Abstract
The application of low-pressure nanofiltration (NF) was investigated for three different applications: water reuse from acid mine drainage (AMD), surface water containing natural organic matter (NOM) and agricultural reuse of microfiltered biologically treated sewage effluent (MF-BTSE). AMD contains many valuable rare earth elements (REEs) and copper (Cu) that can be recovered with fresh water. The NF90 membrane was investigated for recovery of fresh water from synthetic AMD. A steady permeate flux of 15.5 ± 0.2 L/m2h was achieved for pretreated AMD with over 98% solute rejection. NF90 achieved a high dissolved organic carbon (DOC) rejection of 95% from surface water containing NOM where 80% of the organic fraction was hydrophilic, mainly humics. The NF process maintained a high permeate flux of 52 LMH at 4 bars. The MF-BTSE was treated by NTR-729HF for agricultural reuse. NTR-729HF membranes were capable of rejecting DOC and inorganics such as sulfates and divalent ions (SO42−, Ca2+ and Mg2+) from MF-BTSE, with less than 20% rejection of monovalent (Na+ and Cl−) ions. The sodium adsorption ratio (SAR) was significantly reduced from 39 to 14 after treatment through NTR-729HF at 4 bar. The resulting water was found to be suitable to irrigate salt-sensitive crops.
1. Introduction
Membrane filtration technologies like reverse osmosis (RO) and nanofiltration (NF) offer compelling alternatives for water treatment. While RO excels at removing a broad spectrum of dissolved contaminants, both organic and inorganic, NF presents distinct advantages. It boasts faster water flow rates and lower operating pressures, translating to significant energy savings and potentially lower treatment costs [1]. However, NF’s effectiveness in tackling organics, inorganics, and metal removal depends heavily on the membrane’s intrinsic properties. Key factors influencing its performance include the molecular weight cut-off (MWCO)—essentially a measure of the membrane’s pore size—along with its electrical charge and hydrophobicity (water repellency).
Research has identified specific NF membranes, like NF90 and NTR729-HF, as highly effective in removing organics, inorganics, and metal ions while producing clean water [2]. This success hinges on the unique properties of the NF90 and NTR-729HF membranes. Their pore size and surface properties allow them to retain organics and repel positively/negatively charged metal ions found in different types of wastewater, leading to superior rejection rates [3,4]. These characteristics also makes them less prone to fouling compared to other membranes [3]. However, it is important to acknowledge that NF technology does generate a concentrated reject stream containing the filtered-out organics and metals. While this stream may hold potential for resource recovery, its proper management requires further consideration. Thus, NF stands out for its potential in treating wastewater for recovery and reuse purposes. This paper discusses the application of NF membranes in treating three different types of wastewater—acid mine drainage (AMD), surface water containing natural organic matter (NOM), and biologically treated sewage effluent (BTSE)—for irrigation purposes.
Firstly, acid mine drainage (AMD) forms when water interacts with leftover mine waste, exposed mining cuts, or naturally sulfur-rich soils [5]. This acidic cocktail, laden with dissolved heavy metals, rare earth elements (REEs), and sulfates and lacking any buffering capacity, wreaks havoc on downstream waterways. Entire ecosystems are poisoned, and the health of humans and animals who rely on this water is jeopardized. Traditional methods for treating AMD, like chemical precipitation and constructed wetlands, often create new problems. These approaches generate excessive sludge, a secondary pollutant requiring further management. This highlights the urgent need for a paradigm shift in mining practices, one that prioritizes sustainability and resource recovery alongside responsible waste management. As a result, researchers are actively exploring ways to extract valuable resources from waste streams like AMD, driving the development of more efficient and cost-effective treatment solutions [5].
Membrane processes have been studied to recover fresh water from waste sources and to concentrate valuable metals [6,7]. In arid regions, freshwater is a precious commodity; therefore, the recovery of water from AMD is highly beneficial. Many mining operations are located in arid regions. A high operational cost and the generation of concentrate are the major drawbacks. The concentrated reject stream containing metal ions and sulfates may hold potential for resource recovery, but its proper management adds another layer of complexity [8]. The integration of these advanced treatment technologies enables the recovery of more water from waste streams, which can be repurposed for various applications such as irrigation, industrial processes, and even potable use after appropriate treatment. This not only conserves valuable water resources but also contributes to sustainable water management practices.
Secondly, surface water contains a complex mixture of natural organic matter (NOM) derived from decomposing plants and microorganisms. While NOM itself may seem harmless, it can cause unpleasant discoloration, odors, and tastes in drinking water. More importantly, NOM can harbor bacteria and react with chlorine disinfection to form harmful disinfection byproducts (DBPs) during water treatment [9]. Heavy rainfall events further exacerbate this issue by flushing increased amounts of NOM into waterways, elevating its concentration in raw water sources. Strict regulations by the World Health Organization (WHO) for trihalomethanes (THMs) pose a challenge for water treatment plants. While removing NOM directly reduces THM formation, its concentration in source water is steadily rising. This trend threatens the ability of plants to deliver clean drinking water. Traditional methods like flocculation and filtration, while partially removing NOM, often fall short of achieving optimal purification. As a result, exploring more efficient treatment options becomes essential. NF has proven to be an excellent technology to remove NOM, as excellent rejection was reported by previous researchers with minimum fouling effect [4,10].
