HCHO oxidation reaction compared to catalysts without added clove extract. With the decreasing volume ratio of the added clove extract, the HCHO conversion rate of Ag/ATP showed an increasing and then decreasing trend. When the volume ratio of clove extract used reached 10:1, the activity of the Ag/ATP catalyst in the HCHO conversion reaction increased dramatically, and more than 90% HCHO conversion could be achieved at a reaction temperature of 110 °C. In the temperature range of 70–150 °C, the HCHO conversion performance of Ag/ATP catalysts prepared using a 10:1 volume ratio was usually higher than that of the other catalysts in the series under the same reaction temperature conditions, indicating that the moderate amount of clove extract could enhance the oxidizing activity of Ag/ATP on HCHO, because of the fact that the reducing substances in the extract contribute to the formation and loading of Ag, thereby enhancing the performance of the catalyst in the reaction. As seen in Figure 1b,c, the activity of the Ag/ATP 10:1 catalyst was tested for repeatability and stability, and the activity was basically unchanged.
3.2. Structural Properties
3.2.1. ICP Results
The actual metal loadings on the Ag/ATP catalysts were prepared with varying volume ratios of clove extract, as determined by inductively coupled plasma (ICP) analysis. Figure 2 shows that all samples were successfully loaded with silver (Ag), with the Ag loading increasing from the initial 0.096 wt% to 3.02 wt% as the volume of clove extract added increased.
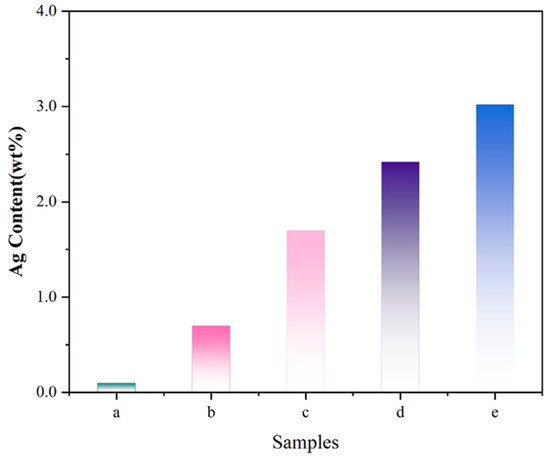
Figure 2.
The actual metal loadings on Ag/ATP catalyst samples prepared with different amounts of clove extract based on ICP results: (a) 0:1, (b) 1:1, (c) 4:1, (d) 10:1, (e) 20:1.
This finding indicates that the biomass present in the clove leaf can facilitate the reduction and dispersion of Ag ions, promoting their formation on the catalyst. Furthermore, the enhancement rate of this promotion exhibits a trend of initially increasing and then decreasing. When the volume ratio of clove extract reached 20:1, the actual loading of Ag in the catalyst reached its highest value of 3.02 wt%, which was still lower than the theoretical loading of Ag at 8 wt%. This discrepancy might be due to the fact that during the synthesis process of the catalyst, some Ag nanoparticles were reduced by the biomass of polyphenols, flavonoids and polysaccharides in the clove leaf. However, these silver nanoparticles were not loaded onto the catalyst successfully, resulting in a lower actual loading compared to the theoretical loading.
3.2.2. XRD Results
The XRD patterns of Ag/ATP catalyst samples prepared with different volume ratios of clove leaves are shown in Figure 3.
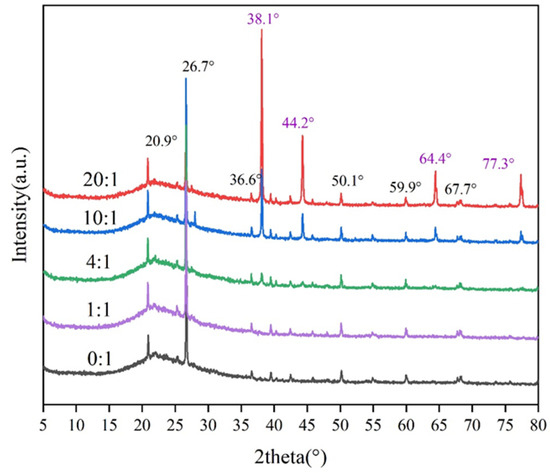
Figure 3.
The XRD pattern of Ag/ATP catalyst samples prepared with different amounts of clove extract.
The diffraction peaks belonging to attapulgite at 2θ = 20.9°, 26.7°, 36.6°, 42.4°, 59.9° and 67.7°, as seen in Figure 3, can be assigned to the SiO2 crystal structure [50,51].
Diffraction peaks belonging to ATP were clearly observed in all Ag/ATP samples. In addition, the diffraction peak at 2θ = 27.9° of the sample prepared with 10:1 volume ratio of clove extract belongs to the characteristic diffraction peak of amorphous SiO2, which is advantageous in the preparation of high-activity loaded catalysts due to its high adsorption and other abilities [52]. Therefore, the catalyst samples prepared in 10:1 were made to exhibit better HCHO catalytic oxidation performance.
In addition to the diffraction peaks belonging to ATP, diffraction peaks of Ag were also observed at 2θ = 38.1° and 44.2°, corresponding to the (111) and (200) planes of the face-centered cubic crystal structure of Ag [53], respectively, indicating that the active metal Ag was successfully loaded onto the surface of the ATP carrier. The diffraction peaks of Ag were very weak in the samples with 0:1 and 1:1 volume ratios of clove leaves, suggesting that the Ag nanoparticles on the carriers were well dispersed or poorly crystallized. With the increasing volume ratio of clove extract, obvious diffraction peaks began to appear at 2θ = 64.4° and 77.3° when the volume ratio reached 10:1, corresponding to the (220) and (311) planes of the face-centered cubic silver structure [54], respectively, and the intensities of the diffraction peaks at 2θ = 38.1° and 44.2° were also significantly enhanced and became sharp. This suggests that the addition of clove extract can, to some extent, promote an increase in the size or/and crystallinity of Ag nanoparticles on the catalyst surface.
3.2.3. FT-IR Results
Figure 4 shows the FT-IR results of Ag/ATP catalyst samples prepared with different additions of clove extract.
