The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
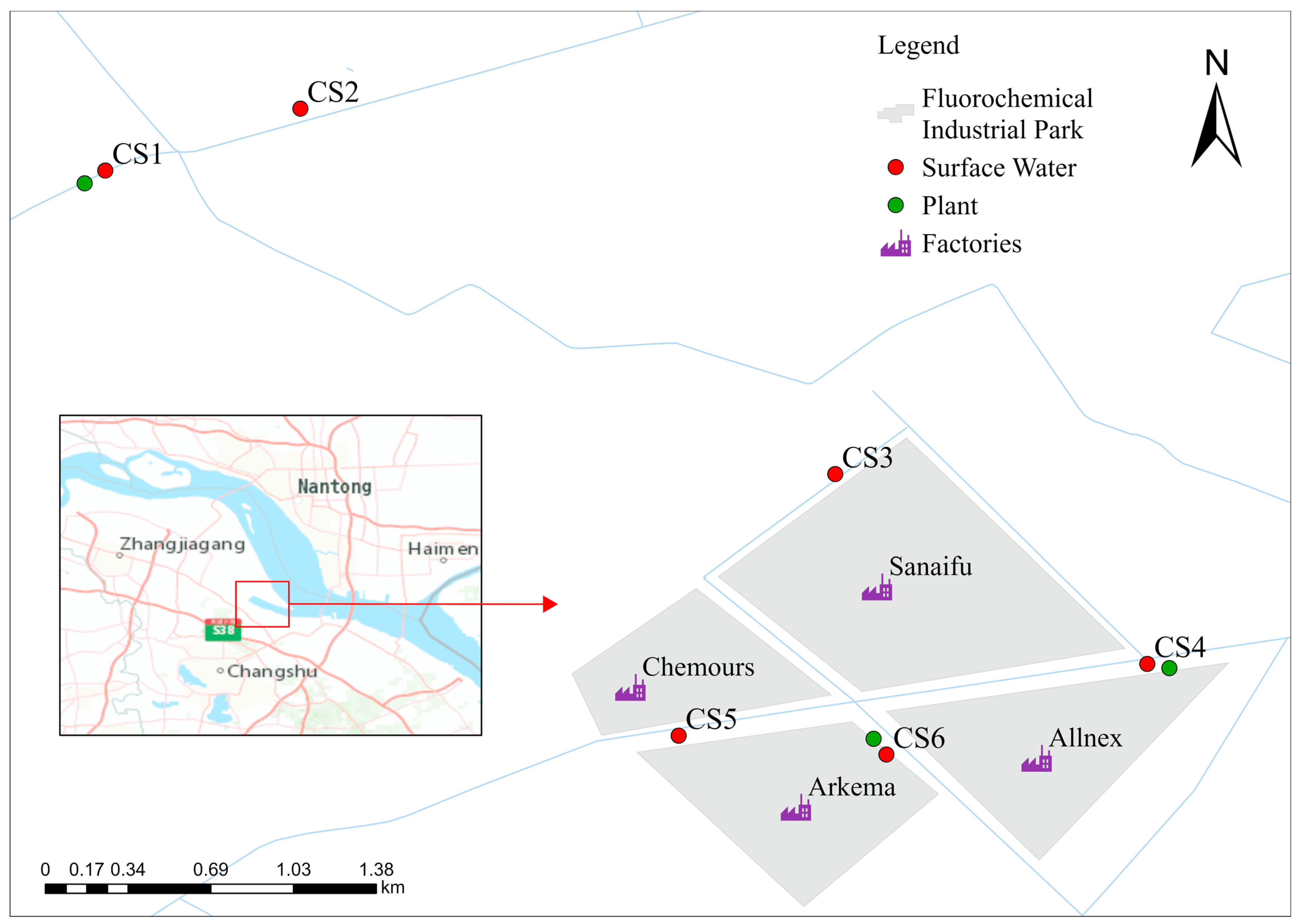
2.2. Sampling Campaign
2.3. PFAS Extraction
2.4. Target Analysis
2.5. Suspect and Nontarget Screening
2.6. Quality Assurance and Quality Control (QA/QC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. PFASs in Surface Water
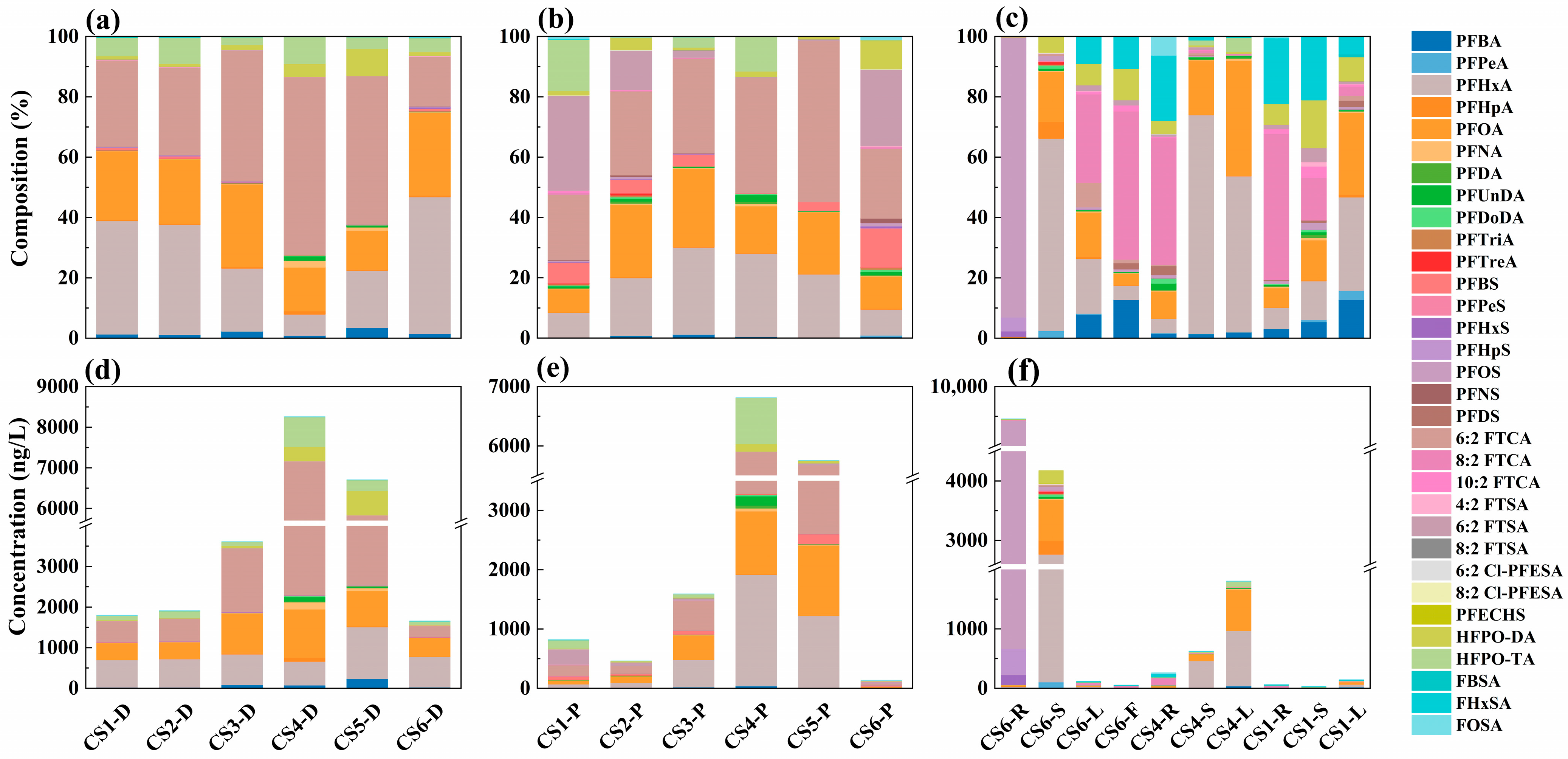
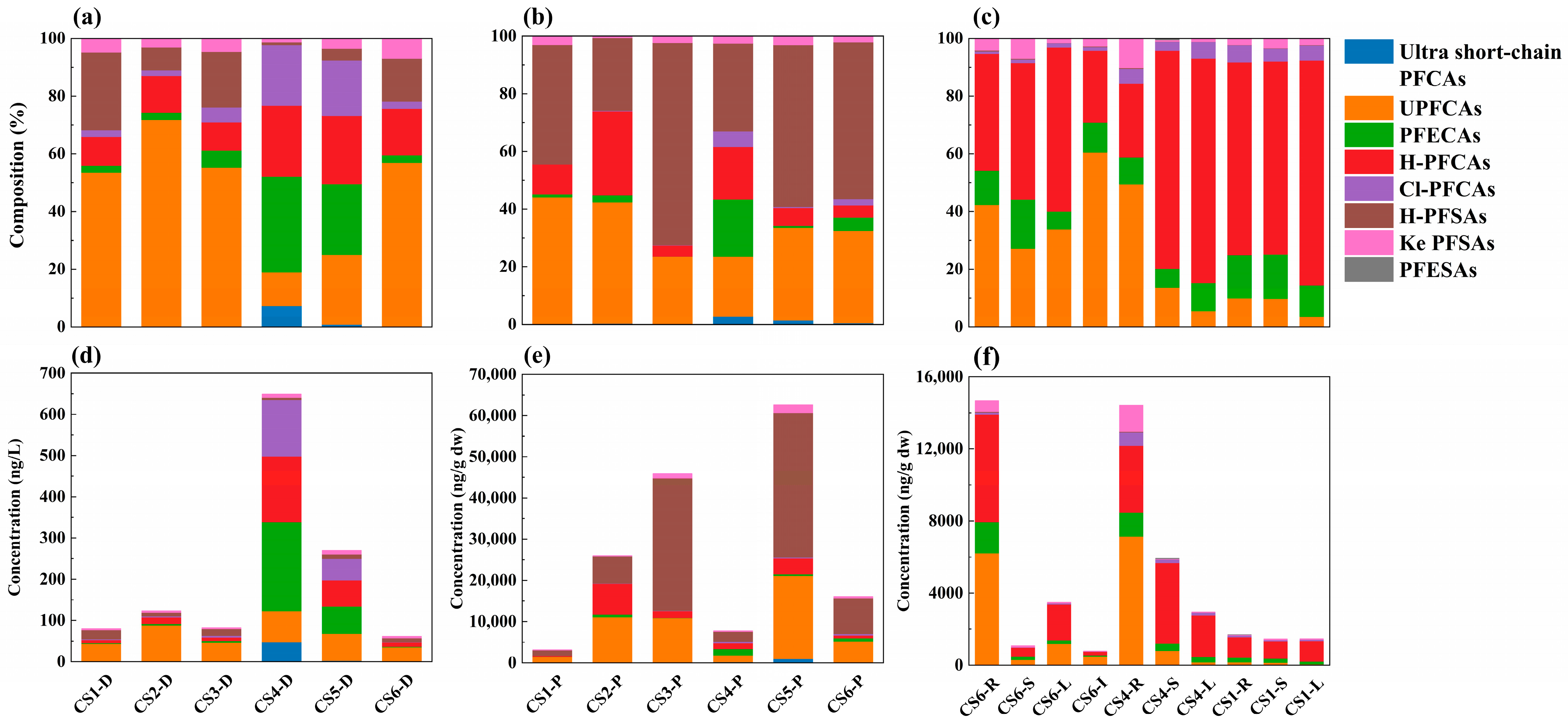
3.1.1. Concentration and Distribution of PFASs in Surface Water
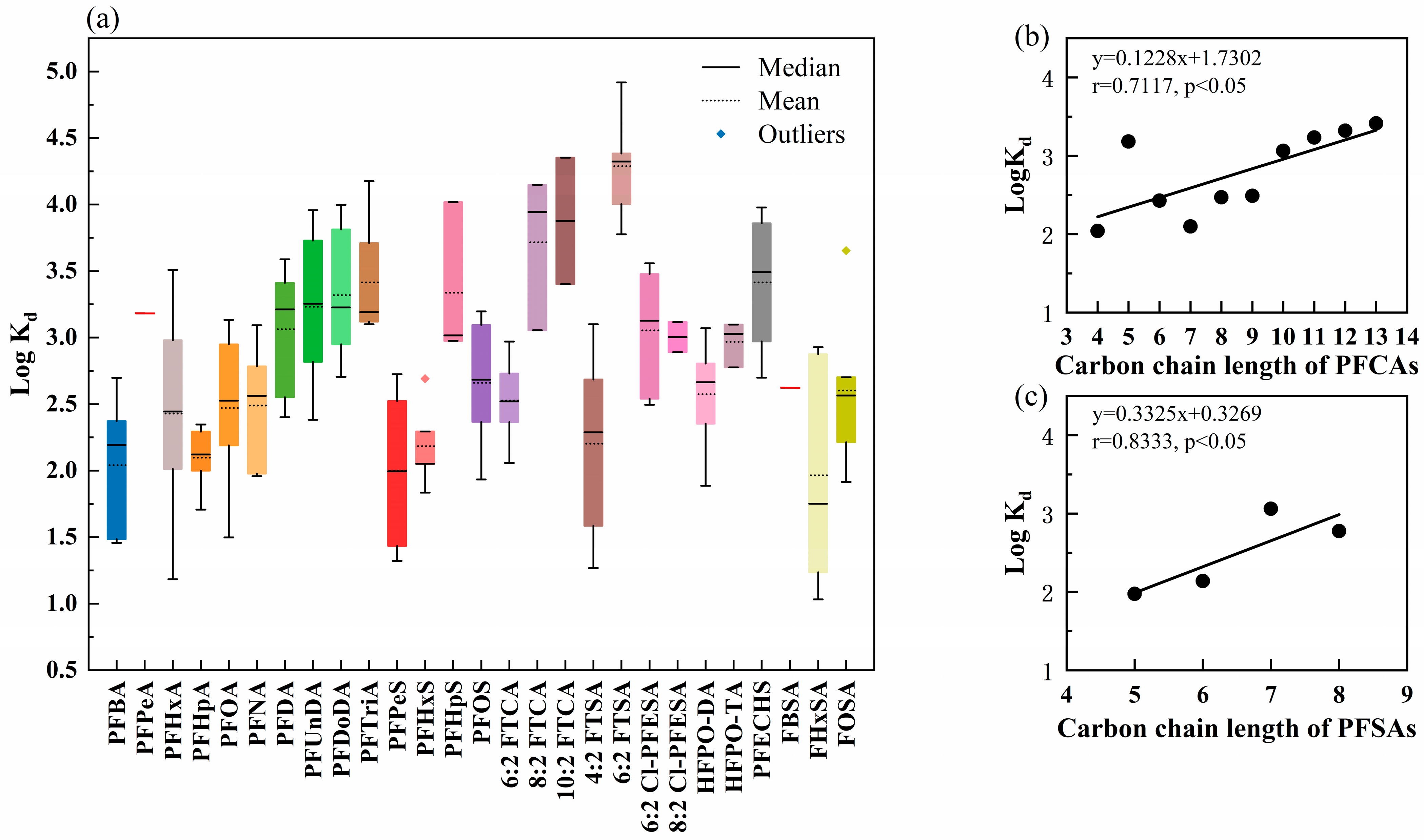
3.1.2. Partitioning of PFASs in Dissolved Phase and Particle Phase
3.2. PFASs in Aquatic Plants
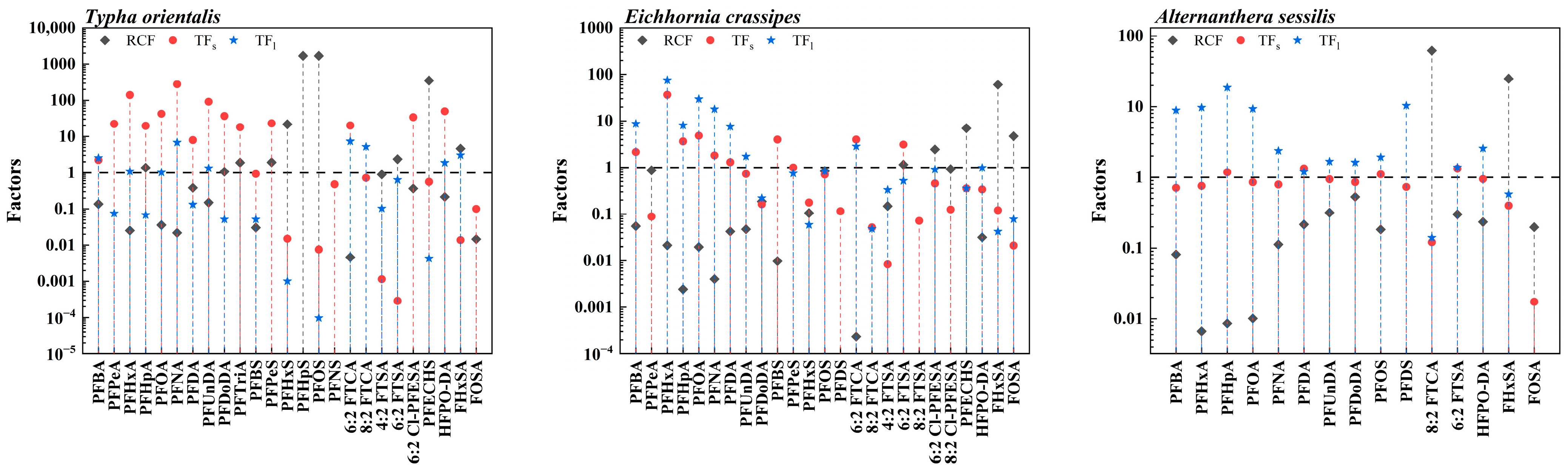
3.2.1. Absorption of PFASs in Aquatic Plants
3.2.2. Transport of PFASs in Aquatic Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhuoma, D. Preparation of Metal-Organic Framework Composite Nanofibers and Application in Perfluoroalkyl and Polyfluoroalkyl Substances Analysis. Master’s Thesis, Wuhan University, Wuhan, China, 2022. [Google Scholar]
- ECHA. Annex Xv Restriction Report—Per- and Polyfluoroalkyl Substances (PFASs); ECHA: Helsinki, Finland, 2023. [Google Scholar]
- He, Q.; Yan, Z.; Zhi, Y.; Qian, S.; Wang, X.; Chen, Y.; Liu, C.; Chen, C.; Hu, X. Research Progress on Bioaccumulation of Per- and Polyfluoroalkyl Compounds by Plants. China Environ. Sci. 2022, 42, 5395–5407. [Google Scholar] [CrossRef]
- Kudo, N.; Kawashima, Y. Toxicity and Toxicokinetics of Perfluorooctanoic Acid in Humans and Animals. J. Toxicol. Sci. 2003, 28, 49–57. [Google Scholar] [CrossRef]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic Acid (PFOA), an Emerging Drinking Water Contaminant: A Critical Review of Recent Literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef]
- Ganesan, S.; Vasudevan, N. Impacts of Perfluorinated Compounds on Human Health. Bull. Environ. Pharmacol. Life Sci. 2015, 4, 183–191. [Google Scholar]
- Rainieri, S.; Conlledo, N.; Langerholc, T.; Madorran, E.; Sala, M.; Barranco, A. Toxic Effects of Perfluorinated Compounds at Human Cellular Level and on a Model Vertebrate. Food Chem. Toxicol. 2017, 104, 14–25. [Google Scholar] [CrossRef]