Thirdly, nanofiltration has emerged as a viable and cost-effective method for treating biologically treated sewage effluent (BTSE) to produce water suitable for irrigation [11]. BTSE contains a notable level of dissolved natural and refractory organics, nutrients, various inorganic ions such as Na+, K+, Ca2+, Mg2+, and Cl−, and pathogens. Though organic matter and nutrients are beneficial for plant growth, trace organics, pathogens, and the elevated levels of salinity of the water (due to Na and Cl ions) can adversely affect soil properties and hinder plant growth. Elevated levels of Na ions, in particular, can induce toxicity in plants, necessitating the determination of appropriate Na ion levels through the sodium adsorption ratio (SAR).
According to the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (2000) [12], the recommended thresholds of SAR, Cl−, and Na+ are 8–18, 175–350 mg/L, and 115–230 mg/L, respectively, especially for sensitive to moderately sensitive crops. Nanofiltration (NF) play a pivotal role in treating BTSE to provide irrigation water that meets these stringent requirements, addressing water scarcity concerns. Previous research has demonstrated that NF can effectively remove trace organic and inorganic ions from BTSE to produce water suitable for irrigation with appropriate SAR and salinity levels [11]. This water can satisfy the requirements of salt-sensitive crops.
The objective of this study was to assess the use of two types of NF membranes (NF90 and NTR-729HF) in treating three different types of wastewater—acid mine drainage (AMD) solution, surface water containing NOM, and MF-BTSE—to produce water for irrigation purposes. The governing mechanisms of the removal of inorganics and organics by the two NF membranes chosen are explained.
2. Materials and Methods
2.1. Preparation of Feedwater
2.1.1. Preparation of AMD Solution
Synthetic wastewater representing actual AMD characteristics was prepared based on previous studies [5]. A known amount of europium (III) nitrate pentahydrate (Eu(NO3)3·5H2O, 99.9%) was used. Na2SO4·10H2O (99%), MgSO4·3H2O (99%), CaSO4·2H2O (99%), CuSO4·5H2O (99%), Ni(NO3)2·6H2O (99%), ZnSO4·7H2O (99%), and Eu(NO3)3·5H2O (99.9%) were dissolved in Milli Q water to prepare synthetic AMD solution for selective adsorption tests. H2SO4 (2 M) was used to adjust the initial pH to 2.0 ± 0.2. All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.1.2. Preparation of Surface Water Containing NOM
Typical leaves from a catchment in Sydney were used to prepare synthetic water containing NOM. Firstly, the leaves were soaked in a tank containing water, simulating dam-like conditions open to the atmosphere. Water in the tank was continuously mixed using a pump, which withdrew water from the bottom of the tank and discharged it to the top to maintain recirculation. The color and dissolved organic carbon (DOC) of the water were measured regularly, typically daily. Synthetic water containing 5.17 mg/L of the DOC mentioned above was prepared by diluting this water with clean water.
2.1.3. Microfiltered Biologically Treated Sewage Effluent (MF-BTSE)
The MF-BTSE was collected from a water reclamation plant located in Sydney, Australia. The purpose of the plant is to produce reusable water for non-potable purposes by treating the BTSE through microfiltration (MF) and then through reverse osmosis (RO). The feedwater used for this study was collected after the MF, and as such, the feedwater is referred to as “microfiltered BTSE” (MF-BTSE).
2.2. NF Membrane and Operation
2.2.1. Selection of NF Membranes
The NF membranes, namely, NF90 and NTR-729HF, were chosen for the experimental studies. As per the study conducted by Jamil et al. [4], the NF90 proved to be the best-performing NF membrane in terms of removing organics from MF-BTSE at low operational pressures ranging from 2 to 5.5 bar compared to the rest of the membranes (NF 270, NP 030, NF-TS 80, NF-Duracid). They stated that NF90 rejected approx. 75% of electrical conductivity (EC) and 88% of dissolved organic carbon (DOC). However, the NTR-729HF was reported to remove more than 95% of the DOC [11].
Considering the better performances in terms of removing organics and inorganics, the above two NF membranes were used in this study with three different types of feedwater. The characteristics of the NF membranes studied are given in Table 1.

Table 1.
Characteristics of NF membrane.
2.2.2. NF Operation
Firstly, the potential of a small-scale nanofiltration (NF) system for treating synthetic AMD was studied using the NF90 membrane, which is commercially available from Sterlitech Corporation (Auburn, WA, USA). The performance of the NF filtration was assessed for its effectiveness in treating the AMD while simultaneously concentrating valuable resources. For the experiments, a feed volume of 2 L was employed alongside a rectangular cross-flow cell with an area of 54 cm2 (9 cm × 6 cm). NF was operated with a low transmembrane pressure of 3 bar and a moderate cross-flow velocity of 0.35 m/s to assess the performance of NF90 for AMD filtration at a temperature of 25 ± 1 °C. It should be noted that previous studies have predominantly utilized transmembrane pressures exceeding 10 bar (1000 kPa) for AMD filtration employing the NF90 membrane [2,3]. The schematic diagram of the NF experimental setup is illustrated in Figure 1.
