Abstract
While several researchers have investigated the anaerobic digestion (AD) of textile wastewater for dye degradation, their studies suffer from lower biogas productivity due to substrate inhibition and the occurrence of secondary pollution from digestate disposal. Hence, this study focuses on using the extract of wheat straw (WS) as a co-substrate to facilitate the dye AD process, followed by recycling the digestate sludge for biochar production. In the first study, the batch digesters were operated at different dye wastewater (DW)/WS ratios (0–50% v/v), substrate-to-inoculum ratio of 0.28–0.50 g/g, pH 7.0 ± 0.2, and 37 °C. The digester operated at a DW/WS fraction of 65/35% (v/v) showed the best chemical oxygen demand (COD) removal efficiency of 68.52 ± 3.40% with bio-CH4 of 270.52 ± 19.14 mL/g CODremoved. About 52.96 ± 3.61% of the initial COD mass was converted to CH4, avoiding inhibition caused by volatile fatty acid (VFA) accumulation. In the second experiment, the dry digestate was thermally treated at 550 °C for 2 h under an oxygen-deprived condition, yielding 0.613 ± 0.031 g biochar/g. This biochar exhibited multiple functional groups, mineral contents, and high stability (O/C = 0.193). The combined digestion/pyrolysis scenario treating 35 m3/d (106.75 kg COD/d) could maintain profits from pollution reduction, biogas, biochar, and carbon trading, obtaining a 6.5-year payback period.
1. Introduction
Textile industries generate large volumes of wastewater containing organic and inorganic pollutants, mainly dyes, surfactants, detergents, solvents, and recalcitrant compounds [1]. The informal disposal of dye wastewater (DW) without proper treatment imposes adverse effects on ecology, agriculture, and public health [2]. For example, a dye layer can float on the water surface and hamper natural processes of sunlight penetration into water bodies [3]. This condition negatively influences the photosynthetic activity of most aquatic plants, further disrupting the availability of dissolved oxygen and nutrients in aquacultures [4]. Furthermore, exposure to toxic pollutants in DW is associated with various health concerns, including vomiting, stomach pain, and damage to the eyes, liver, skin, and kidneys [5]. Moreover, contaminating the water bodies with textile dyes tends to deteriorate the water resource systems available for landscape irrigation; hence, textile wastewater should be adequately treated before reaching the aquatic environment to alleviate the associated risks.
While various physical [6], chemical [7], and electrochemical [1] methods have been employed in DW treatment, these systems entail some drawbacks, especially in low-income countries. For instance, DW filtration using membrane technologies requires high amounts of energy to overcome the osmotic pressure accompanied by the high concentrations of dissolved ions. Moreover, the electrochemical techniques used to capture cationic dyes are infeasible in developing countries with limited access to energy services, and they might generate toxic degradation byproducts [8]. The chemical processes require the addition of reagents that might be expensive and unavailable in some countries, and they could generate sludge containing unfavorable dye byproducts [9]. The biological treatment of DW under anaerobic conditions is considered environmentally friendly because of less chemical consumption and resource (e.g., energy) utilization [10]. Moreover, it could earn profits from bioenergy recovery (based on renewable feedstock resources) owing to dye degradation under the action of some methanogenic activities [11].
The anaerobic digestion (AD) process undergoes multiple biological reactions using microorganisms (e.g., archaea, bacteria) to decompose the biodegradable material of DW under an oxygen-deprived condition [12]. This process can be employed for DW because it involves a consortium of microbes that perform a sequence of biochemical and metabolic pathways [13], including enzymatic hydrolysis, acidogenesis/fermentation, acetogenesis, and methanogenesis [14]. The main products of this AD process include biogas that can be used for electricity production, while the released biogenic CO2 is considered “carbon neutral” [12]. Digestate, including nutrients and undigested biomass, can also be obtained from the biogas systems, and it can be managed to generate fertilizers [15]. Another study showed that the thermal treatment of solid digestate could generate biochar used for carbon storage and the enhancement of soil texture [16]. The marketing of these outputs could gain revenues that could be used to overcome the initial investment of the AD project. More research is required to estimate the time consumed to recoup the construction and equipment costs of the digesters and determine the amount of money that can be earned per year per investment.
Due to the recalcitrant nature of DW, adding a co-substrate to the fermentation medium has been used to enhance the overall biodegradation process [17]. This co-substrate (e.g., lactate and glucose) serves as an electron donor to facilitate the breakdown of complex dye compounds, avoiding volatile fatty acid (VFA) accumulation in the reactors. Some examples of co-substrates/electron donors used in the AD of textile dyes include henna plant [18], honey processing wastewater [15], pineapple wastewater [19], and ethanol [17]. Supplementing the digester with low-cost co-substrates, e.g., extracts of agricultural wastes, would stimulate microbial activity to stabilize sludge and convert more organic dyes into bio-methane (bio-CH4) [20].
Different lignocellulosic waste materials, e.g., wheat straw (WS), paddy straw, sugarcane bagasse, wood shavings, and rice husk, have been reported to enhance the biodegradation of DW under anaerobic conditions [2]. Moreover, these crop residues are rich in multiple macro-nutrients, such as potassium (K), phosphorous (P), nitrogen (N), and aluminum (Al), along with carbohydrates and polysaccharides, which could serve as food for microorganisms’ growth [20]. Because the AD process involves a diversity of microorganisms, optimizing the substrate-to-inoculum ratio (SIR) is a crucial factor for facilitating the hydrolytic acidification of poor biodegradable compounds [21]. The adaptation of SIR not only promotes process efficiency but also avoids the accumulation of inhibitory compounds in the AD systems [10].
The resultant digestate from the anaerobic co-digestion of DW could further cause environmental pollution, such as soil/groundwater contamination via nutrient leaching [22]. Therefore, the thermal treatment of this secondary waste could eliminate the remaining pollutants (e.g., inorganic elements, pathogens, and aromatic compounds) [3]. In addition, this thermal decomposition has been recognized as a waste-to-resource technique, obtaining a final product that has successful environmental applications. For instance, pyrolysis of digestate from the AD of petrochemical wastewater at 500 °C generates biochar with multiple functional groups, improved surface morphology, and a large surface area suitable for adsorbing dyes and heavy metals from aqueous solutions [12]. Biochar has also been applied to improve soil fertility and physical properties, further maximizing the crop yield pattern [23]. Because of its high carbon content, biochar can be a suitable candidate for mitigating greenhouse gas (GHG) emissions via carbon sequestration. As such, the thermal treatment of solid digestate for generating biochar can add profits to the AD process because the market value of biochar would reach up to 1.6 USD/kg [7].
Although previous studies have demonstrated the anaerobic treatment of DW by co-digestion, there is still a research gap in the economic feasibility of the treatment system, as well as the recyclability of the sludge digestate. Therefore, this study focused on combining the co-digestion/pyrolysis approach for the dual benefit of reducing pollution from textile wastewater and maintaining a sustainable strategy from digestate recycling. Methylene blue (MB) was selected as a cationic and primary thiazine dye because it is extensively used in various industries (e.g., as a colorant) and medical applications [9], and direct exposure to MB results in serious health complexities [4]. The study objectives were threefold: (1) use the extract of WS as a co-substrate to enhance biogas productivity from the AD of MB-laden solutions, (2) determine the characteristics, surface morphology, and surface functional groups of biochar derived from the pyrolysis of solid digestate after DW treatment, and (3) estimate the techno-economic feasibility from implementing the digestion/pyrolysis integrated process, regarding biogas recovery, biochar selling, chemical oxygen demand (COD) shadow price, and carbon credit trading.
2. Materials and Methods
2.1. Substrate and Inoculum Preparation
Synthetic DW was prepared to simulate the composition of real textile wastewater [6]. The components of this DW feed included (in mg/L) methylene blue (50), NH4Cl (203), KH2PO4 (22), K2HPO4 (28), CaCl2 (100), NaCl (204), and sodium acetate (205). NaHCO3 solution was used to buffer the fermentation medium and maintain the desirable pH condition, whereas urea was added as a nitrogen source [24]. Trace elements (in mg/L) of CoCl2 (0.1), MgCl2 (4), NiSO4 (0.48), FeCl3·6H2O (0.24), and KI (10) were used for nutrient supply [25]. The solution recorded an initial total COD of 1810 ± 131 mg/L. All the chemicals were of analytical grade.
