Stream Algal Biomass Associations with Environmental Variables in a Temperate Rainforest
Abstract
1. Introduction
- (1)
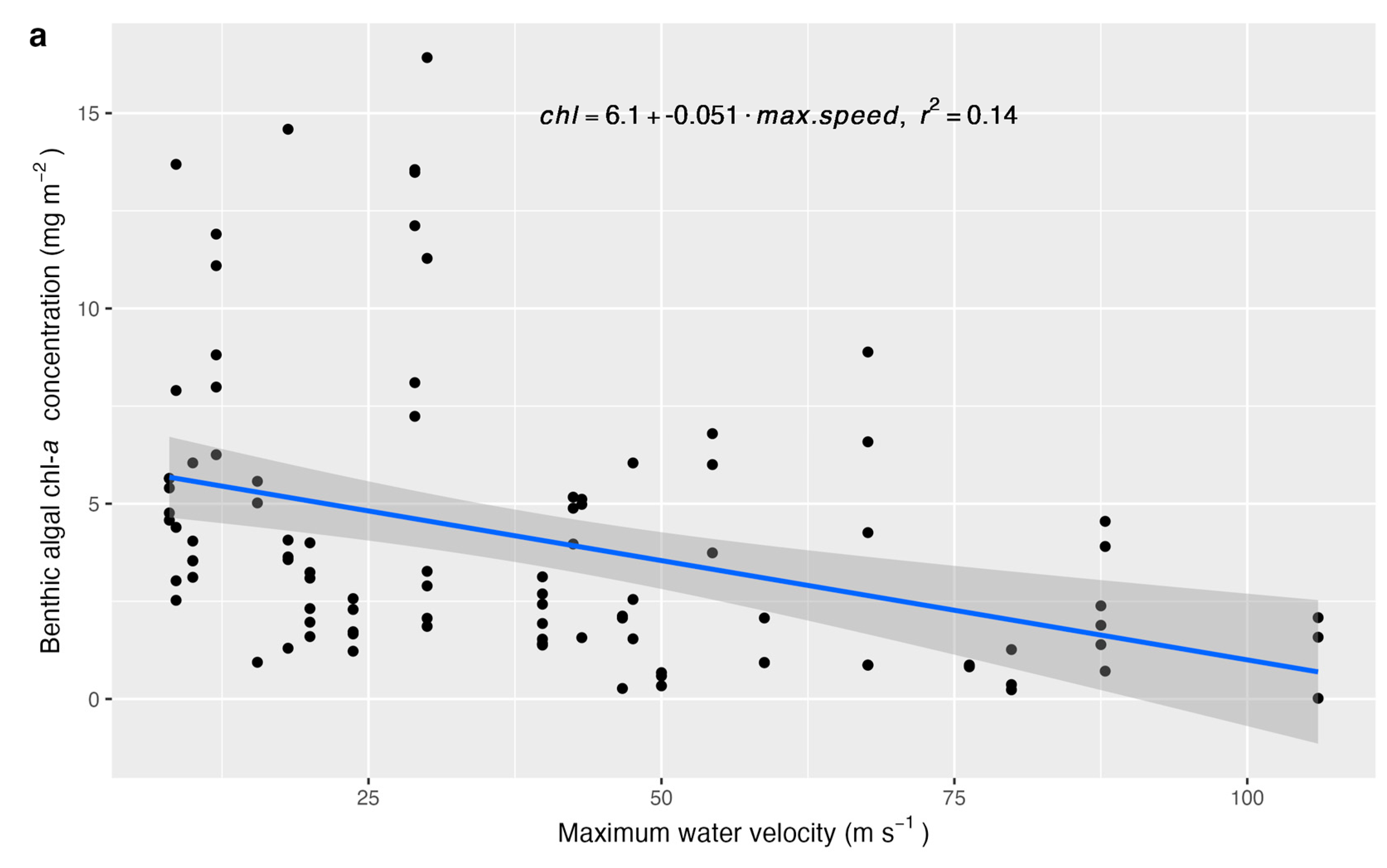
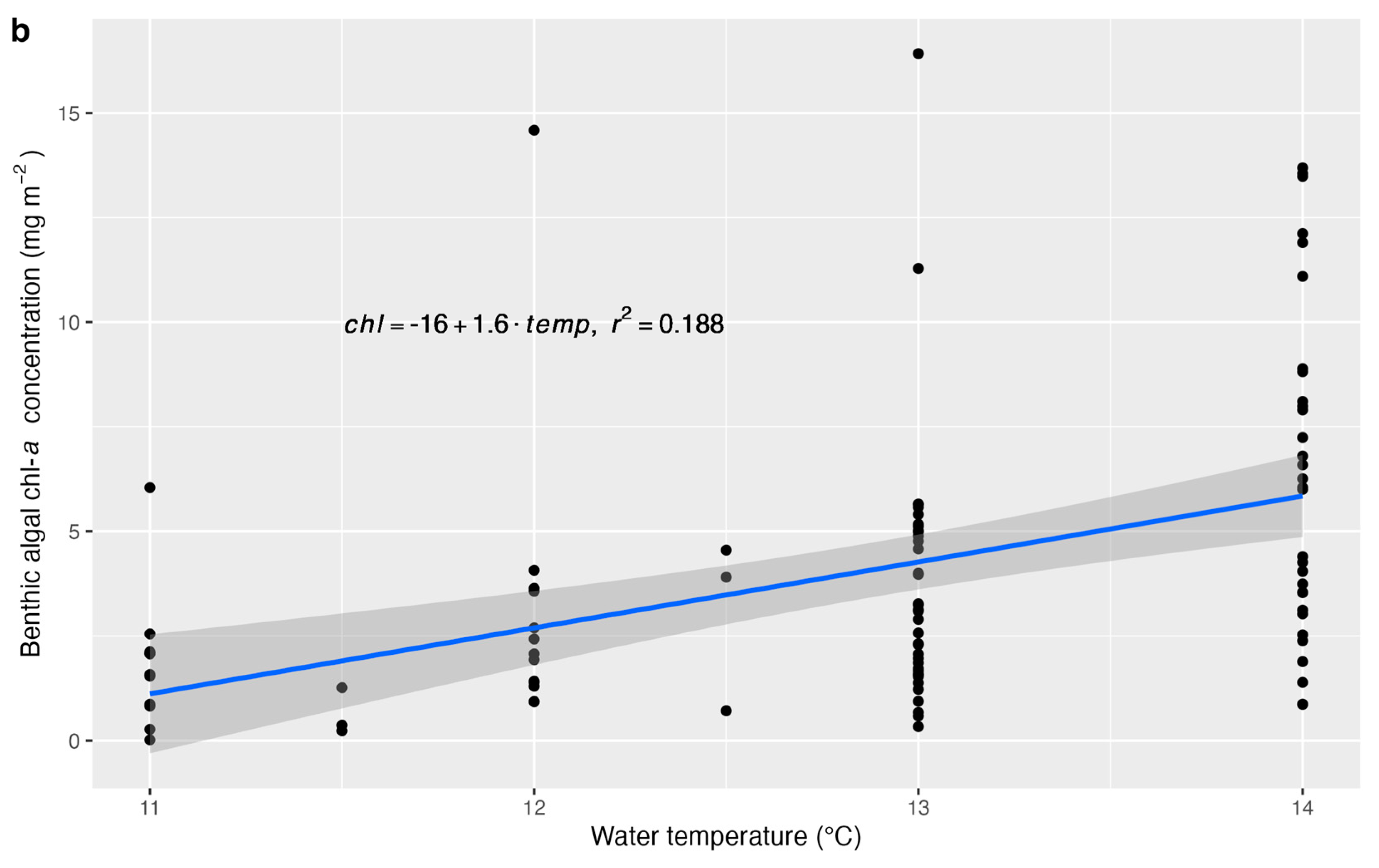
- Benthic algal chl-a concentration would be positively associated with water temperature, bankfull width (related to light availability and stream size), salmonid biomass, macroinvertebrate scraper–grazer abundance (consumers of benthic algae), macroinvertebrate predator abundance, and sample-level canopy cover. The effect of canopy cover would depend on channel orientation, as streams oriented north–south may be exposed to sunlight during midday, whereas canopy cover on the south bank of east–west-oriented streams may block sunlight. Water velocity would be negatively associated with benthic algal chl-a concentration.