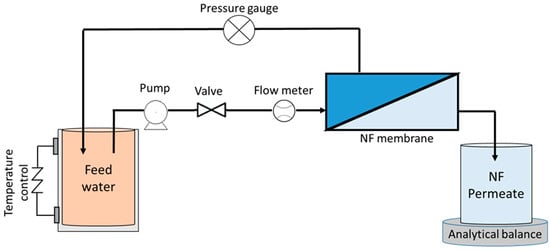
Figure 1.
Schematic diagram of NF setup.
The feed solution was continuously circulated, while permeate was collected and its mass increase was measured using an analytical balance. Permeate flux and water recovery were calculated based on readings obtained from the analytical balance at regular intervals. Upon completion, the initial feed, final feed (concentrated feed), and permeate were analyzed for metal concentrations, pH, and total organic carbon (TOC).
The volume concentrating factor (VCF) of the concentrated feed was determined using the following equation:
where Vi is initial AMD feed volume (L) and Vc is the final concentrated AMD volume (L). The permeate flux of the membrane was calculated by measuring the permeate volume collected at 5 min intervals using the following equation:
where J is permeate flux (L/m2h), A the effective filter area of the membrane (m2), and Δt the time interval (h).
VCF = Vi/Vc
J = V/(A × Δt)
Contaminant or solute rejection was calculated from the concentration of dissolved metals in feed AMD and filtered permeate using Equation (3). The ion concentration was measured using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7900, City of Santa Clara, CA, USA).
where R is solute rejection, Ci initial solute concentration (mg/L), and Cp permeate solute concentration (mg/L).
R(%) = (Ci − Cp)/Ci
Secondly, the performance of the NF90 membrane in removing NOM from synthetic water formulated with representative NOM quality and quantity was evaluated. Duplicate NF experiments were conducted with NF90 membranes continuously for 10 h at filtration flux of 52 ± 2 L/m2h. The average DOC of feedwater was 5.17 mg/L, and the operational pressure was 4 bars.
Thirdly, the NTR-729HF was employed to remove DOC and inorganics from the MF-BTSE to make the water suitable for irrigating sensitive and moderately sensitive crops. This NTR-729HF was selected as it has proved excellent performance in the removal of trace organics that may accumulate into crop tissue. The consumption of such crops by humans may cause toxicity or endocrine disruptive issues. Further, this membrane bears the highest zeta potential (−100 mV)/surface charge, and as such, it can remove ionic charges well through electrostatic interactions (Donnan effect) [15].
2.3. Characterization of Feedwater
2.3.1. Determination of Inorganics/Metals in AMD
The concentration of inorganics/metals (Na, Mg, Al, Ca, Fe, Ni, Cu, Zn and Eu) in the synthetic AMD solution was analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7900).
2.3.2. Determination of Organic Carbon Fraction
Dissolved organic carbon (DOC) content was measured using a liquid chromatography organic carbon detection unit (LC-OCD) from DOC-Labor Dr. Huber, Germany [16]. This technique separates organic carbon in a water sample into five distinct fractions based on molecular weight and polarity. Initially, the LC-OCD separates the sample into two broad categories: hydrophilic and hydrophobic organic carbon (HOC). Hydrophilic organic carbon (HOC) elutes from the column, while HOC binds irreversibly to the column’s solid phase. Further fractionation of the hydrophilic fraction (hydrophilic chromatographable organic carbon, CDOC) reveals four sub-categories: biopolymers, humic substances, building blocks, and low-molecular-weight organics. The difference between DOC and CDOC represents the sample’s hydrophobic fraction.
2.3.3. Determination of DOC, Color, and Inorganic Ions
Followed by filtration through a 0.45 µm filter, the filtrates were analyzed for dissolved organics using a Multi N/C 2000 TOC Analyzer. The color of filtrates was analyzed using a DR 5000 UV-vis spectrophotometer. The analysis of inorganic ions was conducted using a Metrohm ion chromatograph (model 790) (Metrohm Ltd., Herisau, Switzerland) equipped with an auto-sampler and conductivity cell detector.
3. Results
3.1. Characteristics of Feedwater
Synthetic AMD: The composition of the synthetic AMD wastewater is illustrated in Table 2. The pH of the solution was highly acidic, and 2 M H2SO4 was used to adjust the initial pH to 2.0 ± 0.2. The Fe and Mg ions are detected to be high and the Eu is in traces.

Table 2.
Composition of the synthetic AMD wastewater.
Surface water containing NOM: The organic fractions of the synthetic water representing natural surface water containing NOM are presented in Table 3. The DOC of the water is ~5.19 mg/L and consists of about 80% of hydrophilic substances, with the rest hydrophobic substances. Of the hydrophilic substances, 60% are detected to be humics. A similar observation was reported by Krzeminski et al. [17], who conducted organic fractionation in drinking water reservoirs where 82–92% of DOC was detected of a hydrophilic nature, of which 58–73% were humics.

Table 3.