- Pelch, K.E.; Reade, A.; Wolffe, T.A.; Kwiatkowski, C.F. PFAS Health Effects Database: Protocol for a Systematic Evidence Map. Environ. Int. 2019, 130, 104851. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early Life Exposure to Per-and Polyfluoroalkyl Substances (PFAS) and Latent Health Outcomes: A Review Including the Placenta as a Target Tissue and Possible Driver of Peri-and Postnatal Effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef]
- Paul, A.G.; Jones, K.C.; Sweetman, A.J. A First Global Production, Emission, and Environmental Inventory for Perfluorooctane Sulfonate. Environ. Sci. Technol. 2009, 43, 386–392. [Google Scholar] [CrossRef]
- Zhong, H.; Zheng, M.; Liang, Y.; Wang, Y.; Gao, W.; Wang, Y.; Jiang, G. Legacy and Emerging Per- and Polyfluoroalkyl Substances (PFAS) in Sediments from the East China Sea and the Yellow Sea: Occurrence, Source Apportionment and Environmental Risk Assessment. Chemosphere 2021, 282, 131042. [Google Scholar] [CrossRef]
- Dean, W.S.; Adejumo, H.A.; Caiati, A.; Garay, P.M.; Harmata, A.S.; Li, L.; Rodriguez, E.E.; Sundar, S. A Framework for Regulation of New and Existing PFAS by EPA. J. Sci. Policy Gov. 2020, 16, 1–14. [Google Scholar]
- UN Environment Programme (UNEP). SC-9/4: Perfluorooctane Sulfonic Acid, Its Salts and Perfluorooctane Sulfonyl Fluoride. In Proceedings of the 2019 Meetings of the Conferences of the Parties to the Basel, Rotterdam and Stockholm Conventions, Geneva, Switzerland, 29 April–10 May 2019. [Google Scholar]
- UN Environment Programme (UNEP). SC-9/12: Listing of Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-Related Compounds. In Proceedings of the 2019 Meetings of the Conferences of the Parties to the Basel, Rotterdam and Stockholm Conventions, Geneva, Switzerland, 29 April–10 May 2019. [Google Scholar]
- Feng, X. Pollution Characteristics and Human External and Internal Exposure of Novel Per-/Poly-Fluoroalkyl Substances in Typical Environment. Ph.D. Thesis, Nankai University, Tianjin, China, 2021. [Google Scholar]
