Effect of Fermentation Time, pH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Inoculum
2.3. Acidogenic Fermentation Experiments
2.4. Experimental Design and Statistical Analyses
2.5. Analytical Methods
3. Results and Discussion
3.1. Physicochemical Characterization of CWW and Inoculum Solids Concentration
3.2. Effect of Fermentation Time on VFA Production
3.2.1. VFA Production, Yields, and Substrate Uptake
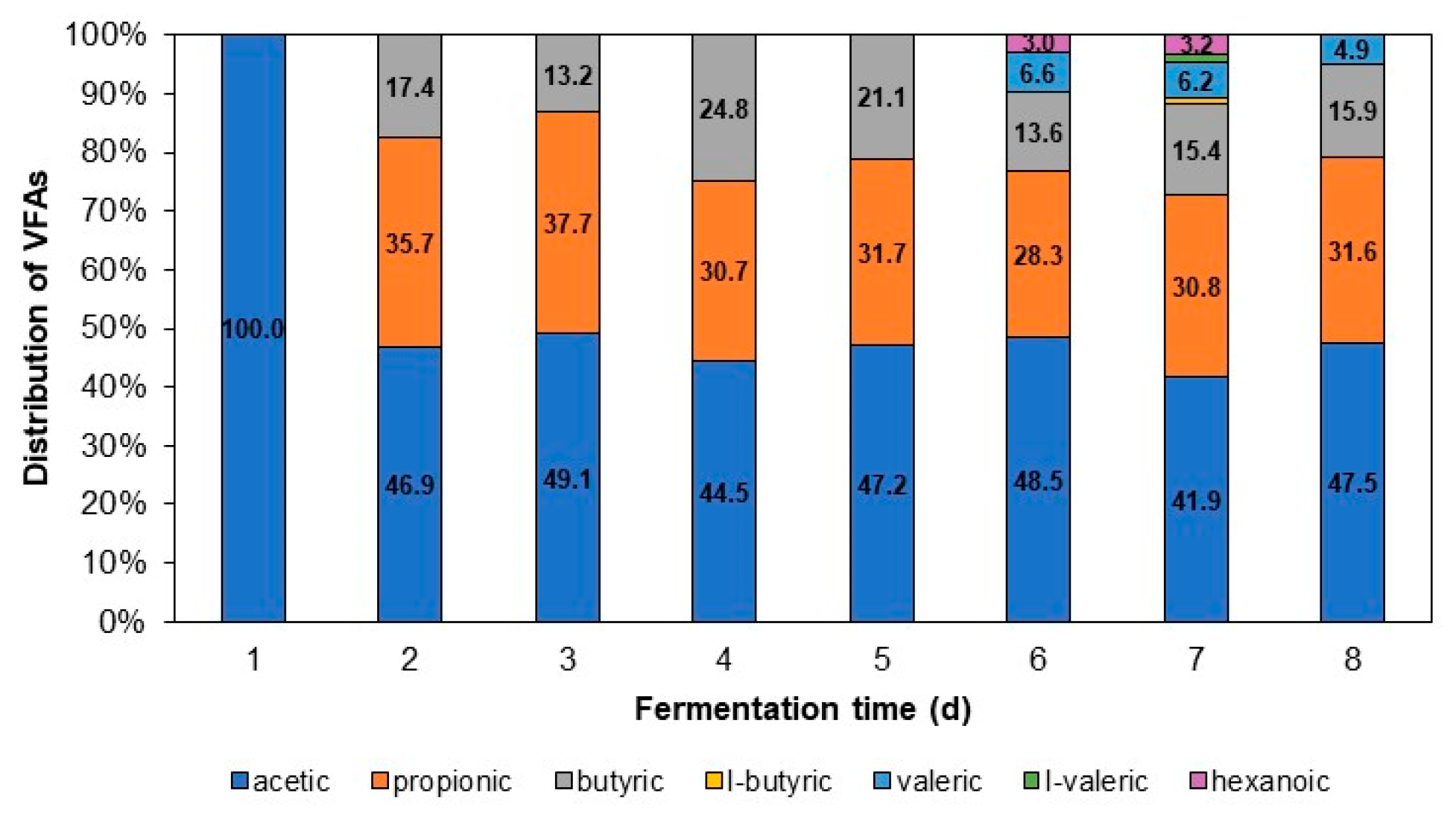
3.2.2. VFA Distribution for Different Fermentation Times
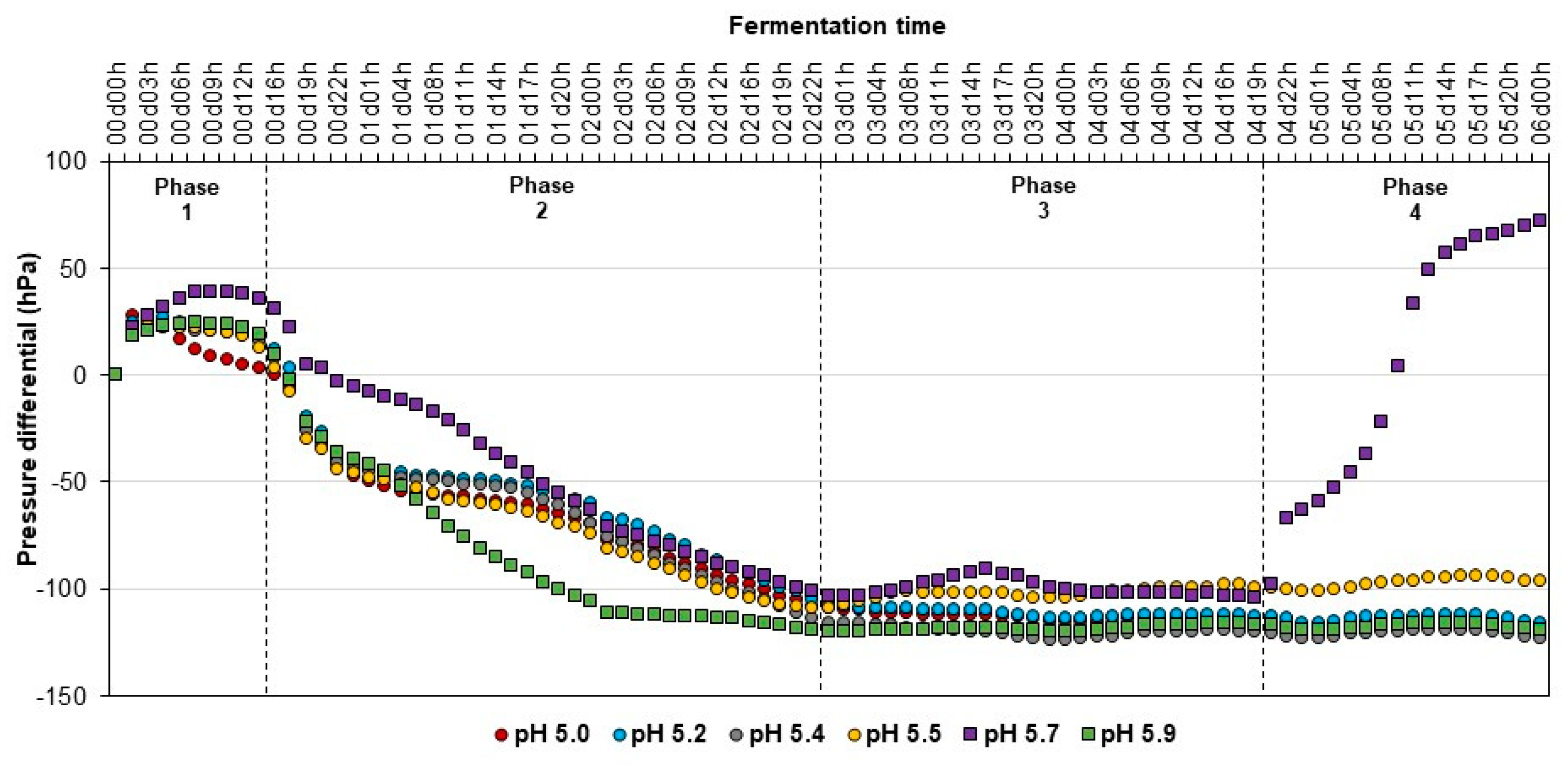
3.2.3. Variation in pH and Internal Pressure in Acidogenic Reactors
3.3. Effect of Initial pH on VFA Production
3.3.1. VFA Production, Yields, and Substrate Uptake
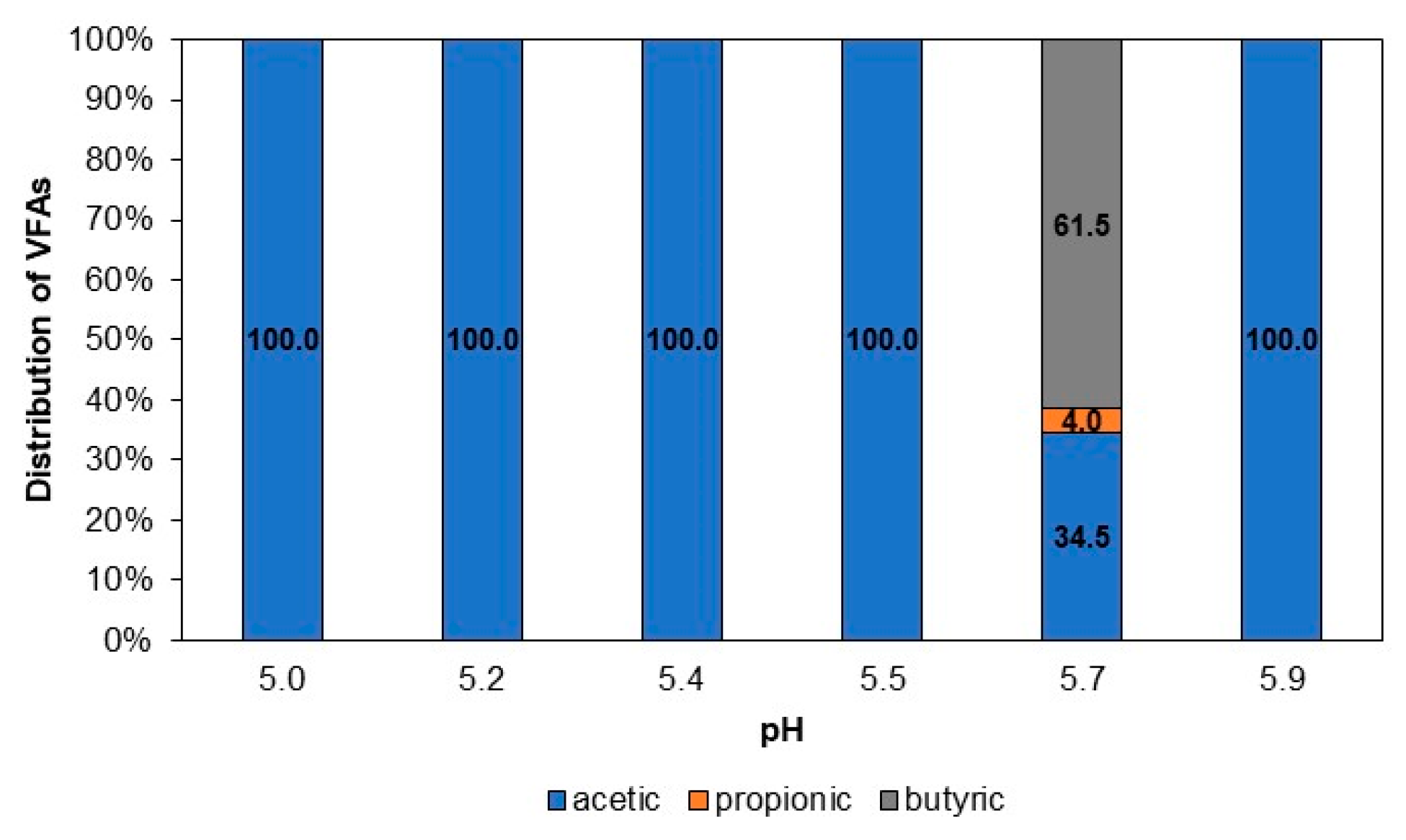
3.3.2. VFA Distribution for Different pH Values
3.3.3. Variation in pH and Internal Pressure in Acidogenic Reactors
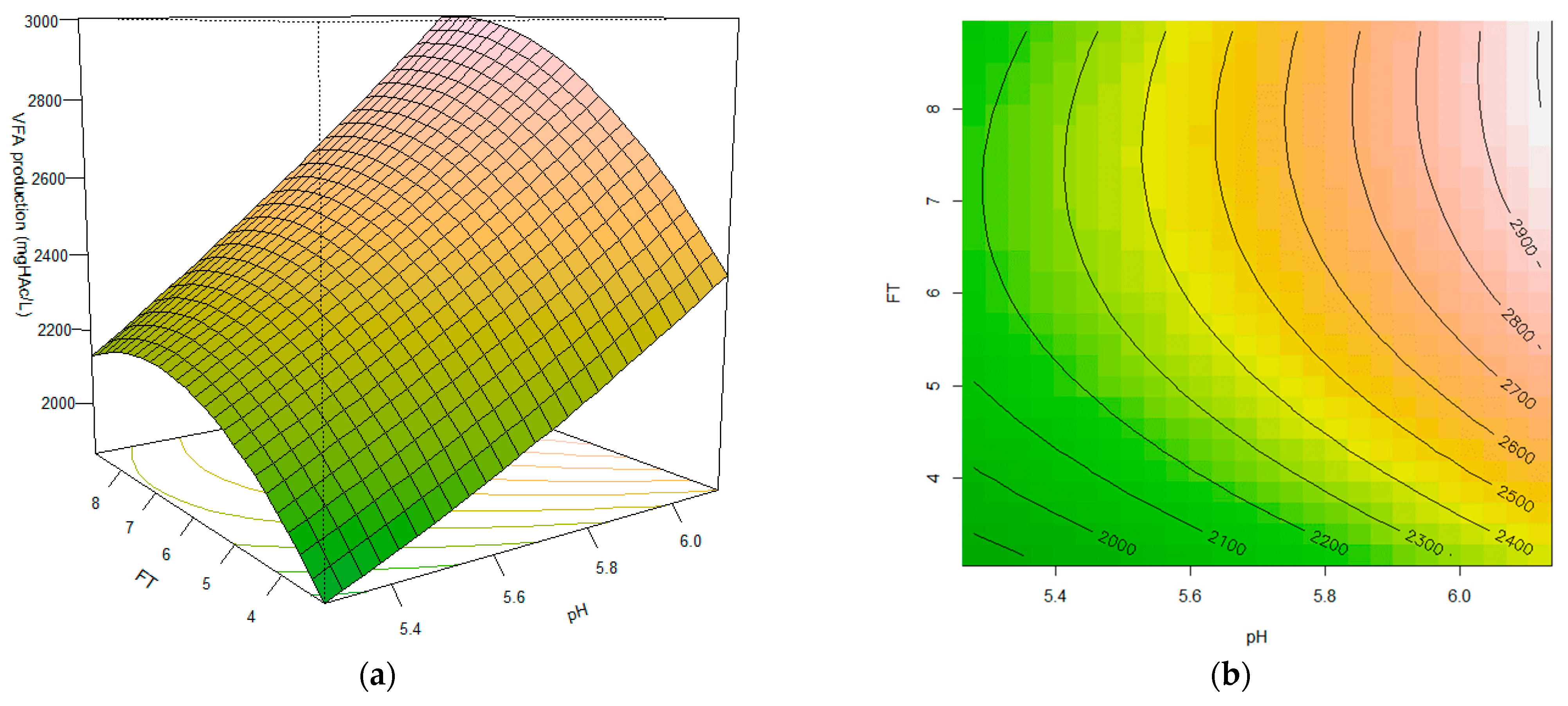
3.4. Effect of the Interaction among Fermentation Time and pH Variables on VFA Production
3.5. Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aristizábal, J.; Sánchez, T. Guía Técnica Para Producción y Análisis de Almidón de Yuca; Organización de las Naciones Unidas para la Agricultura y la Alimentación: Roma, Italy, 2007; Volume 163. [Google Scholar]
- FAOSTAT Cultivos y Productos de Ganadería. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 15 December 2023).
- Nnaji, C.C.; Akanno, C.C. Assessment of Environmental Degradation Due to Processing of Cassava into Garri Flakes Using Pollution Indices. Environ. Process. 2022, 9, 46. [Google Scholar] [CrossRef]
- Torres, P.; Rodríguez, J.; Rojas, O. Extracción de Almidón de Yuca. Manejo Integral y Control de La Contaminación Hídrica. Livest. Res. Rural Dev. 2005, 17, 74. [Google Scholar]
- De Amorim, M.C.C.; De Souza Silva, P.T.; Barbosa, P.S.; Montefusco, N.E. Anaerobic Biodegradation of Cassava Wastewater under Different Temperatures and Inoculums. Comun. Sci. 2019, 10, 65–76. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Saipa, S.; Suaisom, P.; Mai, C.; Mai, C. Methane Recovery from Cassava Starch Wastewater via Anaerobic Digestion: Effect of Inoculum Source and Kinetic Study. Asia-Pac. J. Sci. Technol. 2021, 26, 2. [Google Scholar] [CrossRef]