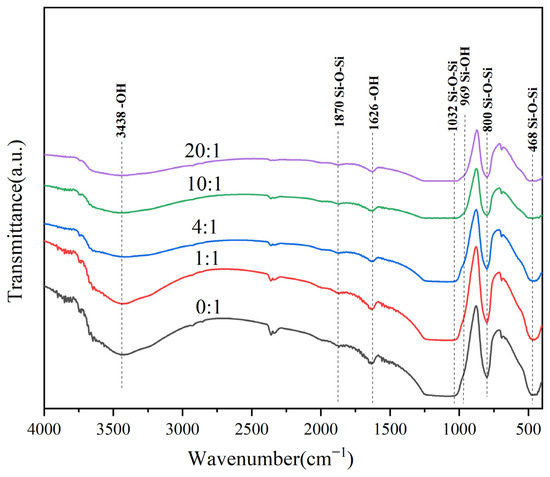
Figure 4.
FT−IR spectra of Ag/ATP catalyst samples prepared with different amounts of clove extract.
The absorption peak at 468 cm−1 is attributed to the Si–O–Si bond [55], and the intensity of the absorption peaks shows a tendency to decrease and move to lower wave numbers with the increasing ratio of clove extract added. This may be due to the entry of Ag nanoparticles into the skeleton structure of the carrier bumpy barite. The absorption peaks near 800 cm−1 and 1032 cm−1 belong to the symmetric and asymmetric Si–O–Si stretching vibrations of the Si–O–Si inorganic skeleton, respectively [53,56], and the intensities of the absorption peaks at the two locations also showed a decreasing trend as the volume ratio of clove extract was increased, which suggests that the addition of clove extract is indeed able to reduce the intensity of S–O–Si bonds. The absorption peak near 969 cm−1 is considered to be the Si–OH stretching vibration [57]; the intensity of the absorption peak decreases with the increasing volume ratio of clove extract, and the peak intensity stabilizes after the volume ratio reaches 10:1. It has been shown in the literature that with the loading of Ag nanoparticles, the Si–O–H intensity decreases, and the Si–O–H intensity increases with the increasing volume ratio of Ag nanoparticles. It has been shown that with the loading of Ag nanoparticles, the Si–O–H groups will be replaced by Si–O–Ag groups, resulting in a decrease in the intensity of the absorption peaks, which is in agreement with the results of the XRD characterization. Absorption peaks at 1626 cm−1 and 3438 cm−1 are attributed to the OH group bending vibration and stretching modes [53], and a large amount of surface adsorbed water and OH groups in the catalyst structure are subsequently reduced under the condition of increasing volume of clove extract. The absorption peak at 1870 cm−1, on the other hand, is attributed to the Si–O–Si symmetric stretching vibration [56].
3.2.4. TEM Results
In order to investigate the effect of clove extract on the reduction and loading of Ag nanoparticles, the series of catalyst samples were characterized by TEM (Figure 5).
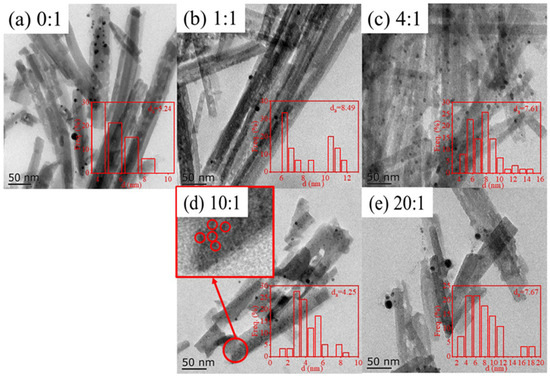
Figure 5.
The TEM image of Ag/ATP catalyst samples prepared with different amounts of clove extract: (a) 0:1, (b) 1:1, (c) 4:1, (d) 10:1 and (e) 10:1.
The ATP carriers of this series of catalysts all showed a typical rod-like fiber architecture with an average diameter between 20 and 40 nm and an average length in the range of 200–400 nm. When the volume ratio of clove extract reached 10:1, the rod-like structure of ATP began to be affected and some blocky and flocculent structures appeared. These phenomena were more pronounced when the volume ratio reached 20:1, which could be attributed to the silica oxides produced as a result of the structural collapse of ATP.
The black particles appearing on the Ag/ATP catalyst might be Ag nanoparticles. Figure 5b shows that the average size of Ag nanoparticles on Ag/ATP catalysts prepared with 1:1 volume ratio of clove extract is 8.49 nm and the number of Ag nanoparticles distributed on the carrier is less, consistent with the ICP results. This is also the reason for the weak intensity of Ag characteristic diffraction peaks in the XRD results. The average particle size of Ag nanoparticles first decreased with the increase in the ratio of clove extract. It reaches the minimum value at a volume ratio of 10:1 with an average particle size of 4.25 nm, and the number of Ag nanoparticles loaded on the catalyst is also enhanced with a more dispersed and uniform distribution. When the volume ratio reaches 20:1, the average particle size increases to 7.67 nm, and it can be clearly seen that there is a larger size of Ag nanoparticles loaded on the catalyst, and the distribution of Ag nanoparticles also appears to be agglomerated, in agreement with the XRD results.
3.2.5. UV–Vis Results
The Figure 6 shows the UV–Vis plots of Ag/ATP catalysts prepared at different volume ratios of clove extract. Compared with raw ATP, absorption peaks near 215–275 nm, 300–360 nm, 419 nm and 520 nm [58] can be observed on the catalyst samples, attributable to the 4d10 to 4d95s1 jumps of Ag+, surface plasmon absorption of Ag, characteristic absorption peaks of nano or larger metallic silver clusters and surface plasmon resonance of AgNPs, respectively [59,60,61].
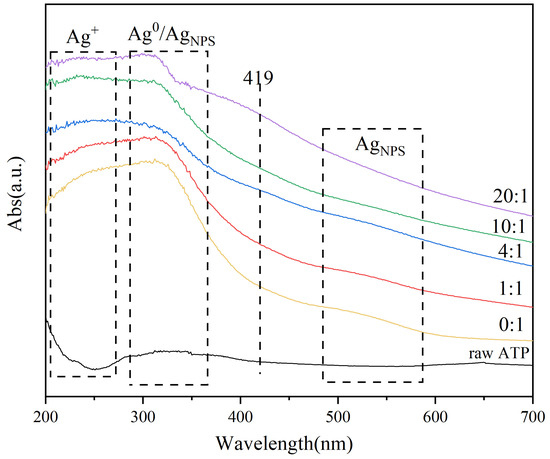
Figure 6.
The UV–Vis plots of Ag/ATP catalyst samples prepared with different amounts of clove extract.
