Effect of Carbon Source on Endogenous Partial Denitrification Process: Characteristics of Intracellular Carbon Transformation and Nitrite Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactors and Operation Procedures
2.2. Wastewaters and Seed Sludge
2.3. Methods for Chemical Analysis
2.4. Calculation of Nitrate-to-Nitrite Transformation Ratio NTR
3. Results and Discussion
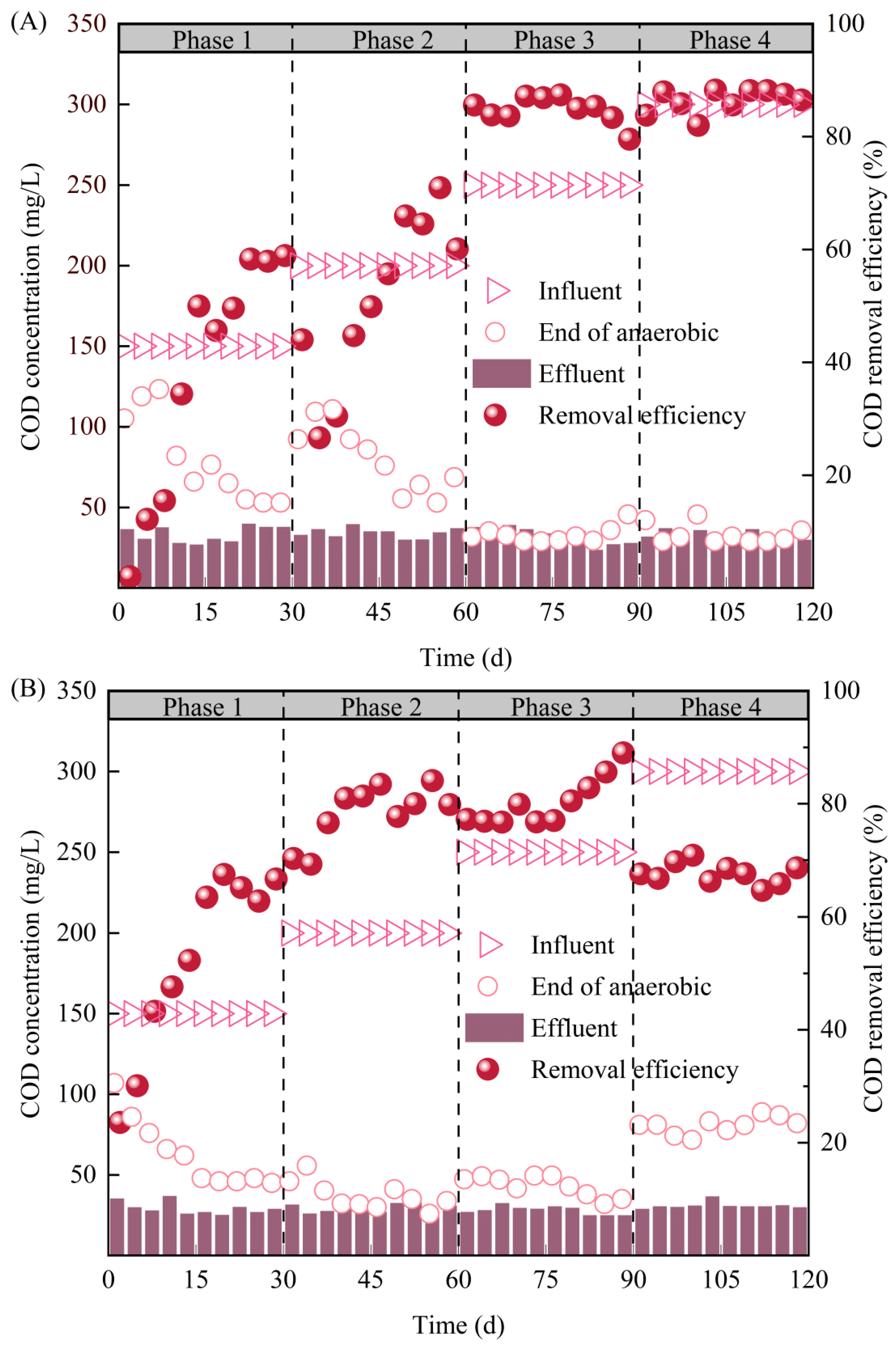
3.1. Effects of Carbon Source on Anaerobic COD Removal in SBR1# and SBR2# under A/O Operation Mode
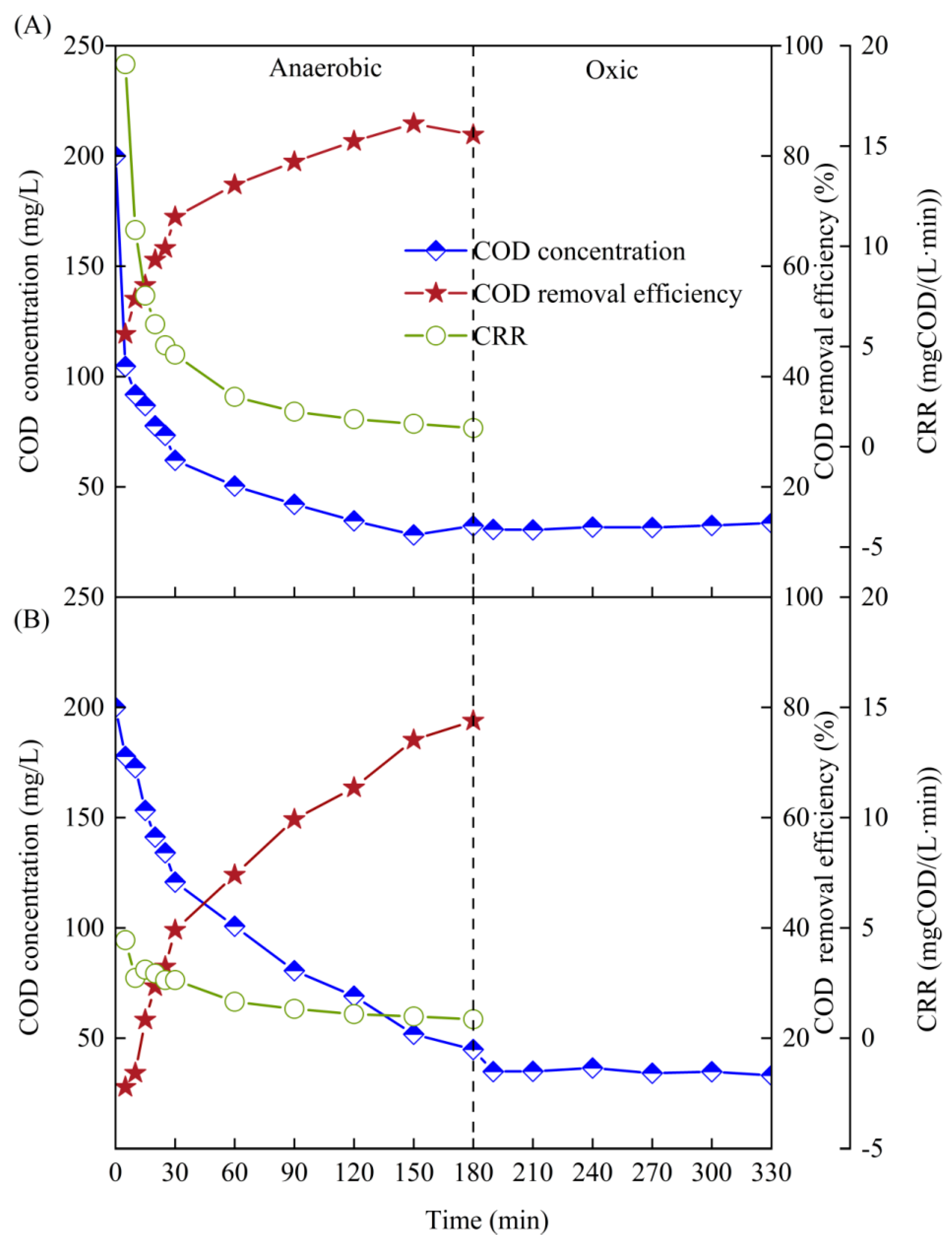
3.2. Mechanisms of COD Removal in SBR1# and SBR2# with Different Carbon Sources
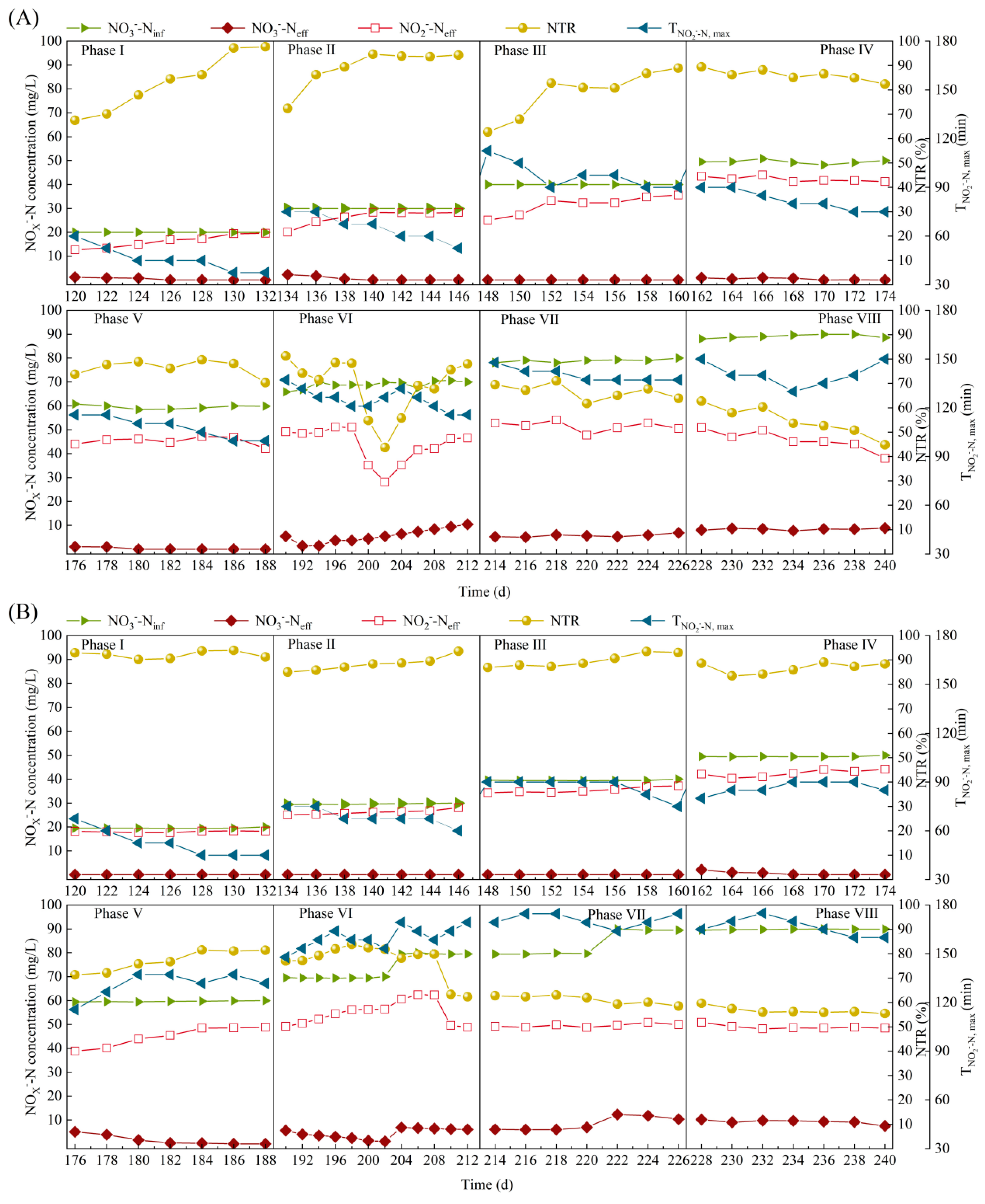
