Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review
Abstract
1. Introduction
2. Nanotechnology-Based Wastewater Treatment Techniques
2.1. Nanomembranes
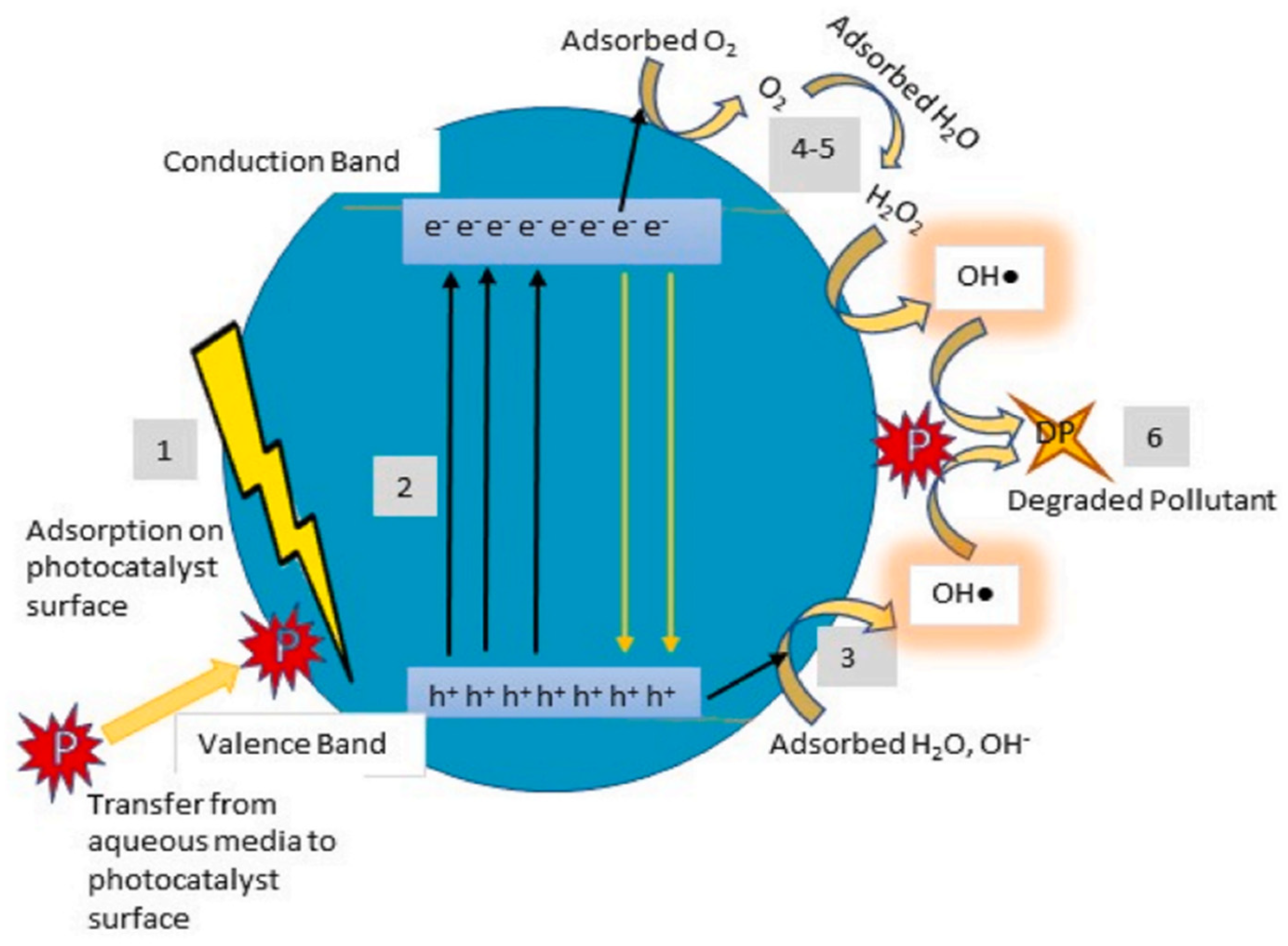
2.2. Nanophotocatalysts
2.3. Nanosorbents
2.4. Nano and Micromotors
2.5. Nanoparticles for Water Disinfection
3. Nanomaterials for Water and Wastewater Management
3.1. Applications of Metal/Metal Oxide Nanoparticles in Water Treatment
3.1.1. Iron Oxide
3.1.2. Silver Nanoparticles
3.1.3. Titanium Nanoparticles
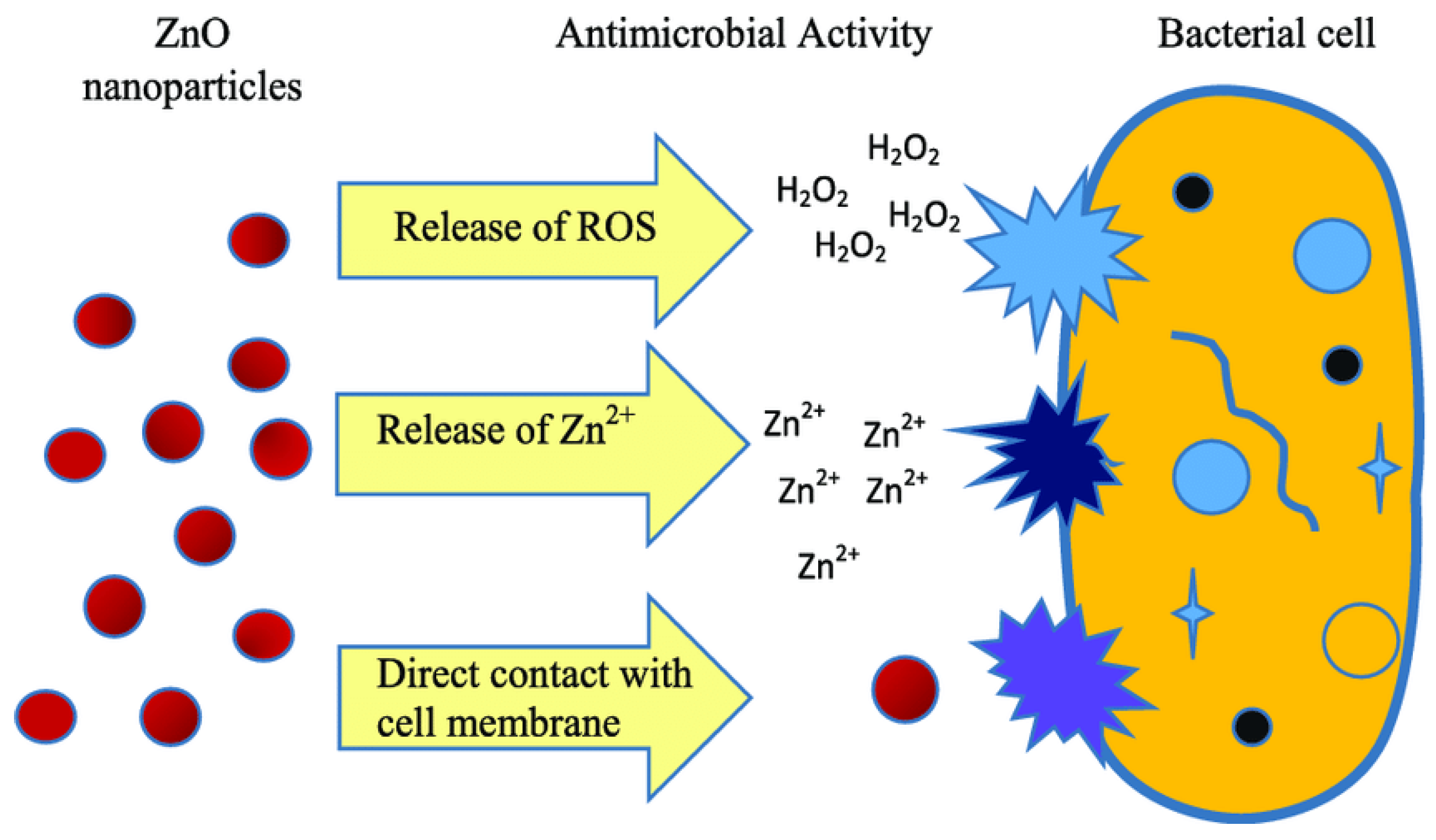
3.1.4. Zinc Oxide
3.1.5. Copper Nanoparticles
3.1.6. Other Metal/Metal Oxide Nanoparticles
3.2. Carbon-Based Nanomaterials
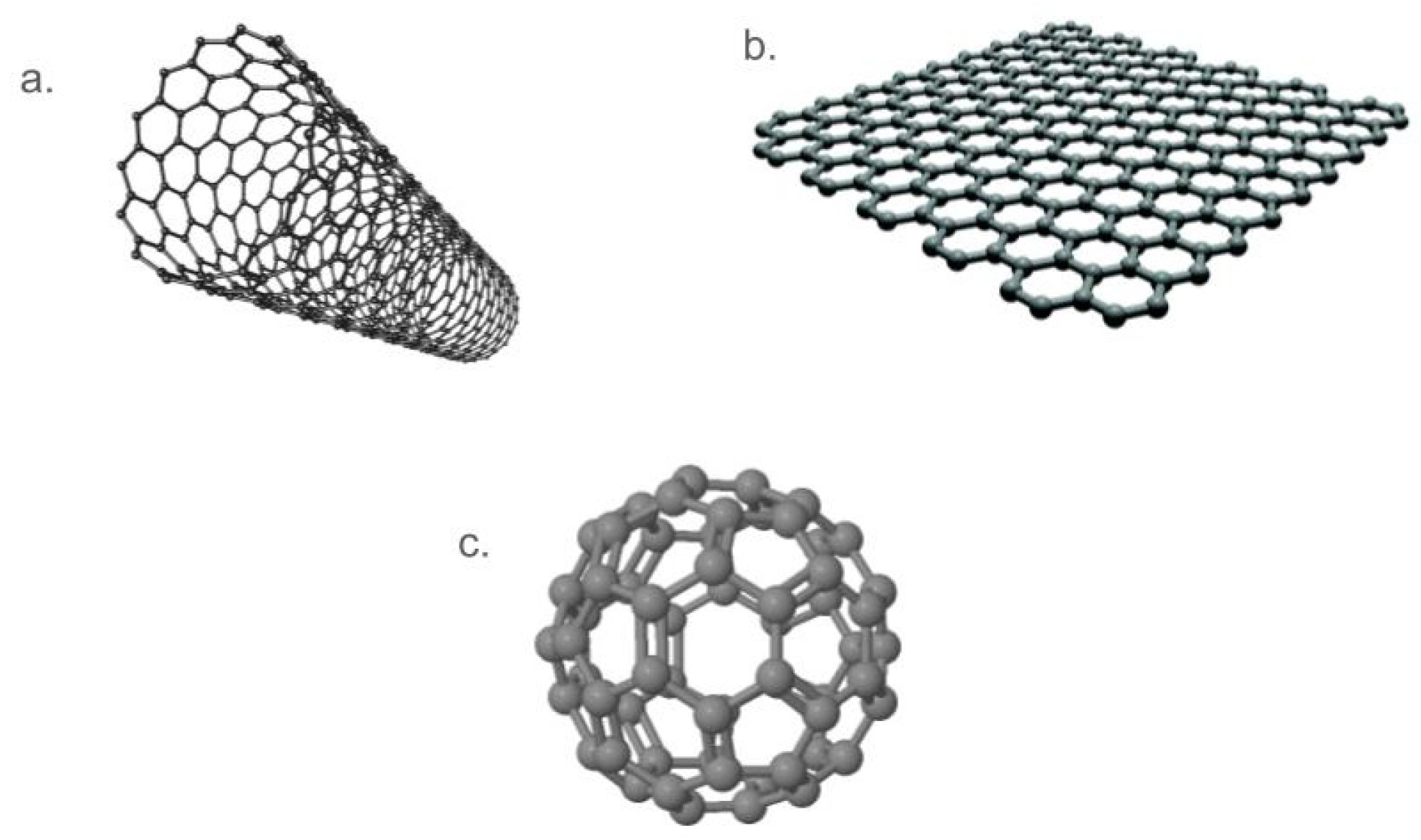
3.2.1. Carbon Nanotubes
3.2.2. Fullerenes
3.2.3. Graphene-Based Nanomaterials
3.2.4. Other Carbon-Based Nanomaterials
3.3. Hybrid Nanocomposites
3.3.1. Metal/Metal Oxide-Based Nanocomposites
3.3.2. Polymer-Based Nanocomposites for Water Treatment
3.4. Miscellaneous
4. Challenges and Future Scope
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-Enabled water and wastewater treatment. Nano Impact 2016, 3, 22–39. [Google Scholar] [CrossRef]
- Gitis, V.; Hankins, N. Water treatment chemicals: Trends and challenges. J. Water Process Eng. 2018, 25, 34–38. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterial. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Larramendy, M.; Soloneski, S. (Eds.) Emerging Pollutants in the Environment: Current and Further Implications; BoD–Books on Demand: Norderstedt, Germany, 2015. [Google Scholar]