- (2)
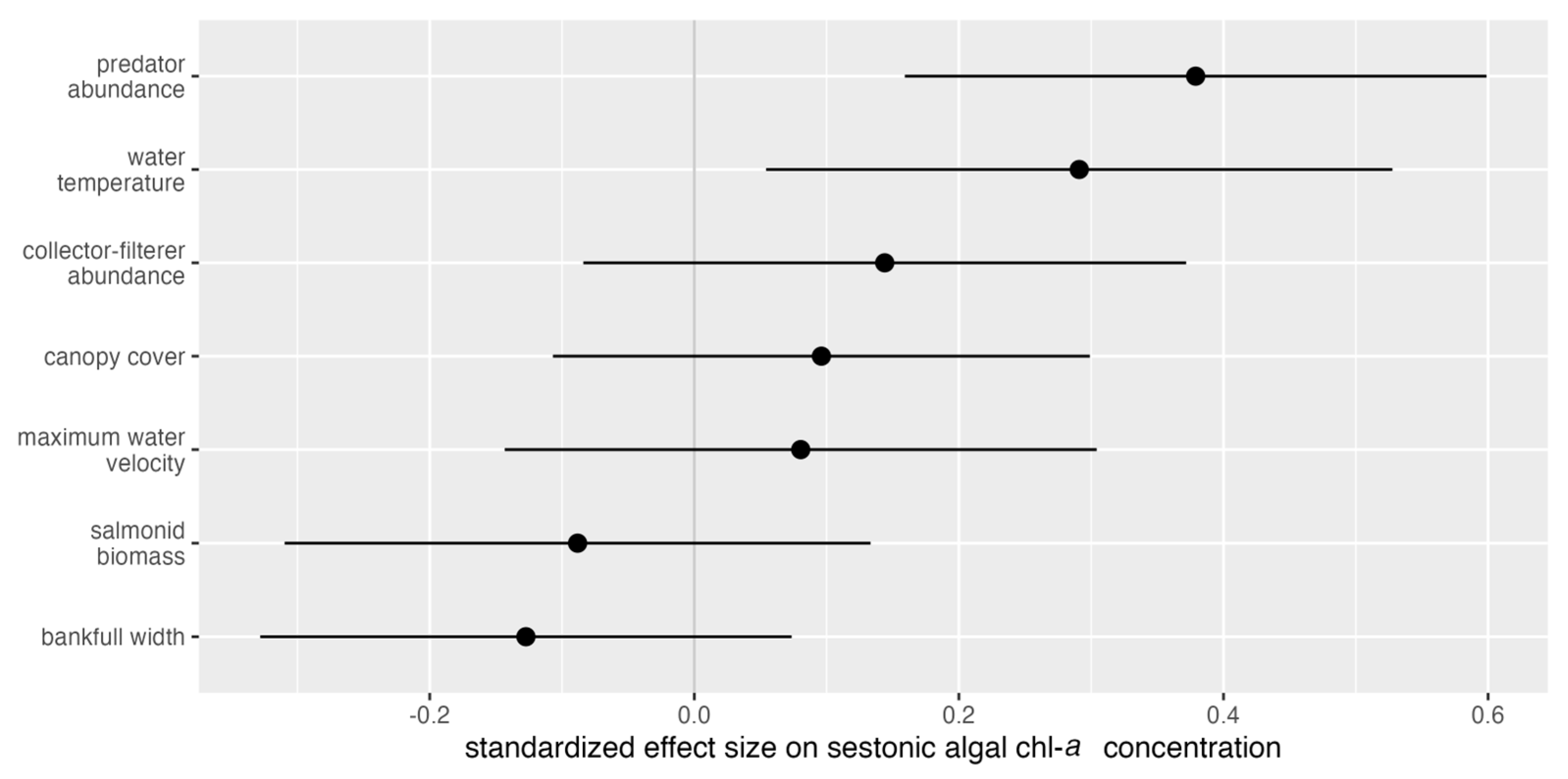
- Sestonic algal biomass would be positively related to water temperature, bankfull width, salmonid biomass, macroinvertebrate collector–filterer abundance, macroinvertebrate predator abundance, and reach-level canopy cover, similarly modulated by stream orientation. Water velocity would be negatively associated with sestonic algal chl-a concentration.