On the catalyst samples prepared without the addition of clove extract, a relatively high intensity of the band at 215–275 nm was observed, indicating the presence of a relatively high content of Ag+ on the samples. On the catalyst samples prepared under the condition of addition of clove extract, the intensity in this band was decreased, indicating that the addition of clove extract promotes the reduction of Ag+. Because the content of Ag+ is reduced, the intensity of the absorption peak is reduced. At a volume ratio of 20:1, the absorption peaks at 300–360 nm are more obviously high, indicating that the Ag nanoparticle content on the sample is relatively high, for the clove leaf extract has sufficiently reduced the Ag+ in AgNO3, and the Ag0 and AgNPs on the catalyst are in the dominant state. Meanwhile, the samples prepared with a volume ratio of 20:1 showed relatively high intensity of absorption peaks in the broad bands centered at 419 nm and 520 nm [62], indicating the presence of Ag nanoparticles of larger sizes on the catalyst carriers, in agreement with the characterization results of XRD and TEM.
3.2.6. BET Results
The specific surface area and pore structure of the Ag/ATP catalyst samples were determined by measuring the adsorption–desorption isotherms of nitrogen (Figure 7).
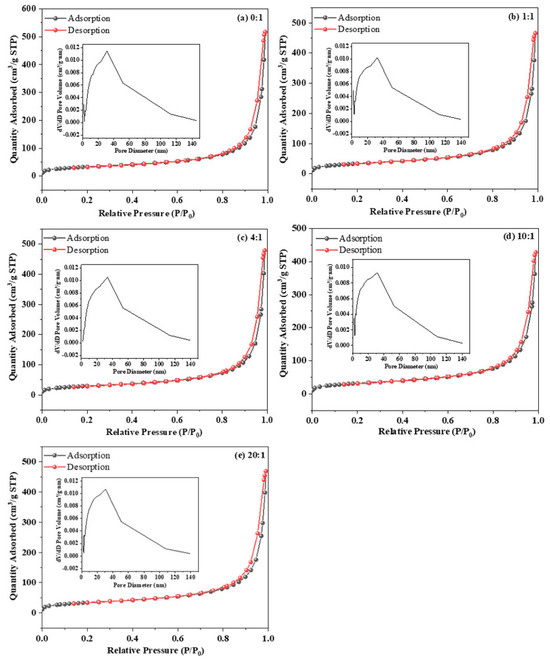
Figure 7.
The N2 adsorption−desorption curve and pore size distribution curves of Ag/ATP catalyst samples prepared with different amounts of clove extract addition: (a) 0:1, (b) 1:1, (c) 4:1, (d) 10:1 and (e) 10:1.
The corresponding specific surface area (SBET), pore volume (Vpore) and average pore size (Dpore) of the samples prepared with different clove extract additions are shown in Table 1.

Table 1.
Analysis of ICP and BET results.
Figure 7 shows that the curves of the Ag/ATP catalyst samples prepared with different clove extract addition ratios at higher pressures all exhibit typical type-IV isotherms with H4 hysteresis lines, indicating that the samples are similar in structure and are all dominated by mesoporous structures [61]. As shown by the pore size distribution curves in Figure 7, the pore distributions of the catalyst samples are all broad.
Table 1 shows the SBET, Vpore and Dpore of the samples changed after the addition of clove extract during the preparation of Ag/ATP. Compared with the catalysts prepared without the addition of clove extract, the Vpore of the remaining samples decreased, with a decrease of about 7–17%, while SBET and Dpore did not seem to be affected by the amount of clove extract, and their sizes did not show obvious regular changes. When the amount of clove extract was gradually increased, the change trend of Vpore roughly showed a decrease and then an increase, which may be due to the ability of clove extract to promote the formation of Ag nanoparticles. With the increase of the addition of clove extract, the number of Ag nanoparticles produced also increased, and these Ag nanoparticles could block or partially fill the pores in the carrier structure, which led to the decrease of Vpore. Combined with the TEM and XRD characterization results, these large-sized Ag nanoparticles on the catalyst samples with an addition ratio of 20:1, although loaded on the ATP carriers, have a relatively larger SBET and Vpore because the larger size does not block the smaller pores. This also explains the higher HCHO catalytic oxidation performance of the catalyst sample with a volume ratio of 10:1 despite the smaller SBET.
3.2.7. XPS Results
In order to further investigate the effect of clove extract on the surface composition, chemical state and metal–carrier interaction of Ag/ATP catalysts, the catalysts prepared with different volume ratios of clove extract were characterized by XPS, along with the Ag 3d and O 1s spectra (Figure 8). The corresponding XPS data are shown in Table 2.
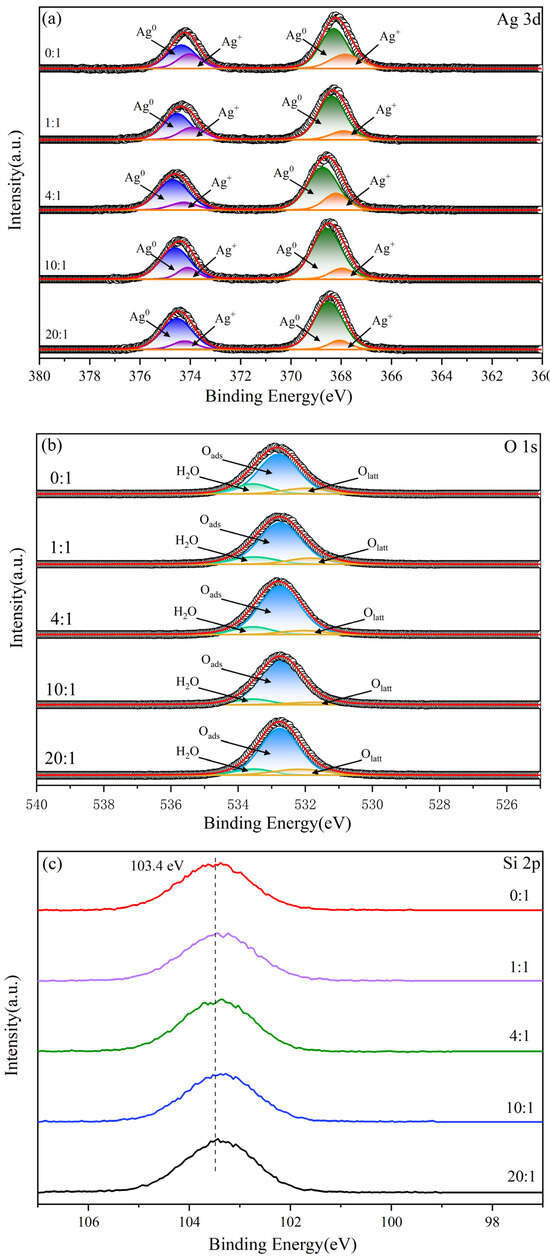
Figure 8.