- Li, X.; Cui, D.; Ng, B.; Ogunbiyi, O.D.; Guerra de Navarro, M.; Gardinali, P.; Quinete, N. Non-Targeted Analysis for the Screening and Semi-Quantitative Estimates of per-and Polyfluoroalkyl Substances in Water Samples from South Florida Environments. J. Hazard. Mater. 2023, 452, 131224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Hu, L.-X.; Liu, T.; Zhao, J.-H.; Yang, Y.-Y.; Liu, Y.-S.; Ying, G.-G. Per- and Polyfluoralkyl Substances (PFAS) in Drinking Water System: Target and Non-Target Screening and Removal Assessment. Environ. Int. 2022, 163, 107219. [Google Scholar] [CrossRef] [PubMed]
- Hensema, T.J.; Berendsen, B.J.A.; van Leeuwen, S.P.J. Non-Targeted Identification of per- and Polyfluoroalkyl Substances at Trace Level in Surface Water Using Fragment Ion Flagging. Chemosphere 2021, 265, 128599. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Vega, R.; Cameron, A.; Clases, D.; Dodgen, T.M.; Doble, P.A.; Bishop, D.P. Simultaneous Targeted and Non-Targeted Analysis of per- and Polyfluoroalkyl Substances in Environmental Samples by Liquid Chromatography-Ion Mobility-Quadrupole Time of Flight-Mass Spectrometry and Mass Defect Analysis. J. Chromatogr. A 2021, 1653, 462423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, N.; Zhu, X.; Guo, H.; Jiang, J.; Wang, X.; Shi, W.; Wu, J.; Yu, H.; Wei, S. Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park. Environ. Sci. Technol. 2018, 52, 11007–11016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Li, J.; Hua, X.; Zhang, B.; Tang, C.; An, X.; Lin, T. Emerging and Legacy Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Surface Water around Three International Airports in China. Chemosphere 2023, 344, 140360. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, M.; Yin, H.; Shen, L.; Wang, Y.; Wu, Q. Analysis of 58 Poly-/Perfluoroalkyl Substances and Their Occurrence in Surface Water in a High-Technology Industrial Park. Environ. Pollut. 2020, 267, 115381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, Y.; Shi, Y.; Xu, L.; Cai, Y. Occurrence and Transport of Perfluoroalkyl Acids (PFAAs), Including Short-Chain PFAAs in Tangxun Lake, China. Environ. Sci. Technol. 2013, 47, 9249–9257. [Google Scholar] [CrossRef]
