Abstract
One method of processing municipal waste biogas plant digestate is to separate it into solid and liquid fractions. Since the digestate can be a potential source of water, it must undergo the appropriate treatment. Pressurised membrane processes preceded by struvite precipitation can be particularly useful in this regard. Experiments were conducted to determine the effectiveness of treating the digestate liquid fraction from a municipal waste biogas plant using an integrated process that combines struvite precipitation with membrane filtration, employing flat ceramic membranes with different cut-off values. The results confirm that this integrated process is effective for digestate treatment. A significantly increased improvement in the final quality of the test solution and a reduction in membrane fouling intensity were observed compared to those of these processes conducted separately. It is noteworthy that the purest solution was obtained when struvite precipitation and filtration through a flat ceramic membrane with a cut-off of 1 kDa were combined. This approach enabled the precipitation of struvite, a valuable fertiliser; the protection of the membranes from fouling; and a high degree of organic compound removal. The recovered water from the digestate (after dilution or removal of excess salts) can be used in agriculture or horticulture.
1. Introduction
There is currently a growing interest in the biogas market worldwide. Biogas plants are a common solution in Western Europe, especially in Germany, Italy, Denmark, Switzerland, and France [1]. Polish companies are following in these countries’ footsteps. Biogas installations have rapidly gained acceptance not only because of their ability to produce safe, environmentally friendly, and economically efficient energy, but also because of their ability to dispose of many environmentally hazardous biodegradable wastes. Biogas is produced by the decomposition of organic biomass using microorganisms. This process takes place in digesters, under anaerobic conditions, and involves four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [2]. In order for the fermentation process to take place properly, the right conditions must be ensured, i.e., temperature, pH, humidity, salinity, and nutrient content. This increases the speed of the process and influences the composition and quality of the biogas produced [3]. Chemically, the biogas produced consists mainly of 50–75% methane and 25–45% carbon dioxide, as well as smaller amounts of hydrogen sulphide, nitrogen, oxygen, and hydrogen [4]. Its composition depends a lot on the type of biomass from which it is produced. The sizes of planned biogas facilities vary depending on the waste collection system that prevails in a given country. In countries where separate waste collection is widespread, e.g., France, the UK, and Spain, the installation capacity is over 100 kt/y. Germany, Italy, and Belgium have installations of 30–50 kt/y. On the contrary, Switzerland, Austria, Sweden, and Norway build smaller biogas plants with a capacity of approximately 8–15 kt/y [1,5].
A key issue associated with the operation of any type of biogas plant is the generation of a large amount of digestate. Generally speaking, the amount of digestate is equal to the weight of the substrates used in the fermentation process [6]. This amount depends on the size of the plant and can be several tens of thousands of tons per year. The varying physical and chemical properties of digestate are characteristic. They depend on the types of raw materials used in biogas production, their sources, and the fermentation technologies used [7]. Digestate consists of undecomposed organic compounds, minerals, and methanogenic bacterial biomass. The raw digestate is in a suspended form with a dry matter content of around 5%. A common characteristic is also an alkaline pH, mostly in the range of 7.5–9 [8,9].
Although the direct use of digestate as fertiliser is the most common solution, other methods of utilisation are constantly being sought. An important measure in the utilisation of digestate is its separation [10]. This process results in two different fractions, solid and liquid. The chemical compositions of the two fractions are quite different (Figure 1).
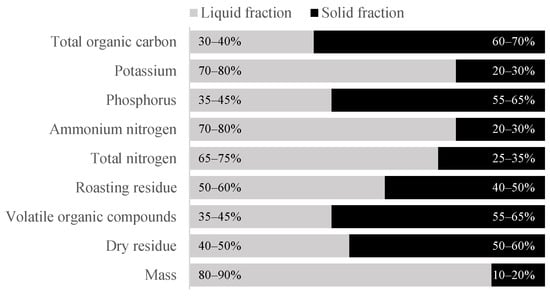
Figure 1.
Distribution of selected components after separating digestate into solid and liquid fractions [11,12].
The separation of digestate from biogas plants is carried out most often using screw presses or sedimentation centrifuges [13]. The separated solid fraction can be stored; used as fertiliser; dried and used as a substrate for the production of pellets and briquettes for heating purposes; used as bedding for farm animals; or used as a protein supplement to animal feedstuffs [7,14]. The liquid fraction, however, can be used for field irrigation, fertiliser preparation, or digester load irrigation [15]. Given the numerous potential applications of the resulting digestate, the concept of its management offers a valuable opportunity for numerous companies that could be involved at this stage in the development of municipal biogas plants.
As a consequence of the progressive deficit of water in agriculture, there is a growing recognition of the potential of the liquid fraction of digestate as a source of water [16]. To achieve this, the liquid fraction must undergo advanced physical and chemical treatment to meet the quality requirements for future use [17]. One possible method for treating the digestate liquid fraction involves membrane processes [18]. Membrane processes are increasingly being used in environmental protection, including water and wastewater treatment, as well as in the recovery of water from contaminated streams for reuse. This is due to the increasing availability of membranes with a wide range of separation properties [19]. This is reflected in the wide range of possibilities for the use of membrane processes, mainly those involving pressurised membranes, both for the pretreatment of water and for the production of water of very high quality. The use of these processes has many advantages. The main advantage is the simplicity of its technological system compared to those of conventional treatment schemes, coupled with the small size of the plant [20]. The use of membranes allows the recovery of not only water, but also other valuable components [21]. Membrane separation is one of the physical separation methods and occurs without phase transformation, enabling energy savings [20]. It also does not require the use of chemicals, which is also an important benefit, both environmentally and economically [22]. Membrane filtration processes experience a decrease in permeate volume flow due to concentration polarisation and fouling phenomena, which is undoubtedly their main disadvantage [23].