XPS spectra of Ag/ATP catalysts samples prepared with different amounts of clove extract: (a) Ag 3d, (b) O 1s and (c) Si 2p.

Table 2.
Analysis of XPS Results.
Figure 8a shows the Ag 3d spectra of the series of Ag/ATP catalysts with two peaks near 368 and 374 eV belonging to Ag 3d5/2 and Ag 3d3/2, respectively, and the bimodal splitting energy of Ag 3d is 6.0 eV, indicating the presence of Ag0 species on the surface of the catalyst carrier [63,64]. Split-peak fitting of Ag 3d5/2 and Ag 3d3/2 peaks showed that the peaks at 374.35 eV and 368.3 eV were attributed to Ag0 species, while the peaks at 374.05 eV and 367.9 eV corresponded to Ag+ species [65]. As the volume ratio of clove extract increased, the peak at Ag 3d5/2 shifted to higher binding energy from 368.2 eV to 368.5 eV, indicating a tendency of Ag to lose electrons, which may be attributed to the ability of the reducing substances in the clove extract to promote the conversion of Ag+ to Ag0, which is in agreement with the ICP results. In addition, the table shows that the ratio of Ag+ to Ag0 on the surface of Ag/ATP catalysts prepared when the volume ratio of clove extract is 20:1 is 0.17, which reaches the minimum value for this series of catalysts. This indicates that Ag in the catalyst is mainly present in the form of Ag0, and that the clove extract has a positive promotion effect on the generation of Ag nanoparticles.
Figure 8b shows the O 1s XPS profile of this series of Ag/ATP catalysts. Its split-peak fitting results showed that 531.9 eV, 532.8 eV and 533.5 eV were attributed to lattice oxygen (Olatt), surface adsorbed oxygen or oxygen vacancy (Osurf) and surface adsorbed water, respectively [65,66], and surface adsorbed oxygen accounted for the major components. According to the ratio of Osurf/(Osurf + Olatt) in the table, the proportion of adsorbed oxygen on the surface of the catalyst samples increased when the volume ratio of clove extract was increasing. Among them, the highest value of adsorbed oxygen ratio on the surface of the sample was found at a volume ratio of 10:1, which suggests that the reducing biocomponent in the clove extract may have a facilitating role in increasing the surface adsorbed oxygen or oxygen vacancies. In the HCHO oxidation reaction, the content of surface adsorbed oxygen has a significant effect on the catalytic reaction activity [67,68], explaining the better catalytic oxidation performance of the catalyst at a volume ratio of 10:1. Meanwhile, the content of surface-bound water showed a significant decreasing trend with the increase of volume ratio, indicating that the clove leaf extract may reduce the surface adsorbed water in the catalyst samples, which is consistent with the FTIR characterization results.
As can be seen from Figure 8c, the Si 2p XPS plots of the series of catalyst samples all showed symmetric peaks near 103.4 eV, due to the Si–O tetrahedral characteristic peaks of SiOx [69,70]. With the increase of the addition of clove extract, the peak gradually shifted to a lower potential energy, decreasing from 103.4 eV to 103.3 eV. From the results of ICP characterization, it can be seen that the addition of clove extract increased the actual loading of Ag, and therefore affected the electronic environment of Si on the ATP carrier, showing a weakening trend with the increase of the actual loading of Ag.
3.2.8. H2-TPR Results
The Figure 9 shows the H2-TPR curves of Ag/ATP catalysts prepared with different volume ratios of clove extract.
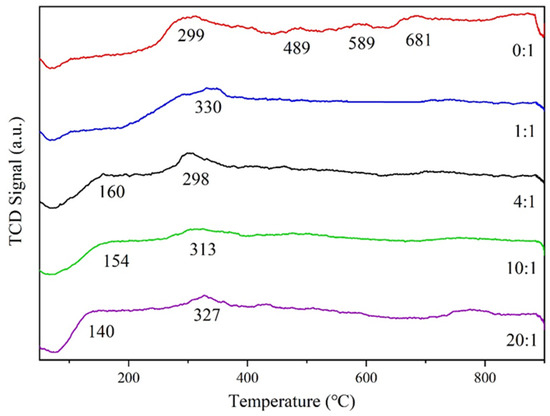
Figure 9.
H2-TPR profiles of Ag/ATP catalyst samples prepared with different amounts of clove extract.
As can be seen from the figure, the reduction peaks of this series of catalysts were mainly two kinds of peaks at low temperature (T < 200 °C) and medium temperature (200 °C < T < 600 °C). The H2 consumption peak at low temperature (140–160 °C) was mainly attributed to the Ag2O cluster, and when the addition of clove extract was increasing, the reduction peak of Ag2O was shifted from an obvious trend to low temperature, indicating that the reducing substances in clove could promote the reduction of Ag2O and the migration of oxygen [71]. The reduction peak at the middle temperature (299–327 °C) was attributed to the reduction of small Ag2O particles [72], and the area of the reduction peak was gradually reduced when the ratio of clove extract was increased from 4:1 to 10:1, which indicated that the size of the Ag2O particles on the catalyst carriers became smaller and more dispersed, whereas the area of the reduction peak was increased when the ratio was increased from 10:1 to 20:1, which indicated that the size of the Ag2O particles was increased, consistent with the XRD and TEM characterization results.
According to the XPS analysis, all the samples contained a certain amount of active surface adsorbed oxygen, so the first peak near 102 °C for the samples with volume ratios of 0:1 and 1:1 might be the depletion of H2 by surface adsorbed OH groups [73]. In addition, the samples with a volume ratio of 0:1 showed reduction peaks at 489, 589 and 681 °C, due to the presence of small amounts of surface oxygen species and bulk-phase lattice oxygen [74]. These results suggest that the addition of clove extract can promote the enhancement of the reduction of adsorbed oxygen on the catalyst surface, thus improving its active performance in the HCHO-catalyzed oxidation reaction.
3.3. Discussion
Figure 10 shows the synthesis reaction mechanism of Ag/ATP prepared using clove extract.