Organic fractions of the surface water synthesized under natural conditions.
MF-BTSE: The MF-BTSE is neutral, detected with 3–7 mg/L of dissolved organics, and the sodium absorption ratio (SAR) is 39, which is influenced by Ca, Mg, Na ions (Table 4). Based on Australian and New Zealand Guidelines (2000) for fresh and marine water quality [12] (as reported by Shanmuganathan et al. [11]), the SAR should be 8–18 to irrigate salt-sensitive crops.

Table 4.
Characteristics of MF-BTSE in terms of organics and inorganic ions (adopted from Shanmuganathan et al. [11]).
3.2. Performance of NF (NF90) in Treating AMD Solution
3.2.1. Permeate Flux and Concentration Factor
The system with NF90 achieved a stable permeate flux and the rate of reusable clean water production was 15.5 L/m2h (LMH) (Figure 2). This indicates efficient water recovery from the contaminated influent solution. It achieved a high volume concentration factor (VCF) of 5. In this case, 80% of the initial volume of the AMD solution was recovered as clean permeate, leaving behind a concentrated retentate enriched with REEs, including Cu.
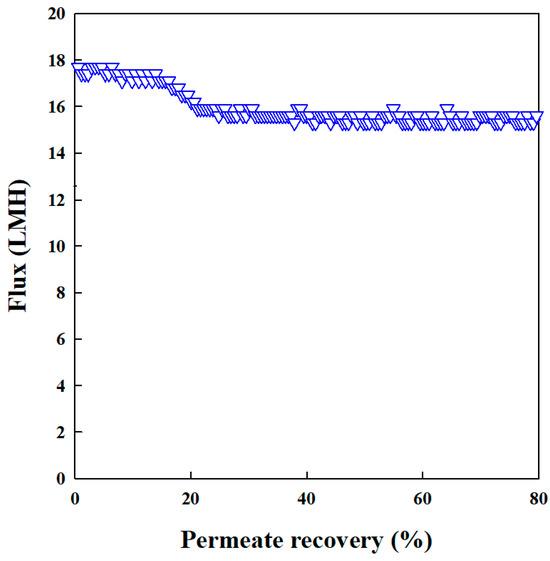
Figure 2.
Permeate flux of NF90 in treating synthetic AMD solution (steady-state flux 15.5 L/m2h; pressure = 3 bar).
3.2.2. Rejection of Solutes from AMD
The system displayed exceptional rejection rates (>98%) for most dissolved solutes present in the AMD, effectively removing them from the treated water (Table 5). However, sodium (Na) was the only exception, exhibiting a lower rejection rate compared to other cations. The concentrated retentate enriched in REEs and Cu can be successfully recovered selectively using functionalized adsorbents packed in columns [18].

Table 5.
Performance of NF90 in terms of the rejection of solutes in AMD solution (steady-state flux 15.5 L/m2h at pressure 3 bar).
As stated by Lim et al. [19], NF membranes reject solutes through a combination of hydrodynamic filtration, the solution-diffusion mechanism, and the Donnan exclusion principle. At the micron level, hydrodynamic filtration occurs when solutes are removed based on the size exclusion principle. As the pore size decreases (to nano level), molecular sieving mechanisms and the interface effect become dominant. When the pore size further decreases (to near-angstrom level), the solute transport is governed by the solution-diffusion mechanism, where the solute dissolves and diffuses faster across the polymer matrix [19].
The NF90 membrane’s ability to selectively reject/transport specific solutes from AMD hinges on two key factors, the nanochannel of the NF90 and the properties of the solutes [19], as the surface friction of the nanochannel and the interactions between the channel cavity and solutes could influence the solute rejection rate from AMD.
The MWCO value essentially indicates the size limit for solutes. Ideally, anything smaller than the MWCO should pass through the membrane into the permeate stream [20]. However, in this case, the picture is more complex. The NF90 membrane’s surface boasts positively charged functional groups. These groups create an electrostatic field (Donnan potential) within the membrane [8,21]. This electrical barrier acts as a deterrent, particularly for positively charged ions (cations), commonly found in AMD. This explains the high rejection rates observed for most cations, despite their potentially lower molecular weights compared to the MWCO. Another phenomenon at play here could be membrane adsorption [22]. In some instances, when electrostatic repulsion and adsorption are stronger than simple size exclusion, even smaller molecules like sodium (Na) can be more effectively rejected compared to larger ones with higher valences, like aluminum (Al). This could explain the observed lower rejection rate for sodium in the experiment.
3.3. Performance of NF90 in the Removal of Organic Fractions from Surface Water
The performance of NF90 membrane in removing NOM from a synthetic water formulated with representative NOM quality and quantity was studied. Duplicate NF experiments were conducted with NF90 membranes for 10 h, and initially these showed a steady filtration flux of 52 ± 2 L/m2h. The average DOC of feedwater was 5.19 mg/L and the operational pressure was 4 bars.