Pressurised membrane processes, including microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO), are the most widely used in water purification processes. The driving force behind pressurised membrane processes is the pressure difference between both sides of the membrane. One of the main factors determining the efficiency of separation during these processes is the type of membrane used [24]. Membranes can be manufactured from organic or inorganic materials. Organic membranes include polymeric membranes, which are manufactured from materials such as polysulphone, polyamide, or cellulose acetate. These membranes have found wide application in many fields, but their main drawback is their limited stability in aggressive environments, including aqueous solutions with low or high pH, and solutions containing organic solvents [25].
An alternative to polymeric membranes is ceramic membranes made of metal oxides, specifically aluminium (Al), titanium (Ti), and zirconium (Zr) [26]. Inorganic membranes, in contrast to organic membranes, are distinguished by their high thermal, chemical, and mechanical resistance and long lifespan [27]. Asymmetric ceramic membranes comprise multiple layers. The support layer is a few millimetres thick and has pore sizes of 1–10 µm. The intermediate layer, which is 10–100 µm thick, is thinner and has pores of a larger size, with diameters ranging from 50–100 µm. The separation layer, which is the thinnest, has a thickness of approximately 1 µm and the smallest pores, with diameters in the range of 2 to 50 nm [28].
In practice, the use of membranes is used to address fundamental technical, economic, and environmental issues [29]. These include (1) the high efficiency of the membrane process in conjunction with the minimisation of fouling, and (2) the adequate quality of the treated streams to allow reuse in the process or discharge to the environment. Achieving high efficiency requires the pretreatment of contaminated streams prior to their direction to the membrane plant [30]. Similarly, the achievement of permeate quality frequently necessitates the implementation of additional non-membrane processes [31]. Consequently, the integration of membrane technology with alternative processes represents a pivotal step in addressing the aforementioned challenges.
The precipitation process of struvite, which is hydrated magnesium ammonium phosphate (MgNH4PO4 · 6 H2O), can be employed to treat the liquid fraction of municipal digestate [32]. It is recommended to precipitate this compound under controlled conditions, as uncontrolled precipitation in both process equipment and pipelines can cause operational problems in biogas plants [33]. Struvite has a crystalline structure. It is white, transparent, or semi-transparent, and exhibits a glassy sheen. Its flakiness ranges from good to poor, its hardness is 2 on the Mohs scale, and its density is 1700 kg/m3 [34]. This mineral can be a valuable fertiliser, with numerous potential applications in agriculture and horticulture [35].
A review of the literature reveals that the topic of treating the agricultural liquid fraction of biogas plant digestate is a subject of interest to numerous authors. However, there is a paucity of literature on the treatment of the digestate liquid fraction from municipal waste biogas plants. This area of research is still relatively under-researched. Given the fundamental differences between the two types of digestate, research on municipal digestate is justified. The introduction of membrane processes into the treatment of municipal digestate and its pretreatment, which constitutes the so-called membrane integrated system, represents a particularly original approach.
In light of these considerations, an investigation was conducted to ascertain the viability of treating the liquid fraction of municipal digestate using an integrated membrane process. This process involves a combination of struvite precipitation as a pretreatment step and a membrane separation process utilising flat ceramic membranes.
2. Materials and Methods
2.1. Materials
The liquid fraction of digestate was used in the study. It was separated from the digestate pulp by using sedimentation centrifuges. This fraction came from a biogas plant that processes the organic fraction of municipal waste, located in the Lower Silesia province (Poland). This biogas plant uses a selected biodegradable fraction of municipal waste, combining a stream from households (so-called kitchen waste) and urban green waste (so-called green waste). The properties of the test liquid are presented in Table 1. The ranges of values reflect the variability of the sampling sessions. The physico-chemical analysis of the test solution was carried out in accordance with Standard Methods for the Examination of Water and Wastewater, 23rd edition.

Table 1.
Characteristics of the liquid fraction of municipal digestate.
In experiments on the struvite precipitation process, 2 chemical reactants (Chempur, Piekary Śląskie, Poland) were used: MgCl2 as the Mg supplement and NaH2PO4 as the P supplement. Their characteristics are given in Table 2. The molar ratio of N:Mg:P in the test solution was 40.1:14.2:1. The dosage of the Mg and P compounds was a prerequisite for ensuring the optimum contribution of these components to the struvite precipitation process. Doses of Mg and P salts were chosen to achieve the most favourable conditions for controlling struvite precipitation.

Table 2.
Characteristics of the chemical reactants used in the struvite precipitation process [36,37].
Six flat ceramic MF and UF membranes from Tami Industries were used in the study. These membranes are characterised by a typical asymmetric structure, consisting of a thin epidermal layer and a thicker support layer. Each can operate at a maximum pressure of 0.4 MPa and a maximum temperature of 350 °C. Their filtration area was 56 cm2. An example SEM image (1000× field) of the selected membrane is shown in Figure 2, while detailed characteristics of all membranes used are provided in Table 3.

Figure 2.
The 5 kDa flat ceramic membrane used in the study.

Table 3.
Characteristics of the flat ceramic membranes used in the experiments [38].
Prior to testing, flat ceramic membranes were subjected to a preparation procedure for proper operation. This included alkaline cleaning by placing the membranes in NaOH solution (15–20 g/dm3) at 80 °C for 30 min, followed by rinsing until neutral pH was reached, acid cleaning, and rinsing again until neutral pH was reached. MF and UF membranes were treated with 58% HNO3 or 75% H3PO4 (5 cm3/dm3) at 50 °C for 15 min. For NF membranes, only a solution of 75% H3PO4 was used at 1 cm3/dm3 and 50 °C for 15 min, as recommended by the membrane manufacturer.