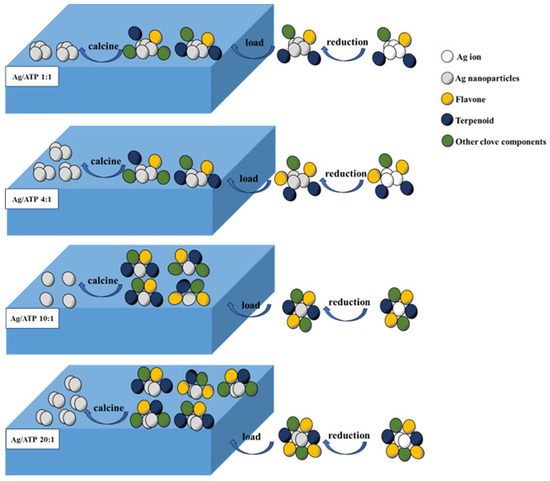
Figure 10.
The synthesis reaction mechanism of Ag/ATP prepared using clove extract.
Most current studies showed that the reducing agents and capping agents in the process of plant synthesis of precious metal nanoparticles are mainly flavonoids, phenols, tannins and terpenoids in extracts, while the reducing components rich in phenols, tannins and terpenoids in cloves play a major role in the preparation of Ag nanoparticles [36].
Although the mechanism of synthesizing nanoparticles from clove extract has been reported, in-depth studies are still needed. We conducted a mechanistic study on the process of nanoparticles synthesized from clove extract based on the report by Singh et al. Eugenol, the main chemical constituent of clove extract, is primarily considered as a reducing agent for metal ions in numerous studies. During synthesis, the -OH group of eugenol is deprotonated, forming an anionic conjugated substance. The electrons of the anionic conjugated substance are oxidized to the cationic conjugated substance. The electrons released from the intermediates are transferred to metal ions, ultimately leading to the reduction of the metal to metal nanoparticles. The entire reaction involves a two-electron reduction of eugenol. In addition, the -OH and two strong electron-withdrawing groups in close proximity and on opposite sides of the -OH group render eugenol in its anionic form, making it an exceptionally effective reducing agent [75,76].
XPS characterization results show that the catalyst prepared without clove extract has a characteristic peak contributed to silver metal at 368.2 eV, and when clove extract is added, the characteristic peak shows a tendency to move toward high binding energy, indicating that Ag+ changed to silver metal Ag0, enhancing the interaction between Ag species and carriers. The interaction between the highly dispersed Ag nanoparticles and the carrier is conducive to improving the reactivity of formaldehyde on the catalyst. When the volume ratio is 10:1, Ag+ → Ag0 generates the most oxygen vacancies on the catalyst surface, showing the highest activity in the catalytic oxidation reaction of HCHO. Although the Ag+/Ag value of the catalyst prepared with a volume ratio of 20:1 was the lowest, the catalytic oxidation performance of HCHO is significantly reduced compared with the sample prepared with a volume ratio of 10:1. This may be due to the larger size of Ag nanoparticles loaded on the surface of ATP, thereby reducing the conversion efficiency of HCHO. The H2-TPR results show that when the amount of clove extract increased, the reducibility of Ag2O and the mobility of oxygen on the catalyst increased, resulting in more oxygen vacancies. This special structure of silver–oxygen plays an active role in many catalytic reactions, thus improving the reaction activity of formaldehyde.
4. Conclusions
A series of Ag/ATP catalysts were prepared by adjusting the volume ratio of clove extract and silver nitrate solution. The HCHO catalytic oxidation performance of Ag/ATP catalyst was related to the amount of clove extract added during preparation. Samples with a volume ratio of 10:1 showed that the addition of a moderate amount of clove extract was beneficial to improving the structural properties of Ag nanoparticles, such as increasing the dispersion and decreasing the average particle size. The sample showed the best catalytic oxidation performance of HCHO, and the conversion efficiency of HCHO was more than 90% at 110 °C. ICP, XRD, TEM, UV–Vis and BET results showed that the addition of clove extract was conducive to Ag reduction and metal Ag loading on the catalyst, and appropriate clove extract was conducive to reducing the size of Ag nanoparticles and improving their dispersion. In addition, the XPS and H2-TPR results indicated that the addition of a moderate amount of clove leaf extract promoted the transformation of Ag+ to Ag0, resulting in an increase in the mobility and activity of the surface oxygen species, and elevated the reductivity of the adsorbed oxygen on the catalyst surface. The promotion of the increase of surface adsorbed oxygen and oxygen vacancies ultimately facilitates the HCHO-catalyzed oxidation reaction.
This study investigated the effects of different volume ratios on the physicochemical state of the active components on the catalysts, metal–carrier interactions and HCHO catalytic oxidation performance, so as to analyze the conformational relationship between the structural morphology of the catalysts and their HCHO catalytic oxidation performance, elucidating the theoretical pathway for the reduction of silver nanoparticles by cloves and providing a new design idea for future green synthesis of nanoparticle-prepared catalysts applied to the effective oxidation of HCHO.
Author Contributions
Y.H.: data curation, writing—original draft, formal analysis, investigation. X.C.: validation, investigation. J.Z.: validation. M.Z.: investigation. L.M.: methodology. D.C.: conceptualization, writing—review and editing, methodology, investigation, formal analysis, writing—original draft, resources, supervision. X.W.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Postdoctoral Science Foundation funded project (Grant No. 2021M702758), the National Nature Science Foundation of China (NO. 21507109) and the Natural Science Fund for colleges and universities in Jiangsu Province (20KJB610013).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank all the members of the research team for their help.
Conflicts of Interest
The authors state that they have no known competing financial interests or personal relationships that could influence the work reported in this article.