Wheat straw (WS) was collected from an agricultural field located in Borg El Arab City in Alexandria, Egypt. Cleaning was performed carefully with distilled water to eliminate any foreign material adhering to the WS surface. The cleaned samples were reduced into small sizes and dried at 105 °C for 24 h to obtain dry biomass. The dried matter was milled and passed through a 0.1 mm sieve, and the resultant fine powder was stored in plastic bags for further utilization. The biomass was subjected to a pretreatment process to increase the bio-availability of monomeric sugars required to promote the formation of CH4, following the previously established hypothesis [26]. For this purpose, the samples were dissolved in an alkaline solution containing 1% NaOH and heated at 120–130 °C for 2 h. After pretreatment, the biomass was filtered and washed with deionized water. The solid biomass was air-dried at room temperature and kept for further utilization in subsequent AD experimentations.
Activated sludge as the inoculum was collected from a sewage treatment plant located in Alexandria, Egypt, treating domestic wastewater. The collected sludge was initially settled for 8 h, and the supernatant was withdrawn to acquire well-concentrated microorganisms. Further, the concentrated sludge passed through a 200 µm pore screen for sieving. The inoculum recorded total solids (TS: 21.35 ± 1.00 g/L) and volatile suspended solids (VSS: 13.88 ± 0.71 g/L), leading to a VSS/TS ratio of 0.65 (Table 1).

Table 1.
Physicochemical characteristics of dye wastewater and sludge inoculum.
2.2. Experiemental Set Up
The experimental procedures included the anaerobic co-digestion of DW and WS for biogas recovery, followed by dry digestate pyrolysis for biochar production (Figure 1).
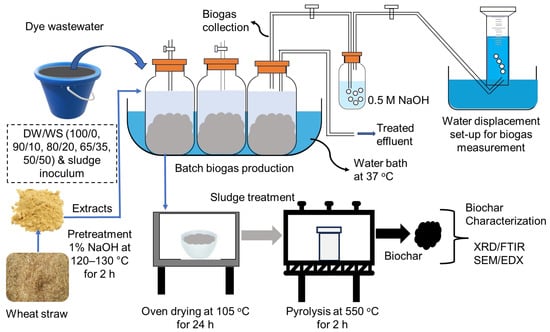
Figure 1.
Experimental setup of the anaerobic co-digestion of dye wastewater (DW) and wheat straw (WS) for bio-CH4 recovery, followed by biomass pyrolysis for biochar production.
The substrates (DW and WS) were mixed according to a previously reported methodology, with a slight modification allowing for up to 50% WS in the mixtures [27]. WS was added to DW in the proportions (%) of 100/0, 90/10, 80/20, 65/35, and 50/50 (DW/WS v/v). The batch experiments were performed using 200 mL working volume in 250 mL serum bottles, allowing for 50 mL headspace. A certain amount of sludge inoculum was added to each reactor, maintaining a SIR range of 0.28–0.50 g COD/g VSS. Both alkaline (0.5 M NaOH) and acidic (0.5 M HCl) solutions were used to adjust the reactors’ pH to 7.0 ± 0.2, which is suitable for dominating methane-producing bacteria [24]. The headspaces of batch reactors were initially purged with nitrogen gas for 3–5 min and sealed with rubber stoppers and aluminum crimp caps to create anaerobic conditions. The batch experimentation assays were performed in a temperature-controlled water bath (37 °C) at a 40-rpm mixing speed. The batch tests were conducted in triplicate to obtain mean ± standard deviation values. Methane-rich biogas was obtained by passing the gas through an alkaline solution (0.5 M NaOH) and then quantified with an inverted measuring cylinder using a water displacement technique following a previous study [12].
In the second experiment, anaerobic digestate was collected after terminating the AD process and oven-dried at 105 °C for 24 h. The dry biomass was pyrolyzed at 550 °C for 2 h under an oxygen-limited condition to obtain biochar, as described elsewhere [28]. The obtained carbon material was ground (<0.1 mm particle size) and further studied for elemental compositions, crystallinity, surface morphology, and main functional groups.
2.3. Analytical Methods
Wastewater samples were analyzed for ammoniacal nitrogen (NH4-N), volatile fatty acids (VFAs), COD, and alkalinity following APHA standard methods [29]. COD was measured using a multiparameter portable calorimeter HACH system (DR900, Loveland, CO, USA). To measure the solution color, the samples were centrifuged at 5000 rpm for 10 min and analyzed according to the procedures described by Bashiri et al. [30]. Decolorization was monitored with UV spectroscopy (Jasco V-630 spectrometer, Jasco Inc., Tokyo, Japan) at the maximum visible absorbance wavelength of 664 nm for MB detection. The sludge inoculum was analyzed for total solids (TSs) and volatile solids (VSs), following the APHA methods [29]. A portable multimeter (Lutron, YK-2001PHA, Taiwan) was used to monitor pH adjustments. The solid phase samples (AD sludge and biochar) were characterized according to previously reported procedures [12]. For example, the variation in surface functional groups was estimated using Fourier transform infrared (FTIR) spectroscopy (Bruker Optics, ALPHA, Ettlingen, Germany) in the 4000–500 cm–1 wavenumber range. The surface morphology was determined using a scanning electron microscope (JCM-6000PLUS Neoscope Benchtop SEM, Tokyo, Japan). The sample’s main elemental compositions were detected using an energy-dispersive X-ray (EDX) spectroscopy (JOEL JSM-6510LV, Tokyo, Japan). The X-ray diffraction (XRD) instrument (XRD-7000, Shimadzu, Tokyo, Japan) was used to determine the mineral phases present in solid substrates and the associated crystallographic structures. The crystallinity index (CrI) was calculated with Equation (1) [31]:
where I200 is the peak intensity of the 002 lattice plane (i.e., the maximum intensity of the crystalline peak), and Iam is the peak intensity of the amorphous phase.
2.4. Determination of the System Performance
The removal efficiency R(%) of each pollutant was calculated with Equation (2) [21]:
where CO and Ct are the initial pollutant concentration and the corresponding value at time t, respectively (mg/L).
The modified Gompertz model (Equation (3)) was used to fit the variation of methane productivity from different reactors following a previous study [24]:
where P is the cumulative methane production (mL), P(t) is the CH4 potential (mL) at an operating time (t in day), R is the maximum CH4 production rate (mL/d), e is Euler’s number (2.71828), and λ is the lag phase to initiate bacterial growth (day) estimated using an optimization algorithm with MATLAB mathematical software (R2019b).
The COD mass balance model was performed to validate the accuracy of data obtained from the biodegradation of organic matter in the bioreactors according to Equation (4), as reported earlier [20]:
where CODsoluble is a soluble COD fraction in the final effluent (g), CODbiomass is the COD equivalent to biomass growth (in g based on 1.42 g COD/g VSS), CODCH₄ is COD-to-CH4 formation (in g based on 1 g COD/350 mL CH4), and tCODinitial is the influent total COD.
2.5. Economic Estimation
The economic feasibility of the AD/pyrolysis scheme was computed based on the data derived from the COD mass balance model. Public sources, supplier quotations, and manufacturer specifications were consulted to obtain the prices for materials and equipment as capital input. Capital and operational costs were computed based on a medium-scale textile industry discharging 35 m3 of wastewater per day (equivalent to 106.75 kg COD/day). The profits related to pollution reduction (environmental benefits) were estimated with Equation (5) [32].
where ENVprofit is the profit related to the prevention of environmental damages (USD/y), CODremoved is the mass of COD removed (kg/y), and PCOD is the shadow price per kg of COD removed (USD/kg).