3.3. Effects of Carbon Sources on Endogenous Partial Denitrification Performance in SBR1# and SBR2# under A/A/(O) Operation Mode
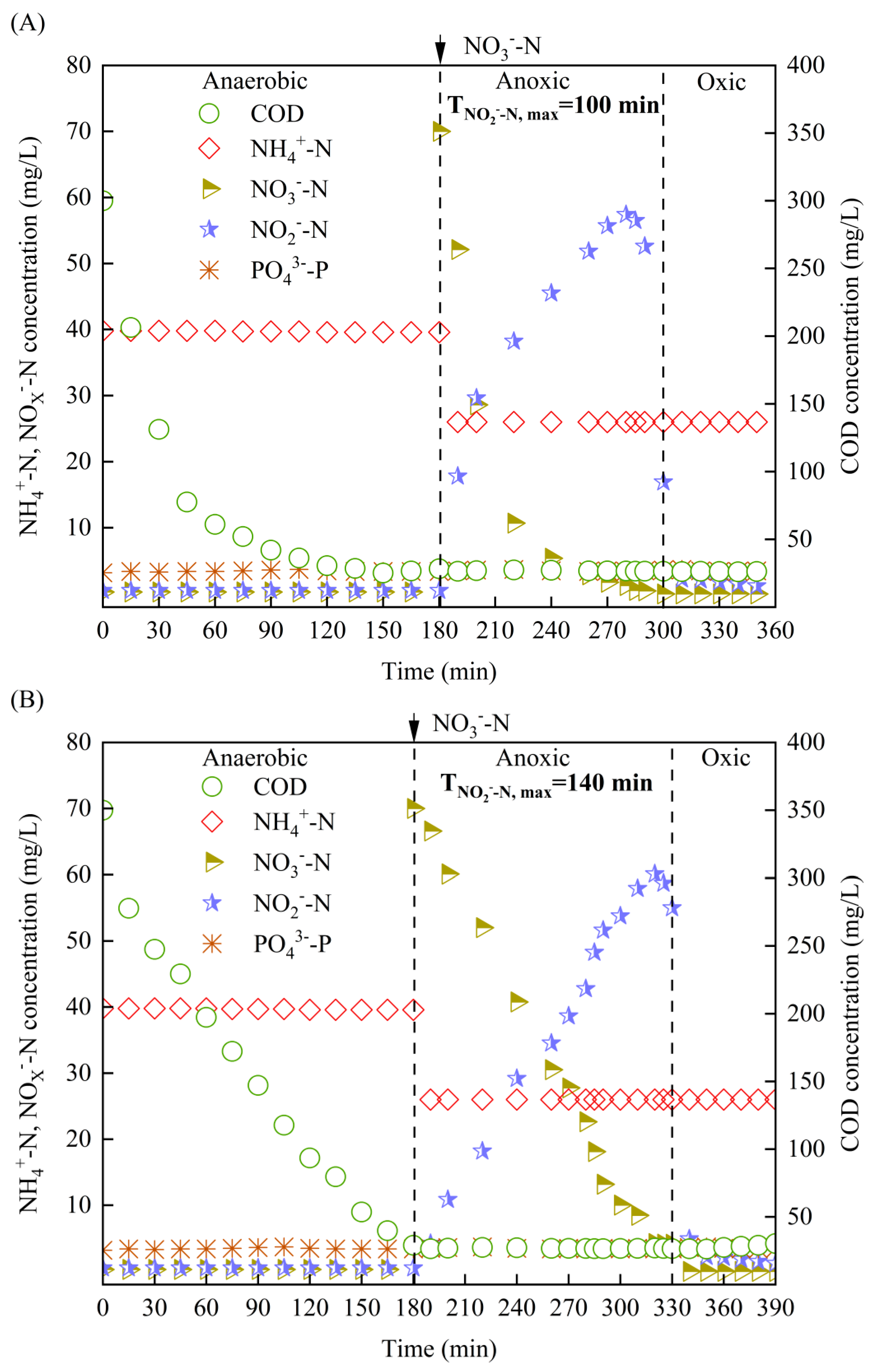
3.4. Characteristics of Carbon and Nitrogen Transformations via EPD with Different Carbon Sources
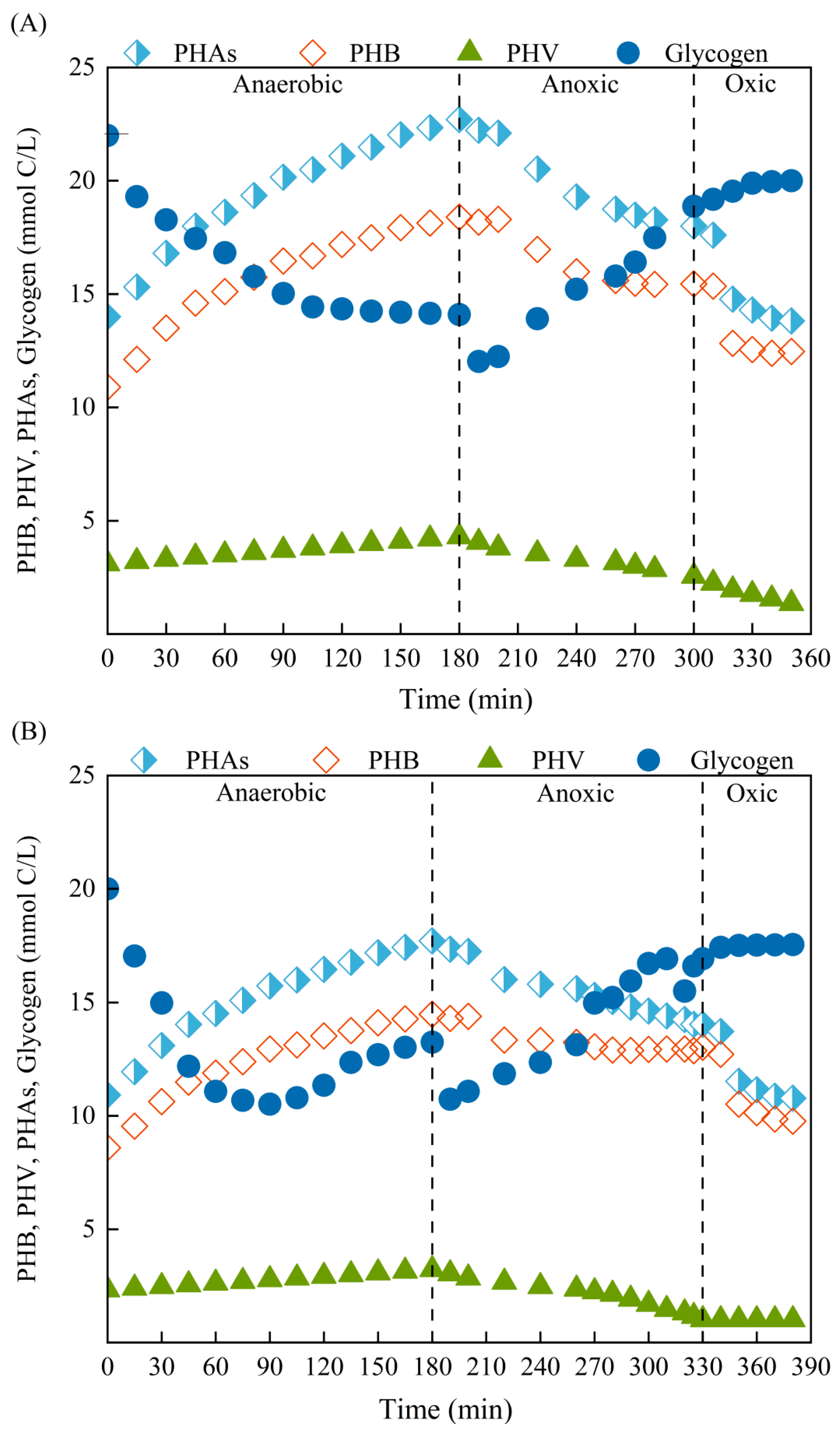