- Huang, D.; Xiao, R.; Du, L.; Zhang, G.; Yin, L.; Deng, R.; Wang, G. Phytoremediation of Poly- and Perfluoroalkyl Substances: A Review on Aquatic Plants, Influencing Factors, and Phytotoxicity. J. Hazard. Mater. 2021, 418, 126314. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T. Removal of PFAS from Water by Aquatic Plants. J. Environ. Manag. 2024, 351, 119895. [Google Scholar] [CrossRef] [PubMed]
- Greger, M. Removal of PFAS from Water by Plants. IJESNR 2021, 28, 556233. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lo, S.-L.; Lee, Y.-C. Distribution and Fate of Perfluorinated Compounds (PFCs) in a Pilot Constructed Wetland. Desalination Water Treat. 2012, 37, 178–184. [Google Scholar] [CrossRef]
- Huff, D.K.; Morris, L.A.; Sutter, L.; Costanza, J.; Pennell, K.D. Accumulation of Six PFAS Compounds by Woody and Herbaceous Plants: Potential for Phytoextraction. Int. J. Phytoremediat. 2020, 22, 1538–1550. [Google Scholar] [CrossRef]
- Battisti, I.; Zambonini, D.; Ebinezer, L.B.; Trentin, A.R.; Meggio, F.; Petit, G.; Masi, A. Perfluoroalkyl Substances Exposure Alters Stomatal Opening and Xylem Hydraulics in Willow Plants. Chemosphere 2023, 344, 140380. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Vidal, P.; Jiang, L.; Mei, W.; Luo, C.; Lacorte, S.; Rigol, A.; Zhang, G. Plant Uptake of Perfluoroalkyl Substances in Freshwater Environments (Dongzhulong and Xiaoqing Rivers, China). J. Hazard. Mater. 2022, 421, 126768. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.K.; Hall, L.M.; Brown, M.A.; Taylor-Manges, A.; Green, T.; Suchanec, K.; Furman, B.T.; Congdon, V.M.; Wilson, S.S.; Osborne, T.Z.; et al. Aquatic Vegetation, an Understudied Depot for PFAS. J. Am. Soc. Mass Spectrom. 2023, 34, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhang, Y.; Wang, J.; Dai, J. Levels and Composition Distribution of Perfluoroalkyl Substances in Water and Biological Samples from Jiangsu Hi-Tech Fluorochemical Industry Park in Changshu, China. Environ. Chem. 2013, 32, 1318–1327. [Google Scholar] [CrossRef]