After filtration of the solutions, the membranes were chemically cleaned with a 0.1 mol/dm3 NaOH solution (Avantor Performance Materials Poland S.A., Gliwice, Poland) and washed with redistilled water until the initial permeate flux values were reached.
2.2. Methods
The pretreatment of the liquid fraction of digestate associated with struvite precipitation was carried out, adopting optimal parameters determined from a literature review [39,40,41,42]. As such, they facilitated the precipitation of struvite. A 500 cm3 digestate sample was placed on a Velp Scientifica FC6S (VELP Scientifica srl, Usmate, Italy) mechanical stirrer. MgCl2 and NaH2PO4 were then dosed. The doses of MgCl2 (12.17 g/dm3) and NaH2PO4 (9.45 g/dm3) were set so that, after taking into account the concentrations of Mg2+, N-NH4+, and PO43− in the test solution, the molar ratio of N:Mg:P was 1:1.1:1.1. According to the literature [43,44], it is recommended to apply MgCl2 because this compound has high solubility, resulting in a shorter reaction time required to dissolve Mg2+ in solution compared to the time required when using other Mg2+ supplements, e.g., MgO or MgSO4. According to [45,46], it is also necessary to ensure the application of P sources due to the large excess of N-NH4+ in relation to Mg and P. The utilised doses of Mg and P salts were chosen to achieve the most favourable conditions to control the precipitation of struvite.
The experiments were carried out at pH 9.0. Adjustment of the pH value was carried out after dosing the reactants, using 0.1 mol/dm3 NaOH. The temperatures of the solutions were in the range of 20–23 °C. After dosing both reactants, the samples were stirred for 5 min at 160 rpm, followed by sedimentation for 30 min.
Experiments on the membrane filtration process using flat ceramic membranes were carried out using a Sterlitech laboratory plant with a 316 SS pressure chamber of 3.8 dm3 capacity. The process was carried out in a dead-end system at a transmembrane pressure of 0.3 MPa. All samples of the liquid fraction of the digestate subjected to membrane filtration were pretreated by sedimentation for 72 h. A diagram of the installation used in the study is shown in Figure 3.
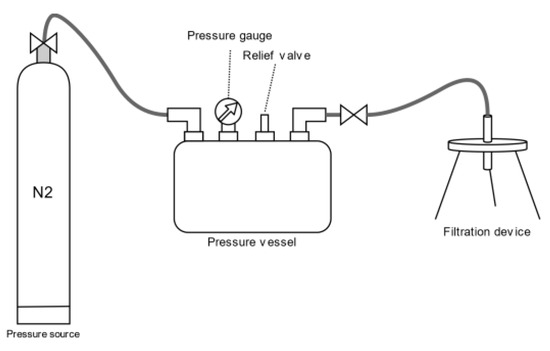
Figure 3.
Sterlitech membrane filtration laboratory unit diagram.
Separation efficiencies (R) were calculated using the following formula:
where:
- cp—concentration of impurities in the treated solution, g/m3,
- cn—initial concentration of impurities in the solution to be purified, g/m3.
R > 90% values were determined with an error of less than 1%.
The transport properties of the membranes were assessed by calculating the permeate flux J:
where:
- V—volume of permeate, m3,
- A—membrane surface area, m2,
- t—filtration time, d.
The intensity of membrane blocking was also assessed by calculating the values of relative membrane permeability J/J0, expressed as the ratio of permeate flux J to the redistilled water flux of the new membrane J0.
The unit processes of struvite precipitation and membrane filtration, which make up the integrated process, were carried out by subjecting raw liquid municipal digestate to the precipitation of struvite, and then the clarified liquid was directed to flat ceramic membranes. The pretreated solution from which the struvite precipitated was the feed for the next purification process, membrane filtration. The selection of parameters for the integrated process was made on the basis of tests with individual processes carried out independently.
The effectiveness of the processes was determined by measuring the concentration of organic compounds expressed as COD, BOD5, and DOC, contained in the effluents before and after treatment. COD and BOD5 were measured using standard bichromate and dilution methods, respectively. On the other hand, DOC concentration was measured with a Hach IL550 (Hach Company, Loveland, CO, USA) carbon analyser. All experiments were duplicated.
The particle size distribution was measured using a Mastersizer 2000 laser diffractometer (Malvern, UK), equipped with a HydroMu dispersion device (Malvern, UK) with a particle size measurement range of 0.1 to 2000 µm. During the measurement procedure, depending on the particle concentration, approximately 3 cm3 of the suspension was poured into a 700 cm3 beaker filled with water that circulated in the measuring cell. The particle size distribution was measured without ultrasound (the suspension circulated through the measuring cell, but no ultrasound was generated), and then during ultrasonication of the suspension (sonication took place in the beaker from which the suspension was pumped and circulated through the measuring cell), until the particle size distribution stabilised (disintegration of any agglomerates).
The determination of particle size distributions was also performed using a Nicomp 380 DLS instrument (Nicomp Particle Sizing Systems, Santa Barbara, CA, USA). This analyser uses the DLS method to obtain particle size distributions for samples with particle sizes from 1 nm to 5 µm. The measurement was carried out by placing approximately 3.5 cm3 of diluted suspension into the measuring chamber. Using the Nicomp analysis algorithm, complex multimodal distributions were analysed with the highest resolution and repeatability.
In the experiments conducted, ζ-potential measurements were also carried out using a ζ-potential analyser (Malvern Zetasizer 2000, Malvern Panalytical, Malvern, UK). The diluted suspension was conditioned in a beaker for 10 min at 25 °C, at a specific pH adjusted with NaOH or HCl. Then, using a syringe, the suspension was placed into an electrophoretic chamber. The value of the ζ-potential was determined as the average of five consecutive measurements.