- da Silva, D.B.; Fenandes, B.S.; da Silva, A.J. Effect of Initial PH and Substrate Concentration on the Lactic Acid Production from Cassava Wastewater Fermentation by an Enriched Culture of Acidogenic Microorganisms. Water Environ. Res. 2021, 93, 1925–1933. [Google Scholar] [CrossRef]
- Niz, M.Y.K.; Formagini, E.L.; Boncz, M.À.; Paulo, P.L. Acidogenic Fermentation of Cassava Wastewater for Volatile Fatty Acids Production. Int. J. Environ. Waste Manag. 2020, 25, 245–261. [Google Scholar] [CrossRef]
- Hasan, S.D.M.; Giongo, C.; Fiorese, M.L.; Gomes, S.D.; Ferrari, T.C.; Savoldi, T.E. Volatile Fatty Acids Production from Anaerobic Treatment of Cassava Waste Water: Effect of Temperature and Alkalinity. Environ. Technol. 2015, 36, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- de Sousa e Silva, A.; Morais, N.W.S.; Coelho, M.M.H.; Pereira, E.L.; dos Santos, A.B. Potentialities of Biotechnological Recovery of Methane, Hydrogen and Carboxylic Acids from Agro-Industrial Wastewaters. Bioresour. Technol. Rep. 2020, 10, 100406. [Google Scholar] [CrossRef]
- Dionisi, D.; Silva, I.M.O. Production of Ethanol, Organic Acids and Hydrogen: An Opportunity for Mixed Culture Biotechnology? Rev. Environ. Sci. Biotechnol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Sun, L.; Gong, M.; Lv, X.; Huang, Z.; Gu, Y.; Li, J.; Du, G.; Liu, L. Current Advance in Biological Production of Short-Chain Organic Acid. Appl. Microbiol. Biotechnol. 2020, 104, 9109–9124. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Lingam, Y.; Venkata Mohan, S. Understanding Acidogenesis towards Green Hydrogen and Volatile Fatty Acid Production—Critical Analysis and Circular Economy Perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Nizzy, A.M.; Kannan, S. A Review on the Conversion of Cassava Wastes into Value-Added Products towards a Sustainable Environment. Environ. Sci. Pollut. Res. 2022, 29, 69223–69240. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Cetecioglu, Z. The Effects of PH on the Production of Volatile Fatty Acids and Microbial Dynamics in Long-Term Reactor Operation. J. Environ. Manag. 2022, 319, 115700. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, F.; Wang, Z.; Wu, D.; Xing, T.; Kong, X.; Sun, Y. Impact of PH, Temperature, and Hydraulic Residence Time on the Acidogenic Fermentation of Fruit and Vegetable Waste and Microbial Community Analysis. J. Chem. Technol. Biotechnol. 2023, 98, 819–828. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, R.; Huang, L.; Wang, X.; Chou, S.; Zhu, J. Acidogenic Fermentation of Potato Peel Waste for Volatile Fatty Acids Production: Effect of Initial Organic Load. J. Biotechnol. 2023, 374, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. PH and Hydraulic Retention Time Regulation for Anaerobic Fermentation: Focus on Volatile Fatty Acids Production/Distribution, Microbial Community Succession and Interactive Correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile Fatty Acids Production during Mixed Culture Fermentation—The Impact of Substrate Complexity and PH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Infantes, D.; Gonzáles del Campo, A.; Villaseñor, J.; Fernández, F.J. Kinetic Model and Study of the Influence of PH, Temperature and Undissociated Acids on Acidogenic Fermentation. Biochem. Eng. J. 2012, 66, 66–72. [Google Scholar] [CrossRef]
- Tamis, J.; Joosse, B.M.; van Loosdrecht, M.C.M.; Kleerebezem, R. High-Rate Volatile Fatty Acid (VFA) Production by a Granular Sludge Process at Low PH. Biotechnol. Bioeng. 2015, 112, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Mañunga, T. Acople Entre Un Reactor Anaerobio de Medio Suspendido y Un Reactor Anaerobio de Crecimiento Adherido Para La Producción de Hidrógeno y Metano a Partir de Agua Residual Del Proceso de Extracción de Almidón de Yuca. Ph.D. Thesis, Universidad del Valle, Cali, Colombia, 2019. [Google Scholar]
- Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- Bolaji, I.O.; Dionisi, D. Acidogenic Fermentation of Vegetable and Salad Waste for Chemicals Production: Effect of PH Buffer and Retention Time. J. Environ. Chem. Eng. 2017, 5, 5933–5943. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- DiLallo, R.; Albertson, O. Volatile Acids by Direct Titration. Water Pollut. Control Fed. 1961, 33, 356–365. [Google Scholar]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of PH and Retention Time on Volatile Fatty Acids Production during Mixed Culture Fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, J.I.; Shimizu, T.; Tada, K.; Kanno, T.; Kobayashi, M. Selective Production of Organic Acids in Anaerobic Acid Reactor by PH Control. Bioresour. Technol. 