Rejection of Organic Fractions
In terms of the larger organic molecules, hydrodynamic filtration is the primary mechanism that rejects organics from passing through the membrane’s narrow pores due to steric hindrance [23]. The low MWCO value (<200 Da) of the NF90 membrane indicates its tight pore structure, effectively excluding larger organic molecules. Kim et al. [24] reported that there was a strong correlation between the MW of organics and their rejection by NF membranes, where 90% of the rejection was observed with organics bearing MW of more than 200. The results obtained from this study (Table 6) support the above phenomenon: 96.6% of DOC rejection was noticed with the NF90 bearing MWCO of 90–180 Da. Another study by Sadrzadeh et al. [25] reported that NF90 removed more than 98% of the organics (from 500 mg/L to 10 mg/L).

Table 6.
Performance of NF90 in rejecting organic fractions (LCOCD).
The hydrophobics were removed (95%, from 1.17 to 0.06 mg/L), which could be explained by the hydrophobic interactions. The low hydrophobicity of the NF90 membrane allows hydrophobic organic molecules to approach the membrane surface more closely, enhancing adsorption via hydrophobic interactions and hydrogen bonding [26]. The rejection of hydrophobic DOC, despite its lower feed concentration, can be attributed to hydrophobic interactions between the DOC and the moderately hydrophobic NF membrane surface [4,26].
The feedwater also contained a significant portion (~80%) of hydrophilic organic matter where humic substances constituted a major fraction. Biopolymers are polysaccharides with significantly higher MW (>20,000 Da), and are likely also adsorbed primarily on the membrane surface rather than within pores. The highest rejection of biopolymers (100%) observed in this study can be explained mainly due to this removal mechanism–size exclusion [24]. Electrostatic repulsion and membrane adsorption are also other mechanisms that further contribute to DOC rejection.
As stated by Hwang et al. [27], humics, the major fraction of organics in the feedwater, exist as aromatic ring structures and various types of hydrophilic functional groups (OOH and OCOOH) that are transformed into ionic states at different pH. Here, the rejection is governed by electrostatic repulsion (Donnan exclusion), where the molecules with higher ionic strength show better rejection [27]. This may subsequently lead more organics to adsorb onto the membrane and further increases the negative charge of membrane. As reported by Bellona et al. [28], it is not yet completely understood whether organics are absorbed onto the surface or inside the pores of the nanopores during the rejection process.
Smaller DOC fractions, including building blocks and low-molecular-weight organics (LMWOs) with sizes below 500 Da, exhibited high rejection rates by the NF membrane. The primary mechanism for this removal is likely adsorption within the membrane pores and the valleys of its rough surface [26,29]. Several forces likely contribute to this adsorption: (1) hydrogen bonding between the membrane surface (or water molecules attached to it) and the hydrophilic LMWO/building block molecules; (2) π–π bonding and van der Waals forces may also play a role. In this context, excellent removal of fractions such as humics (97.7%), building blocks (98.5%), and LMWOs (91.7) are observed in this study with NF90. This is also supported by Kim et al. [24], where in terms of organic removal, the compounds with MW > 200 were rejected by NF up to 90%.
3.4. Performance of NTR-729HF in Treating MF-BTSE to Produce Water for Irrigation
As reported by Jamil et al. [4], the NF 90 membrane gave rise to a DOC removal of 88.2% and conductivity rejection of 75.4%, whereas NTR-729HF membranes gave rise to 95–99% of DOC removal. Thus, a detailed study was performed with NTR-729HF membranes for MF-BTSE water to remove organics as well as inorganic ions to produce irrigation water suitable for sensitive to moderately sensitive crops.
The NTR-729HF was operated at permeate flux of 48.5 L/m2.h and pressure of 400 kPa, and its performance in the removal of DOC and inorganic ions are shown in Table 7. The NTR-729HF membrane was found to reject 95–99% of the DOC, and its performance was primarily governed by the sieving effect. However, other factors such as molecular charge of the solute, zeta potential of the membrane surface, and hydrophilicity/hydrophobicity also influence the removal of organics from feedwater [30].

Table 7.
Rejection of dissolved organic and inorganic ions by NF (NTR-729 HF) (modified from Shanmuganathan et al. [11]).
In terms of the removal of inorganic ions, the NTR-729 HF achieved the highest rejection of sulfate ions (99%) followed by nitrate ions (88%). This is consistent with the findings of Suhalim et al. [31], who elucidated that the elevated rejection of sulfate ions can be attributed to their higher negative charge (SO42−) in comparison to nitrate ions (NO3−), which possess a lower negative charge. Furthermore, this phenomenon is explained by the Donnan exclusion mechanism, where cations are attracted towards the NF whilst the anions (SO42− and NO3−) are repelled by the NF.
The attraction of the cations (divalents) towards the NF reduced the rejection by the NF. As shown in Table 7, the rejection of 62% of divalents (Ca and Mg ions) and 11–19% of monovalent (Na and Cl ions) was observed, where the latter is insignificant. The notable rejection of divalents could be explained by their larger hydrated size and charge effects compared to monovalents [32,33]. In connection to this, Kim et al. [24] stated that the presence of divalent cations also lowers the removal of other cations in a mixed solution compared to cations in a single solution [24]. Therefore, in addition to the sieving effect, the electrostatic interactions (Donnan exclusion) highly influence the rejection of ionic compounds by the NF. Khettaf et al. [34] and Virga et al. [35] also reported a significant removal of divalents by negatively charged NF. The insignificant removal of monovalents (Na+ and Cl− ions) could be due to their weaker electrostatic interactions compared to divalents [31]. As the concentrations of Na and Cl ions in the feedwater do not significantly exceed the tolerance levels, concerns regarding rejection rates of such ions are not necessary.