3. Results
A study dedicated to assessing the suitability of the struvite digestion process was carried out, with the objective of finding a way to improve the final quality of the liquid fraction of digestate and reduce the fouling of the membranes used in the subsequent purification step. It was observed that after dosing the liquid fraction of the digestate with MgCl2 (as an external source of Mg) and NaH2PO4 (as an external source of P) and correcting the pH, the liquid became turbid. This was clear evidence of the start of struvite precipitation. It was also easy to notice the large amount of light brown precipitate formed in the solution. The remaining supernatant in the sample was also much paler than the raw fraction of the digestate.
The removal efficiency of selected macronutrients from samples of the liquid fraction of municipal digestate, from which the struvite was precipitated by dosing with the Mg and P compounds, is shown in Figure 4. Analysing the results obtained, it was observed that the removal rate of organic compounds was low—COD, BOD5, and DOC concentrations decreased by 17% (reduction from 11,450 to 9860 g O2/m3), 11% (reduction from 3600 to 3210 g O2/m3), and 13% (reduction from 4210 to 3670 g C/m3), respectively, compared to the concentrations in the initial sample. On the contrary, the removal efficiency of N-NH4+ was much higher at around 48% (reduction from 776 to 404 g/m3). This may have been due to the release of gaseous NH3, which at pH 9 can account for approximately 30% of the ammonium nitrogen in the solution.
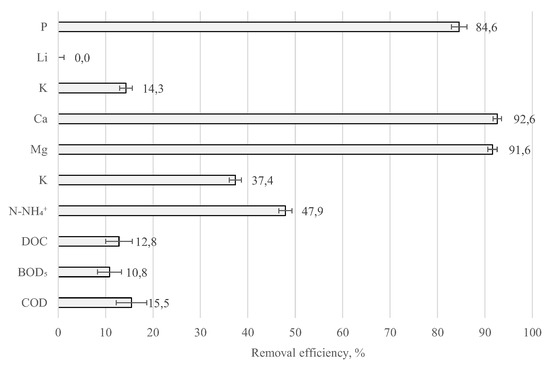
Figure 4.
The removal rates of selected factors in the analysed sample during struvite precipitation.
Despite external dosing of MgCl2 and NaH2PO4, the concentrations of Mg and Ca ions in the treated solution were 19.8 g/m3 and 31 g/m3, respectively, and were much lower than the initial concentrations. In a previous publication [47], it was shown that the presence of Ca2+ in a solution with a pH greater than 9.5 leads to increased concentrations of, among others, CaPO4 and CaHPO4. As the pH was lower in this study, no precipitation of calcium salts was observed. A key factor in the precipitation of struvite is maintaining an appropriate Ca2+:Mg2+ ratio. According to [48], this value should not exceed 1. In the study we carried out, this value was also below 1, so there should not be any risk of precipitation of hydroxyapatite. Therefore, it can be assumed with high probability that the precipitation of struvite from the test solution did occur.
The K concentration in the treated digestate was also observed to be slightly reduced (approximately 15%—reduction from 1980 to 1697 g K/m3) compared to the concentration in the initial sample. In addition, a marginal removal of Li was observed. On the other hand, the significant reduction in the concentration of P ions (approximately 86%—reduction from 21.4 to 3.3 g P/m3) confirms that the applied process conditions favoured the precipitation of this element in its crystalline form, that is, as MgKPO4 · H2O.
The sediment formed during the struvite precipitation process was further investigated. Macroscopic images (Figure 5) show that the precipitate obtained after the addition of the Mg and P compounds is made up of crystals similar in shape to chestnut leaves, consisting of 4–6 members that each resemble a coffin outline. The 4-membered shapes assume the shape of a large letter X. The struvite structure obtained during the study finds confirmation in the literature [49]. Such branching, or taking the shape of the letter X, is attributed to the uneven occurrence of supersaturation in the surrounding crystals. This can lead to the growth of structures in one or two planes and the formation of elongated or branched structures.
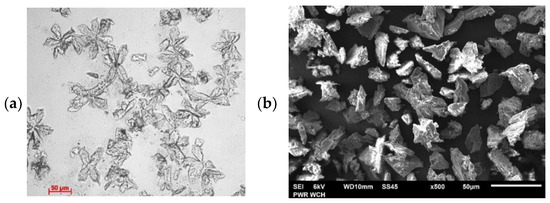
Figure 5.
Microscopic image (a) and SEM image (b) of the precipitate after struvite precipitation.
The evaluation of the efficiency of municipal digestate liquid fraction purification by membrane filtration using flat ceramic membranes was based on assessing the effect of the membrane cut-off on the change in organic compound content in the tested solution. Analysis of the test results obtained (Figure 6) shows that the tested membranes can be used in digestate purification; however, a deterioration in quality could be observed as the cut-off value increased. A sieve mechanism, based on the relationship between the size of the dissolved or colloidal particles present in the solution and the pore size of the membrane, is responsible for the separation of contaminants in pressurised membrane processes [50]. According to our own research [51], the pore diameter of a 1 kDa membrane is approximately 35.5 nm. In contrast, during the study it was about 37.7 nm for the 5 kDa membrane and about 52.5 nm and 67.1 nm for the 15 kDa and 50 kDa membranes, respectively. The highest organic compound content in the permeate was obtained with 0.14 µm and 0.45 µm MF membranes with pore diameters of 0.12 µm and 0.29 µm, respectively. Comparing the obtained purification efficiencies of the permeate, it was observed that the higher the compactness of the membranes, the better the efficiencies were. The best results were obtained from a membrane with a cut-off of 1 kDa. The RCOD, RBOD₅, and RDOC values obtained for it were 43% (drop from 5875 to 3365 g O2/m3), 51% (from 1910 to 930 g O2/m3), and 55% (from 2910 to 1300 g C/m3), respectively. The least effective separation was recorded in tests where a 0.45 µm membrane was used. The retention rates of COD, BOD5, and DOC obtained were 12% (to 5160 g O2/m3), 13% (to 1666 g O2/m3), and 18% (to 2400 g C/m3), respectively, and are indicative of the significant penetration of organic pollutants into the permeate.