The revenues from biogas and biochar selling were computed with Equation (6) and Equation (7), respectively [6].
where Biogasprofit and Biocharprofit are the incomes derived from biogas and biochar selling (USD/y), CODin is the influent COD (kg/y), Ybiogas is the biogas yield (m3/kg COD), Md is the annual dry sludge for biochar production (kg/y), Ychar is the biochar yield (kg/kg), and Pbiogas (USD/m3) and Pchar (USD/kg) are the biogas and biochar selling prices, respectively.
The biogenic carbon footprint was computed for biogas (Equation (8)) and biochar (Equation (9)), as reported elsewhere [20]:
where CCbiogas and CCbiochar represent the carbon credit related to the biogenic CO2 of biogas and biochar (USD/y), respectively, CH4 is the annual methane production (m3/y), GWP is the global warming potential for CH4 (i.e., the 100 year GWP for CH4 is 25), CH4 density = 0.717 kg/m3, CO2,trading is the global carbon trading price (USD/kg CO2), Charmass is the annual biochar production (kg/y), Carboncontent is the carbon fraction in biochar (w/w%), and Ec is the carbon emission coefficient (CO2/C = 44/12).
The time taken to recover the initial investment was calculated with Equation (10) [33]:
where PBP is the payback period (years).
3. Results and Discussion
3.1. Performance of Anaerobic Batch Reactor toward Pollutant Removal and Bio-CH4 Production from Dye Wastewater (Results of the First Experiment)
3.1.1. Effect of Mixing Ratio on Color and COD Removal Efficiencies
Increasing the co-substrate fraction in the feed wastewater from 10% to 35% v/v positively impacted color R(%) from 65.30 ± 3.13% to 83.70 ± 4.12%, respectively. A similar correlation between color removal and co-substrate supplementation was also observed for the treatment of real alkaline textile wastewater using pineapple wastewater as a co-substrate [19]. Their study revealed that elevating the co-substrate fraction was associated with the supply of more electron donors for catalyzing dye bond degradation [19]. A further increase in co-substrate fraction to 50% v/v insignificantly (p > 0.05) improved color R(%) to 89.46 ± 4.47% (see Figure 2a). It is suggested that a co-substrate fraction between 35% and 50% v/v could be suitable for supplying sufficient electron donors to enhance the dye decolorization efficiency. In a comparable study, glucose was used as an electron donor to improve MB biodegradation was initiated through the demethylation cleavage by an isolated bacterium [4]. However, this microbe’s capacity to break down the heterocyclic and aromatic structure of MB dropped significantly (p < 0.05) to 28.31 ± 1.73% at 100/0 (% v/v) DW/WS mixing ratio. This pattern could be because of the limited substrate available to generate the required electron donors and the recalcitrant nature of the MB phenothiazine ring [5]. This inadequate electron transfer pattern suggests the occurrence of adsorption as another pathway for color removal. As such, some functional groups (e.g., —OH, —COOH, and —NH2) on microbial cell surfaces could be involved in making complexations with the dye molecules [34]. This theory has been demonstrated previously [35], where the functional groups (e.g., C=O, —NH2, and P—OH) on the Klebsiella oxytoca strains provided binding sites with MB cations to facilitate color removal.
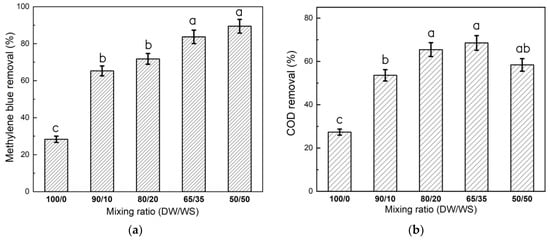
Figure 2.
Effect of DW/WS mixing ratios on the removal efficiencies of (a) MB color and (b) COD. The statistical significance between DW/WS mixing ratios was derived by one-way ANOVA followed by Tukey’s post hoc test estimated using MATLAB mathematical software. Groups sharing the common letter do not statistically differ from each other at a significant level of alpha = 0.05.
The reduction in COD value followed a similar pattern for the degradation of MB dye molecules, where the COD R(%) remained almost stable between 65.41 ± 3.27% and 68.52 ± 3.40% by adapting the co-substrate fractions in the 20–35% v/v range (Figure 2b). The enzymatic hydrolysis of feedstock straw could produce monosaccharides, like xylose, glucose, and galactose, utilized by microorganisms as electron donors to form colorless aromatic amines [17]. Moreover, some microbial species could utilize dye molecules and their metabolites as C/N sources [4]. However, the microbial’s ability to convert COD dropped to 58.38 ± 2.92% (see Figure 2b) when the co-substrate fraction in the feed wastewater increased beyond 35% (v/v). Although the 50/50% (v/v) contained the largest fraction of readily biodegradable organics, microbial inhibition could occur due to altered nutrient balance in the culture. This condition showed a C/N ratio of 33/1, which was beyond the ratio of 30/1 reported during the treatment of textile wastewater [36]. Similarly, Malik et al. [15] noted that excessive carbon supplementation would disrupt the bacterial activity, e.g., phylum Halobacteriota, Euryacrhaeota, Desulfobacteriota, and Actinobacteriota, under anaerobic conditions to degrade dye Remazol blue because of nutrient imbalance in the mixtures. The lowest R(%) of 27.35 ± 1.37% was recorded for the batch run with no co-substrate addition (i.e., 100/0 v/v), thus elucidating microbial inhibition to degrade textile compounds at high dye concentrations. The COD removal pattern by this group reflects the microbial’s ability to utilize organic matter from decaying biomass as a substrate via endogenous metabolism, a similar pathway reported previously [18]. It has also been reported that long-term exposure to textile-dyeing wastewater stress would encourage microorganisms to secrete extracellular polymeric substances (EPSs), e.g., exopolysaccharides, for soluble COD utilization [25]. In particular, using 35% v/v of WS in the DW/WS digestion process could provide a suitable environment for enabling microbial consortia to degrade the MB recalcitrant compound.
3.1.2. Effect of Mixing Ratio on Methane Production from Dye Wastewater and WS Substrate
The cumulative amount of bio-CH4 for about 40 d was successfully fitted with the modified Gompertz model (Figure 3a), with R2 values between 0.97 and 0.99. The onset of CH4 production experienced an initial delay lasting 10–12 d (lag phase) required for microbial acclimatization to the dye-related environment. Following this adaptation phase, there was a gradual rise in CH4 production, indicating an augmentation in microbial activity to facilitate substrate conversion into CH4. The maximum cumulative CH4 production of 113.08 ± 7.35 mL was achieved at the 65/35% v/v (DW/WS) mixing ratio. This finding was also consistent with the highest organic matter degradation (given as COD removal 68.52 ± 3.40%, as discussed in Section 3.1.1; see Figure 2b; Supplementary Table S1). This condition was also accompanied by exhibiting the maximum CH4 production rate (R) of 5.20 ± 0.26 mL/d, with a lag period (λ) of 10.1 ± 0.1 days, indicating favorable physiological methanogenic activity for readily converting the substrate into CH4. An extended λ was observed for the 100/0 v/v group, also corresponding to the lowest R of 1.30 ± 0.07 mL/d. This finding demonstrated that microbes needed a more extended period to acclimatize to the toxicity induced by textile-dyeing effluents.
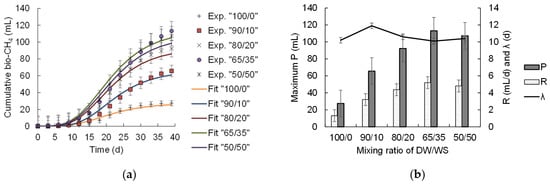
Figure 3.
Cumulative bio-CH4 patterns at various DW/WS mixing ratios of 100/0, 90/10, 80/20, 65/35, and 50/50 (v/v), (a) experimental data “Exp.” Compared with fitting the Gompertz model to data “Fit”, and (b) Gompertz model parameters, where Maximum P is the cumulative methane production at the end of the anaerobic digestion period (mL), and R is the maximum CH4 production rate (mL/d).