2002, 82, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic Fermentation of Industrial Wastewaters: Effects of Chemostat Retention Time and PH on Volatile Fatty Acids Production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Colin, X.; Farinet, J.L.; Rojas, O.; Alazard, D. Anaerobic Treatment of Cassava Starch Extraction Wastewater Using a Horizontal Flow Filter with Bamboo as Support. Bioresour. Technol. 2007, 98, 1602–1607. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Archilla, J.; Papirio, S.; Lens, P.N.L. Two Step Process for Volatile Fatty Acid Production from Brewery Spent Grain: Hydrolysis and Direct Acidogenic Fermentation Using Anaerobic Granular Sludge. Process Biochem. 2021, 100, 272–283. [Google Scholar] [CrossRef]
- Cohen, A.; Van Gemert, J.M.; Zoetemeyer, R.J.; Breure, A.M. Main Characteristics and Stoichiometric Aspects of Acidogenesis of Soluble Carbohydrate Containing Wastewaters. Process Biochem. 1984, 19, 228–232. [Google Scholar]
- Liu, C.; Ren, L.; Yan, B.; Luo, L.; Zhang, J.; Awasthi, M.K. Electron Transfer and Mechanism of Energy Production among Syntrophic Bacteria during Acidogenic Fermentation: A Review. Bioresour. Technol. 2021, 323, 124637. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Energetics and Application of Heterotrophy in Acetogenic Bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Salerno, M.B.; Rittmann, B.E. Thermodynamic Evaluation on H2 Production in Glucose Fermentation. Environ. Sci. Technol. 2008, 42, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Feng, L.; Zhang, C.; Wisniewski, C.; Zhou, Q. Ultrasonic Enhancement of Waste Activated Sludge Hydrolysis and Volatile Fatty Acids Accumulation at PH 10.0. Water Res. 2010, 44, 3329–3336. [Google Scholar] [CrossRef]
- de Oliveira Schmidt, V.K.; de Vasconscelos, G.M.D.; Vicente, R.; de Souza Carvalho, J.; Della-Flora, I.K.; Degang, L.; de Oliveira, D.; de Andrade, C.J. Cassava Wastewater Valorization for the Production of Biosurfactants: Surfactin, Rhamnolipids, and Mannosileritritol Lipids. World J. Microbiol. Biotechnol. 2023, 39, 65. [Google Scholar] [CrossRef]
- Yu, H.G.; Fang, H.H. Acidogenesis of Dairy Wastewater at Various PH Levels. Water Sci. Technol. 2002, 45, 201–206. [Google Scholar] [CrossRef]
- Fernández, F.J.; Villaseñor, J.; Infantes, D. Kinetic and Stoichiometric Modelling of Acidogenic Fermentation of Glucose and Fructose. Biomass Bioenergy 2011, 35, 3877–3883. [Google Scholar] [CrossRef]