3.5. Characteristics of Intracellular Carbons Transformations via EPD with Different Carbon Sources
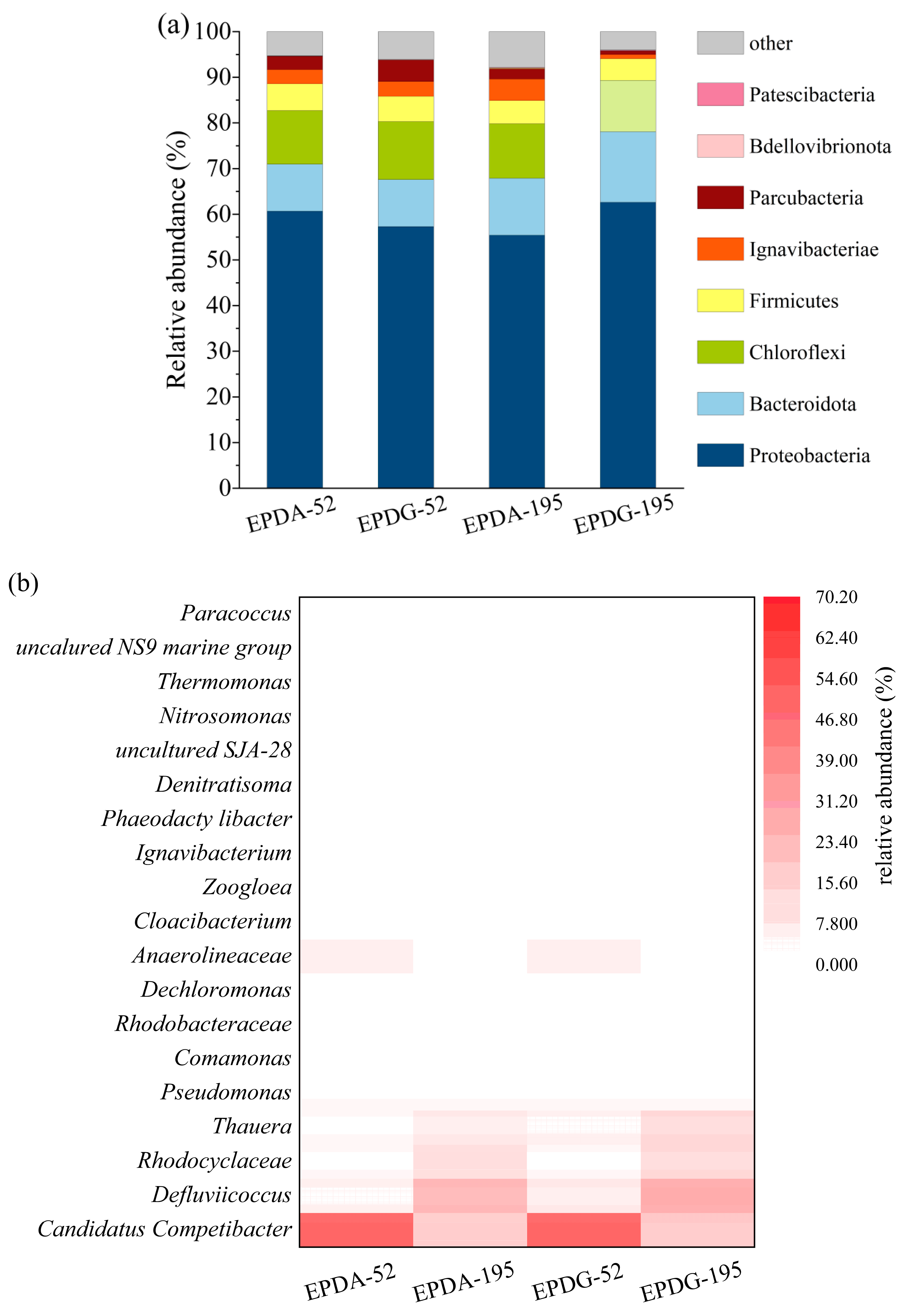
3.6. Analysis of the Variation in Microbial Community under Different Carbon Sources
4. Conclusions
- (1)
- During the A/O operation, both SBR1# and SBR2# achieved good anaerobic COD removal performances with COD removal efficiency higher than 85% and CODeff lower than 35 mg/L, but the performance of SBR2# was significantly affected by high COD (250~350 mg/L). Specially, COD mainly removed in the first 15 min in SBR1# with CRR reaching 7.54 mgCOD/(L·min), whereas in SBR2#, COD removal occurred in the whole anaerobic phase (180 min) with an average CRR of 2.22 mgCOD/(L·min).
