CuFeS2/MXene-Modified Polyvinylidene Fluoride Membrane for Antibiotics Removal through Peroxymonosulfate Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CuFeS2/MXene–PVDF Membrane
2.3. Membrane Characterization Method
2.4. Experimental Procedure
2.5. Analysis Methods
2.6. Computational Methods
3. Results and Discussion
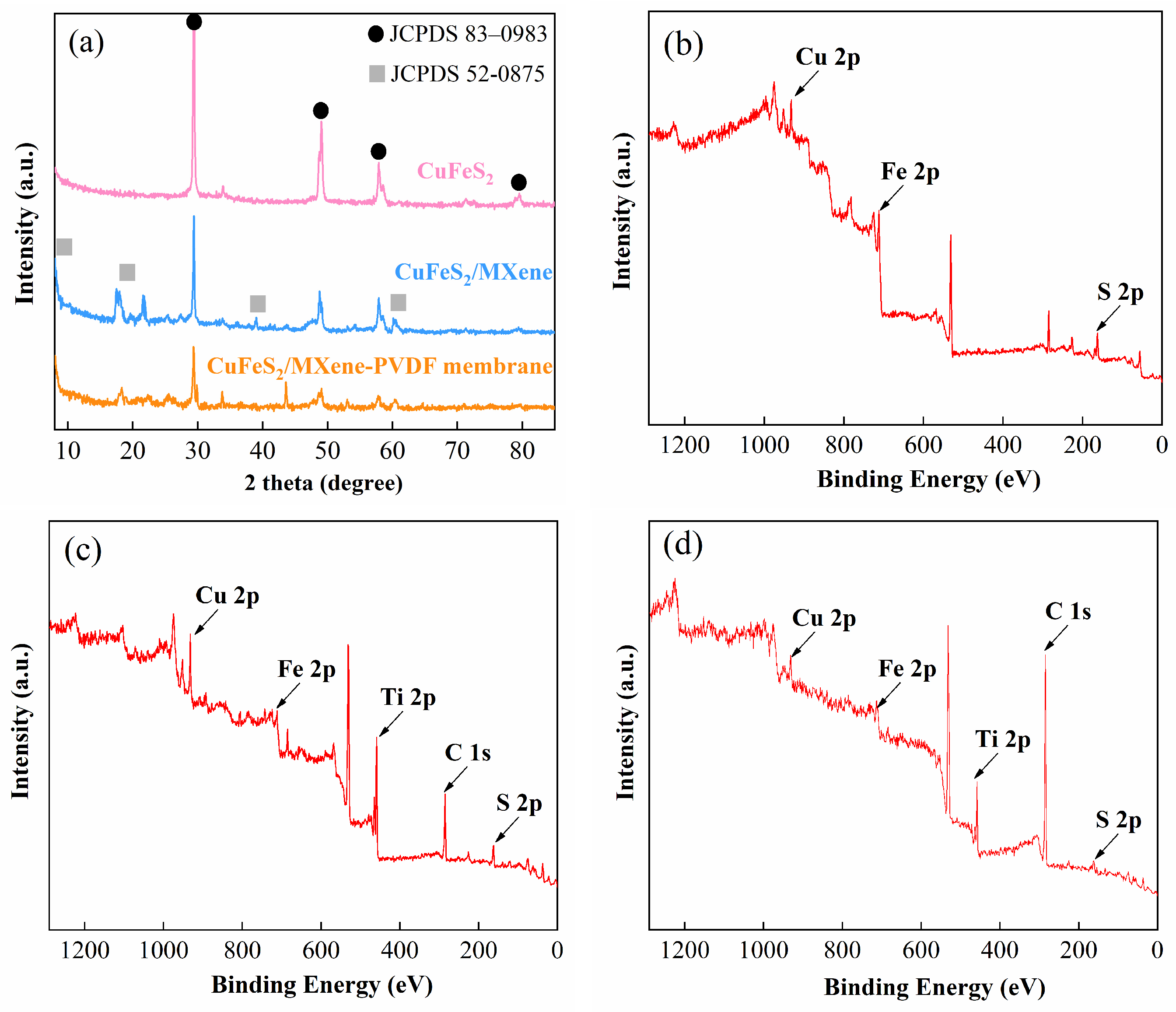
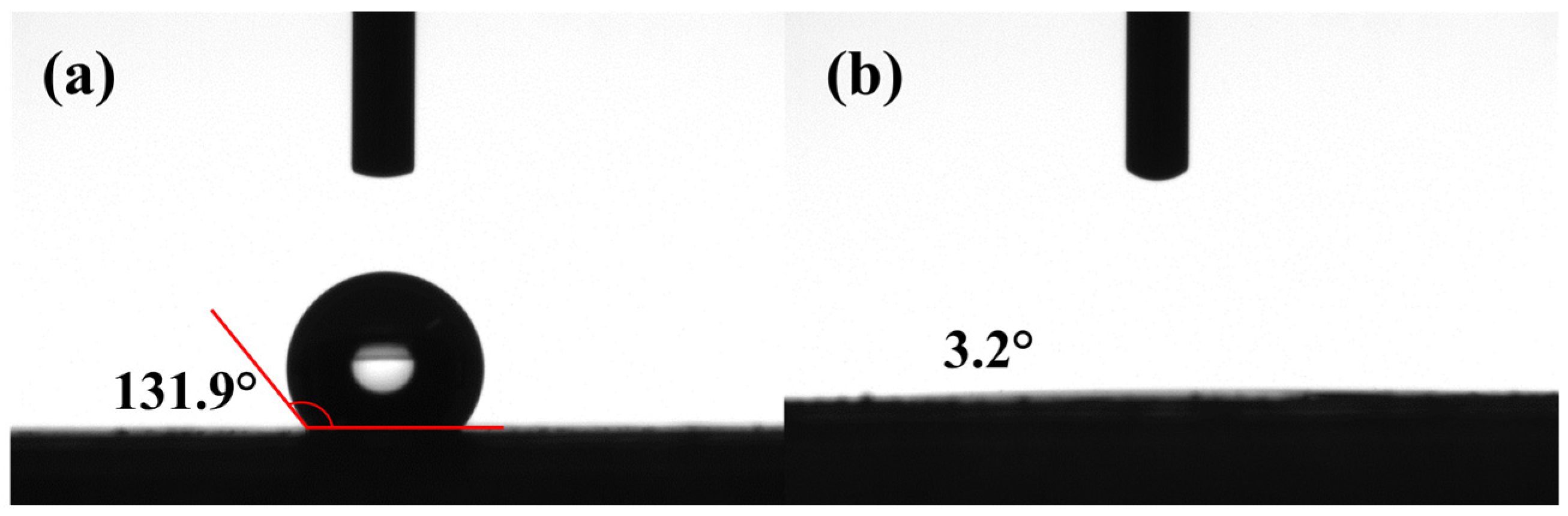
3.1. Membrane Characterization
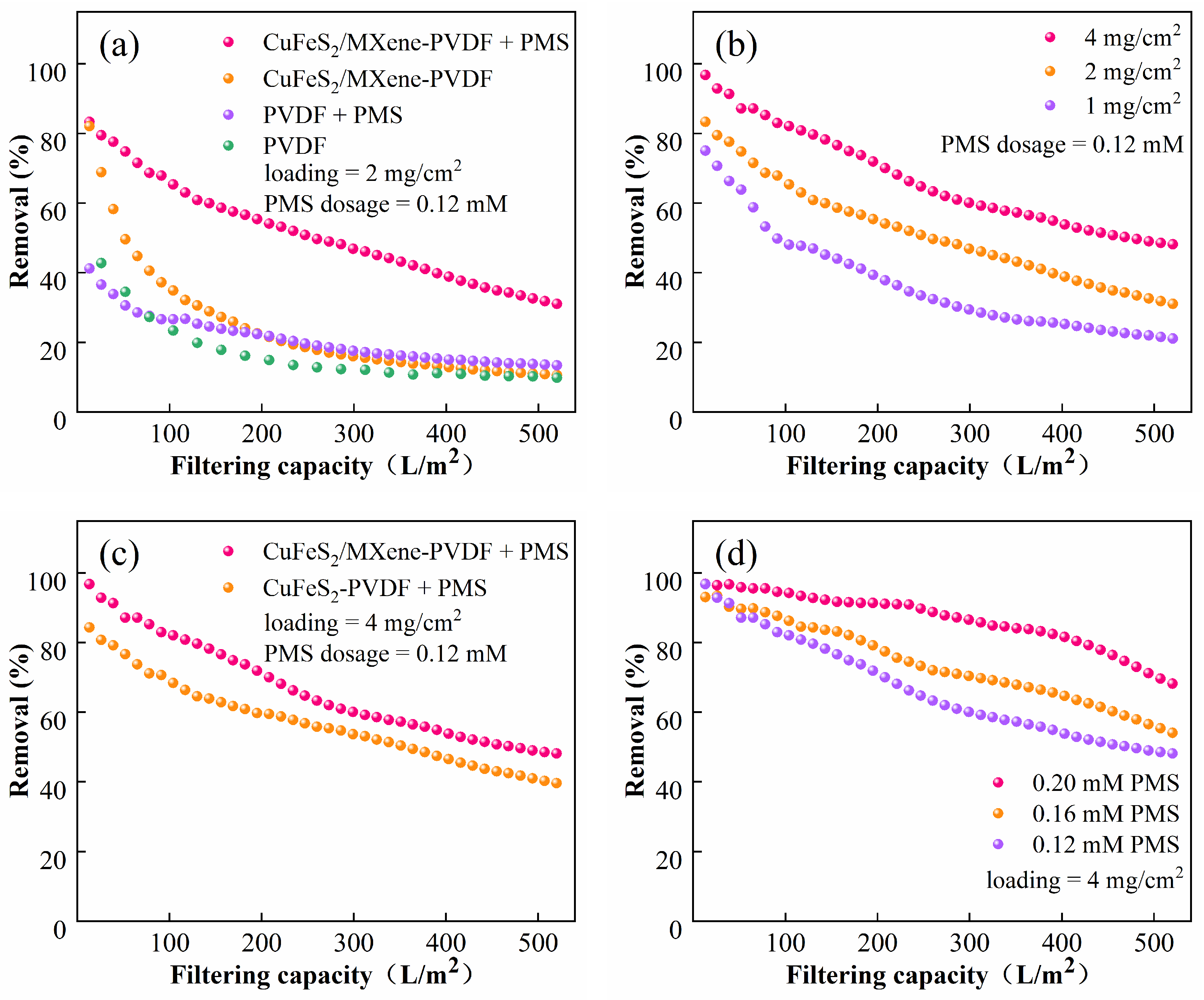
3.2. Parameter Optimization of the Catalytic Membrane/PMS System
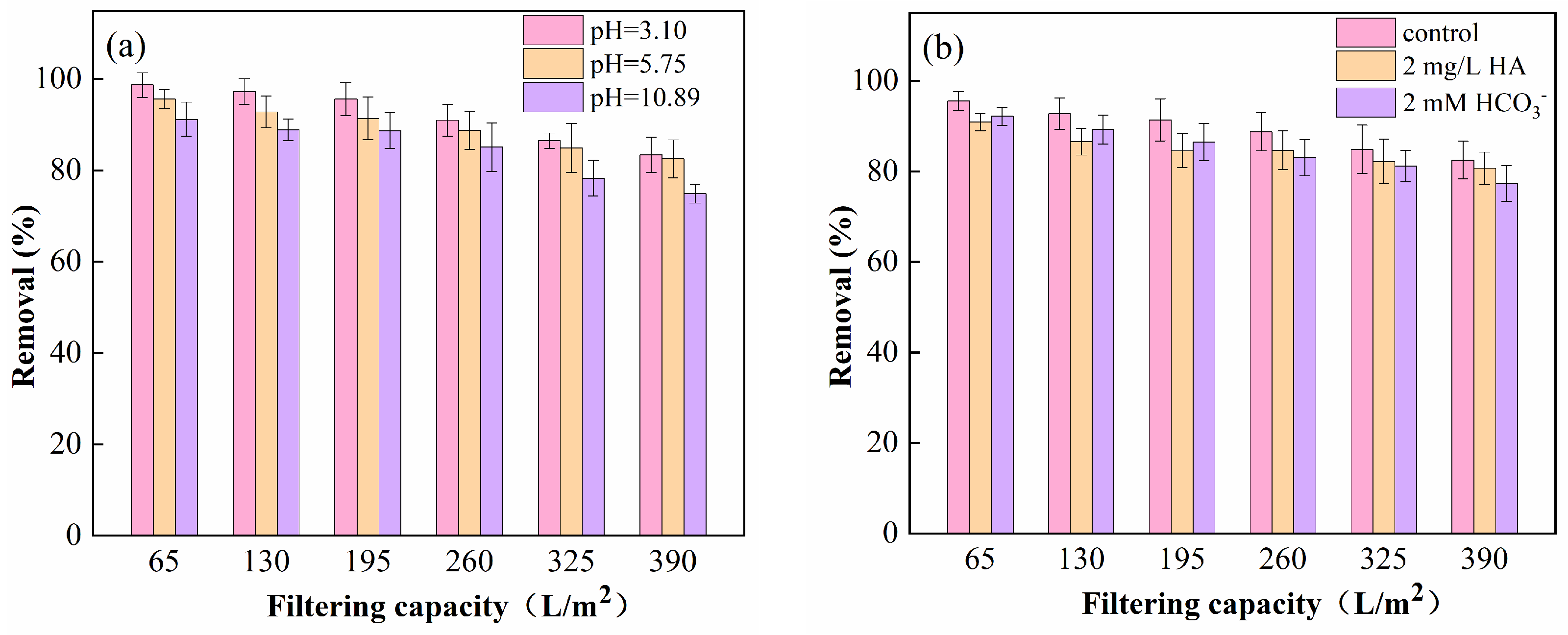
3.3. Removal Efficiency under Varying Conditions
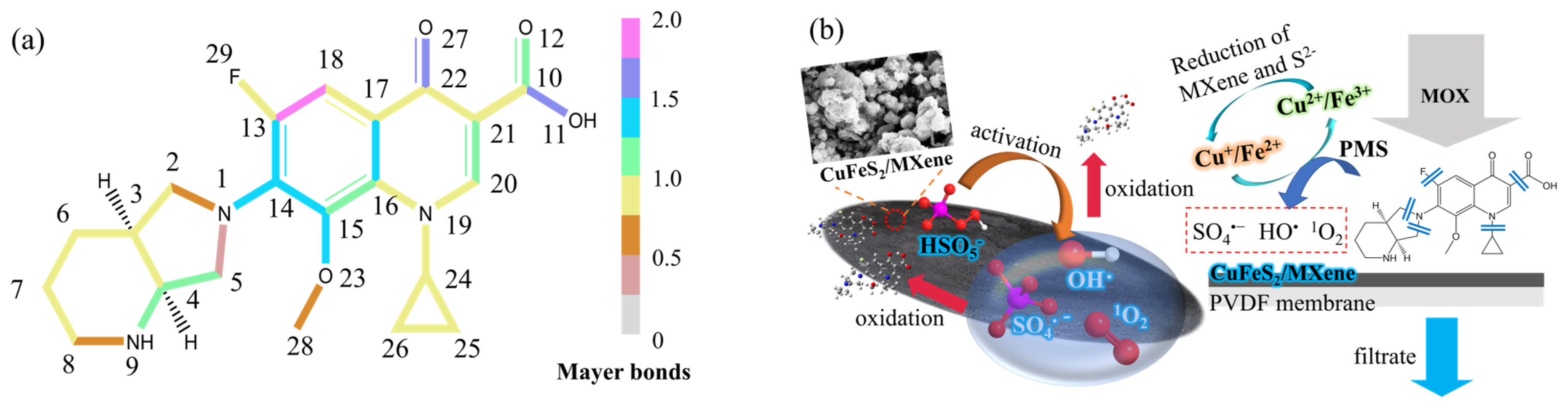
3.4. Mechanism of MOX Removal by Catalytic Membrane
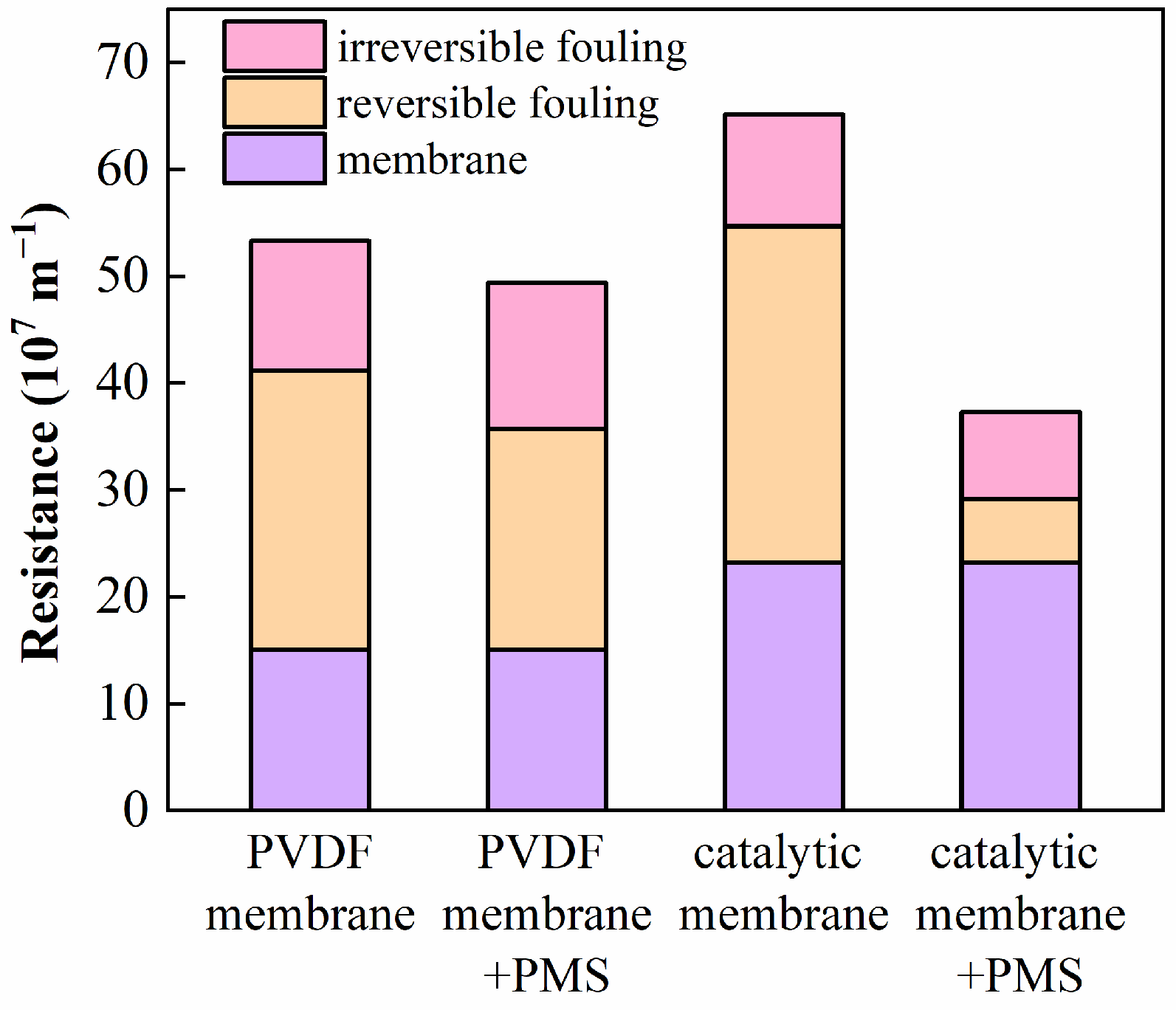
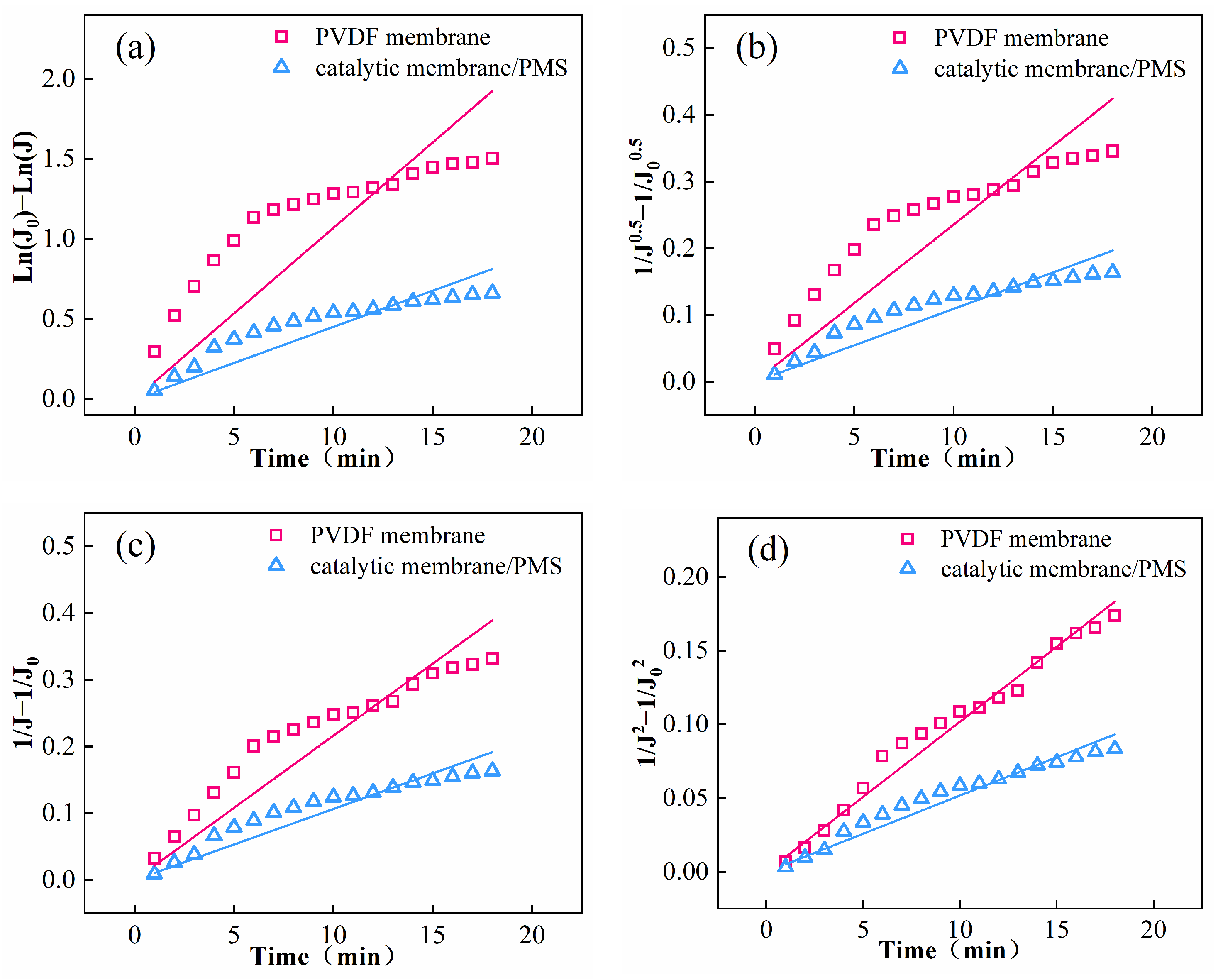
3.5. Anti-Fouling Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.R.M.; Li, K. Poly (vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funct. Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, Y.; Wang, D.; Zhu, M.; Sun, X.; Jiang, S.; Fan, Y.; Zhang, D.; Zhang, L. Surface-functionalized PVDF membranes by facile synthetic Cu-MOF-74 for enhanced contaminant degradation and antifouling performance. Colloids Surf. A. 2022, 651, 129640. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Ren, L.; Lei, T.; Li, J.; Jin, J.; Luo, S.; Qin, S.; Gao, C. Properties and preparation of TiO2-HAP@PVDF composite ultrafiltration membranes. Polym. Compos. 2023, 44, 7499–7509. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Dong, X.; Wu, C.; Lichtfouse, E. Removal of antibiotics from black water by a membrane filtration-visible light photocatalytic system. J. Water Process Eng. 2023, 53, 103605. [Google Scholar] [CrossRef]