Cumulative CH4 production significantly increased from 65.64 ± 4.25 to 113.08 ± 7.33 mL by increasing the WS co-substrate fraction from 10% to 35%, respectively (Figure 3b). Raising the WS fraction in the digesters could offer more carbon sources to offset the nutrient limitation in DW, making the entire digestion process simpler for microbes. Digestion at 65/35 (v/v) DW/WS maintained a C/N ratio of 24.12, complying with the preferable range (C/N of 21–25) for maximizing biogas from textile wastewater with food waste and dairy effluent [37]. Similarly, the addition of 40% of food waste to garden waste and tofu waste digestion enhanced CH4 productivity (370.54 mL/g VS) under a balanced C/N ratio (16.68–22.04), favoring the enrichment of genus Methanosarcina archaea species to utilize H2/CO2 to produce CH4 [38]. Further, increasing the WS fraction beyond 35% showed a slight drop in P to 107.16 ± 7.25 mL observed at a 50/50 (v/v) DW/WS mixing ratio, indicating substrate underutilization (i.e., the amount of biodegradable organics could exceed the microbe utilization capacity). It is also suggested that elevating feeding substrate concentration over a certain threshold could lead to a shift in SIR to an unfavorable value, dominating hydrogenotrophic methanogenic and syntrophic bacteria [39]. Their study also demonstrated that the unpreferable SIR value caused a shift in the methanogenesis pathway (i.e., from acetoclastic methanogenesis to syntrophic pathways) with an about 60% reduction in the genus Aminobacterium species responsible for CH4 production [39].
3.1.3. COD Mass Balance for Validation of Bio-CH4 Production Data
The conversion of total influent COD into CH4 and other end products showed a mass balance of over 85% (Table 2). Some portions of COD mass, such as non-degradable organic matter, COD stored in bacterial cells, and dissolved CH4, were undetected in this mass balance model [12].

Table 2.
COD mass balance at varying DW/WS mixing ratios of 100/0, 90/10, 80/20, 65/35, and 50/50 (v/v), showing the transformation of CODinitial to CH4 and biomass accumulation.
Increasing co-substrate concentration in the feed provided a favorable environment for microbial growth, probably due to the availability of easily degradable organics in WS, which also justifies the highest biomass growth (CODbiomass/CODinitial = 16.69 ± 1.37%) at 65/35 mixing ratio. In particular, WS could be microbially consumed and fermented into glucose, VFAs, reducing sugars, soluble proteins, and other low molecular weight monomers to induce metabolic enzymes and co-enzymes [18]. Moreover, WS addition maintained a proper medium’s buffering capacity (VFA/alkalinity = 0.19) with a pH condition of 6.85 ± 0.29, which is within a favorable range (pH 6.6–7.3) for acetoclastic methanogens growth [40]. Likely, methane production returned the highest yield (CODCH₄/CODinitial) of 50–53% (Table 2) at 80/20 and 65/35 (v/v) DW/WS, probably due to the adaptation of methanogenic consortia to the balanced SIR range. Similarly, the methanogens grown under the optimum pH range (6.4–7.2) during garden waste (GW) digestion maintained better CH4 potential, assigning to the quick adaptation of the genus Methanosaeta archaea species to the balanced SIR range [38].
For the group with no co-substrate (100/0 v/v), most of the feed COD remained in soluble form (CODsoluble/CODinitial = 54.53 ± 3.95%). This poor COD conversion efficiency could be attributed to the high fraction of DW in the mixture and the lack of interaction between C—O as electron donors to the aromatic ring of MB (electron acceptor) [41]. Some microorganisms are encouraged to activate physiological antioxidative defense systems (release more metabolic secretions) during exposure to the toxic effects of textile dyes, increasing the COD soluble fraction in the final effluent [42]. Moreover, higher dye concentrations could be associated with faster cell lysis and death (i.e., biomass decay that inhibits methanogenesis), giving the lowest CODbiomass/CODinitial of 9.84 ± 0.72%. This condition was also associated with the smallest CODCH₄/CODinitial (21.69 ± 1.58%) fraction, possibly due to substrate limitation to supply the required nutrients to microbial consortia. The insufficient CODCH₄/CODinitial percentage could also be validated by a pH drop to 5.19 ± 0.23, attributed to the accumulation of VFAs in the digester (i.e., a high portion of CODfeed was acidified and not converted to CH4). Similarly, lower methane conversion efficiency was observed under pH-stressed AD (pH< 6.0) due to the dominance of acidogenesis over methanogenesis responsible for VFA accumulation and subsequently raising CODsoluble in the final supernatant [43].
3.2. Biochar Production (Results of the Second Experiment)
3.2.1. Morphology and Elemental Composition of Raw Sludge, Exhausted Sludge, and Biochar
The pyrolysis of textile sludge yielded 61.3% biochar (0.613 g/g sludge), complying with the 0.40–0.73 g/g dry sludge range reported earlier [44]. Moreover, this value closely aligns with the biochar yield (0.60 g/g sludge) obtained from the pyrolysis of petrochemical digestate [12]. The percentage loss in weight could be associated with the dehydration (release of moisture) and volatilization of some organic compounds [45]. The main elements in the ash were Si, Al, Fe, P, Ca, Mg, and K, showing ash contents of 28.38%wt for sludge and 47.78%wt for biochar. These values agree with the enrichment of inorganics (ash) in the biochar by the order of 1.4–2.4 times the ash fraction in the raw sludge [44].
The SEM morphology of raw sludge was relatively compact, with well-arranged layers and almost vacant pores (Figure 4a). Some bright white spots appeared on the exhausted sludge surface, probably because various bacterial species were more metabolically active within the surface layer to form granular structures [46]. In a comparable study, granular sludge containing decolorization and degradation microbial communities was responsible for dye mineralization, generating aromatic amines [47]. The folding structure on the sludge surface subsequently disappeared in biochar, depending on the decomposition and carbonization degree of organic matter at higher temperatures. The gradual increase in the concentration of inorganic components (e.g., minerals and metals) during pyrolysis could make the biochar pores smaller, further increasing the associated surface area [22]. This pattern, in addition to the increased decomposition of organic substances after pyrolysis, could increase the ash content and other residues occupying the pores initially present in the digestate.
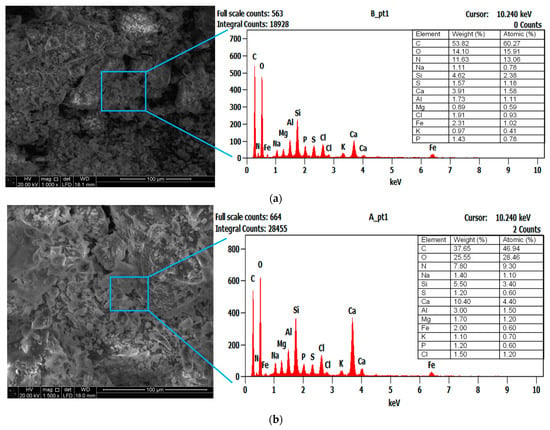
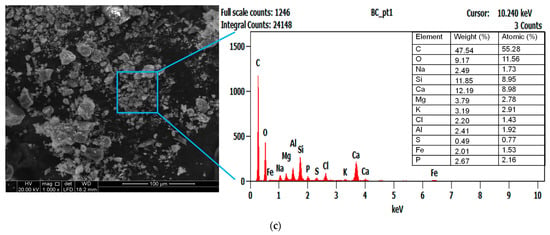
Figure 4.
SEM/EDX characterization of (a) raw sludge, (b) exhausted sludge, and (c) anaerobic sludge biochar.