The rejection trend of inorganics (Ca, Mg, and Na ions) by NF90 and NTR-729HF vary significantly and are unable to explain due to different experimental conditions, different characteristics of the NF membrane, and more importantly different pH levels. As shown by Kim et al. [24], feedwater pH highly influences the rejection of inorganics by NF membranes. They found that the removal of divalent cations (Ca and Mg ions) by NTR-729HF at pH 3 was extremely high (100%), but the same at pH 7 was in the range of 60–80%. This agrees with the results obtained in this study, where the removal of divalents was 62% in feedwater of pH 6.8–7.6. Furthermore, the higher rejection of the sulfate ions on the membrane surface due to steric effects may also reduce the rejection of monovalents, as stated by Nocolini et al. [15].
The performance of the NTR-729HF in terms of the removal of inorganic ions plays a key role in treating microfiltered MF-BTSE to produce water suitable for irrigation with appropriate ionic levels and SAR in a cost-effective manner.
Apart from the excellent role of NF membranes in water recovery and reuse, managing NF concentrate is crucial for minimizing environmental impact and ensuring the sustainable operation of treatment plants. Managing NF concentrates by recovering valuable metals has been explored in one of our previous studies [6]. Here, a chromium-based metal–organic framework modified with N-(phosphonomethyl)-iminodiacetic acid (PMIDA) and amine grafted mesoporous silica (SBA15-NH2) adsorbents was successfully used to sequentially recover REEs and Cu from concentrated AMD. Cr-MIL-PMIDA was found to selectively adsorb over 90% of REE available in the AMD concentrate at optimum dosage of 3.2 g/L [6]. Then, an optimum dosage of 3.2 g/L of SBA15-NH2 was used to recover over 90% of Cu present in the concentrate [6]. Recovering valuable metals like europium and copper not only mitigates environmental impact but also adds significant value to the treatment process. Hence, combining low-pressure NF with adsorption for treating AMD offers a robust approach to sustainable water management and resource recovery. However, further treatment is necessary to handle any remaining contaminants and ensure environmentally safe disposal or reuse of the treated effluent.
Our previous studies focused on treating RO (or NF) concentrate using a microfiltration (MF) membrane hybrid integrated with adsorption and ion-exchange techniques using granular activated carbon (GAC) and a commercially available ion-exchange resin named Purolite A502PS. Shanmuganathan et al. [36] reported that a membrane–GAC hybrid system with an initial GAC dosage of 10 g/L significantly reduced dissolved organic carbon (DOC) by 80%. The system operated for 10 days with a daily GAC replacement of 10%. During the operation of the MF–GAC hybrid system, there was a significant removal of DOC (50–80%) and most trace organics (90–99%). Another study by Devaisy et al. [37] investigated the performance of the MF–Purolite A502PS hybrid system in terms of removing DOC and trace organics. Results showed that DOC was reduced by 45–60%, with significant removal of hydrophilic substances. This system also effectively reduced trace organics from RO concentrate. Since the quality of RO concentrate and NF concentrate is similar, the above systems can be used to treat NF concentrate produced from this system. Purolite A502PS can remove ions from feedwater more effectively than GAC. Depending on the level of ions present and the sodium adsorption ratio (SAR), the resulting water can be used for beneficial reuse purposes, such as irrigating salt-sensitive plants.
4. Conclusions
Low-pressure nanofiltration (NF) emerged as a promising technology for treating different types of feedwater, such as synthetic acid mine drainage (AMD) and surface water containing NOM and MF-BTSE, to produce water for irrigation. The NF90 was excellent, with solute rejection of 95% and water recovery of 80% from AMD and DOC rejection of 95% from surface water. The NTR-729HF rejected ions from the BTSE to produce irrigation water suitable for salt-sensitive crops. The NF provided high permeate flux at low pressure (3–4 bar). The NF process achieved a dual benefit: recovering a significant portion of clean water and removing solute/contaminants effectively. The recovery of valuable resources such as REEs and Cu is also possible when treating AMD with NF membranes.
The NF concentrate can be treated using several techniques, including volume reduction for resource recovery, employing membrane-hybrid systems, and beneficial water reuse purposes, such as irrigating salt-tolerant crops. See Figure 3.
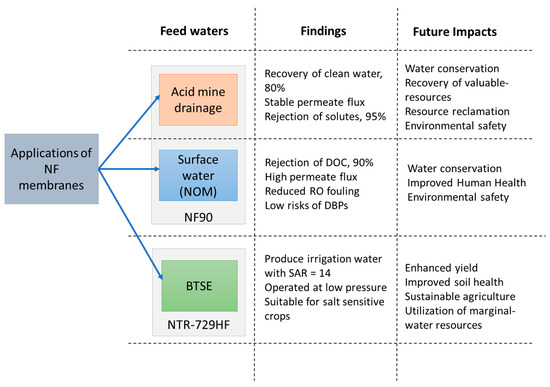
Figure 3.