- Alpatova, A.; Meshref, M.; McPhedran, K.N.; Gamal El-Din, M. Composite polyvinylidene fluoride (PVDF) membrane impregnated with Fe2O3 nanoparticles and multiwalled carbon nanotubes for catalytic degradation of organic contaminants. J. Membr. Sci. 2015, 490, 227–235. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Ji, W.; Li, S.; Nian, P.; Xu, N.; Ye, N.; Wei, Y. Visible-light-driven photocatalytic ZnO@Ti3C2Tx MXene nanofiltration membranes for enhanced organic dyes removal. Sep. Purif. Technol. 2023, 323, 124420. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, J.; Wang, Z.; Yang, Z.; Yang, W.; Yin, Z. Defective MOFs-based electrocatalytic self-cleaning membrane for wastewater reclamation: Enhanced antibiotics removal, membrane fouling control and mechanisms. Water Res. 2022, 220, 118635. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Sun, X. A Self-cleaning, catalytic titanium carbide (MXene) membrane for efficient tetracycline degradation through peroxymonosulfate activation: Performance evaluation and mechanism study. Sep. Purif. Technol. 2021, 279, 119796. [Google Scholar] [CrossRef]
- Li, X.; Jie, B.; Lin, H.; Deng, Z.; Qian, J.; Yang, Y.; Zhang, X. Application of sulfate radicals-based advanced oxidation technology in degradation of trace organic contaminants (TrOCs): Recent advances and prospects. J. Environ. Manag. 2022, 308, 114664. [Google Scholar] [CrossRef]
- Liu, Y.; Du, N.; Liu, X.; Yao, D.; Wu, D.; Li, Z.; Mao, S. ZnS-embedded porous carbon for peroxydisulfate activation: Enhanced electron transfer for bisphenol A degradation. Environ. Sci. Ecotechnol. 2024, 19, 100338. [Google Scholar] [CrossRef]
- Yang, J.; Jia, K.; Lu, S.; Cao, Y.; Boczkaj, G.; Wang, C. Thermally activated natural chalcopyrite for Fenton-like degradation of Rhodamine B: Catalyst characterization, performance evaluation, and catalytic mechanism. J. Environ. Chem. Eng. 2024, 12, 111469. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Deng, S.; Shangguan, Y.; Wang, R.; Ge, Q.; Feng, X.; Yang, Z.; Ji, Y.; Fan, T.; et al. Photo-assisted reductive cleavage and catalytic hydrolysis-mediated persulfate activation by mixed redox-couple-involved CuFeS2 for efficient trichloroethylene oxidation in groundwater. Water Res. 2022, 222, 118885. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Zhang, M.; Ying, G.; Feng, Y. Highly Efficient Degradation of Sulfisoxazole by Natural Chalcopyrite-Activated Peroxymonosulfate: Reactive Species and Effects of Water Matrices. Water-Sui 2022, 14, 3450. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, H.; Liu, W.; Ao, Z.; Ji, H.; Liu, Y.; Su, S.; Yao, G.; Lai, B. Insights into heterogeneous catalytic activation of peroxymonosulfate by natural chalcopyrite: pH-dependent radical generation, degradation pathway and mechanism. Chem. Eng. J. 2020, 397, 125387. [Google Scholar] [CrossRef]
- Zuo, S.; Jin, X.; Wang, X.; Lu, Y.; Zhu, Q.; Wang, J.; Liu, W.; Du, Y.; Wang, J. Sandwich structure stabilized atomic Fe catalyst for highly efficient Fenton-like reaction at all pH values. Appl. Catal. B Environ. 2021, 282, 119551. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Lai, L.; Zhang, Y.; Chen, Y.; Li, X.; Liu, B.; Wang, H. Peroxymonosulfate activation using MnFe2O4 modified biochar for organic pollutants degradation: Performance and mechanisms. Sep. Purif. Technol. 2023, 308, 122886. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Sun, Y.; Zhu, Y.; Wang, L.; Lin, F.; Ma, Y.; Ji, W.; Li, Y.; Wang, L. Enhancing deep mineralization of refractory benzotriazole via carbon nanotubes-intercalated cobalt copper bimetallic oxide nanosheets activated peroxymonosulfate process: Mechanism, degradation pathway and toxicity. J. Colloid Interface Sci. 2022, 628, 448–462. [Google Scholar] [CrossRef]