- Li, P.; Oyang, X.; Zhao, Y.; Tu, T.; Tian, X.; Li, L.; Zhao, Y.; Li, J.; Xiao, Z. Occurrence of Perfluorinated Compounds in Agricultural Environment, Vegetables, and Fruits in Regions Influenced by a Fluorine-Chemical Industrial Park in China. Chemosphere 2019, 225, 659–667. [Google Scholar] [CrossRef]
- Shan, G.; Wei, M.; Zhu, L.; Liu, Z.; Zhang, Y. Concentration Profiles and Spatial Distribution of Perfluoroalkyl Substances in an Industrial Center with Condensed Fluorochemical Facilities. Sci. Total Environ. 2014, 490, 351–359. [Google Scholar] [CrossRef]
- Song, D.; Qiao, B.; Yao, Y.; Zhao, L.; Wang, X.; Chen, H.; Zhu, L.; Sun, H. Target and Nontarget Analysis of Per- and Polyfluoroalkyl Substances in Surface Water, Groundwater and Sediments of Three Typical Fluorochemical Industrial Parks in China. J. Hazard. Mater. 2023, 460, 132411. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hua, Z.; Li, X. Distribution, Sources, and Dietetic-Related Health Risk Assessment of Perfluoroalkyl Acids (PFAAs) in the Agricultural Environment of an Industrial-Agricultural Interaction Region (IAIR), Changshu, East China. Sci. Total Environ. 2022, 809, 152159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Zhang, W.; Jin, Y.; Dai, J. Association of Perfluorooctanoic Acid with HDL Cholesterol and Circulating miR-26b and miR-199–3p in Workers of a Fluorochemical Plant and Nearby Residents. Environ. Sci. Technol. 2012, 46, 9274–9281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, H.; Liang, Y. Plant Uptake and Soil Fractionation of Five Ether-PFAS in Plant-Soil Systems. Sci. Total Environ. 2021, 771, 144805. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. PFAS Master List of PFAS Substances (Version 2); United States Environmental Protection Agency: Washington, DC, USA, 2021.