The raw sludge particles contained elements, e.g., carbon (C: 53.82%), oxygen (O: 14.10%), and nitrogen (N: 11.63%). It also compressed various inorganic microelements, e.g., Al, Si, Na, Cl, Ca, Mg, P, K, and Fe (Figure 4a). The elemental contents of C and N reduced by 30% and 33%, respectively, after the AD process (see exhausted sludge in Figure 4b). These elements could have been utilized by the methanogenic microorganisms. Pyrolysis temperatures enriched most of the inorganic elements present in the sludge (see biochar in Figure 4c), possibly due to the gradual loss of volatile organic contents (C, H, N, and O) during the sludge thermal treatment. These features are comparable with the characteristics of biochar prepared from the fermenter digestate, showing enrichment of the Ca, Mg, Na, Si, S, P, K, and Fe elements [48]. The increased amount of cationic elements (e.g., Na+, Mg2+, Ca2+, and K+) supports the use of biochar for heavy metal adsorption from wastewater via the ion exchange mechanism [12]. The presence of K, Mg, Ca, S, and P elemental composition makes biochar suitable for soil amendment applications. The reduction in N and S contents suggests their volatilization, releasing gaseous products as NOX, NH3, and SO2 [45]. The C content presented a significant increase of 26%, and the O/C ratio reached ≈0.2 after pyrolysis (Figure 4c). It is supposed that the amorphous regions of digestate were partially disintegrated, and the degree of carbonization and aromatization was enhanced, yielding a stable biochar material against the biological decomposition in the soil environment [16]. This low O/C ratio could also be linked to the decomposition of oxygen functional groups, e.g., –C=O, –COOH, and O–H, in the digestate during the decarbonylation and decarboxylation processes [49]. Based on the aforementioned properties, the prepared biochar possesses a high carbon sequestration potential (O/C atomic ratio < 0.2), with an estimated half-life of over 1000 years [50].
3.2.2. Crystallographic Structure of Raw Sludge, Exhausted Sludge, and Biochar
The XRD of the raw sludge (Figure 5a) showed a broad non-crystalline peak at 2θ = 20.93°, corresponding to the (002) plane. Other diffraction peaks appeared at 2θ ≈ 26.67° (101), 29.39° for calcite [51], and 42.65° for quartz [52], representing the sludge major component. However, more peaks appeared in the exhausted sludge, possibly explaining the reduction in the amorphous cellulose content [53]. Incrementing the crystalline cellulose was further validated by having a greater CrI value of 44.5% for exhausted sludge compared with 32.5% for raw sludge. As such, some microbes tended to cleave the amorphous fraction of cellulose during the AD process. Disrupting the amorphous cellulose through the enzymatic hydrolysis of water hyacinth was also accompanied by increasing CrI from 50.13% to 60.21% [3].
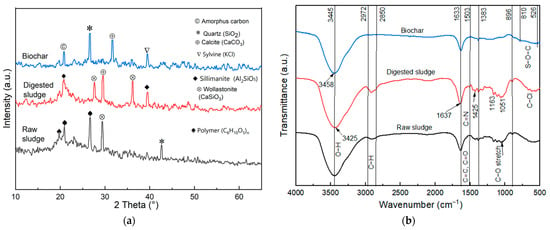
Figure 5.
Characterization of raw sludge, exhausted sludge, and anaerobic sludge biochar for (a) crystallinity and (b) main functional groups.
The crystalline phases of the digestate denoted the presence of silicates, aluminosilicates, and carbonates, e.g., wollastonite (CaSiO3, PDF#00-001-0720), sillimanite (Al2SiO5), and calcium carbonate as calcite (CaCO3, PDF#01-072-1650) [54], due to sufficient Si, Ca, and Al contents of WS. The intensity of these crystalline peaks decreased in biochar, indicating a shift toward a more amorphous structure due to the decomposition of crystalline domains within the solid digestate under pyrolysis temperatures [55]. The CrI reduced from 44.51% to 35.65% after pyrolysis, elucidating the formation of more amorphous carbon [56].
The mineral phases of the biochar could be monitored by the diffraction peaks at 2θ = 26.61° for SiO2 (silica quartz structure) [57], confirming the high ash content of biochar. Other peaks at 2θ = 31.65° (dellaite) and 39.47° (crystalline components of sylvine, KCl) [58] were noticed for the biochar XRD pattern. This pattern also comprised other inorganic minerals related to magnetite (Fe3O4 crystalline, PDF#01-076-0955) and aluminum oxide II (Al2O3, PDF#01-075-0278). In a comparable study [59], sludge-derived biochar also contained quartz and kaolinite as the major crystalline minerals and major elements of Ca, Al, Fe, and Si.
3.2.3. Main Functional Groups of Raw Sludge, Exhausted Sludge, and Biochar
FTIR spectra of the raw sludge exhibited dominant peaks at 3445 cm–1 (–OH) for the hydroxyl group and 2970–2850 cm–1 (C–H) in the aliphatic CH2 and CH groups (Figure 5b). The intensity of the sludge FTIR peaks showed a slight shift after AD, assigned to the microbial degradation of the substrate structure into CH4, CO2, and other metabolites. For instance, the shift in band for the O–H group around 3425 cm–1 could be assigned to the binding of MB with the sludge biomass due to N–H stretching [60]. The hydrogen atoms on the adsorbent hydroxyl groups could form hydrogen bonds with nitrogen atoms in MB. A previous article also demonstrated that a shift in transmittance peak from 3407 to 3411 cm−1 could describe the occurrence of hydrogen bonding between the nitrogen atoms (N +) in the MB molecule and OH− (as negatively charged hydroxyl groups) [3]. The absorption peaks between 2850 and 2972 cm–1 for the aliphatic C–H stretching bonds were slightly widened and shifted, probably due to the activity of bacterial species able to decompose aliphatic fraction contents accompanied by the conversion of VFAs into CH4 and CO2 [61]. The peak at ≈2900 cm−1 could represent the C–H stretching vibration in the methylene group [62], suggesting that MB biodegradation initiated through the demethylation cleavage by some bacterial species [4]. Some peaks appeared in the treated sample, as compared with the raw sludge, suggesting the dye degradation scheme (e.g., the C=N and C—S bonds in the MB molecular structure could be oxidized to generate small molecular substances [9]). The appearance of a new vibration band at 1503 cm–1 agrees with the bending vibrations of MB molecule adsorbed into sludge, while narrowing the band characteristic of OH vibration suggests MB molecule fixation [63]. In a similar study, the adsorption of MB onto the bacterial cell wall of sludge could be supported by C=O electrostatic attraction, –NH2, P-OH hydrogen bonding, and van der Waals forces [35]. Similarly, the appearance of peaks in the 820–557 cm–1 region, describing the C-Cl stretching [64], could justify the interaction of MB molecules with the sludge matrix.
Some peaks at 2972–2850 cm–1 and 1503 cm–1 disappeared after sludge pyrolysis, indicating the thermal decomposition of the organic compounds into gaseous products. The decrease in the intensity of multiple peaks at 1383–1051 cm–1 could also indicate the transformation of the remaining organic fatty hydrocarbons of sludge into volatiles under the thermal treatment process. The peaks in the 1633–1503 cm–1 range could represent the conjugated aromatic ring stretching, where the C=C bonds in biochar suggested the formation of stable aromatic ring systems [65]. The bands at the 1163–900 cm–1 region could be related to the polysaccharide components [60]. The weak peaks between 810526 cm–1 could reveal the existence of Si–O–Si and Si–C, consistent with the presence of quartz in the as-prepared biochar (see Figure 5a). These functional groups would promote the cation-exchange capacity of the soil and improve crop nutrition during the land application of biochar [49].
3.3. Economic Analysis for the Anaerobic Co-Digestion System Treating Dye Wastewater
The economic feasibility of the biogas and biogas/biochar scenarios was performed based on the optimized mixing ratio of 65/35 (v/v) DW/WS for a medium-sized scheme treating 35 m3/day of DW (Table 3). The operational parameters of sludge retention time (SRT) = 14.6–16.2 d and food-to-microorganism (F/M) ratio of 0.68–0.71 g COD/g VSS/d complied with a previous study [66], avoiding VFA accumulation and maintaining balance between the faster-growing bacteria and the slower-growing groups. The computation of capital costs was based on elements like construction facilities, mechanical and electrical apparatus, piping and fittings, and site footprint, following the previous study [32]. The total capital cost of scenario#2 was estimated at USD 28,500 and reduced by ≈22% due to the exclusion of the pyrolysis unit. Contingencies maintained USD 2400, which was estimated at ≈9% of the capital costs. The prices of construction materials for digesters with a volume of 15 m3 (hydraulic retention time of 10.29 h) were based on manufacturer specifications, local contractors, and supplier quotes.