2. Materials and Methods
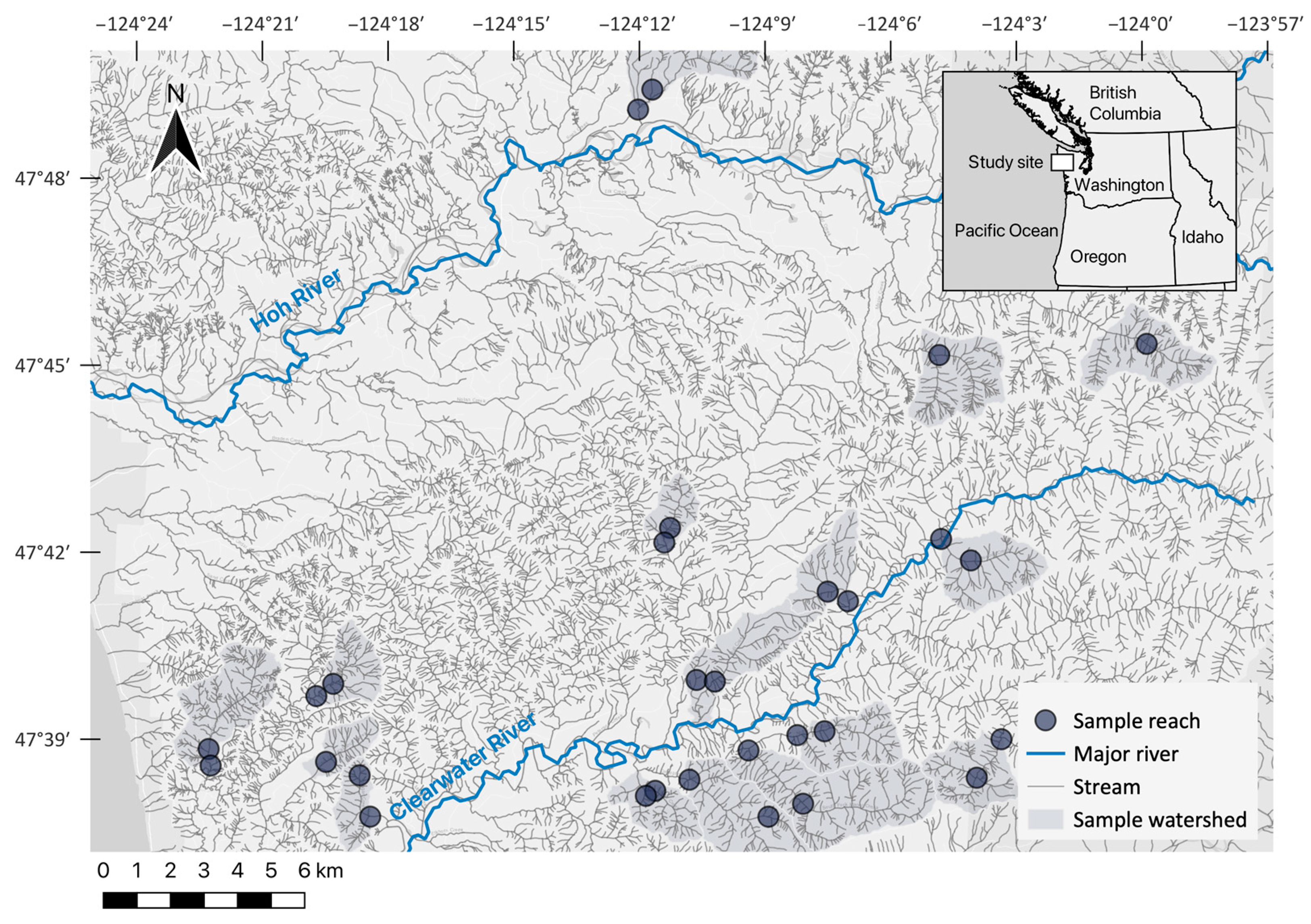
2.1. Study Sites
2.2. Field Data Collections
2.3. Lab Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finlay, J.C.; Khandwala, S.; Power, M.E. Spatial Scales of Carbon Flow in a River Food Web. Ecology 2002, 83, 1845–1859. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; Wehr, J.D.; Perrone, A.A. Trophic Relations in a Stream Food Web: Importance of Fatty Acids for Macroinvertebrate Consumers. J. N. Am. Benthol. Soc. 2007, 26, 509–522. [Google Scholar] [CrossRef]
- Giller, P.S.; Malmqvist, B. The Biology of Streams and Rivers; Biology of habitats; Oxford University Press: Oxford, UK; New York, NY, USA; Toronto, ON, Canada, 1998; ISBN 978-0-19-854977-2. [Google Scholar]
- Hill, W.R.; Smith, J.G.; Stewart, A.J. Light, Nutrients, and Herbivore Growth in Oligotrophic Streams. Ecology 2010, 91, 518–527. [Google Scholar] [CrossRef]
- Veldboom, J.A.; Haro, R.J. Stoichiometric Relationship between Suspension-Feeding Caddisfly (Trichoptera: Brachycentridae) and Seston. Hydrobiologia 2011, 675, 129–141. [Google Scholar] [CrossRef]
- Guo, F.; Kainz, M.J.; Valdez, D.; Sheldon, F.; Bunn, S.E. The Effect of Light and Nutrients on Algal Food Quality and Their Consequent Effect on Grazer Growth in Subtropical Streams. Freshw. Sci. 2016, 35, 1202–1212. [Google Scholar] [CrossRef]
- Wipfli, M.S. Terrestrial Invertebrates as Salmonid Prey and Nitrogen Sources in Streams: Contrasting Old-Growth and Young-Growth Riparian Forests in Southeastern Alaska, U.S.A. Can. J. Fish. Aquat. Sci. 1997, 54, 1259–1269. [Google Scholar] [CrossRef]
- Fellows, C.S.; Clapcott, J.E.; Udy, J.W.; Bunn, S.E.; Harch, B.D.; Smith, M.J.; Davies, P.M. Benthic Metabolism as an Indicator of Stream Ecosystem Health. Hydrobiologia 2006, 572, 71–87. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Hendricks, S.P. A Mixing Model Analysis of Stream Solute Dynamics and the Contribution of a Hyporheic Zone to Ecosystem Function*. Freshw. Biol. 2003, 48, 995–1014. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Baattrup-Pedersen, A.; Riis, T.; Friberg, N. Stream Ecosystem Properties and Processes along a Temperature Gradient. Aquat. Ecol. 2011, 45, 231–242. [Google Scholar] [CrossRef]