Schematic diagram of trajectory of NF membranes for water reuse applications and their future implications.
Author Contributions
Conceptualization, S.V. and S.D.; methodology, S.V.; formal analysis, C.F.; investigation, C.F. and S.R.; resources, S.V. and H.R.; data curation, C.F.; writing—original draft preparation, S.D. and C.F.; writing—review and editing, S.V., L.M. and J.K.; supervision, S.V. and J.K.; project administration, S.V.; funding acquisition, S.V. and H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by University of Technology Sydney grants obtained by S. Vigneswaran. Support from EU ERA-NET Water JPI-2018 grant 776692 (Closing the Water Cycle Gap—Sustainable Management of Water Resources (Water Harmony)) and Norwegian Research Council grant 322529 (MEMPREX-II) to Harsha Ratnaweera is also acknowledged.
Data Availability Statement
The data used in this paper are contained within it. They have been supplemented with data from previous studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, S.; Ba, C.; Yao, Y.; Zheng, W.; Economy, J.; Wang, P. Removal of Antibiotics Using Polyethylenimine Cross-Linked Nanofiltration Membranes: Relating Membrane Performance to Surface Charge Characteristics. Chem. Eng. J. 2018, 335, 101–109. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Hayes, T.; Lokare, O.R.; Mittal, D.; Vidic, R.D. Laboratory and Pilot-Scale Nanofiltration Treatment of Abandoned Mine Drainage for the Recovery of Products Suitable for Industrial Reuse. Ind. Eng. Chem. Res. 2017, 56, 7355–7364. [Google Scholar] [CrossRef]
- Pino, L.; Beltran, E.; Schwarz, A.; Ruiz, M.C.; Borquez, R. Optimization of Nanofiltration for Treatment of Acid Mine Drainage and Copper Recovery by Solvent Extraction. Hydrometallurgy 2020, 195, 105361. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Comparing Nanofiltration Membranes Effectiveness for Inorganic and Organic Compounds Removal from a Wastewater-Reclamation Plant’s Micro-Filtered Water. Mater. Today Proc. 2021, 47, 1389–1393. [Google Scholar] [CrossRef]
- Vass, C.R.; Noble, A.; Ziemkiewicz, P.F. The Occurrence and Concentration of Rare Earth Elements in Acid Mine Drainage and Treatment By-Products: Part 1—Initial Survey of the Northern Appalachian Coal Basin. Min. Metall. Explor. 2019, 36, 903–916. [Google Scholar] [CrossRef]
- Fonseka, C.; Ryu, S.; Naidu, G.; Kandasamy, J.; Vigneswaran, S. Recovery of Water and Valuable Metals Using Low Pressure Nanofiltration and Sequential Adsorption from Acid Mine Drainage. Environ. Technol. Innov. 2022, 28, 102753. [Google Scholar] [CrossRef]
- Lopez, J.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Evaluation of NF Membranes as Treatment Technology of Acid Mine Drainage: Metals and Sulfate Removal. Desalination 2018, 440, 122–134. [Google Scholar] [CrossRef]
- Saha, S.; Sinha, A. Review on Treatment of Acid Mine Drainage with Waste Materials: A Novel Approach. Glob. NEST J. 2018, 20, 512–528. [Google Scholar] [CrossRef]
- Mohiuddin, A.; Cox, P.; Blayney, B. The Impact of the Millennium Drought on Water Filtration Plants. Water E-J. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xiao, K.; Wang, X.; Xie, Y.F. Key Foulants and Their Interactive Effect in Organic Fouling of Nanofiltration Membranes. J. Membr. Sci. 2020, 610, 118252. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Vigneswaran, S.; Nguyen, T.V.; Loganathan, P.; Kandasamy, J. Use of Nanofiltration and Reverse Osmosis in Reclaiming Micro-Filtered Biologically Treated Sewage Effluent for Irrigation. Desalination 2015, 364, 119–125. [Google Scholar] [CrossRef]
- Australian and New Zealand Environment and Conservation Council. Australian and New Zealand Guidelines for Fresh and Marine Water Quality 2000; Australian and New Zealand Environment and Conservation Council: Canberra, ACT, Australia, 2000. [Google Scholar]
- Ramdani, A.; Deratani, A.; Taleb, S.; Drouiche, N.; Lounici, H. Performance of NF90 and NF270 Commercial Nanofiltration Membranes in the Defluoridation of Algerian Brackish Water. Desalination Water Treat. 2021, 212, 286–296. [Google Scholar] [CrossRef]