- Trier, X.; Lunderberg, D. S9|PFASTRIER|PFAS Suspect List: Fluorinated Substances. Zenodo Dataset 2015. Available online: https://zenodo.org/records/2621989 (accessed on 15 November 2023).
- Place, B. Suspect List of Possible Per-And Polyfluoroalkyl Substances (PFAS) Version 1.5.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021. [Google Scholar]
- Liu, Y.; Qian, M.; Ma, X.; Zhu, L.; Martin, J.W. Nontarget Mass Spectrometry Reveals New Perfluoroalkyl Substances in Fish from the Yangtze River and Tangxun Lake, China. Environ. Sci. Technol. 2018, 52, 5830–5840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, N.; Qian, Y.; Shi, W.; Zhang, X.; Geng, J.; Yu, H.; Wei, S. Non-Target and Suspect Screening of per- and Polyfluoroalkyl Substances in Chinese Municipal Wastewater Treatment Plants. Water Res. 2020, 183, 115989. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Guo, H.; Yang, J.; Jin, L.; Wang, X.; Shi, W.; Zhang, X.; Yu, H.; Wei, S. Non-Target and Suspect Screening of Per- and Polyfluoroalkyl Substances in Airborne Particulate Matter in China. Environ. Sci. Technol. 2018, 52, 8205–8214. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Li, J.; Wu, N.; Li, W.; Niu, Z. Distribution, Partitioning Behavior and Positive Matrix Factorization-Based Source Analysis of Legacy and Emerging Polyfluorinated Alkyl Substances in the Dissolved Phase, Surface Sediment and Suspended Particulate Matter around Coastal Areas of Bohai Bay, China. Environ. Pollut. 2019, 246, 34–44. [Google Scholar] [CrossRef]
- Li, H.; Lao, Z.; Liu, Y.; Feng, Y.; Song, A.; Hu, J.; Liao, Z.; Zhang, L.; Liu, M.; Liu, Y.; et al. Uptake, Accumulation, and Translocation of Organophosphate Esters and Brominated Flame Retardants in Water Hyacinth (Eichhornia crassipes): A Field Study. Sci. Total Environ. 2023, 874, 162435. [Google Scholar] [CrossRef]
- Zhu, Y. Occurrence Characteristic and Risk Assessment of Per- and Polyfluorinated Substances around Fluorine Chemical Park. Master’s Thesis, Liaoning Technical University, Liaoning, China, 2023. [Google Scholar]
- Li, W.; Liu, X.; Mao, H.; Wang, S. Concentration, Distribution, and Bioconcentration of Short- and Long-Chain Perfluoroalkyl Substances in the Water, Suspended Particulate Matter, and Surface Sediment of a Typical Semi-Enclosed Bay. Sci. Total Environ. 2023, 890, 164416. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, X. Evidence of Large-Scale Deposition of Airborne Emissions of per- and Polyfluoroalkyl Substances (PFASs) near a Fluoropolymer Production Plant in an Urban Area. Chemosphere 2023, 337, 139407. [Google Scholar] [CrossRef] [PubMed]
- Dalmijn, J.; Glüge, J.; Scheringer, M.; Cousins, I.T. Emission Inventory of PFASs and Other Fluorinated Organic Substances for the Fluoropolymer Production Industry in Europe. Environ. Sci. Process. Impacts 2024, 26, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, G.; Kemball-Cook, S.; Keinath, M.; Waterland, R.L.; Korzeniowski, S.H.; Buck, R.C.; Russell, M.H.; Washburn, S.T. High-Resolution Atmospheric Modeling of Fluorotelomer Alcohols and Perfluorocarboxylic Acids in the North American Troposphere. Environ. Sci. Technol. 2007, 41, 5756–5762. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Guo, Y.; Sun, Y.; Dai, J. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol. 2017, 51, 9553–9560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Gu, L.; Hua, Z.; Zhang, Y.; Xue, H. Distribution and Release of Perfluorinated Compounds (PFCs) in Water-Sediment Systems: The Effect of Confluence Channels. Sci. Total Environ. 2021, 775, 145720. [Google Scholar] [CrossRef]
- Kotthoff, M.; Müller, J.; Jürling, H.; Schlummer, M.; Fiedler, D. Perfluoroalkyl and Polyfluoroalkyl Substances in Consumer Products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Lindström, M.; Håkanson, L. On the Modelling of the Particulate and Dissolved Fractions of Substances in Aquatic Ecosystems—Sedimentological and Ecological Interactions. Ecol. Model. 2001, 137, 225–240. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, X.; Dong, J.; Xia, N.; Jiang, X.; Li, Y.; Zhu, Y. Short- and Long-Chain Perfluoroalkyl Substances in the Water, Suspended Particulate Matter, and Surface Sediment of a Turbid River. Sci. Total Environ. 2016, 568, 57–65. [Google Scholar] [CrossRef]