- Myrstener, M.; Rocher-Ros, G.; Burrows, R.M.; Bergström, A.; Giesler, R.; Sponseller, R.A. Persistent Nitrogen Limitation of Stream Biofilm Communities along Climate Gradients in the Arctic. Glob. Chang. Biol. 2018, 24, 3680–3691. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Feminella, J.W.; Power, M.E. Biotic Interactions in Freshwater Benthic Habitats. J. N. Am. Benthol. Soc. 2010, 29, 220–244. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Callisto, M.; Barbosa, J.E.L.; Firmiano, K.R.; França, J.; Júnior, J.F.G. Top-down and Bottom-up Control of Epilithic Periphyton in a Tropical Stream. Freshw. Sci. 2018, 37, 857–869. [Google Scholar] [CrossRef]
- Effenberger, M.; Diehl, S.; Gerth, M.; Matthaei, C.D. Patchy Bed Disturbance and Fish Predation Independently Influence the Distribution of Stream Invertebrates and Algae: Disturbance, Fish Exclusion and Stream Invertebrates. J. Anim. Ecol. 2011, 80, 603–614. [Google Scholar] [CrossRef]
- Cederholm, C.J.; Kunze, M.D.; Murota, T.; Sibatani, A. Pacific Salmon Carcasses: Essential Contributions of Nutrients and Energy for Aquatic and Terrestrial Ecosystems. Fisheries 1999, 24, 6–15. [Google Scholar] [CrossRef]
- Kaylor, M.J.; White, S.M.; Sedell, E.R.; Sanders, A.M.; Warren, D.R. Carcass Additions Influence Food Webs Through Bottom-Up and Direct Consumption Pathways Along a Fish Species Assemblage Gradient. Ecosystems 2021, 24, 168–184. [Google Scholar] [CrossRef]
- Townsend, S.A.; Garcia, E.A.; Douglas, M.M. The Response of Benthic Algal Biomass to Nutrient Addition over a Range of Current Speeds in an Oligotrophic River. Freshw. Sci. 2012, 31, 1233–1243. [Google Scholar] [CrossRef]
- Elsaholi, M. Nutrient and Light Limitation of Algal Biomass in Selected Streams in Ireland. Inland Waters 2011, 1, 74–80. [Google Scholar] [CrossRef]
- Warren, D.R.; Collins, S.M.; Purvis, E.M.; Kaylor, M.J.; Bechtold, H.A. Spatial Variability in Light Yields Colimitation of Primary Production by Both Light and Nutrients in a Forested Stream Ecosystem. Ecosystems 2017, 20, 198–210. [Google Scholar] [CrossRef]
- Burrows, R.M.; Jonsson, M.; Fältström, E.; Andersson, J.; Sponseller, R.A. Interactive Effects of Light and Nutrients on Stream Algal Growth Modified by Forest Management in Boreal Landscapes. For. Ecol. Manag. 2021, 492, 119212. [Google Scholar] [CrossRef]
- Cummins, K.W.; Klug, M.J. Feeding Ecology of Stream Invertebrates. Annu. Rev. Ecol. Syst. 1979, 10, 147–172. [Google Scholar] [CrossRef]