- Kumar, V.; Singh, S.; Kashyap, N.; Singla, S.; Bhadrecha, P.; Kaur, P. Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with industrial wastewater. Orient. J. Chem. 2015, 31, 357–361. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Datta, S.; Singh, D.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Umar, K.; Haque, M.M.; Mir, N.A.; Muneer, M. Titanium dioxide-Mediated photocatalyzed mineralization of two Selected organic pollutants in aqueous suspensions. J. Adv. Oxid. Technol. 2013, 16, 252–260. [Google Scholar]
- Umar, K.; Ibrahim, M.N.M.; Ahmad, A.; Rafatullah, M. Synthesis of Mn-Doped TiO2 by novel route and photocatalytic mineralization/intermediate studies of organic pollutants. Res. Chem. Intermediat. 2019, 45, 2927–2945. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Riffat, R. Fundamentals of Wastewater Treatment and Engineering; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Gautam, S.; Saini, G. Use of natural coagulants for industrial wastewater treatment. Glob. J. Environ. Sci. Manag. 2020, 6, 553–578. [Google Scholar]
- Dutta, A.; Sarkar, S. Sequencing batch reactor for wastewater treatment: Recent advances. Curr. Pollut. Rep. 2015, 1, 177–190. [Google Scholar] [CrossRef]
- Bassin, J.P.; Castro, F.D.; Valério, R.R.; Santiago, E.P.; Lemos, F.R.; Bassin, I.D. The impact of wastewater treatment plants on global climate change. In Water Conservation in the Era of Global Climate Change; Elseviers: Amsterdam, The Netherlands, 2021; pp. 367–410. [Google Scholar]
- Gerba, C.P.; Pepper, I.L. Municipal wastewater treatment. In Environmental and Pollution Science; Academic Press: Cambridge, MA, USA, 2019; pp. 393–418. [Google Scholar]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Sudesh, K. Smart and innovative nanotechnology applications for water purification. Hybrid Adv. 2023, 3, 