- Amorim, N.C.S.; Amorim, E.L.C.; Kato, M.T.; Florencio, L.; Gavazza, S. The Effect of Methanogenesis Inhibition, Inoculum and Substrate Concentration on Hydrogen and Carboxylic Acids Production from Cassava Wastewater. Biodegradation 2018, 29, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; De Wever, H.; Buisman, C.J.N.; Hamelers, H.V.M. Effect of Hydrogen and Carbon Dioxide on Carboxylic Acids Patterns in Mixed Culture Fermentation. Bioresour. Technol. 2012, 118, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, X.; Chen, H.; Zhou, Y.; Zhou, Y.; Wang, D. Advances in Enhanced Volatile Fatty Acid Production from Anaerobic Fermentation of Waste Activated Sludge. Sci. Total Environ. 2019, 694, 133741. [Google Scholar] [CrossRef]
- Pabón Pereira, C.P.; Castañares, G.; Van Lier, J.B. An OxiTop® Protocol for Screening Plant Material for Its Biochemical Methane Potential (BMP). Water Sci. Technol. 2012, 66, 1416–1423. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological Hydrogen Production: Effects of PH and Intermediate Products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, J.; Tan, M.; Du, J.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced Carboxylic Acids Production by Decreasing Hydrogen Partial Pressure during Acidogenic Fermentation of Glucose. Bioresour. Technol. 2017, 245, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Soccol, V.T.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Biohydrogen Production in Cassava Processing Wastewater Using Microbial Consortia: Process Optimization and Kinetic Analysis of the Microbial Community. Bioresour. Technol. 2020, 309, 123331. [Google Scholar] [CrossRef] [PubMed]
- Souto, T.F.; Aquino, S.F.; Silva, S.Q.; Chernicharo, C.A.L. Influence of Incubation Conditions on the Specific Methanogenic Activity Test. Biodegradation 2010, 21, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Hilkiah Igoni, A.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of Anaerobic Digesters for Producing Biogas from Municipal Solid-Waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Dinamarca, S.; Aroca, G.; Chamy, R.; Guerrero, L. The Influence of PH in the Hydrolytic Stage of Anaerobic Digestion of the Organic Fraction of Urban Solid Waste. Water Sci. Technol. 2003, 48, 249–254. [Google Scholar] [CrossRef]