Table 3.
Economic analysis for the AD and AD/pyrolysis scenarios treating dye wastewater.
Electrical energy demands varied among the two scenarios and dominated most of the operational costs, accounting for 34.02% and 40.84% for scenario#1 and scenario#2, respectively. This cost comprised the energy consumed by electrical appliances and a source of heat to maintain mesophilic conditions for the effective digestion of DW. It also considered the power required to operate a screw press for sludge dewatering, further facilitating the pyrolysis of dry sludge [67]. The energy consumed by drying sludge (assuming 105 °C for 24 h) was computed based on the previous study [68]. Energy demand for pyrolysis at 550 °C was estimated from 150 kWh/t total solids [68], assuming 4 h of daily operation. Elevating the electrical energy costs for scenario#2 was due to additional energy demands for maintaining pyrolysis temperatures.
In both scenarios, the methanogenic microbes could convert 52.96 ± 3.61% of the feed COD into CH4 (see COD mass balance in Table 2), giving a daily biogas production of 19.79 m3. CH4 production would positively reduce the carbon footprint from the uncontrolled decomposition of waste organics. Thus, the carbon credit earned from biogenic CH4 was estimated at 355 kg CO2/d, contributing 1277 USD/y in environmental benefits. This system was also responsible for reducing COD pollution up to 68.52 ± 3.40% to give a yearly benefit of USD 2107, expressed as a profit of 0.08 USD/kg CODremoved [12]. This shadow profit reflects the benefit of avoiding environmental damages from the uncontrolled disposal of untreated DW. Although installing a pyrolysis unit raised capital and operating costs of scenario#2, biochar production could add extra revenues to the cash flow. Sludge accumulation was determined from the COD conversion efficiency (CODbiomass = 16.69 ± 1.37% of CODinitial; see Table 2), which could yield 3.76 kg/d of solid digestate (estimated from 1.42 g COD/g biomass conversion ratio). The pyrolysis scheme would increase the profitability of scenario#2, where the accumulated sludge would produce 2.31 kg biochar/d estimated from 3.76 kg solid digestate/d multiplied by a biochar yield of 0.163 kg/kg dry sludge. The GHG offset benefit was estimated from the amount of carbon stored in biochar, giving 1.11 kg/d (using the C fraction of 48% in EDX; Figure 4c). With the carbon trading price of 10 USD/ton CO2 and the biochar price of 1.5 USD/kg, the pyrolysis scheme could deliver an extra 2616 USD/y to the cash flow of scenario#2. The biochar price is within the comparable range of the global biochar price (0.09–8.85 USD/kg), as reported elsewhere [69]. Conversely, the net profits of scenario#1 suffered from sludge disposal expenses compared with scenario#2.
Incorporating a pyrolysis unit would recover the initial investment capital in 6.5 years compared with 8.4 years for the AD alone scenario. Moreover, the profits of scenario#2 would maintain a positive net present value (NPV) with an internal rate of return greater than the project discount rate (8%). These findings demonstrated that the study outputs could pave the way toward the circular economy of recalcitrant textile dyes and residual sludge, meeting several sustainable development goal (SDG) targets (see Supplementary Figure S1). Further studies are required to consider the liquid (bio-oil) and gases (e.g., non-condensable gases with a high-calorie value) generated from the co-pyrolysis of sludge with wood biomass and estimate the profitability criteria with the NPV.
4. Conclusions
This study successfully developed a sustainable strategy that could cost-effectively manage recalcitrant dye wastewater (DW) based on biological and thermal treatment processes. Adding extracts of wheat straw (WS) to DW under anaerobic conditions enhanced the methylene blue (MB) degradation efficiency by ≈3-fold higher than the mono-digestion assays (only DW). The 35% v/v WS supplementation showed a better synergistic interaction with DW, in which 52.96 ± 3.61% of the influent COD was transformed to bio-CH4 production. Moreover, the thermal treatment of solid digestate depicted a promising route for sludge management, getting biochar characterized by a high degree of aromaticity (O/C ≈ 0.2). The profits related to pollution reduction, biogas, biochar, and carbon trading showed that the integrated digestion/pyrolysis scenario would overcome capital costs in a time less than the project lifetime (<10 y). The profitability scenario of the digestion/pyrolysis project also revealed a positive net present value, with an internal rate of return greater than the discount rate (8%). Further studies are required to determine the best post-treatment phase that could achieve complete mineralization of the aromatic compounds to meet the discharge standards. Moreover, some future studies should aim to identify the microbial strains and enzymatic activities involved in the degradation processes and carry out phytotoxicity analysis of the biodegradation products.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16142025/s1, Table S1. Data of CH4 recovery from the anaerobic digestion of dye wastewater compared with the current study; Figure S1: Correlating the study output from the anaerobic digestion (scenario-1) and anaerobic digestion/pyrolysis (scenario-2) treating dye wastewater to the sustainable development goals (SDGs).
Author Contributions
Conceptualization, M.N., M.F. and M.G.I.; methodology, A.T.; software, A.T.; formal analysis, A.T.; writing—original draft preparation, A.T.; writing—review and editing, M.N., M.F. and M.G.I.; visualization, M.N., M.F. and M.G.I.; supervision, M.N., M.F. and M.G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TICAD7, the Egypt-Japan University of Science and Technology (E-JUST), and the Japanese International Corporation Agency (JICA).
Data Availability Statement
The data that support the findings of this study are within the article (and or) its Supplementary Materials.