100044. [Google Scholar]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sher, F.; Hameed, M.; Bashir, O.; Kumar, R.; Vo, D.V.; Ahmad, P.; Lima, E.C. Sustainable nanotechnology-based wastewater treatment strategies: Achievements, challenges and future perspectives. Chemosphere 2022, 288, 132606. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Khan, M.N.; Dutta, J. Perspectives and applications of nanotechnology in water treatment. Environ. Chem. Lett. 2016, 14, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation—A comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef] [PubMed]
- Soren, S.; Panda, P.; Chakroborty, S. Nanotechnology in water and wastewater treatment. In Agricultural and Environmental Nanotechnology: Novel Technologies and Their Ecological Impact; Springer Nature: New York, NY, USA, 2023; pp. 127–143. [Google Scholar]
- Umar, K. Water Contamination by Organic-Pollutants: TiO2 Photocatalysis. In Modern Age Environmental Problems and Their Remediation; Springer International Publishing: Cham, Switzerland, 2018; pp. 95–109. [Google Scholar]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Wang, L. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; Du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-Fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Hogen-Esch, T.; Pirbazari, M.; Ravindran, V.; Yurdacan, H.M.; Kim, W. High Performance Membranes for Water Reclamation Using Polymeric and Nanomaterials. U.S. Patent 10,456,754, 29 October 2019. [Google Scholar]