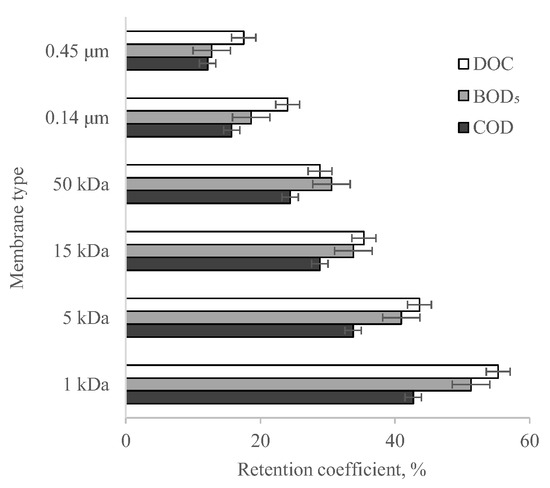
Figure 6.
Reduction efficiencies of COD, BOD5, and DOC from the digestate liquid fraction, depending on the type of ceramic membrane used in the membrane filtration process.
The decrease in membrane hydraulic performance is one of the operational problems encountered when implementing membrane processes. Therefore, when deciding on the suitability of particular membranes for the purification of the investigated solution, attention should be paid not only to their separation properties, but also to their transport properties. The influence of the tested membrane type on permeate flux for the redistilled water and the liquid fraction of the digestate is shown in Figure 7a, while the relative permeability of the ceramic membranes obtained during liquid digestate purification is shown in Figure 7b. When the flux values during digestate liquid fraction filtration were analysed, they were observed to be significantly lower than the flux values obtained during redistilled water filtration. This is probably due to an increase in the flux resistance values as a result of membrane fouling. In addition, the obtained results clearly show that the tested membranes differed not only in absolute hydraulic efficiency, which was mainly due to differences in pore diameters, but also in susceptibility to fouling. It was observed that an increase in the cut-off value of each membrane—and, thus, an increase in the radius of its pores—resulted in a decrease in relative permeability except for the 0.14 µm and 0.45 µm MF membranes. The J/J0 value for these membranes was 0.01. Of all the membranes tested, the MF membranes were the least resistant to fouling. The obtained results correspond to the data from the literature. According to [52], a significantly higher susceptibility to fouling is observed for membranes with larger pore diameters (in this case MF), where fouling due to blocking of the membrane pores by particles from the feed penetrating the membrane is more dominant than it is for more compact membranes such as UF.
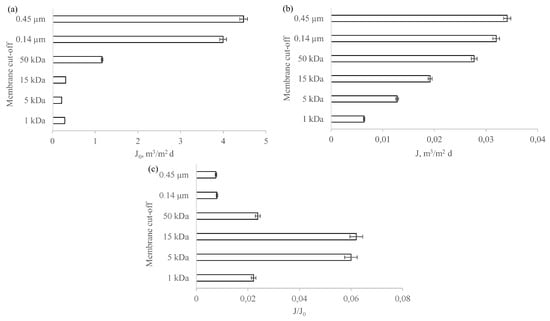
Figure 7.
Permeate flux for the redistilled water (a) and liquid fraction of the digestate (b), and the relative permeability (c) in relation to the cut-off of the tested flat ceramic membranes.
As shown in the earlier stages of the study, neither struvite precipitation nor membrane separation yields a purified solution of sufficient quality, e.g., in terms of organic content. Therefore, the quality of the purified digestate was assessed in the following test series using an integrated process combining struvite precipitation and filtration with ceramic membranes (Figure 8).
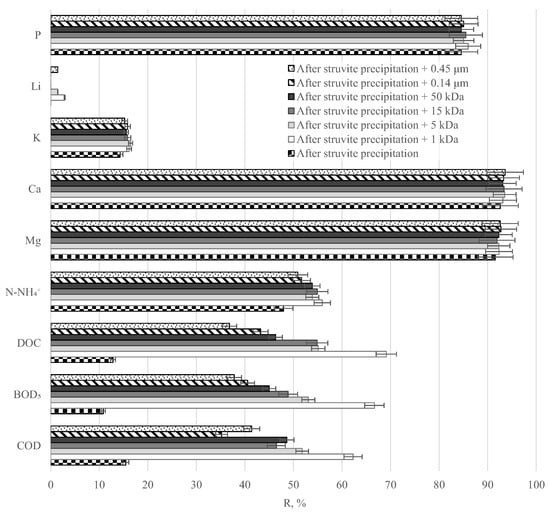
Figure 8.
Influence of the type of ceramic membrane used in an integrated process combining struvite precipitation and membrane separation on changes in the removal degrees of selected indicators.