- Ambrose, H.E.; Wilzbach, M.A.; Cummins, K.W. Periphyton Response to Increased Light and Salmon Carcass Introduction in Northern California Streams. J. N. Am. Benthol. Soc. 2004, 23, 701–712. [Google Scholar] [CrossRef]
- Gjerløv, C.; Richardson, J.S. Experimental Increases and Reductions of Light to Streams: Effects on Periphyton and Macroinvertebrate Assemblages in a Coniferous Forest Landscape. Hydrobiologia 2010, 652, 195–206. [Google Scholar] [CrossRef]
- Yoshimura, M.; Kubota, T. Evaluation of Sunlight Penetration through Riparian Forest and Its Effects on Stream Biota. Glob. Ecol. Conserv. 2022, 34, e02043. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Richardson, J.S.; Bull, J.P. Responses of Periphyton and Insects to Experimental Manipulation of Riparian Buffer Width along Forest Streams. J. Appl. Ecol. 2003, 40, 1060–1076. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Richardson, J.S.; Bull, J.P. Establishing Light as a Causal Mechanism Structuring Stream Communities in Response to Experimental Manipulation of Riparian Buffer Width. J. N. Am. Benthol. Soc. 2004, 23, 542–555. [Google Scholar] [CrossRef]
- Kiffney, P.M. Response of Lotic Producer and Consumer Trophic Levels to Gradients of Resource Supply and Predation Pressure. Oikos 2008, 117, 1428–1440. [Google Scholar] [CrossRef]
- Minkova, T.V.; Devine, W.D.; Martens, K.D. T3 Watershed Experiment in the Olympic Experimental State Forest: 2016-2023 Implementation Report; Washington State Department of Natural Resources: Forest Resources Division, Olympia, WA, USA, 2024. [Google Scholar]
- Naiman, R.J.; Bilby, R.E.; Bisson, P.A. Riparian Ecology and Management in the Pacific Coastal Rain Forest. BioScience 2000, 50, 996. [Google Scholar] [CrossRef]
- Parker, S. AOS Protocol and Procedure: ALG—Periphyton and Phytoplankton Sampling; NEON.DOC.003045vE. 2020.
- Peterson, C.G. Mechanisms of Lotic Microalgal Colonization Following Space-Clearing Disturbances Acting at Different Spatial Scales. Oikos 1996, 77, 417. [Google Scholar] [CrossRef]
- Lemmon, P.E. A Spherical Densiometer For Estimating Forest Overstory Density. For. Sci. 1956, 2, 314–320. [Google Scholar] [CrossRef]
- Comeau, P.G.; Gendron, F.; Letchford, T. A Comparison of Several Methods for Estimating Light under a Paper Birch Mixedwood Stand. Can. J. For. Res. 1998, 28, 1843–1850. [Google Scholar] [CrossRef]
- Parker, S. AOS Protocol and Procedure: INV—Aquatic Macroinvertebrate Sampling. NEON.DOC.003046vE. 2019.