- Felizeter, S.; McLachlan, M.S.; De Voogt, P. Root Uptake and Translocation of Perfluorinated Alkyl Acids by Three Hydroponically Grown Crops. J. Agric. Food Chem. 2014, 62, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, D.; Zhou, S.; Lin, J.; Liu, Y.; Huang, X.; Lin, Q.; Morel, J.L.; Ni, Z.; Wang, S.; et al. New Insights into the Accumulation, Transport, and Distribution Mechanisms of Hexafluoropropylene Oxide Homologues, Important Alternatives to Perfluorooctanoic Acid, in Lettuce (Lactuca sativa L.). Environ. Sci. Technol. 2023, 57, 9702–9712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, L.; Yang, X.; Zhao, X.; Li, M.; Zhao, S.; Zhu, L.; Zhan, J. Degradation of 8:2 Fluorotelomer Carboxylic Acid (8:2 FTCA) by Plants and Their Co-Existing Microorganisms. J. Hazard. Mater. 2023, 451, 131129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, B.; Hu, X.; Wu, Y.; Luo, L.; Chen, Z.; Zhang, S. Determination of Fluorotelomer Alcohols and Their Degradation Products in Biosolids-Amended Soils and Plants Using Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1404, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Lei, G.; Yang, B.; Hu, T.; Ye, Y.; Li, W.; Zhou, Y.; Yang, X.; Xu, H. Current Progress on Fluoride Occurrence in the Soil Environment: Sources, Transformation, Regulations and Remediation. Chemosphere 2023, 341, 139901. [Google Scholar] [CrossRef] [PubMed]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hyland, K.C.; Stushnoff, C.; Dickenson, E.R.V.; Higgins, C.P. Perfluoroalkyl Acid Uptake in Lettuce (Lactuca sativa) and Strawberry (Fragaria ananassa) Irrigated with Reclaimed Water. Environ. Sci. Technol. 2014, 48, 14361–14368. [Google Scholar] [CrossRef] [PubMed]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended Soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Y.; Shi, Y.; Wang, P.; Jones, K.; Sweetman, A.J.; Johnson, A.C.; Zhang, M.; Zhou, Y.; Lu, X.; et al. Crop Bioaccumulation and Human Exposure of Perfluoroalkyl Acids through Multi-Media Transport from a Mega Fluorochemical Industrial Park, China. Environ. Int. 2017, 106, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Lu, H.; Grieger, K.D.; Munoz, G.; Li, W.; Wang, X.; He, Q.; Qian, S. Bioaccumulation and Translocation of 6:2 Fluorotelomer Sulfonate, GenX, and Perfluoroalkyl Acids by Urban Spontaneous Plants. ACS EST Eng. 2022, 2, 1169–1178. [Google Scholar] [CrossRef]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationships between Lipophilicity and Root Uptake and Translocation of Non-Ionised Chemicals by Barley. Pestic. Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Felizeter, S.; McLachlan, M.S.; de Voogt, P. Uptake of Perfluorinated Alkyl Acids by Hydroponically Grown Lettuce (Lactuca sativa). Environ. Sci. Technol. 2012, 46, 11735–11743. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, C.; Chen, L.; Li, Y.; Huang, X.; Wang, S.; Qiu, R.; Tang, C. Accumulation and Associated Phytotoxicity of Novel Chlorinated Polyfluorinated Ether Sulfonate in Wheat Seedlings. Chemosphere 2020, 249, 126447. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Qian, S.; Zhi, Y.; Li, Z.; Yan, Z.; Li, H.; Lu, X. Bioaccumulation Characteristics of Perfluorocarboxylic Alkyl Compounds by Ferns. China Environ. Sci. 2023, 43, 2440–2449. [Google Scholar] [CrossRef]
- Mc, K.; Pegman, A.P.; Ogden, J. Productivity-decomposition Dynamics of Typha Orientalis at Kaitoke Swamp, Great Barrier Island, New Zealand. N. Z. J. Bot. 2005, 43, 779–789. [Google Scholar] [CrossRef]
- Lei, M.; He, J.; Zhang, H.; Liu, W.; Fang, S. Pollution Characteristics and Risk Assessment of Perfluoroalkyl Substances in the Typical Wetland of Chengdu City. Environ. Chem. 2024, 43, 1–11. [Google Scholar]
- Wang, T.-T.; Ying, G.-G.; Shi, W.-J.; Zhao, J.-L.; Liu, Y.-S.; Chen, J.; Ma, D.-D.; Xiong, Q. Uptake and Translocation of Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) by Wetland Plants: Tissue- and Cell-Level Distribution Visualization with Desorption Electrospray Ionization Mass Spectrometry (DESI-MS) and Transmission Electron Microscopy Equipped with Energy-Dispersive Spectroscopy (TEM-EDS). Environ. Sci. Technol. 2020, 54, 6009–6020. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.V.; Nguyen, T.D.; Nguyen, T.N.; Truong, T.K.; Pham, H.V.; Duong, H.A. Removal of Perfluoroalkyl Acids (PFAAs) from Aqueous Solution by Water Hyacinth (Eichhornia crassipes): Uptake, Accumulation, and Translocation. Sci. Total Environ. 2024, 926, 172029. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, J.; Tan, T.; Li, Y.; Xiang, B. Contaminant characteristic of per- and poly-fluorinated substances in maize in the surrounding of fluorine chemical park. Ecol. Environ. Sci. 2023, 32, 1001–1006. [Google Scholar] [CrossRef]