References
- Qin, D.; Guo, B.; Zhou, J.; Cheng, H.; Chen, X. Indoor air formaldehyde (HCHO) pollution of urban coach cabins. Sci. Rep. 2020, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, D.; Mocho, P.; Desauziers VPlaisance, H. Formaldehyde emission behavior of building materials: On-site measurements and modeling approach to predict indoor air pollution. J. Hazard. Mater. 2014, 280, 164–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; He, X.; Yang, P.; Zong, T.; Sun, P.; Sun, R.C.; Yu, T.; Jiang, Z. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J. Cell. Mol. Med. 2021, 25, 5358–5371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ou, B.; Wang, Y.; Zhang, R. α-Ni(OH)2 Surface Hydroxyls Synergize Ni3+ Sites for Catalytic Formaldehyde Oxidation. J. Inorg. Mater. 2023, 38, 1216. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Mo, J.; Li, X. Indoor Formaldehyde Removal by Thermal Catalyst: Kinetic Characteristics, Key Parameters, and Temperature Influence. Environ. Sci. Technol. 2011, 45, 5754–5760. [Google Scholar] [CrossRef]
- Desiccant wheels as gas-phase absorption (GPA) air cleaners: Evaluation by PTR-MS and sensory assessment. Indoor Air 2008, 18, 375–385. [CrossRef]
- Obee, T.N. Photooxidation of Sub-Parts-per-Million Toluene and Formaldehyde Levels on Titania Using a Glass-Plate Reactor. Environ. Sci. Technol. 1996, 30, 3578–3584. [Google Scholar] [CrossRef]
- Srisuda, S.; Virote, B. Adsorption of formaldehyde vapor by amine-functionalized mesoporous silica materials. J. Environ. Sci. 2008, 20, 379–384. [Google Scholar] [CrossRef]
- Park, J.H.; Byeon, J.H.; Yoon, K.Y.; Hwang, J. Lab-scale test of a ventilation system including a dielectric barrier discharger and UV-photocatalyst filters for simultaneous removal of gaseous and particulate contaminants. Indoor Air 2008, 18, 44–50. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Kim, W.-K.; Younis, S.A.; Kim, K.-H. The control on adsorption kinetics and selectivity of formaldehyde in relation to different surface-modification approaches for microporous carbon bed systems. Sep. Purif. Technol. 2022, 283, 120178. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Dong, F.; Boukhvalov, D.W.; Choi, W. Deep oxidation of gaseous formaldehyde at room-temperature by a durable catalyst formed through the controlled addition of potassium to platinum supported on waste eggshell. Chem. Eng. J. 2022, 428, 131177. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Cao, J.; Lee, S. Improved Oxygen Activation over a Carbon/Co3O4 Nanocomposite for Efficient Catalytic Oxidation of Formaldehyde at Room Temperature. Environ. Sci. Technol. 2021, 55, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yu, H.; Liu, T.; Li, Y.; Wang, Z.; Xiao, Y.; Dong, X. Efficiently photothermal conversion in a MnOx-based monolithic photothermocatalyst for gaseous formaldehyde elimination. Chin. Chem. Lett. 2022, 33, 2564–2568. [Google Scholar] [CrossRef]
- Ying, Z.; Fan, D.; Bang-Xin, L.I.; Zi-Yan, Z.; Fan, W.U. Interfacial Oxygen Vacancy of Bi2O2CO3/PPy and its Visible-light Photocatalytic NO Oxidation Mechanism. J. Inorg. Mater. 2019, 35, 541–548. [Google Scholar] [CrossRef]
- Wu, X.; Sun, S.; Wang, R.; Huang, Z.; Shen, H.; Zhao, H.; Jing, G. Pt single atoms and defect engineering of TiO2-nanosheet-assembled hierarchical spheres for efficient room-temperature HCHO oxidation. J. Hazard. Mater. 2023, 454, 131434. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Meng, M.; Huang, H.; Wang, H.; Ding, H.; Zhang, Q. Ag-promoted Cr/MnO2 catalyst for catalytic oxidation of low-concentration formaldehyde at room temperature. Phys. Chem. Chem. Phys. 2023, 25, 10155–10165. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Chen, Y.; Qin, Q.; Li, Y.; He, H. Formaldehyde oxidation on Pd/USY catalysts at room temperature: The effect of acid pretreatment on supports. J. Environ. Sci. 2023, 125, 811–822. [Google Scholar] [CrossRef]
- Li, H.; Fang, S.; Jiang, G.; Zhang, Z. Enhanced oxygen activation on an atomically dispersed Au catalyst with dual active sites for room-temperature formaldehyde oxidation. Environ. Sci. Nano 2023, 10, 80–91. [Google Scholar] [CrossRef]
- Soni, V.; Goel, V.; Singh, P.; Garg, A. Abatement of formaldehyde with photocatalytic and catalytic oxidation: A review. Int. J. Chem. React. Eng. 2021, 19, 1–29. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, L.; Li, C.; Zheng, Y.; Pan, L. Highly porous CuO/MnO2 catalyst prepared by gas release-assisted technology and its enhancement of formaldehyde removal efficiency. Res. Chem. Intermed. 2022, 48, 1971–1988. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Liu, L.; Chu, X.; Wang, X.; Song, S.; Zhang, H. Construction of strongly-coupled CeO2/MnO2 heterogeneous catalysts for highly-efficient removal of formaldehyde. New J. Chem. 2023, 47, 6282–6286. [Google Scholar] [CrossRef]
- Zhang, C.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catal. Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Lee, S.; Cao, J. A Review of Co3O4-based Catalysts for Formaldehyde Oxidation at Low Temperature: Effect Parameters and Reaction Mechanism. Aerosol Sci. Eng. 2020, 4, 147–168. [Google Scholar] [CrossRef]
- Li, D.; Liu, P.; Zheng, Y.; Wu, Y.; Ling, L.; Chen, L.; Hao, F.; Lv, Y.; Xiong, W.; Luo, H.a. Chitosan-promoted sepiolite supported Ag as efficient catalyst for catalytic oxidative degradation of formaldehyde at low temperature. J. Environ. Chem. Eng. 2022, 10, 108510. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhan, J.; Zhou, H.; Niu, M.-S.; Yang, H.-H.; Zhou, X.; Yi, X.; Liu, Y. Lithium promotes Ag-CoOx composite for formaldehyde oxidation at ambient temperature: Chemically adsorbed oxidative oxygen formed by the interaction between AgCoO2 and catalyst parent. J. Environ. Chem. Eng. 2022, 10, 108844. [Google Scholar] [CrossRef]