The application of the analysed variant for the treatment of digestate liquid fraction makes it possible to obtain permeate of significantly better quality in comparison with the results obtained when each process was applied alone. The degrees of reduction for COD, BOD5, and DOC ranged from 35 to 62% (reduction with respect to raw digestate from 11,450 to 4320–7420 g O2/m3), 38 to 67% (from 3600 to 1200–2240 g O2/m3), and 37 to 69% (from 4210 to 1300–2660 g C/m3) (depending on the membrane cut-off). The best purification effect of the liquid digestate in the described process was obtained by directing the solution after struvite precipitation to a ceramic membrane plant with a cut-off of 1 kDa. This is probably due to the compact structure of the most compact membrane tested (average pore size 35.53 nm), which allowed the highly effective removal of organic macromolecules from the treated digestate. In the case of the other membranes, as their limiting resolution increased, the separation efficiency of the organic compounds decreased, as larger contaminant particles entered the purified solution. The removal efficiency of the remaining macronutrients from samples of the liquid fraction of the digestate, which were subjected to membrane filtration after struvite precipitation, did not depend on the cut-off of the membranes used for this purpose. In the experiments carried out, a reduction rate of 53 ± 3% was achieved in the concentration of N-NH4+ (reduction from 776 to 342–381 g/m3). It can be concluded that this may have been partly due to the release of gaseous NH3 during the integrated process, which at pH 9 can account for about 30% of the ammonium nitrogen in the solution. In contrast, the removal efficiency of Mg and Ca ions from the digestate did not change despite the additional use of membrane separation. The degree of reduction in the concentration of Mg ions in the treated solution was approximately 92% (from 235 to 18.1 g/m3). A similar effect was observed for Ca and K ions, which were removed with efficiencies of 93% (from 420 to 27.9 g/m3) and 16% (from 1980 to 1100 g/m3), respectively. A similar trend can be observed when analysing changes in the concentrations of P and Li ions. Their removal efficiencies remained constant at 85% (from 21.4 to 3.1 g/m3) and 2% (from 7.0 to 6.8 g/m3), respectively.
Analysis of the obtained results shows that supporting the membrane separation process with the precipitation of struvite has a major impact only on the efficiency of organic compound removal from the municipal digestate liquid fraction. On the contrary, it is not significant for components such as N-NH4+, K, Mg, Ca, Li, or P.
The integration of membrane separation techniques with other unit processes aims, in addition to increasing separation efficiency, to reduce the intensity of membrane fouling by substances present in the treated solution. As demonstrated earlier in this study, the intensity of this phenomenon during the process is significant. The results we obtained show that, by precipitating struvite, it is possible to eliminate from the digestate a certain amount of the impurities responsible for the membrane permeability decrease. The changes in relative membrane permeability during filtration with ceramic membranes and as a result of the integrated process are shown in Figure 9. From these test results it can be concluded that pretreatment of the liquid fraction of digestate through the precipitation of struvite has the effect of significantly reducing the intensity of membrane fouling. This effect was observed independently of the cut-off of the tested membranes. It can be assumed that struvite precipitation allows compounds to be removed from the solution that, in the absence of pretreatment, would settle on the membrane surface or in the membrane pores. Furthermore, the J/J0 values clearly indicate that the MF membranes, used both alone and in the integrated process, were more susceptible to fouling than the other membranes. However, supporting membrane filtration with the precipitation of struvite did not completely eliminate concentration polarisation, one consequence of which is membrane fouling. Agglomerates of organic macromolecules formed a filter cake on the membrane surface, resulting in a decrease in the relative permeability of the membranes.
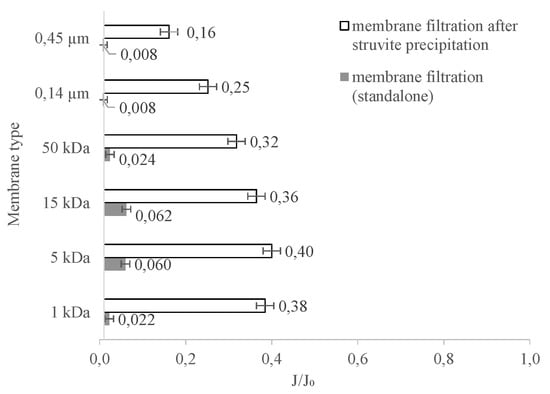
Figure 9.
The dependence of relative permeability on the type of ceramic membrane used in the integrated process involving struvite precipitation/membrane separation.
To better understand the phenomena occurring during municipal digestate treatment through the integrated struvite precipitation/filtration process using ceramic membranes, the particle size distributions in the obtained permeates were determined (Figure 10). The use of the LD and DLS methods did not take into account the possibility of the formation of larger agglomerates. It is likely that their size was determined instead of the sizes of individual particles. Therefore, the results obtained cannot be compared with the determined pore sizes of the tested membranes [51]. The determined average particle diameter of the raw liquid digestate (after 72 h of sedimentation) was approximately 46 μm, while the lower and upper deciles were 1.5 and 260 μm, respectively. From the results obtained for the digestate solution after the precipitation of struvite crystals and after the subsequent filtration with ceramic membranes with different cut-offs, it is clear that the combination of these two processes into an integrated process allows a significant number of larger particles to be removed from the analysed solution. By precipitating struvite from the digestate liquid alone, it is possible to remove the largest particle fraction. The membrane with the smallest pore diameter (1 kDa) was found to be the most effective in eliminating the turbidity of the digestate solution after precipitation, which is also confirmed by photos of the digestate samples after the precipitation of struvite and after the membrane process (Figure 10). The samples after ultrafiltration with the 1 kDa cut-off membrane are the brightest. It was observed that as the cut-off resolutions of the tested membranes increased, the average particle sizes remaining in the solution after membrane filtration were larger. Interestingly, the analysis of the average particle sizes indicates that the absence of a thicker fraction in the solution after precipitation causes the average particle size after membrane filtration to decrease only slightly relative to the feed, with a beneficial effect only for the most compact membrane. The greater the observed proportion of larger particles in the solution undergoing membrane separation, the more the particle distribution in the permeate shifted towards finer particles.
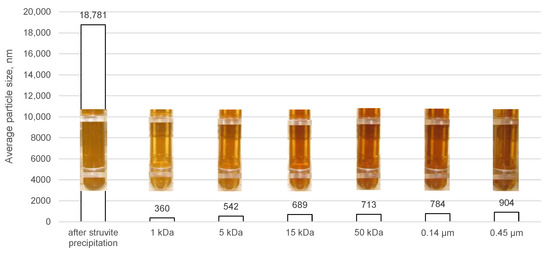
Figure 10.
Average particle sizes and visual effects of samples after struvite precipitation and after integrated struvite precipitation/membrane filtration using ceramic membranes.