- Martens, K.D.; Connolly, P.J. Juvenile Anadromous Salmonid Production in Upper Columbia River Side Channels with Different Levels of Hydrological Connection. Trans. Am. Fish. Soc. 2014, 143, 757–767. [Google Scholar] [CrossRef]
- Minkova, T.V.; Foster, A.D. Status and Trends Monitoring of Riparian and Aquatic Habitat in the Olympic Experimental State Forest: Monitoring Protocols; Washington State Department of Natural Resources, Forest Resources Division: Olympia, WA, USA, 2017; 363p. [Google Scholar]
- Arar, E.J.; Collins, G.B. Method 445.0, In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence. In Methods for the Determination of Chemical Substances in Marine and Estuarine Environmental Matrices; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Morin, A.; Lamoureux, W.; Busnarda, J. Empirical Models Predicting Primary Productivity from Chlorophyll a and Water Temperature for Stream Periphyton and Lake and Ocean Phytoplankton. J. N. Am. Benthol. Soc. 1999, 18, 299–307. [Google Scholar] [CrossRef]
- Wu, N.; Qu, Y.; Guse, B.; Makarevičiūtė, K.; To, S.; Riis, T.; Fohrer, N. Hydrological and Environmental Variables Outperform Spatial Factors in Structuring Species, Trait Composition, and Beta Diversity of Pelagic Algae. Ecol. Evol. 2018, 8, 2947–2961. [Google Scholar] [CrossRef]
- Wu, S.; Dong, Y.; Stoeck, T.; Wang, S.; Fan, H.; Wang, Y.; Zhuang, X. Geographic Characteristics and Environmental Variables Determine the Diversities and Assembly of the Algal Communities in Interconnected River-Lake System. Water Res. 2023, 233, 119792. [Google Scholar] [CrossRef]
- Kanth Tiwary, R.; Kumar Maiti, S.; Chowdhury, A.; Dora, S.L. Assessment of Pollution Load and Identifying Bioindicator Algal Species Using Multivariate Statistical Techniques: A Case Study from Damodar River, India. Int. J. Environ. Pollut. 2022, 69, 151–178. [Google Scholar] [CrossRef]
- Roesler, C.; Uitz, J.; Claustre, H.; Boss, E.; Xing, X.; Organelli, E.; Briggs, N.; Bricaud, A.; Schmechtig, C.; Poteau, A.; et al. Recommendations for Obtaining Unbiased Chlorophyll Estimates from in Situ Chlorophyll Fluorometers: A Global Analysis of WET Labs ECO Sensors. Limnol. Oceanogr. Methods 2017, 15, 572–585. [Google Scholar] [CrossRef]
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested Classification of Stream Trophic State: Distributions of Temperate Stream Types by Chlorophyll, Total Nitrogen, and Phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. (Eds.) An Introduction to the Aquatic Insects of North America, 5th ed.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2019; ISBN 978-1-5249-6854-0. [Google Scholar]
- Iacobucci, D.; Schneider, M.J.; Popovich, D.L.; Bakamitsos, G.A. Mean Centering Helps Alleviate “Micro” but Not “Macro” Multicollinearity. Behav. Res. Methods 2016, 48, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Modelling for Nested Data. In Mixed Effects Models and Extensions in Ecology with R; Zuur, A.F., Ieno, E.N., Walker, N., Saveliev, A.A., Smith, G.M., Eds.; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; pp. 101–142. ISBN 978-0-387-87458-6. [Google Scholar]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel Inference in Ecology and Evolution: Challenges and Solutions: Multimodel Inference. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect Size, Confidence Interval and Statistical Significance: A Practical Guide for Biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.J.F. Patterns in Benthic Algae of Streams. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. (Eds.) Stream Ecology: Structure and Function of Running Waters, 3rd ed.; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-61285-6. [Google Scholar]
- Volk, C.; Kiffney, P. Comparison of Fatty Acids and Elemental Nutrients in Periphyton, Invertebrates, and Cutthroat Trout (Oncorhynchus clarki) in Conifer and Alder Streams of Western Washington State. Aquat. Ecol. 2012, 46, 85–99. [Google Scholar] [CrossRef]
- Martens, K.D.; Devine, W.D.; Minkova, T.V.; Foster, A.D. Stream Conditions after 18 Years of Passive Riparian Restoration in Small Fish-Bearing Watersheds. Environ. Manag. 2019, 63, 673–690. [Google Scholar] [CrossRef]