Acknowledgments
The author is very grateful to TICAD7 for providing financial support in the form of an MSc. scholarship. Also, thanks to JICA—Japan International Corporation Agency for providing all the facilities and equipment needed to conduct this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nnaji, C.; Ume, S.; Obasi, U.; Anadebe, C.; Ezemagu, G.; Okeke, U.; Onukwuli, D. Machine learning-based performance evaluation and sludge characterization studies of oxidized starch-aluminum electrode assisted by direct current treatment of dye laden wastewater. Results Eng. 2023, 20, 101576. [Google Scholar] [CrossRef]
- Saratale, G.R.; Gandhi, S.A.; Purankar, V.M.; Kurade, B.M.; Govindwar, P.S.; Oh, E.S.; Saratale, D.G. Decolorization and detoxification of sulfonated azo dye C.I. Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS J. Biosci. Bioeng. 2013, 115, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Msemwa, G.; Ibrahim, M.; Manabu, F.; Nasr, M. Phytomanagement of textile wastewater for dual biogas and biochar production: A techno-economic and sustainable approach. J. Environ. Manag. 2022, 322, 116097. [Google Scholar] [CrossRef] [PubMed]
- Kishor, R.; Saratale, D.; Saratale, G.; Ferreira, R.; Bilal, M.; Iqbal, N.; Bharagava, N. Efficient degradation and detoxification of methylene blue dye by a newly isolated ligninolytic enzyme producing bacterium Bacillus albus MW407057. Colloids Surf. B Biointerfaces 2021, 206, 111947. [Google Scholar] [CrossRef] [PubMed]
- Ogunlaja, A.; Nwankwo, N.; Omaliko, E.; Olukanni, D. Biodegradation of Methylene Blue as an Evidence of Synthetic Dyes Mineralization during Textile Effluent Biotreatment by Acinetobacter pittii. Environ. Process. 2020, 7, 931–947. [Google Scholar] [CrossRef]
- Kalengyo, B.R.; Ibrahim, G.M.; Fujii, M.; Nasr, M. Utilizing orange peel waste biomass in textile wastewater treatment and its recyclability for dual biogas and biochar production: A techno economic sustainable approach. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Demarema, S.; Nasr, M.; Ookawara, S.; Abdelhaleem, A. Enhanced synergistic system for the persulfate activation under visible light using novel N–ZnO photocatalyst supported on Lantana camara-based biochar. Chemosphere 2024, 349, 140840. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, S.; Lin, Z.; Yang, J.; Li, C.; Gu, R. Combination of plasma oxidation process with microbial fuel cell for mineralizing methylene blue with high energy efficiency. J. Hazard. Mater. 2020, 384, 121307. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Shi, M.; Pan, X.; Zhang, J.; Zhang, X.; Shen, T.; Tian, Y. Decourization and biodegradation of methylene blue dye by ligninolytic enzyme-producing Bacillus thuringiensis: Deradation products and pathway. Enzym. Microb. Technol. 2022, 156, 109999. [Google Scholar] [CrossRef]
- Labayen, J.J.; Yanac, K.; Yuan, Q. Effect of substrate-to-inoculum ratio on anaerobic digestion of treated and untreated cotten textile waste. Int. J. Environ. Sci. Technol. 2021, 18, 287–296. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, C.; Chaudhari, K. Anaerobic biological treatment of dye bearing water in anaerobic sequencing batch reactor: Performance and kinetics studies. J. Indian Chem. Soc. 2022, 99, 10. [Google Scholar] [CrossRef]
- Atukunda, A.; Ibrahim, G.M.; Fujii, M.; Ookawara, S.; Nasr, M. Dual biogas/biochar production from anaerobic co digestion of petrochemical and domestic wastewater: A techno economic and sustainable approach. Biomass Convers. Biorefin. 2024, 14, 8793–8803. [Google Scholar] [CrossRef]
- Rahman, U.W.; Khan, D.M.; Khan, Z.M. Anaerobic biodegradation of benzene-laden wastewater under mesophilic environment and simultaneous recovery of methane-rich biogas. J. Environ. Chem. Eng. 2018, 6, 2957–2964. [Google Scholar] [CrossRef]
- Ikram, M.M.; Zahoor, M.; Hanafiah, M.; Oyekanmi, A.; Ullah, R.; Farraj, D.; Gulfam, N. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Malik, A.R.; Vistanty, H.; Suhardi, H.S. Performance of anaerobic co-digestion with honey processing wastewater as co-substrate for treating synthetic wastewater containing commercial anthraquinone dye Remazol blue RSP: Effect of C:N ratio and HRT. Bioresour. Technol. Rep. 2022, 19, 101157. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, C.; Siyal, A.A.; Song, Y.; Jiang, Z.; Dai, J.; Zhang, T. Comperative study for fluidized bed pyrolysis of textile dyeing sludge and municipal sewage sludge. J. Hazard. Mater. 2020, 396, 122619. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Mahmoud, A.K.; Lee, S.D. Influence of co-substrate on textile wastewater treatment and microbial community changes in the anaerobic biological sulfate reduction process. J. Hazard. Mater. 2015, 299, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chu, S.; Chen, J.; Chen, Y.; Xie, Z. Enhanced reduction of an azo dye using henna plant biomass as a solid-phase electron donor, carbon source, and redox mediator. Bioresour. Technol. 2014, 161, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Nuid, M.; Aris, A.; Krishnen, R.; Chelliapan, S.; Muda, K. Pineapple wastewater as co-substrate in treating real alkaline, non-biodegradable textile wastewater using biogranulation technology. J. Environ. Manag. 2023, 344, 118501. [Google Scholar] [CrossRef] [PubMed]
- Tugume, M.; Ibrahim, G.; Fujii, M.; Nasr, M. Management of Cheese Whey Wastewater and Greywater for Dual Biogas and Biochar Production: A Techno Economic and Sustainable Approach. Waste Biomass Valorization 2024, 15, 4373–4393. [Google Scholar] [CrossRef]
- Elreedy, A.; Fujii, M.; Tawfik, A. Factors affecting on hythane bio-generation via anaerobic digestion of mono-ethylene glycol contaminated wastewater: Inoculum-to-substrate ratio, nitrogen-to-phosphorus ratio and pH. Bioresour. Technol. 2017, 223, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Alghashm, S.; Song, L.; Liu, L.; Ouyang, C.; Zhou, J.; Li, X. Improvement of Biogas Production Using Biochar from Digestate at Different Pyrolysis Temperatures during OFMSW Anaerobic Digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- Khan, R.; Shukla, S.; Kumar, M.; Zuorro, A.; Pandey, A. Sewage sludge derived biochar and its potential for sustainable environment in circular economy: Advantages and challenges. J. Chem. Eng. 2023, 471, 144495. [Google Scholar] [CrossRef]
- Tawfik, A.; Ali, M.; Danial, A.; Zhao, S.; Meng, F.; Nasr, M. 2-Biofuels (H2 nad CH4) production from anaerobic digestion of biscuits wastewater: Experimental study and techno-economic analysis. J. Water Process Eng. 2021, 39, 101736. [Google Scholar] [CrossRef]
- Barathi, S.; Aruljothi, N.; Karthik, C.; Padikasan, A.; Ashokkumar, V. Biofilm mediated decolorization and degradation of reactive red 170 dye by the bacterial consortium isolated from the dyeing industry wastewater sediments. Chemosphere 2022, 286, 131914. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.A.; Tyagi, K.V.; Gunjyal, N.; Kazmi, A.A.; Ojha, P.S.; Moustakas, K. Hydrothermal and Thermal-alkali pretreatments of wheat straw: Co-digestion, subtrate solubilization, biogas yield and kinetic study. Environ. Res. 2023, 216, 114436. [Google Scholar] [CrossRef]
- Jadhav, U.U.; Dawkar, V.V.; Telke, A.A.; Govindwar, P.S. Decolorization of Direct Blue GLL with enhanced lignin peroxidase enzyme production in Comamonas sp UVS. J. Chem. Technol. Biotechnol. 2009, 84, 126–132. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Ni, Q.J.; Zhuo, G.; Chen, W.; Zheng, Y.; Zhen, G. Effect of municipal sludge-based biochar produced at different pyrolysis temperatures on humification and oxytetracycline degradation of pig manure composting. Sci. Total Environ. 2024, 906, 167816. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Bashiri, B.; Fallah, N.; Bonakdarpour, B.; Elysai, S. The development of aerobic granules from slaughterhouse wastewater in treating real dyeing wastewater by Sequencing Batch Reactor (SBR). J. Environ. Chem. Eng. 2018, 6, 5536–5543. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Ansari, A.; Ravindran, B.; Gupta, K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Bakr, H.M.; Nasr, M.; Haider, J.; k.Al Mesfer, M.; Lim, H.; Qyyum, M.A.; Lam, S.S. Economic and environmental sustainability for anaerobic biological treatment of wastewater from paper and cardboard manufacturing industry. Chemosphere 2022, 289, 133166. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; Arroussi, E.H.; Bahafid, W.; Ghachtouli, E.N. An overview of the biosorption mechanism for the bioremediation of synthetic dyes using yeast cells. Environ. Technol. Rev. 2021, 10, 58–76. [Google Scholar] [CrossRef]