- Xia, X.; Deng, L.; Yang, L.; Shi, Z. Facile synthesis of CoOOH@MXene to activate peroxymonosulfate for efficient degradation of sulfamethoxazole: Performance and mechanism investigation. Environ. Sci. Pollut. Res. 2022, 29, 52995–53008. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Li, S.; Lin, Y.; Zhang, S.; Guan, Q. Activation of sodium percarbonate with Fe3O4@MXene composite for the efficient removal of bisphenol A. J. Environ. Chem. Eng. 2022, 10, 108702. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, H.; Deng, L.; Amin, R.S.; Yu, D.; Fetohi, A.E.; Maximov, M.Y.; Li, L.; El-Khatib, K.M.; Peng, S. Anchoring stable FeS2 nanoparticles on MXene nanosheets via interface engineering for efficient water splitting. Inorg. Chem. Front. 2022, 9, 662–669. [Google Scholar] [CrossRef]
- Li, R.; Ren, Y.; Zhao, P.; Wang, J.; Liu, J.; Zhang, Y. Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J. Hazard. Mater. 2019, 365, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Yu, X. Peracetic acid integrated catalytic ceramic membrane filtration for enhanced membrane fouling control: Performance evaluation and mechanism analysis. Water Res. 2022, 220, 118710. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Gao, S.; Yu, H.; Liu, X.; Tian, J. Layered metal oxides loaded ceramic membrane activating peroxymonosulfate for mitigation of NOM membrane fouling. Water Res. 2022, 222, 118928. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, B.; Wang, W.; Zhang, W.; Du, P.; Liu, W.; Xing, X.; Ding, D.; Lv, G.; Lv, Q.; et al. In situ produced hydrogen peroxide by biosynthesized palladium nanoparticles and natural clay mineral for highly-efficient carbamazepine degradation. Chem. Eng. J. 2021, 426, 131567. [Google Scholar] [CrossRef]
- Xu, X.; Tang, D.; Cai, J.; Xi, B.; Zhang, Y.; Pi, L.; Mao, X. Heterogeneous activation of peroxymonocarbonate by chalcopyrite (CuFeS2) for efficient degradation of 2,4-dichlorophenol in simulated groundwater. Appl. Catal. B Environ. 2019, 251, 273–282. [Google Scholar] [CrossRef]