- Rosa, J.; Ferreira, V.; Canhoto, C.; Graça, M.A.S. Combined Effects of Water Temperature and Nutrients Concentration on Periphyton Respiration—Implications of Global Change. Int. Rev. Hydrobiol. 2013, 98, 14–23. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, J.; An, R.; Zhang, L.; Chen, M. Influences of Hydrodynamic Conditions on the Biomass of Benthic Diatoms in a Natural Stream. Ecol. Indic. 2018, 92, 51–60. [Google Scholar] [CrossRef]
- Francoeur, S.N.; Biggs, B.J.F. Short-Term Effects of Elevated Velocity and Sediment Abrasion on Benthic Algal Communities. Hydrobiologia 2006, 561, 59–69. [Google Scholar] [CrossRef]
- Saravia, L.A.; Momo, F.; Boffi Lissin, L.D. Modelling Periphyton Dynamics in Running Water. Ecol. Model. 1998, 114, 35–47. [Google Scholar] [CrossRef]
- Horner, R.R.; Welch, E.B.; Seeley, M.R.; Jacoby, J.M. Responses of Periphyton to Changes in Current Velocity, Suspended Sediment and Phosphorus Concentration. Freshw. Biol. 1990, 24, 215–232. [Google Scholar] [CrossRef]
- McIntire, C.D. Some Effects of Current Velocity on Periphyton Communities in Laboratory Streams. Hydrobiologia 1966, 27, 559–570. [Google Scholar] [CrossRef]
- Whitford, L.A. The Current Effect and Growth of Freshwater Algae. Trans. Am. Microsc. Soc. 1960, 79, 302–309. [Google Scholar] [CrossRef]
- Choudhury, M.I.; Yang, X.; Hansson, L.-A. Stream Flow Velocity Alters Submerged Macrophyte Morphology and Cascading Interactions among Associated Invertebrate and Periphyton Assemblages. Aquat. Bot. 2015, 120, 333–337. [Google Scholar] [CrossRef]
- Stevenson, R.J. Effects of Current and Conditions Simulating Autogenically Changing Microhabitats on Benthic Diatom Immigration. Ecology 1983, 64, 1514–1524. [Google Scholar] [CrossRef]
- Labiod, C.; Godillot, R.; Caussade, B. The Relationship between Stream Periphyton Dynamics and Near-Bed Turbulence in Rough Open-Channel Flow. Ecol. Model. 2007, 209, 78–96. [Google Scholar] [CrossRef]
- Smith, R.A.; Duncan, M.J. Velocity and Sediment Disturbance of Periphyton in Headwater Streams: Biomass and Metabolism. J. N. Am. Benthol. Soc. 1999, 18, 222–241. [Google Scholar] [CrossRef]
- Irving, K.; Taniguchi-Quan, K.T.; Aprahamian, A.; Rivers, C.; Sharp, G.; Mazor, R.D.; Theroux, S.; Holt, A.; Peek, R.; Stein, E.D. Application of Flow-Ecology Analysis to Inform Prioritization for Stream Restoration and Management Actions. Front. Environ. Sci. 2022, 9, 787462. [Google Scholar] [CrossRef]
- Hillebrand, H. Top-down versus Bottom-up Control of Autotrophic Biomass—A Meta-Analysis on Experiments with Periphyton. J. N. Am. Benthol. Soc. 2002, 21, 349–369. [Google Scholar] [CrossRef]
- Honeyfield, D.C.; Maloney, K.O. Seasonal Patterns in Stream Periphyton Fatty Acids and Community Benthic Algal Composition in Six High-Quality Headwater Streams. Hydrobiologia 2015, 744, 35–47. [Google Scholar] [CrossRef]
- Pniewski, F.; Sylwestrzak, Z. Influence of Short Periods of Increased Water Temperature on Species Composition and Photosynthetic Activity in the Baltic Periphyton Communities. Biologia 2018, 73, 1067–1072. [Google Scholar] [CrossRef]


| Parameter | β | p-Value |
|---|---|---|
| Water temperature | 0.3459 | 0.0085 |
| Maximum water velocity | −0.0139 | 0.0053 |
| Parameter | β | p-Value |
|---|---|---|
| Water temperature | 0.2979 | 0.0160 |
| Macroinvertebrate predator abundance | 0.0119 | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toskey, E.K.; Bollens, S.M.; Rollwagen-Bollens, G.; Kiffney, P.M.; Martens, K.D.; Bormann, B.T. Stream Algal Biomass Associations with Environmental Variables in a Temperate Rainforest. Water 2024, 16, 1533. https://doi.org/10.3390/w16111533
Toskey EK, Bollens SM, Rollwagen-Bollens G, Kiffney PM, Martens KD, Bormann BT. Stream Algal Biomass Associations with Environmental Variables in a Temperate Rainforest. Water. 2024; 16(11):1533. https://doi.org/10.3390/w16111533
Chicago/Turabian StyleToskey, Elsa K., Stephen M. Bollens, Gretchen Rollwagen-Bollens, Peter M. Kiffney, Kyle D. Martens, and Bernard T. Bormann. 2024. "Stream Algal Biomass Associations with Environmental Variables in a Temperate Rainforest" Water 16, no. 11: 1533. https://doi.org/10.3390/w16111533
APA StyleToskey, E. K., Bollens, S. M., Rollwagen-Bollens, G., Kiffney, P. M., Martens, K. D., & Bormann, B. T. (2024). Stream Algal Biomass Associations with Environmental Variables in a Temperate Rainforest. Water, 16(11), 1533. https://doi.org/10.3390/w16111533