- Norli, I.; Ho, Y.C.; Fischer, K.; Kranert, M.; Boley, A. Biodegradability Assessment of Bio-Flocculant via Anaerobic and Aerobic Test. In Proceedings of the International Conference on Environment Science and Engineering (ICESE), Bali, Indonesia, 1–3 April 2011; Volume 7, pp. 189–193. [Google Scholar]
- Barrios Pérez, J.D. Influencia Del PH Sobre La Producción Biológica de Hidrógeno a Partir de Agua Residual Agroindustrial. Bachelor’s Thesis, Universidad del Valle, Cali, Colombia, 2015. [Google Scholar]
- Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T.; De Gioannis, G.; Muntoni, A. Continuous Biohydrogen Production from Cheese Whey—Part 1: New Insights into Process Stability. J. Clean. Prod. 2022, 47, 21044–21059. [Google Scholar] [CrossRef]
- Torres Lozada, P.; Pérez Vidal, A.; Cajigas, Á.A.; Otero, A.M.; González, M. Selección de Acondicionadores Químicos Para El Tratamiento Anaerobio de Aguas Residuales Del Proceso de Extracción de Almidón de Yuca; Universidad del Valle: Cali, Colombia, 2008. [Google Scholar]
- Huang, Y.X.; Guo, J.; Zhang, C.; Hu, Z. Hydrogen Production from the Dissolution of Nano Zero Valent Iron and Its Effect on Anaerobic Digestion. Water Res. 2016, 88, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of PH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Barana, A.C.; Cereda, M.P. Cassava Wastewater (Manipueira) Treatment Using a Two-Phase Anaerobic Biodigestor. Ciência e Tecnol. Aliment. 2000, 20, 1–7. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile Fatty Acids Production via Mixed Culture Fermentation: Revealing the Link between PH, Inoculum Type and Bacterial Composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Temudo, M.F.; Kleerebezem, R.; van Loosdrecht, M. Influence of the PH on (Open) Mixed Culture Fermentation of Glucose: A Chemostat Study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Fuess, L.T.; Bovio-Winkler, P.; Etchebehere, C.; Sakamoto, I.K.; Zaiat, M. Optimization of Volatile Fatty Acid Production by Sugarcane Vinasse Dark Fermentation Using a Response Surface Methodology. Links between Performance and Microbial Community Composition. Sustain. Energy Technol. Assess. 2022, 53, 102764. [Google Scholar] [CrossRef]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J.O. Resource Recovery from Wastewater by Biological Technologies: Opportunities, Challenges, and Prospects. Front. Microbiol. 2017, 7, 2106. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of Food Waste to Volatile Fatty Acids: Impact of Microbial Community, PH and Retention Time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef]