- Huang, F.; Li, Q.; Ji, G.; Tu, J.; Ding, N.; Qu, Q.; Liu, G. Oil/water separation using a lauric acid-modified, superhydrophobic cellulose composite membrane. Mater. Chem. Phys. 2021, 266, 124493. [Google Scholar] [CrossRef]
- Li, N.; Xiao, C.; An, S.; Hu, X. Preparation and properties of PVDF/PVA hollow fiber membranes. Desalination 2010, 250, 530–537. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Escudey, M.; Vyhmeister, E.; Higueras, P.; Godoy-Faúndez, A.; Salazar, J.L.; Valdés-González, H.; Wolf-Sepúlveda, G.; Herrera-Urbina, R. Adsorption of biosolids and their main components on chalcopyrite, molybdenite and pyrite: Zeta potential and FTIR spectroscopy studies. Miner. Eng. 2015, 78, 128–135. [Google Scholar] [CrossRef]
- Yu, H.; Xu, J.; Hu, Y.; Zhang, H.; Zhang, C.; Qiu, C.; Wang, X.; Liu, B.; Wei, L.; Li, J. Synthesis and characterization of CuFeS2 and Se doped CuFeS2−xSex nanoparticles. J. Mater. Sci.-Mater. Electron. 2019, 30, 12269–12274. [Google Scholar] [CrossRef]
- Jun, B.; Kim, S.; Rho, H.; Park, C.M.; Yoon, Y. Ultrasound-assisted Ti3C2Tx MXene adsorption of dyes: Removal performance and mechanism analyses via dynamic light scattering. Chemosphere 2020, 254, 126827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Ren, L.; Zhang, B.; Shao, J. Facile preparation of COF-LZU1 modified membrane via electrostatic spraying for emerging contaminants treatment in direct contact membrane distillation. Sep. Purif. Technol. 2024, 337, 126411. [Google Scholar] [CrossRef]
- Xiang, S.; Dong, H.; Li, Y.; Xiao, J.; Dong, Q.; Hou, X.; Chu, D. Novel flower-like Fe-Mo composite for peroxydisulfate activation toward efficient degradation of carbamazepine. Sep. Purif. Technol. 2023, 305, 122487. [Google Scholar] [CrossRef]