- Han, M.; Li, Z.; Xu, L.; Li, Z.; Wen, X.; Shi, Q.; Hu, X.; Qin, C. The migration, transformation and toxicity of per- and polyfluoroalkyl substances in organisms. China Environ. Sci. 2024, 44, 1638–1647. [Google Scholar] [CrossRef]
- Wu, Z. Study on Leaching of Perfluoroalkyl Substances in Soil. Master’s Thesis, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, China, 2020. [Google Scholar]
- Marzban, L.; Akhzari, D.; Ariapour, A.; Mohammadparast, B.; Pessarakli, M. Effects of Cadmium Stress on Seedlings of Various Rangeland Plant Species (Avena fatua L., Lathyrus sativus L., and Lolium temulentum L.): Growth, Physiological Traits, and Cadmium Accumulation. J. Plant Nutr. 2017, 40, 2127–2137. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Florida Site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Mayakaduwage, S.; Ekanayake, A.; Kurwadkar, S.; Rajapaksha, A.U.; Vithanage, M. Phytoremediation Prospects of Per- and Polyfluoroalkyl Substances: A Review. Environ. Res. 2022, 212, 113311. [Google Scholar] [CrossRef] [PubMed]
| Dissolved Phase | Particle Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | DF (%) | Mean | Median | Range | DF (%) | |
| PFBA | 79.1 | 52.0 | 24.0–237 | 100% | 14.1 | 7.23 | n.d.–38.9 | 83% |
| PFPeA | 0.430 | 0.430 | <LOQ–0.460 | 33% | 0.930 | 0.720 | n.d.–1.65 | 67% |
| PFHxA | 789 | 724 | 583–1270 | 100% | 620 | 273 | 11.5–1880 | 100% |
| PFHpA | 26.4 | 12.8 | 7.75–95.1 | 100% | 3.31 | 2.01 | 0.440–9.52 | 100% |
| PFOA | 721 | 663 | 407–1190 | 100% | 474 | 261 | 14.3–1180 | 100% |
| PFNA | 42.6 | 5.26 | 1.89–174 | 100% | 10.4 | 3.02 | 0.250–48.5 | 100% |
| PFDA | 7.72 | 2.07 | 0.840–23.6 | 100% | 11.2 | 3.00 | 0.630–53.9 | 100% |
| PFUnDA | 23.2 | 1.88 | 0.470–106 | 100% | 28.7 | 4.34 | 1.19–152 | 100% |
| PFDoDA | 5.18 | 0.770 | 0.230–24.2 | 100% | 5.44 | 2.34 | 0.980–22.9 | 100% |
| PFTriA | 2.13 | 1.32 | <LOQ–5.69 | 67% | 3.01 | 2.81 | 0.700–7.17 | 100% |
| PFTreA | <LOQ | <LOQ | <LOQ | 0% | 1.75 | 1.88 | 0.240–3.12 | 100% |
| PFBS | 9.15 | 10.9 | 0.790–14.3 | 100% | <LOQ | <LOQ | <LOQ | 0% |
| PFPeS | 1.19 | 1.72 | <LOQ–1.91 | 83% | 0.0900 | 0.0700 | 0.0400–0.170 | 100% |
| PFHxS | 5.82 | 5.55 | 3.14–9.69 | 100% | 0.960 | 0.870 | <LOQ–1.54 | 83% |
| PFHpS | 0.400 | 0.260 | <LOQ–0.720 | 83% | 0.570 | 0.220 | <LOQ–1.77 | 67% |
| PFOS | 3.41 | 3.27 | 2.35–5.20 | 100% | 2.04 | 1.71 | 0.310–3.75 | 100% |
| PFNS | 0.750 | 0.750 | <LOQ–0.870 | 33% | <LOQ | <LOQ | <LOQ | 0% |
| PFDS | <LOQ | <LOQ | <LOQ | 0% | 0.780 | 0.780 | <LOQ–1.12 | 33% |
| 6:2 FTCA | 1840 | 1060 | 272–4870 | 100% | 1090 | 338 | 31.0–3070 | 100% |
| 8:2 FTCA | 0.600 | 0.500 | <LOQ–1.02 | 83% | 2.10 | 1.16 | <LOQ–4.40 | 83% |
| 10:2 FTCA | 0.230 | 0.240 | <LOQ–0.280 | 50% | 2.25 | 0.580 | n.d.–5.63 | 50% |
| 4:2 FTSA | 1.35 | 1.29 | 0.730–1.96 | 67% | 0.370 | 0.310 | 0.0300–0.920 | 100% |
| 6:2 FTSA | 2.36 | 2.35 | 1.28–3.61 | 100% | 71.5 | 35.2 | 9.37–258 | 100% |
| 8:2 FTSA | <LOQ | <LOQ | <LOQ | 0% | 0.540 | 0.170 | 0.0400–2.09 | 100% |
| 6:2 Cl-PFESA | 0.290 | 0.300 | 0.0900–0.490 | 100% | 0.410 | 0.320 | 0.100–0.840 | 100% |
| 8:2 Cl-PFESA | 0.160 | 0.160 | <LOQ–0.180 | 67% | 0.210 | 0.150 | 0.100–0.540 | 100% |
| PFECHS | 0.0400 | 0.0200 | <LOQ–0.0800 | 67% | 0.130 | 0.150 | 0.0100–0.190 | 100% |
| HFPO-DA | 179 | 40.6 | 16.4–601 | 100% | 38.2 | 16.5 | 11.3–126 | 100% |
| HFPO-TA | 240 | 138 | 76.8–731 | 100% | 324 | 139 | n.d.–779 | 50% |
| FBSA | 2.06 | 1.84 | 1.53–3.45 | 100% | 0.720 | 0.720 | <LOQ–0.720 | 17% |
| FHxSA | 0.730 | 0.650 | 0.560–0.990 | 100% | 0.200 | 0.0400 | n.d.–0.500 | 83% |
| FOSA | 2.68 | 2.69 | 1.46–3.44 | 100% | 1.80 | 1.01 | 0.280–6.58 | 100% |
| ΣPFAS | 3990 | 2760 | 1650–8250 | – | 1140 | 2540 | 113–6800 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhao, Z.; Liu, J.; Li, S. The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park. Water 2024, 16, 1513. https://doi.org/10.3390/w16111513
Huang J, Zhao Z, Liu J, Li S. The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park. Water. 2024; 16(11):1513. https://doi.org/10.3390/w16111513
Chicago/Turabian StyleHuang, Jingqi, Zhen Zhao, Jing Liu, and Shiyue Li. 2024. "The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park" Water 16, no. 11: 1513. https://doi.org/10.3390/w16111513
APA StyleHuang, J., Zhao, Z., Liu, J., & Li, S. (2024). The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park. Water, 16(11), 1513. https://doi.org/10.3390/w16111513