The electrokinetic potential of the samples was also measured. The results obtained show that in the pH range of the raw digestate solution and the solution after struvite precipitation and membrane separation, the curve of the ζ-potential value, which becomes negative, does not change significantly as a result of the previous struvite precipitation process. The shift of the IEP point with a pH value oscillating around 1.75 is also not observed. In Figure 11, showing the changes in the ζ-potential value determined at natural pH (9.2–9.5) for the solutions together after struvite precipitation and membrane separation, it can be seen that the value of the electrokinetic potential is negative and oscillates in the range 40–16 mV. In the case of the raw digestate, when the pH was natural (about 7.5), the ζ-potential was −26 mV and increased slightly at pH 9 to −30 mV. After struvite precipitation, the ζ-potential increased slightly, while after further membrane treatment it decreased (except for in a few cases) compared to the values obtained for the feed. This may indicate the removal of a specific group of compounds from solutions with a certain potential value.
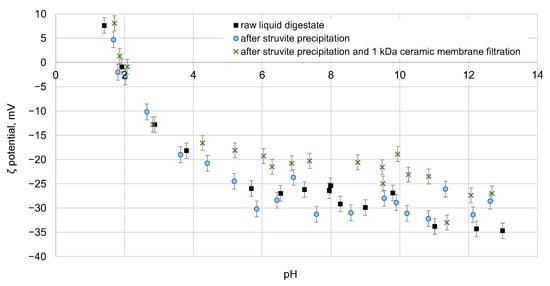
Figure 11.
ζ-potential of raw digestate, digestate after struvite precipitation, and after an integrated process combining struvite precipitation with membrane separation using a 1 kDa ceramic membrane.
4. Conclusions
On the basis of the experiments carried out, it was found that:
- The introduction of a suitable amount of MgCl2 and NaH2PO4 into the digestion fluid creates the possibility to precipitate struvite from the analysed solution. The adoption of process parameters—i.e., pH 9.0, a temperature in the range 20–23 °C, a molar ratio of N:Mg:P = 1:1.1:1.1, a reaction time of 5 min with a stirring rate of 160 rpm—ensures the high efficiency of struvite precipitation from the digestate.
- The separation of organic contaminants from the digestate is possible in pressurised membrane processes, and the purification effect depends on the cut-off resolution of the membranes (as the cut-off value increases, a deterioration in permeate quality can be observed). The best separation of contaminants was achieved by a ceramic membrane with a cut-off of 1 kDa.
- The transport properties of ceramic membranes significantly depend on the cut-off resolution of the membrane. An increase in membrane cut-off results in an increase in permeate flux values.
- Membranes with larger pores (MF membranes) are much more susceptible to fouling than more compact membranes, e.g., NF membranes.
- The use of an integrated process for the purification of the liquid fraction of digestate—struvite precipitation/membrane filtration with flat ceramic membranes allows a much more effective improvement of the final quality of the test solution and a greater reduction in the intensity of membrane fouling than has been observed for these processes when carried out individually.
- The best final quality of the treated digestate was obtained using a combination of struvite precipitation and filtration through a flat ceramic membrane with a cut-off of 1 kDa.
Author Contributions
Conceptualization, A.U.; methodology, A.U. and I.P.; validation, A.U.; formal analysis, A.U.; investigation, A.U. and I.P.; resources, A.U. and I.P.; data curation, A.U. and I.P.; writing—original draft preparation, A.U.; writing—review and editing, A.U.; visualization, A.U. and I.P.; supervision, A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and Perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic Digestion in Global Bio-Energy Production: Potential and Research Challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for Transformation of Biogas to Biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Fagerström, A.; Al Seadi, T.; Rasi, S.; Briseid, T. The Role of Anaerobic Digestion and Biogas in the Circular Economy; IEA Bioenergy: Cork, Ireland, 2018; Volume 8. [Google Scholar]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A Critical Review of Ammonia Recovery from Anaerobic Digestate of Organic Wastes via Stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the Fertiliser Potential of Digestates from Farm and Agroindustrial Residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Gómez, X.; Cuetos, M.J.; García, A.I.; Morán, A. An Evaluation of Stability by Thermogravimetric Analysis of Digestate Obtained from Different Biowastes. J. Hazard. Mater. 2007, 149, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Pognani, M.; D’Imporzano, G.; Scaglia, B.; Adani, F. Substituting Energy Crops with Organic Fraction of Municipal Solid Waste for Biogas Production at Farm Level: A Full-Scale Plant Study. Process Biochem. 2009, 44, 817–821. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and Liquid Fractionation of Digestate: Mass Balance, Chemical Characterization, and Agronomic and Environmental Value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; IEA Bioenergy: Dublin, Ireland, 2015. [Google Scholar]
- Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. Detailed Monitoring of Two Biogas Plants and Mechanical Solid–Liquid Separation of Fermentation Residues. J. Biotechnol. 2009, 142, 56–63. [Google Scholar] [CrossRef]
- Makdi, M.; Tomcsik, A.; Orosz, V. Digestate: A New Nutrient Source-Review. In Biogas; InTech: London, UK, 2012. [Google Scholar]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K. Anaerobic Digestate Management for Carbon Neutrality and Fertilizer Use: A Review of Current Practices and Future Opportunities. Biomass Bioenergy 2024, 180, 106991. [Google Scholar] [CrossRef]