- (2)
- By gradually increasing CODinf (150~250 mg/L) and NO3−−Ninf (20~40 mg/L), both SBR1# and SBR2# maintained good partial denitrification performance with high NTR and (rNaR) of 88.4~90%, 2.41~2.38 mgN/(gVSS·h), respectively, but high CODinf (250~350 mg/L) and NO3−−Ninf (50~60 mg/L) facilitated stable NO2−−N accumulation in SBR1# using sodium acetate as the carbon source. Both SBR1# and SBR2# reach the maximum NO2−−N accumulation of 54.7 and 62.5 mg/L, respectively, under NO3−−Ninf reaching 70~80 mg/L.
- (3)
- Using sodium acetate and glucose as carbon sources to drive EPD, similar anaerobic and anoxic internal carbon transformations were observed, but higher and faster carbon transformation was achieved with sodium acetate as carbon source than glucose. Precise control of anoxic time at TNi,max was still the key to achieve high NO2−−N accumulation.
- (4)
- The differences in carbon sources (sodium acetate and glucose) would affect the microbial community structure in the EPD system. Both sodium acetate and glucose as carbon sources could achieve EPD systems successfully; glucose as a carbon source was more beneficial to the enrichment of partial denitrifying bacterium compared to sodium acetate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhao, J.; Yu, D.; Chen, G.; Du, S.; Zhen, J.; Yuan, M. Stable nitrite accumulation and phosphorous removal from nitrate and municipal wastewaters in a combined process of endogenous partial denitrification and denitrifying phosphorus removal (EPDPR). Chem. Eng. J. 2019, 355, 560–571. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Z.; Ishida, K.; Urasaki, K.; Kubota, K.; Li, Y.Y. Fast formation of anammox granules using a nitrification-denitrification sludge and transformation of microbial community. Water Res. 2022, 221, 118751. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Zhao, J.; Xiang, H.; Wang, J.; Gu, R.; Zhang, Y.; Shen, C.; Yu, D.; Wang, X. A glimpse of the re-granulation process after artificially crushing anammox granular sludge. J. Water Process Eng. 2023, 56, 104375. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Han, F.; Mao, Z.; Hu, L.; Wang, W.; Zhang, J. Enhancement of anaerobic ammonia oxidation process composed of mixed sludge without additional deoxidation by sulfur autotrophic denitrification under mainstream conditions. J. Environ. Chem. Eng. 2023, 11, 110902. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Li, B.; Wang, S.; Ren, N.; Peng, Y. Nitrite production from partial-denitrification process fed with low carbon/nitrogen (C/N) domestic wastewater: Performance, kinetics and microbial community. Chem. Eng. J. 2017, 326, 1186–1196. [Google Scholar] [CrossRef]
- Ding, J.; Gao, X.; Peng, Y.; Peng, Y.; Zhang, Q.; Li, X.; Wang, S. Anaerobic duration optimization improves endogenous denitrification efficiency by glycogen accumulating organisms enhancement. Bioresour. Technol. 2022, 348, 126730. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, L.; Zhang, Q.; Li, X.; Huang, Y.; Peng, Y. Advanced nitrogen removal from low C/N municipal wastewater by combining partial nitrification-anammox and endogenous partial denitrification-anammox (PN/A-EPD/A) process in a single-stage reactor. Bioresour. Technol. 2021, 339, 125501. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Peng, Y.; Wang, B.; Li, X.; Zhang, Q. A novel SNPR process for advanced nitrogen and phosphorus removal from mainstream wastewater based on anammox, endogenous partial-denitrification and denitrifying dephosphatation. Water Res. 2020, 170, 115363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, Y.; Zhao, C.; Zhang, C.; Zhang, Z.; Wang, L.; Wang, S.; Wang, J. Exploration of the metabolic flexibility of glycogen accumulating organisms through metatranscriptome analysis and metabolic characterization. J. Environ. Sci. 2023, 126, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zeng, W.; Fan, Z.; Li, S.; Peng, Y. Sulfide inhibition on polyphosphate accumulating organisms and glycogen accumulating organisms: Cumulative inhibitory effect and recoverability. J. Hazard. Mater. 