- Xiao, B.; Han, Y.; Liu, J. Evaluation of Biohydrogen Production from Glucose and Protein at Neutral Initial PH. Renew. Energy 2010, 35, 6152–6160. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A.; O-Thong, S. Biohydrogen Production from Dual Digestion Pretreatment of Poultry Slaughterhouse Sludge by Anaerobic Self-Fermentation. Int. J. Hydrogen Energy 2010, 35, 13427–13434. [Google Scholar] [CrossRef]
- Pittmann, T.; Steinmetz, H. Influence of Operating Conditions for Volatile Fatty Acids Enrichment as a First Step for Polyhydroxyalkanoate Production on a Municipal Waste Water Treatment Plant. Bioresour. Technol. 2013, 148, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Chávez, B. Evaluación Del Desarrollo de La Actividad Sulfatorreductora En Un Lodo Granular Metanogénico de Diferentes Tamaños. Master’s Thesis, Instituto Potosino de Investigación Científica y Tecnológica, San Luis Potosí, Mexico, 2007. [Google Scholar]




| Experiment | E1 | E2 |
|---|---|---|
| Variable evaluated | FT | pH |
| Evaluated conditions | 1, 2, 3, 4, 5, 6, 7 and 8 d | 5.0, 5.2, 5.4, 5.5, 5.7 and 5.9 |
| Adjusted conditions | S/M: 4 gCOD/gVS pH: 5.4 T: 34 ± 1 °C | S/M: 4 gCOD/gVS T: 34 ± 1 °C FT: 6 d |
| Replicates | 3 | 3 |
| Treatment | Factors | Codified Factors | Replicates | ||
|---|---|---|---|---|---|
| pH | FT | pH | FT | ||
| 1 | 5.40 | 4.00 | −1 | −1 | 2 |
| 2 | 6.00 | 4.00 | 1 | −1 | 2 |
| 3 | 5.40 | 8.00 | −1 | 1 | 2 |
| 4 | 6.00 | 8.00 | 1 | 1 | 2 |
| 5 | 5.70 | 6.00 | 0 | 0 | 4 |
| 6 | 5.70 | 8.83 | 0 | 1.414 | 2 |
| 7 | 5.70 | 3.17 | 0 | −1.414 | 2 |
| 8 | 6.12 | 6.00 | 1.414 | 0 | 2 |
| 9 | 5.28 | 6.00 | −1.414 | 0 | 2 |
| Parameter | Units | E1 | E2 | E3 |
|---|---|---|---|---|
| pH | 4.29 | 4.44 | 4.47 | |
| TCOD | mg/L | 5000.00 | 4775.00 | 5000.00 |
| SCOD | mg/L | 4580.00 | 4580.00 | 4700.00 |
| VFAs | mgHAc/L | 1749.58 | 1553.31 | 1265.57 |
| Carbohydrates | mg/L | 546.34 | 1658.54 | 1776.42 |
| Total alkalinity | mgCaCO3/L | 0 | 162.72 | 100.12 |
| Bicarbonate alkalinity | mgCaCO3/L | 0 | 0 | 0 |
| Total acidity | mgCaCO3/L | 1173.55 | 822.60 | 853.67 |
| TS | mg/L | 6310.00 | 5520.00 | 5230.00 |
| VS | mg/L | 4480.00 | 4010.00 | 3715.00 |
| Ammonia nitrogen | mgNH4+/L | 60.05 | 57.85 | 76.10 |