- Utomo, D.P.; Kusworo, T.D.; Kumoro, A.C.; Othman, M.H.D. Developing a versatile and resilient PVDF/CeO2@GO-COOH photocatalytic membrane for efficient treatment of antibiotic-contaminated wastewater. J. Water Process Eng. 2023, 56, 104353. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Lin, L.; Wang, Z.; Hou, X.; Wang, L.; Zhang, F.; Li, Y.; Xie, H. Activation of peroxymonosulfate for ciprofloxacin degradation by a novel oxygen-deficient CoTiO3/TiO2NTs/Ti composite catalytic membrane: Electron transfer pathway and underlying mechanism. Sep. Purif. Technol. 2024, 330, 125511. [Google Scholar] [CrossRef]
- Neves, P.; Leite, A.; Rangel, M.; de Castro, B.; Gameiro, P. Influence of structural factors on the enhanced activity of moxifloxacin: A fluorescence and EPR spectroscopic study. Anal. Bioanal. Chem. 2007, 387, 1543–1552. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Fu, D.; Deng, J.; Deng, L. Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep. Purif. Technol. 2017, 175, 47–57. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Leiknes, T. Oxidation of refractory benzothiazoles with PMS/CuFe2O4: Kinetics and transformation intermediates. Environ. Sci. Technol. 2016, 50, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gan, X.; Cao, S.; Shang, J.; Cheng, X. Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies. Water-Sui 2023, 15, 735. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Yao, G.; Zhang, Y.; Li, X.; Lai, B. Improving the degradation of atrazine in the three-dimensional (3D) electrochemical process using CuFe2O4 as both particle electrode and catalyst for persulfate activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- Xu, P.; Wang, P.; Li, X.; Wei, R.; Wang, X.; Yang, C.; Shen, T.; Zheng, T.; Zhang, G. Efficient peroxymonosulfate activation by CuO-Fe2O3/MXene composite for atrazine degradation: Performance, coexisting matter influence and mechanism. Chem. Eng. J. 2022, 440, 135863. [Google Scholar] [CrossRef]