- Chen, D.; He, X.; Chen, X.; Wang, Z.; Wang, X. Bimetallic Au-Ag catalysts in HCHO catalytic oxidation: No synergetic effect? Sep. Purif. Technol. 2022, 301, 121930. [Google Scholar] [CrossRef]
- Mu, B.; Wang, Q.; Wang, A. Preparation of magnetic attapulgite nanocomposite for the adsorption of Ag+ and application for catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2013, 1, 7083–7090. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Q.; Xu, D. Hydrogen generation from catalytic hydrolysis of alkaline sodium borohydride solution using attapulgite clay-supported Co-B catalyst. J. Power Sources 2010, 195, 2136–2142. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, K.; Li, H.; Fan, W.; Zhao, F.; Zhang, Y.; Ji, H. A highly durable catalyst based on CoxMn3–xO4 nanosheets for low-temperature formaldehyde oxidation. Nano Res. 2016, 9, 3881–3892. [Google Scholar] [CrossRef]
- Fan, J.; Niu, X.; Teng, W.; Zhang, P.; Zhang, W.-x.; Zhao, D. Highly dispersed Fe–Ce mixed oxide catalysts confined in mesochannels toward low-temperature oxidation of formaldehyde. J. Mater. Chem. A 2020, 8, 17174–17184. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, C.; Zhang, T.; Li, L.; Yang, Q.; Zhao, H. Sodium doped SrTi1-xBxO3 (BMn, Co) for formaldehyde catalytic oxidation: Flame spray pyrolysis fabrication and reaction mechanism elaboration. Fuel Process. Technol. 2023, 247, 107763. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, W.; Li, X.; Qi, X.; Yu, J.; Liu, G.; Wang, J.; Zhang, P. Efficient Removal of Formaldehyde by Nanosized Gold on Well-Defined CeO2 Nanorods at Room Temperature. Environ. Sci. Technol. 2014, 48, 9702–9708. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Shixing, W.; Libo, Z.; Jinhui, P.; Gengwei, Z. The application of ultrasound technology in the field of the precious metal. Russ. J. Non-Ferr. Met. 2015, 56, 417–427. [Google Scholar] [CrossRef]
- Murali Krishna, I.; Bhagavanth Reddy, G.; Veerabhadram, G.; Madhusudhan, A. Eco-friendly green synthesis of silver nanoparticles using salmalia malabarica: Synthesis, characterization, antimicrobial, and catalytic activity studies. Appl. Nanosci. 2015, 6, 681–689. [Google Scholar] [CrossRef]
- Su, H.; Chen, T.-H. Preparation of PtSn2–SnO2/C nanocatalyst and its high performance for methanol electro-oxidation. Chin. Chem. Lett. 2016, 27, 1083–1086. [Google Scholar] [CrossRef]
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977. [Google Scholar] [CrossRef]
- Tian, W.; Ding, X.; Jiang, F.; Du, X.; Shi, J.; Zhang, J. Green Preparation of Cu Nanoparticles via Gallic Acid Applied to H2O2 Detection. J. Electron. Mater. 2022, 51, 1752–1758. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Guang, T.; Hu, M.; Cheng, R.; Wang, R.; Shi, C.; Chen, J.; Hou, P.; Zhu, K.; et al. Green synthesis of high-performance LiFePO4 nanocrystals in pure water. Green Chem. 2018, 20, 5215–5223. [Google Scholar] [CrossRef]
- Mahajan, A.; Arya, A.; Chundawat, T.S. Green synthesis of silver nanoparticles using green alga (Chlorella vulgaris) and its application for synthesis of quinolines derivatives. Synth. Commun. 2019, 49, 1926–1937. [Google Scholar] [CrossRef]
- Battersby, A.R.; Hall, E.S.; Southgate, R. Alkaloid biosynthesis. Part XIII. The structure, stereochemistry, and biosynthesis of loganin. J. Chem. Soc. C Org. 1969, 721–728. [Google Scholar] [CrossRef]
- Shen, S.; Chen, Y.; Zhou, J.; Zhang, H.; Xia, X.; Yang, Y.; Zhang, Y.; Noori, A.; Mousavi, M.F.; Chen, M.; et al. Microbe-Mediated Biosynthesis of Multidimensional Carbon-Based Materials for Energy Storage Applications. Adv. Energy Mater. 2023, 13, 2204259. [Google Scholar] [CrossRef]
- Magdi, H.M.; Bhushan, B. Extracellular biosynthesis and characterization of gold nanoparticles using the fungus Penicillium chrysogenum. Microsyst. Technol. 2015, 21, 2279–2285. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Gusha, C.; Mujuru, M.; Guyo, U.; Nyoni, S. Synthesis of silver nanoparticles using plant extracts from Erythrina abyssinica aerial parts and assessment of their anti-bacterial and anti-oxidant activities. Results Chem. 2022, 4, 100402. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Murtaza, G.; Mehmood, A.; Bhatti, T.M. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: Characterization and antibacterial activity. Mater. Lett. 2015, 153, 10–13. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Chen, R.; Shar, A.H.; Chand, K.; Shah, A.H.; Ahmed, M.; Ali, I.; Ahmed, R.; Liu, J.; Takahashi, K.; et al. Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and antidiatom activity. J. Microbiol. Methods 2020, 173, 105934. [Google Scholar] [CrossRef]
- Rojluechai, S.; Chavadej, S.; Schwank, J.W.; Meeyoo, V. Catalytic activity of ethylene oxidation over Au, Ag and Au–Ag catalysts: Support effect. Catal. Commun. 2007, 8, 57–64. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Evdou, A.P.; Lemonidou, A.A.; Vasalos, I.A. Ag/alumina catalysts for the selective catalytic reduction of NOx using various reductants. Appl. Catal. A Gen. 2004, 274, 179–189. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Shi, P.; Chen, D.; Song, L.; He, H.; Frost, R.L. CO 2 reforming of toluene as model compound of biomass tar on Ni/Palygorskite. Fuel 2013, 107, 699–705. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Chen, M.; Tang, Z.; Yang, Z.; Hu, J.; Zhang, H. Hydrogen production from steam reforming ethanol over Ni/attapulgite catalysts—Part I: Effect of nickel content. Fuel Process. Technol. 2019, 192, 227–238. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Peng, F.; Yang, H.; Xiong, L.; Wang, C.; Huang, C.; Chen, X.; Ma, L. Effects of Cu/Fe ratio on structure and performance of attapulgite supported CuFeCo-based catalyst for mixed alcohols synthesis from syngas. Appl. Catal. A Gen. 2015, 503, 51–61. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Zhang, D.; Zhang, W.; Chen, S.; Li, M.; Liang, P. Nanoconfinement of Ag nanoparticles inside mesoporous channels of MCM-41 molecule sieve as a regenerable and H2O resistance sorbent for Hg0 removal in natural gas. Chem. Eng. J. 2019, 361, 139–147. [Google Scholar] [CrossRef]