- He, T.; Hua, J.; Chen, R.; Yu, L. Adsorption characteristics of methylene blue by a dye-degrading and extracellular polymeric substance -producing strain. J. Environ. Manag. 2021, 288, 112446. [Google Scholar] [CrossRef] [PubMed]
- Gadow, I.S.; Estrada, L.A.; Li, Y.Y. Characterization and potential of two different anaerobic mixed microflora for bioenergy recovery and decolorization of textile wastewater: Effect of C/N ratio, dye concentration and pH. Bioresour. Technol. Rep. 2022, 17, 100886. [Google Scholar] [CrossRef]
- Ghosh, S.; Kunnoth, B.; Pilli, S.; Rao, V.; Tyagi, D. Novel hybrid system for organic matter removal and energy production from dairy and textile wastewaters: Anaerobic digestion and electrocoagulation approach. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Song, Y.; Meng, S.; Chen, G.; Yan, B.; Zhang, Y.; Tao, J.; Li, J. Co-digestion of garden waste, food waste, and tofu residue: Effects of mixing ratio on methane production and microbial community structure. J. Environ. Chem. Eng 2021, 9, 105901. [Google Scholar] [CrossRef]
- Xiao, Y.; Zan, F.; Zhang, W.; Hao, T. Alleviating nutrient imbalance of low carbon-to-nitrogen ratio food waste in anaerobic digestion by controlling the inoculum-to-substrate ratio. Bioresour. Technol. 2022, 346, 126342. [Google Scholar] [CrossRef] [PubMed]
- Ciotola, J.; Martin, F.; Tamkin, A.; Castańo, M.; Rosenblum, J.; Bisesi, S.; Lee, J. The Influence of Loading Rate and Variable Temperatures on Microbial Communities in Anaerobic Digesters. Energies 2014, 7, 785–803. [Google Scholar] [CrossRef]
- Anfar, Z.; El Haouti, R.; Lhanafi, S.; Benafqir, M.; Azougarh, Y.; El Alem, N. Treated digested residue during anaerobic co-digestion of Agri-food organic waste: Methylene blue adsorption, mechanism and CCD-RSM design. J. Environ. Chem. Eng. 2017, 5, 5857–5867. [Google Scholar] [CrossRef]
- Ramzan, U.; Shakoori, R.; Shakoori, R.; Abbas, Z.; Wabaidur, M.; Eldesoky, E.; Rafatullah, M. Biodegradation and decolorization of Reactive Red 2 azo dye by Paramecium jenningsi and Paramecium multimicronucleatum in industrial wastewater. Biomass Convers. Biorefin. 2024, 14, 7753–7761. [Google Scholar] [CrossRef]
- Lackner, N.; Wagner, O.; Markt, R.; Illmer, P. pH and Phosphate Induced Shifts in Carbon Flow and Microbial Community during Thermophilic Anaerobic Digestion. Microorganisms 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Tarelho, L.; Hauschild, T.; Vilas-Boas, A.; Silva, D.; Matos, M. Biochar from pyrolysis of biological sludge from wastewater treatment. Energy Rep. 2020, 6 (Suppl. S1), 757–763. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef] [PubMed]
- Krysiak-Baltyn, K.; Cavalida, R.; Thwaites, B.; Reeve, P.; Scales, P.; Van den Akker, B.; Gras, S. Comparison of physical characteristics and dewatering behaviour between granular and floccular sludges generated from the same sewage source. J. Water Process Eng. 2019, 29, 100785. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Ni, J.; Hu, B. Cultivation of granules containing anaerobic decolorization and aerobic degradation cultures for the complete mineralization of azo dyes in wastewater. Chemosphere 2020, 246, 125753. [Google Scholar] [CrossRef] [PubMed]
- Eraky, M.; Nasr, M.; Elsayed, M.; Ai, P.; Tawfik, A. Synergistic interaction of tween 20 and magnesium@ functionalized graphene oxide nano-composite for dual productivity of biohydrogen and biochar from onion peel waste. Renew. Energy 2023, 216, 119082. [Google Scholar] [CrossRef]
- Nasr, M.; Tawfik, A.; Awad, H.M.; Galal, A.; El-Qelish, M.; Qyyum, M.A.; Ali Khan, M.M.; Rehan, M.; Nizami, A.-S.; Lee, M. Dual production of hydrogen and biochar from industrial effluent containing phenolic compounds. Fuel 2021, 301, 121087. [Google Scholar] [CrossRef]
- Ullah, H.; Abbas, Q.; Ali, U.M.; Cheema, I.A.; Yousaf, B.; Rinklebe, J. Synergistic effects of low-/medium-vacuum carbonization on physicochemical properties and stability characteristics of biochars. Chem. Eng. J. 2019, 373, 44–57. [Google Scholar] [CrossRef]
- Li, M.; Ren, Z.; Gao, H.; Zhang, A.; Sun, Z. Preparation of tobermorite onto flux-calcinated diatomite surface and the adsorption properties and mechanism of methylene blue. Desalin. Water Treat. 2020, 188, 247–256. [Google Scholar] [CrossRef]
- Fan, H.; Li, L.; Li, Z.; Shang, S. Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release. Materials 2022, 15, 1855. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Chahal, H.; Matthews, S.; Jones, M. Fabrication of Calcium Phosphate Coatings by the Inflight Reaction of Precursor Feedstocks Using Plasma Spraying. J. Therm. Spray Technol. 2023, 32, 1465–1481. [Google Scholar] [CrossRef]
- Ma, Q.; Song, W.; Wang, R.; Zou, J.; Yang, R.; Zhang, S. Physicochemical properties of biochar derived from anaerobically digested dairy manure. Waste Manag. 2018, 79, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Das, K.S.; Ghosh, K.G.; Avasthe, R.; Sinha, K. Morpho-mineralogical exploration of crop, weed and tree derived biochar. J. Hazard. Mater. 2021, 407, 124370. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Cheng, X.; Shi, Y.; Chen, Z.; Liu, Y.; Han, X.; Liu, Y.; Zhang, Z. Insights into lead removal in water using a novel carbonized material derived from the by-product of oil refining: Action mechanism and performance optimization. J. Chem. Technol. Biotechnol. 2021, 96, 3224–3236. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.; Chakraborty, S.; Owens, G.; Naushad, M. Valorization of fruit waste-based biochar for arsenic removal in soils. Environ. Res. 2022, 213, 113710. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Tang, Y.; Lu, X.-Y.; Meng, Z. Mechanisms of copper stabilization by mineral constituents in sewage sludge biochar. J. Clean. Prod. 2018, 193, 185–193. [Google Scholar] [CrossRef]
- Martínez, E.; Fierro, J.; Rosas, J.; Lobato, A.; Otero, M.; Gómez, X. Assessment of Cationic Dye Biosorption onto Anaerobic Digested Sludge: Spectroscopic Characterization. Environ. Prog. Sustain. Energy 2016, 35, 1330–1337. [Google Scholar] [CrossRef]
- Provenzano, M.; Malerba, A.; Pezzolla, D.; Gigliotti, G. Chemical and spectroscopic characterization of organic matter during the anaerobic digestion and successive composting of pig slurry. Waste Manag. 2014, 34, 653–660. [Google Scholar] [CrossRef]
- Wang, J.; Kou, L.; Huang, Z.; Zhao, L. One-pot preparation of MnOx impregnated cotton fibers for methylene blue dye removal. RSC Adv. 2018, 8, 21577–21584. [Google Scholar] [CrossRef] [PubMed]
- Ettahiri, Y.; Bouna, L.; Hanna, J.; Benlhachemi, A.; Pilsworth, H.; Bouddouch, A.; Bakiz, B. Pyrophyllite clay-derived porous geopolymers for removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2023, 296, 127281. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Pan, X.; Lin, Z.; Guan, X. Investigation of Methylene Blue Biosorption and Biodegradation by Bacillus thuringiensis 016. Water Air Soil Pollut. 2015, 226, 146. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Zhang, Z.; Zhang, L.; Gao, J.; Sun, S. Synergetic effects of biochar structure and AAEM species on reactivity of H2O-activated biochar from cyclone air gasification. Int. J. Hydrogen Energy 2017, 42, 16045–16053. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, K.; Jiang, X.; Shen, N.; Zeng, R.; Zhou, Y. Long solid retention time (SRT) has minor role in promoting methane production in a 65 °C single-stage anaerobic sludge digester. Bioresour. Technol. 2018, 247, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, K. Life cycle assessment of the environmental impacts and energy efficiency of an integration of sludge anaerobic digestion and pyrolysis. J. Clean. Prod. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Antoniou, N.; Barakat, A.; Zabaniotou, A. A new concept for enhancing energy recovery from agricultural residues by coupling anaerobic digestion and pyrolysis process. Appl. Energy 2015, 148, 32–38. [Google Scholar] [CrossRef]
- Saharudin, M.D.; Jeswani, K.H.; Azapagic, A. Biochar from agricultural wastes: Environmental sustainability, economic viability and the potential as a negative emissions technology in Malaysia. Sci. Total Environ. 2024, 919, 170266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).