- Liu, L.; Mi, H.; Zhang, M.; Sun, F.; Zhan, R.; Zhao, H.; He, S.; Zhou, L. Efficient moxifloxacin degradation by CoFe2O4 magnetic nanoparticles activated peroxymonosulfate: Kinetics, pathways and mechanisms. Chem. Eng. J. 2021, 407, 127201. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Chu, M.; Gao, H.; Zhang, D. Biochar composite derived from cellulase hydrolysis apple branch for quinolone antibiotics enhanced removal: Precursor pyrolysis performance, functional group introduction and adsorption mechanisms. Environ. Pollut. 2022, 313, 120104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, P.; Dou, X.; Liu, C.; Zhang, Y.; Song, Z.; Chen, Z.; Xu, B.; Qi, F. Degradation of benzophenone-4 by peroxymonosulfate activated with microwave synthesized well-distributed CuBi2O4 microspheres: Theoretical calculation of degradation mechanism. Appl. Catal. B Environ. 2021, 290, 120048. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Q.; Zhang, M.; Nie, Y.; Tian, X.; Yang, C.; Li, Y. pH-dependent oxidation mechanisms over FeCu doped g-C3N4 for ofloxacin degradation via the efficient peroxymonosulfate activation. J. Clean. Prod. 2021, 315, 128207. [Google Scholar] [CrossRef]
- Song, H.; Pan, S.; Wang, Y.; Cai, Y.; Zhang, W.; Shen, Y.; Li, C. MXene-mediated electron transfer in Cu(II)/PMS process: From Cu(III) to Cu(I). Sep. Purif. Technol. 2022, 297, 121428. [Google Scholar] [CrossRef]
- Mo, F.; Liu, Y.; Xu, Y.; He, Q.; Sun, P.; Dong, X. Photocatalytic elimination of moxifloxacin by two-dimensional graphitic carbon nitride nanosheets: Enhanced activity, degradation mechanism and potential practical application. Sep. Purif. Technol. 2022, 292, 121067. [Google Scholar] [CrossRef]