| Orthophosphates | mgPO43−/L | 0.62 | 1.07 | 0.80 |
| Parameter | Units | E1 | E2 | E3 |
|---|---|---|---|---|
| TS | g/L | 57.63 | 105.73 | 63.93 |
| VS | g/L | 41.31 | 52.56 | 53.52 |
| Source | df | Sum of Squares | Mean Square | F-Value | ρ-Value |
|---|---|---|---|---|---|
| Treatment | 7 | 1,869,472 | 267,067 | 28.087 | 5.064 × 10−5 |
| Residuals | 8 | 76,068 | 9508 |
| Source | df | Sum of Squares | Mean Square | F-Value | ρ-Value |
|---|---|---|---|---|---|
| Treatment | 5 | 461,052 | 92,210 | 4.0958 | 0.0211 |
| Residuals | 12 | 270,162 | 22,513 |
| Treatment | Factors | VFA Production (mgHAc/L) | Yields gCODVFA/gCOD | |
|---|---|---|---|---|
| pH | FT | |||
| 1 | 5.40 | 4.00 | 1980.40 | 0.21 |
| 2 | 6.00 | 4.00 | 2428.92 | 0.34 |
| 3 | 5.40 | 8.00 | 2202.23 | 0.27 |
| 4 | 6.00 | 8.00 | 2820.41 | 0.46 |
| 5 | 5.70 | 6.00 | 2486.98 | 0.36 |
| 6 | 5.70 | 8.83 | 2601.37 | 0.39 |
| 7 | 5.70 | 3.17 | 2165.37 | 0.26 |
| 8 | 6.12 | 6.00 | 2913.59 | 0.48 |
| 9 | 5.28 | 6.00 | 2248.12 | 0.29 |
| Source | Sum of Squares | F-Value | ρ-Value |
|---|---|---|---|
| Model | 6.855 | 0.001982 | |
| Linear terms | 1,386,605 | 15.9045 | 0.000249 |
| Interaction term | 14,393 | 0.3302 | 0.574671 |
| Quadratic terms | 93,047 | 1.0673 | 0.370346 |
| Lack of fit | 65,337 | 0.4396 | 0.729221 |
| Pure error | 544,947 | ||
| R2 | 0.71 | ||
| Adjusted R2 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Ledesma, L.M.; Rodríguez-Victoria, J.A.; Ramírez-Malule, H. Effect of Fermentation Time, pH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater. Water 2024, 16, 1514. https://doi.org/10.3390/w16111514
Sanchez-Ledesma LM, Rodríguez-Victoria JA, Ramírez-Malule H. Effect of Fermentation Time, pH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater. Water. 2024; 16(11):1514. https://doi.org/10.3390/w16111514
Chicago/Turabian StyleSanchez-Ledesma, Lina Marcela, Jenny Alexandra Rodríguez-Victoria, and Howard Ramírez-Malule. 2024. "Effect of Fermentation Time, pH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater" Water 16, no. 11: 1514. https://doi.org/10.3390/w16111514
APA StyleSanchez-Ledesma, L. M., Rodríguez-Victoria, J. A., & Ramírez-Malule, H. (2024). Effect of Fermentation Time, pH, and Their Interaction on the Production of Volatile Fatty Acids from Cassava Wastewater. Water, 16(11), 1514. https://doi.org/10.3390/w16111514