- Mandi, U.; Kundu, S.K.; Salam, N.; Bhaumik, A.; Islam, S.M. Ag@polypyrrole: A highly efficient nanocatalyst for the N-alkylation of amines using alcohols. J. Colloid Interface Sci. 2016, 467, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, Q.; Luo, J.; He, X. Baeyer–Villiger oxidation of ketones with hydrogen peroxide catalyzed by Sn-palygorskite. Tetrahedron Lett. 2005, 46, 3505–3508. [Google Scholar] [CrossRef]
- Motevalizadeh, S.F.; Alipour, M.; Ashori, F.; Samzadeh-Kermani, A.; Hamadi, H.; Ganjali, M.R.; Aghahosseini, H.; Ramazani, A.; Khoobi, M.; Gholibegloo, E. Heck and oxidative boron Heck reactions employing Pd(II) supported amphiphilized polyethyleneimine-functionalized MCM-41 (MCM-41@aPEI-Pd) as an efficient and recyclable nanocatalyst. Appl. Organomet. Chem. 2017, 32, e4123. [Google Scholar] [CrossRef]
- Kunchakara, S.; Ratan, A.; Dutt, M.; Shah, J.; Kotnala, R.K.; Singh, V. Impedimetric humidity sensing studies of Ag doped MCM-41 mesoporous silica coated on silver sputtered interdigitated electrodes. J. Phys. Chem. Solids 2020, 145, 109531. [Google Scholar] [CrossRef]
- Ding, Q.; Li, R.; Chen, M.; Sun, M. Ag nanoparticles-TiO2 film hybrid for plasmon-exciton co-driven surface catalytic reactions. Appl. Mater. Today 2017, 9, 251–258. [Google Scholar] [CrossRef]
- Encina, E.R.; Coronado, E.A. Near Field Enhancement in Ag Au Nanospheres Heterodimers. J. Phys. Chem. C 2011, 115, 15908–15914. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, J.; Gao, J.; Li, Y.; Bao, S.; Li, K.; Ning, P.; Wang, F. “Reduction-aggregation” strategy to construct a low-cost and high-efficiency Ag/Al2O3 catalyst for NH3-SCO. Sep. Purif. Technol. 2023, 317, 123881. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Doan, H.V.; Nguyen, T.T.-B.; Pham, X.N. Nanoarchitectonics of Ag-modified g-C3N4@halloysite nanotubes by a green method for enhanced photocatalytic efficiency. Adv. Powder Technol. 2022, 33, 103862. [Google Scholar] [CrossRef]
- Radoń, A.; Łukowiec, D. Silver nanoparticles synthesized by UV-irradiation method using chloramine T as modifier: Structure, formation mechanism and catalytic activity. CrystEngComm 2018, 20, 7130–7136. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Hou, F.; Li, H.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Ismail, A.; Alsouz, M.A.K.; Almashhadani, H.A.; Khan, M.F.; Zahid, M. An efficient Ag decorated CeO2 synergetic catalyst for improved catalytic reduction of lethal 4-nitrophenol. Chem. Phys. Impact 2023, 6, 100173. [Google Scholar] [CrossRef]
- Yu, W.; Chen, S.; Zhu, J.; He, Z.; Song, S. A highly dispersed and surface-active Ag-BTC catalyst with state-of-the-art selectivity in CO2 electroreduction towards CO. J. CO2 Util. 2023, 70, 102457. [Google Scholar] [CrossRef]
- Kong, H.; Wang, J.; Zhang, G.; Shen, F.; Li, Q.; Huang, Z. Synthesis of three-dimensional porous lanthanum modified attapulgite chitosan hydrogel bead for phosphate removal: Performance, mechanism, cost-benefit analysis. Sep. Purif. Technol. 2023, 320, 124098. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, R.; Wang, Y.; Zhang, X. Microwave catalytic activities of supported perovskite catalysts MOx/LaCo0.5Cu0.5O3@CM (M = Mg, Al) for salicylic acid degradation. J. Colloid Interface Sci. 2020, 564, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, J.; Zhao, Z.; Chen, Y.; Xu, C.; Duan, A.; Jiang, G.; He, H. Highly Active Catalysts of Gold Nanoparticles Supported on Three-Dimensionally Ordered Macroporous LaFeO3 for Soot Oxidation. Angew. Chem.-Int. Ed. 2011, 50, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Sheng, C.; Guo, Z.; Dai, L.; Yuan, C. A novel finding on tribological, emission, and vibration performances of diesel engines linking to graphene-attapulgite lubricants additives under hot engine tests. Renew. Sustain. Energy Rev. 2023, 182, 113366. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Zhang, J.; Myshkin, N.K.; Zhang, G. Significant friction and wear-reduction role of attapulgite nanofibers compounded in PEEK-Based materials. Compos. Sci. Technol. 2022, 230, 109449. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Song, Z.; Wu, Y.; Liu, W.; Wang, K.; Li, H. Catalytic Behavior of Ag-Mn Catalyst for Efficient Toluene Removal at Low Temperature: Effect of Redox Property. Chem. Phys. Impact 2022, 5, 100133. [Google Scholar] [CrossRef]
- Hu, Y.; Lü, W.; Liu, D.; Liu, J.; Shi, L.; Sun, Q. Effect of ZnO on the performance of Ag/SiO2 catalyst for the vapor-phase synthesis of 3-methylindole. J. Nat. Gas Chem. 2009, 18, 445–448. [Google Scholar] [CrossRef]
- Hu, W.; Guo, T.; Ma, K.; Li, X.; Luo, W.; Wu, M.; Guo, H.; Zhang, Y.; Shangguan, W. Promoted catalytic performance of Ag-Mn bimetal catalysts synthesized through reduction route. J. Environ. Sci. 2024, 137, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, D.; Li, J.; Bai, B.; Fu, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B Environ. 2014, 148–149, 36–43. [Google Scholar] [CrossRef]
- Gul, A.; Fozia; Shaheen, A.; Ahmad, I.; Khattak, B.; Ahmad, M.; Ullah, R.; Bari, A.; Ali, S.S.; Alobaid, A.; et al. Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts. Biomolecules 2021, 11, 206. [Google Scholar] [CrossRef]
- Singh, D.; Tiwari, A.; Singh, R.P.; Singh, A.K. Clove bud extract mediated green synthesis of bimetallic Ag–Fe nanoparticles: Antimicrobial, antioxidant and dye adsorption behavior and mechanistic insights of metal ion reduction. Mater. Chem. Phys. 2024, 311, 128529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).