- Xu, A.; Wu, D.; Zhang, R.; Fan, S.; Lebedev, A.T.; Zhang, Y. Bio-synthesis of Co-doped FeMnOx and its efficient activation of peroxymonosulfate for the degradation of moxifloxacin. Chem. Eng. J. 2022, 435, 134695. [Google Scholar] [CrossRef]
- Yang, H.; Xue, W.; Liu, M.; Yu, K.; Yu, W. Carbon doped Fe3O4 peroxidase-like nanozyme for mitigating the membrane fouling by NOM at neutral pH. Water Res. 2020, 174, 115637. [Google Scholar] [CrossRef]
- Jacob, J.; Prádanos, P.; Calvo, J.I.; Hernández, A.; Jonsson, G. Fouling kinetics and associated dynamics of structural modifications. Colloids Surf. A. 1998, 138, 173–183. [Google Scholar] [CrossRef]
- Bolton, G.; Lacasse, D.; Kuriyel, R. Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids. J. Membr. Sci. 2006, 277, 75–84. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Changes in surface properties and separation efficiency of a nanofiltration membrane after repeated fouling and chemical cleaning cycles. Sep. Purif. Technol. 2013, 113, 42–50. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, K.; Fang, L.; Chen, H. CuFeS2/MXene-Modified Polyvinylidene Fluoride Membrane for Antibiotics Removal through Peroxymonosulfate Activation. Water 2024, 16, 1504. https://doi.org/10.3390/w16111504
Zhang D, Li K, Fang L, Chen H. CuFeS2/MXene-Modified Polyvinylidene Fluoride Membrane for Antibiotics Removal through Peroxymonosulfate Activation. Water. 2024; 16(11):1504. https://doi.org/10.3390/w16111504
Chicago/Turabian StyleZhang, Dongyang, Kunfu Li, Lei Fang, and Huishan Chen. 2024. "CuFeS2/MXene-Modified Polyvinylidene Fluoride Membrane for Antibiotics Removal through Peroxymonosulfate Activation" Water 16, no. 11: 1504. https://doi.org/10.3390/w16111504
APA StyleZhang, D., Li, K., Fang, L., & Chen, H. (2024). CuFeS2/MXene-Modified Polyvinylidene Fluoride Membrane for Antibiotics Removal through Peroxymonosulfate Activation. Water, 16(11), 1504. https://doi.org/10.3390/w16111504