- Ozaki, H.; Ikejima, N.; Matsui, S.; Terashima, Y.; Takeda, S.; Tari, I.; Li, H. The Role of Membrane ξ-Potential in Solute Rejection by Low-Pressure Reverse Osmosis Membrane. Water Supply 2002, 2, 321–328. [Google Scholar] [CrossRef]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective Rejection of Ions and Correlation with Surface Properties of Nanofiltration Membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of Aquatic Humic and Non-Humic Matter with Size-Exclusion Chromatography—Organic Carbon Detection—Organic Nitrogen Detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Krzeminski, P.; Vogelsang, C.; Meyn, T.; Köhler, S.J.; Poutanen, H.; De Wit, H.A.; Uhl, W. Natural Organic Matter Fractions and Their Removal in Full-Scale Drinking Water Treatment under Cold Climate Conditions in Nordic Capitals. J. Environ. Manag. 2019, 241, 427–438. [Google Scholar] [CrossRef]
- Fonseka, C.; Ryu, S.; Choo, Y.; Naidu, G.; Kandasamy, J.; Thiruvenkatachari, R.; Foseid, L.; Ratnaweera, H.; Vigneswaran, S. Selective Recovery of Europium from Real Acid Mine Drainage by Using Novel Amine Based Modified SBA15 Adsorbent and Membrane Distillation System. J. Water Process Eng. 2023, 56, 104551. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Wang, R. The Coming of Age of Water Channels for Separation Membranes: From Biological to Biomimetic to Synthetic. Chem. Soc. Rev. 2022, 51, 4537–4582. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Quist-Jensen, C.A.; Giorno, L. Molecular weight cutoff. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar] [CrossRef]
- Mehiguene, K.; Garba, Y.; Taha, S.; Gondrexon, N.; Dorange, G. Influence of Operating Conditions on the Retention of Copper and Cadmium in Aqueous Solutions by Nanofiltration: Experimental Results and Modelling. Sep. Purif. Technol. 1999, 15, 181–187. [Google Scholar] [CrossRef]
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient Removal of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Treatment: Nanofiltration Combined with Active Carbon or Anion Exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843. [Google Scholar] [CrossRef]
- Soyekwo, F.; Zhang, Q.; Gao, R.; Qu, Y.; Lin, C.; Huang, X.; Zhu, A.; Liu, Q. Cellulose Nanofiber Intermediary to Fabricate Highly-Permeable Ultrathin Nanofiltration Membranes for Fast Water Purification. J. Membr. Sci. 2017, 524, 174–185. [Google Scholar] [CrossRef]
- Kim, S.; Ozaki, H.; Kim, J. Effect of pH on the Rejection of Inorganic Salts and Organic Compound Using Nanofiltration Membrane. Korean J. Chem. Eng. 2006, 23, 28–33. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Hajinasiri, J.; Bhattacharjee, S.; Pernitsky, D. Nanofiltration of Oil Sands Boiler Feed Water: Effect of pH on Water Flux and Organic and Dissolved Solid Rejection. Sep. Purif. Technol. 2015, 141, 339–353. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Pinnau, I. Fouling of Reverse Osmosis Membranes by Biopolymers in Wastewater Secondary Effluent: Role of Membrane Surface Properties and Initial Permeate Flux. J. Membr. Sci. 2007, 290, 173–181. [Google Scholar] [CrossRef]
- Hwang, J.; Jegal, J.; Lee, K. Separation of Humic Acid with Nanofiltration Polyamide Composite Membranes. J. Appl. Polym. Sci. 2002, 86, 2847–2853. [Google Scholar] [CrossRef]
- Bellona, C.; Marts, M.; Drewes, J.E. The Effect of Organic Membrane Fouling on the Properties and Rejection Characteristics of Nanofiltration Membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Dang, H.Q.; Nghiem, L.D.; Price, W.E. Factors Governing the Rejection of Trace Organic Contaminants by Nanofiltration and Reverse Osmosis Membranes. Desalination Water Treat. 2014, 52, 589–599. [Google Scholar] [CrossRef]
- Yu, W.; Liu, T.; Crawshaw, J.; Liu, T.; Graham, N. Ultrafiltration and Nanofiltration Membrane Fouling by Natural Organic Matter: Mechanisms and Mitigation by Pre-Ozonation and pH. Water Res. 2018, 139, 353–362. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Khettaf, S.; Boumaraf, R.; Benmahdi, F.; Bouhidel, K.-E.; Bouhelassa, M. Removal of the Neutral Dissolved Organic Matter (NDOM) from Surface Water by Coagulation/Flocculation and Nanofiltration. Anal. Lett. 2021, 54, 2713–2726. [Google Scholar] [CrossRef]
- Virga, E.; De Grooth, J.; Žvab, K.; De Vos, W.M. Stable Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes for Produced Water Treatment. ACS Appl. Polym. Mater. 2019, 1, 2230–2239. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Loganathan, P.; Kazner, C.; Johir, M.A.H.; Vigneswaran, S. Submerged Membrane Filtration Adsorption Hybrid System for the Removal of Organic Micropollutants from a Water Reclamation Plant Reverse Osmosis Concentrate. Desalination 2017, 401, 134–141. [Google Scholar] [CrossRef]
- Devaisy, S.; Kandasamy, J.; Aryal, R.; Johir, M.A.H.; Ratnaweera, H.; Vigneswaran, S. Removal of Organics with Ion-Exchange Resins (IEX) from Reverse Osmosis Concentrate. Membranes 2023, 13, 136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).