- Camilleri-Rumbau, M.S.; Briceño, K.; Fjerbæk Søtoft, L.; Christensen, K.V.; Roda-Serrat, M.C.; Errico, M.; Norddahl, B. Treatment of Manure and Digestate Liquid Fractions Using Membranes: Opportunities and Challenges. Int. J. Environ. Res. Public Health 2021, 18, 3107. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-Based Water and Wastewater Treatment Technologies: Issues, Current Trends, Challenges, and Role in Achieving Sustainable Development Goals, and Circular Economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 202400011. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Murcia, M.D. Membranes for Water and Wastewater Treatment. Membranes 2021, 11, 295. [Google Scholar] [CrossRef]
- Nascimbén Santos, É.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic Membrane Filtration and Its Advantages over Conventional Approaches in the Treatment of Oily Wastewater: A Review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development, and Application. Env. Sci. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Aristizábal, S.L.; Lively, R.P.; Nunes, S.P. Solvent and Thermally Stable Polymeric Membranes for Liquid Molecular Separations: Recent Advances, Challenges, and Perspectives. J. Memb. Sci. 2023, 685, 121972. [Google Scholar] [CrossRef]
- Averina, Y.M.; Kurbatov, A.Y.; Sakharov, D.A.; Subcheva, E.N. Development of Nanofiltration Ceramic Membrane Production Technology. Glass Ceram. 2020, 77, 98–102. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Gitis, V.; Rothenberg, G. Ceramic Membranes: New Opportunities and Practical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Drioli, E.; Macedonio, F.; Tocci, E. Membrane Science and Membrane Engineering for a Sustainable Industrial Development. Sep. Purif. Technol. 2021, 275, 119196. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for Low Pressure Membranes in Water Treatment: A Review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C. Integrated Membrane Systems and Processes; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lin, H.; Gan, J.; Rajendran, A.; Reis, C.E.R.; Hu, B. Phosphorus Removal and Recovery from Digestate after Biogas Production. In Biofuels-Status and Perspective; InTech: London, UK, 2015. [Google Scholar]
- Marti, N.; Bouzas, A.; Seco, A.; Ferrer, J. Struvite Precipitation Assessment in Anaerobic Digestion Processes. Chem. Eng. J. 2008, 141, 67–74. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Zhong, Y.; Liu, W.; Zhang, J.; Yu, X.; Ou, R.; Zeng, G. A Review of Struvite Crystallization for Nutrient Source Recovery from Wastewater. J. Environ. Manag. 2023, 344, 118383. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Abbatantuono, F.; Ruiz-García, J.L.; Parra, M.; Martínez, R.M.; Intrigliolo, D.S. Struvite as a Reliable and More Environmental Friendly Alternative of Nutrients for Vegetable Crops. Acta Hortic. 2023, 1375, 337–342. [Google Scholar] [CrossRef]
- Chempur Karta Charakterystyki Substancji Chemicznej-Chlorek Magnezu. Available online: http://chempur.pl/pliki/karty_charakterystyk/magnezu_chlorek_6h.pdf (accessed on 16 August 2021).
- Chempur Karta Charakterystyki Substancji Chemicznej-Sodu Diwodorofosforan Bezwodny. Available online: http://chempur.pl/pliki/karty_charakterystyk/sodu_fosforan_I_bezwodny.pdf (accessed on 16 August 2021).
- Sterlitech Ceramic Membrane Filters. Available online: https://www.sterlitech.com/media/wysiwyg/pdfs/Sterlitech_Catalog2016_Ceramic_.pdf (accessed on 14 June 2024).
- Ye, Z.; Shen, Y.; Ye, X.; Zhang, Z.; Chen, S.; Shi, J. Phosphorus Recovery from Wastewater by Struvite Crystallization: Property of Aggregates. J. Environ. Sci. 2014, 26, 991–1000. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite Formation and Decomposition Characteristics for Ammonia and Phosphorus Recovery: A Review of Magnesium-Ammonia-Phosphate Interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Dong, R.; Ahring, B.K.; Zhang, W. Properties of Plant Nutrient: Comparison of Two Nutrient Recovery Techniques Using Liquid Fraction of Digestate from Anaerobic Digester Treating Pig Manure. Sci. Total Environ. 2016, 544, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Çelen, I.; Türker, M. Recovery of Ammonia as Struvite from Anaerobic Digester Effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.T.; Moody, L.B.; Celen, I.; Buchanan, J.R. Optimization of Phosphorus Precipitation from Swine Manure Slurries to Enhance Recovery. Water Sci. Technol. 2003, 48, 139–146. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X. Nutrient Removal from Anaerobically Digested Cattle Manure by Struvite Precipitation. J. Environ. Eng. Sci. 2006, 5, 285–294. [Google Scholar] [CrossRef]
- Siciliano, A. Assessment of Fertilizer Potential of the Struvite Produced from the Treatment of Methanogenic Landfill Leachate Using Low-Cost Reagents. Environ. Sci. Pollut. Res. 2016, 23, 5949–5959. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Rosa, S. De Recovery of Ammonia in Digestates of Calf Manure through a Struvite Precipitation Process Using Unconventional Reagents. Environ. Technol. 2014, 35, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of Slow Release Crystal Fertilizer from Wastewaters through Struvite Crystallization–A Review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential Phosphorus Recovery by Struvite Formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.-Z.; Wang, Y.-Y.; Li, Y.-L.; Zhou, G.-T. Biomimetic Synthesis of Struvite with Biogenic Morphology and Implication for Pathological Biomineralization. Sci. Rep. 2015, 5, 7718. [Google Scholar] [CrossRef]
- Verma, B.; Balomajumder, C.; Sabapathy, M.; Gumfekar, S.P. Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes 2021, 9, 752. [Google Scholar] [CrossRef]
- Agnieszka, U.; Małgorzata, K.K. Properties of Flat Ceramic Membranes and Their Application for Municipal Digestate Liquid Fraction Purification. J. Membr. Sci. Res. 2023, 9, 556692. [Google Scholar] [CrossRef]
- Field, R. Fundamentals of Fouling. In Membrane Technology; Wiley Online Books; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; Volume 4, pp. 1–23. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).