2023, 451, 131157. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, W.; Meng, Q.; Fan, Z.; Peng, Y. Phosphorus removal performance, intracellular metabolites and clade-level community structure of Tetrasphaera-dominated polyphosphate accumulating organisms at different temperatures. Sci. Total. Environ. 2022, 842, 156913. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Huh, M.J.; Nam, I.; Park, J.H.; Lee, D.H.; Lee, M.W.; Park, I.K. Morphological and biological characteristics of the Korean population of Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae), an invasive insect pest of conifer cones. J. Asia-Pac. Entomol. 2023, 26, 102057. [Google Scholar] [CrossRef]
- Dong, K.; Qiu, Y.; Wang, X.; Yu, D.; Yu, Z.; Feng, J.; Wang, J.; Gu, R.; Zhao, J. Towards low carbon demand and highly efficient nutrient removal: Establishing denitrifying phosphorus removal in a biofilm-based system. Bioresour. Technol. 2023, 372, 128658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, Y.; Wang, J.; Gu, R.; Ding, Z.; Liu, Z.; Zhang, Y.; Yu, D.; Wang, X. Nitrite soaking pretreatment induced initial denitrifying nitrite accumulation. Bioresour. Technol. 2023, 387, 129605. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liu, C.; Zhang, W.; Yin, Z. Phosphorus release and uptake behavior and microbial metabolic activity in reversed A2O process: Importance of influent VFAs/COD and anaerobic duration. Biochem. Eng. 2024, 200, 109098. [Google Scholar] [CrossRef]
- Zhang, C.; Guisasola, A.; Baeza, J.A. Exploring the stability of an A-stage-EBPR system for simultaneous biological removal of organic matter and phosphorus. Chemosphere 2023, 313, 137576. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cheng, W.; Ren, J.; Zhang, X.; Wang, M.; Wan, T.; Lv, T. Relationship of phosphorus removal, extracellular polymeric substances characteristics, and microbial community diversity in an aerobic moving bed biofilm reactor: Effect of carbon sources. J. Environ. Chem. Eng. 2022, 10, 108555. [Google Scholar] [CrossRef]
- Chu, G.; Yu, D.; Wang, X.; Wang, Q.; He, T.; Zhao, J. Comparison of nitrite accumulation performance and microbial community structure in endogenous partial denitrification process with acetate and glucose served as carbon source. Bioresour. Technol. 2021, 320, 124405. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Denver, CO, USA, 2012. [Google Scholar]
- Zhao, J.; Wang, X.; Li, X.; Jia, S.; Wang, Q.; Peng, Y. Improvement of partial nitrification endogenous denitrification and phosphorus removal system: Balancing competition between phosphorus and glycogen accumulating organisms to enhance nitrogen removal without initiating phosphorus removal deterioration. Bioresour. Technol. 2019, 281, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yuan, H.; Luo, Q.; Fan, Z.; Jiang, L.; Jiang, W.; Li, Z. Carbon coating with different carbon sources on rare earth hydrogen storage alloy. Int. J. Hydrogen Energy. 2023, 48, 30868–30876. [Google Scholar] [CrossRef]
- Shang, T.; Zhu, X.; Gong, X.; Guo, J.; Li, X.; Zhang, Q.; Peng, Y. Efficient nitrogen removal in a total floc sludge system from domestic wastewater with low C/N: High anammox nitrogen removal contribution driven by endogenous partial denitrification. Bioresour. Technol. 2023, 378, 128995. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, J.; Wang, J.; Gu, R.; Qu, Y.; Yin, J.; Yu, D.; Yu, Z.; Feng, J.; Wang, X. Elucidating performance failure in the use of an Anaerobic-Oxic-Anoxic (AOA) plug-flow system for biological nutrient removal. Sci. Total Environ. 2023, 880, 163320. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Sun, R.; Hu, S.; Qiao, Z.; Wang, S.; Mi, X. NH4+-N/NO3−-N ratio controlling nitrogen transformation accompanied with NO2−-N accumulation in the oxic-anoxic transition zone. Environ. Res. 2020, 189, 109962. [Google Scholar] [CrossRef] [PubMed]
- Dan, Q.; Peng, Y.; Wang, B.; Wang, Q.; Sun, T. Side-stream phosphorus famine selectively strengthens glycogen accumulating organisms (GAOs) for advanced nutrient removal in an anaerobic-aerobic-anoxic system. Chem. Eng. J. 2021, 420, 129554. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, J.; Yang, P.; Jing, Y.; Yu, D.; Yu, Z.; Qiu, Y.; Wang, X. Forming densified activated sludge in a modified simultaneous nitrification–denitrification and phosphorus removal (SNDPR) system by introducing shift work mode. Chem. Eng. J. 2024, 492, 153333. [Google Scholar] [CrossRef]
- Kang, D.; Zhao, X.; Yuan, J.; Wang, N.; Suo, Y.; Peng, Y. Nitrite accumulation in activated sludge through cyclic anaerobic exposure with acetate. J. Environ. Manag. 2023, 346, 119005. [Google Scholar] [CrossRef] [PubMed]
- Maszenan, A.M.; Bessarab, I.; Williams, R.B.H.; Petrovski, S.; Seviour, R.J. The phylogeny, ecology and ecophysiology of the glycogen accumulating organism (GAO) Defluviicoccus in wastewater treatment plants. Water Res. 2022, 221, 118729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, P.; Yu, Z.; Qiu, Y.; Yu, D.; Wang, X. Ultra-rapid achievement of denitrifying nitrite accumulation using anoxic starvation treatment. Chem. Eng. J. 2024, 492, 152205. [Google Scholar] [CrossRef]

| Phase | Operating Time (d) | Operating Mode | Duration (min) | Influent (mg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaerobic | Anoxic | Oxic | COD | NO3−-N | PO43−-P | |||||||||||
| SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | SBR1# | SBR2# | |
| 1 | 1~30 | A/O | 180 | — | 150 | 150 | — | 0 | ||||||||
| 2 | 31~60 | A/O | 180 | — | 150 | 200 | — | 0 | ||||||||
| 3 | 61~90 | A/O | 180 | — | 150 | 250 | — | 0 | ||||||||
| 4 | 91~120 | A/O | 180 | — | 150 | 300 | — | 0 | ||||||||
| I | 121~132 | A/A/O | 180 | 30 | 30 | 50 | 150 | 200 | 20 | 4 | ||||||
| II | 133~146 | A/A/O | 180 | 40 | 45 | 50 | 200 | 200 | 30 | 4 | ||||||
| III | 147~160 | A/A/O | 180 | 60 | 60 | 50 | 250 | 250 | 40 | 4 | ||||||
| IV | 161~174 | A/A/O | 180 | 80 | 90 | 50 | 250 | 250 | 50 | 4 | ||||||
| V | 175~188 | A/A/O | 180 | 90 | 120 | 50 | 250 | 300 | 60 | 4 | ||||||
| VI | 189~212 | 189~202 | A/A/O | 180 | 100 | 140 | 0 | 30 | 300 | 350 | 70 | 4 | ||||
| VII | 213~226 | 203~220 | A/A/O | 180 | 120 | 150 | 0 | 20 | 350 | 350 | 80 | 4 | ||||
| VIII | 227~240 | 221~240 | A/A | 180 | 150 | 160 | — | 350 | 350 | 90 | 4 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Li, J.; You, Z.; Qiu, Y.; Feng, J.; Zhao, J.; Chu, G.; Wang, X. Effect of Carbon Source on Endogenous Partial Denitrification Process: Characteristics of Intracellular Carbon Transformation and Nitrite Accumulation. Water 2024, 16, 1645. https://doi.org/10.3390/w16121645
Xiang H, Li J, You Z, Qiu Y, Feng J, Zhao J, Chu G, Wang X. Effect of Carbon Source on Endogenous Partial Denitrification Process: Characteristics of Intracellular Carbon Transformation and Nitrite Accumulation. Water. 2024; 16(12):1645. https://doi.org/10.3390/w16121645
Chicago/Turabian StyleXiang, Han, Juan Li, Zhipeng You, Yanling Qiu, Juan Feng, Ji Zhao, Guangyu Chu, and Xiaoxia Wang. 2024. "Effect of Carbon Source on Endogenous Partial Denitrification Process: Characteristics of Intracellular Carbon Transformation and Nitrite Accumulation" Water 16, no. 12: 1645. https://doi.org/10.3390/w16121645
APA StyleXiang, H., Li, J., You, Z., Qiu, Y., Feng, J., Zhao, J., Chu, G., & Wang, X. (2024). Effect of Carbon Source on Endogenous Partial Denitrification Process: Characteristics of Intracellular Carbon Transformation and Nitrite Accumulation. Water, 16(12), 1645. https://doi.org/10.3390/w16121645