- Saleh, A.; Parthasarathy, P.; Irfan, M. Advanced functional polymer nanocomposites and their use in water ultra-purification. Trends. Environ. Anal. 2019, 24, 67–78. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Hirata, K.; Watanabe, H.; Kubo, W. Nanomembranes as a substrate for ultra-thin lightweight devices. Thin Solid Films 2019, 676, 8–11. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; Al-saadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology; air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Rehman, W.U.; Khan, I.U.; Ahmad, S.; Ayoub, M.; Usmani, M.A. Nanocomposite membrane for environmental remediation. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 407–440. [Google Scholar]
- Muntha, S.T.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. Polym.-Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Ji, K.; Liu, C.; He, H.; Mao, X.; Wei, L.; Wang, H.; Zhang, M.; Shen, Y.; Sun, R.; Zhou, F. Research progress of water treatment technology based on nanofiber membranes. Polymers 2023, 15, 741. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels—Combining polymers with low-MOLECULAR-Weight gelators to yield modified self-Assembling smart materials with high-Tech applications. Mater. Horiz. 2015, 2, 279–293. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Z.; Petrini’c, I.; Fane, A.G.; Hélix-Nielsen, C. Biomimetic aquaporin membranes coming of age. Desalination 2015, 368, 89–105. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Mostafa, E. Nanofiltration Membranes for the Removal of Heavy Metals from Aqueous Solutions: Preparations and Applications. Membranes 2023, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-Based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Q.; Tian, S.; Zhao, X. Exposed facet dependent stability of ZnO micro/nano crystals as a photocatalyst. Appl. Surf. Sci. 2019, 470, 807–816. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Umar, K.; Aris, A.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.B.; Talib, J. Synthesis, Characterization of Mo and Mn doped Zno and their photocatalytic activity for the decolorization of two different chromophoric dyes. Appl. Catal. A 2015, 505, 507–514. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.B.; Jaafar, J.; Majid, Z.A.; Aris, A.; Umar, K.; Talib, J.; Madhavi, G. Relative efficiency comparison of carboxymethyl cellulose (cmc) stabilized Fe0 and Fe0/Ag nanoparticles for rapid degradation of chlorpyrifos in aqueous solutions. Dig. J. Nanomater. Bios. 2015, 10, 331–340. [Google Scholar]
- Samanta, H.S.; Das, R.; Bhattachajee, C. Influence of Nanoparticles for Wastewater Treatment-A Short Review. Austin Chem. Eng. 2016, 3, 1036–1045. [Google Scholar]
- Sherman, J. Nanoparticulate Titanium Dioxide Coatings, and Processes for the Production and Use Thereof. U.S. Patent No. 6653356B2, 25 November 2003. [Google Scholar]
- Ali, I.; Ghamdi, K.A.; Wadaani, F.T.A. Advances in iridium nano catalyst preparation, characterization and applications. J. Mol. Liq. 2019, 280, 274–284. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on grapheme-TiO2 and doped grapheme-TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Yamakata, A.; Junie Jhon, M.V. Curious behaviors of photogenerated electrons and holes at the defects on anatase, rutile, and brookite TiO2 powders: A review. J. Photochem. Photobiol. C Phtotochem. Rev. 2019, 40, 234–243. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci. Rep. 2017, 7, 40–95. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.F.; Elhadidy, H. Effect of Zr+4 doping on characteristics and sono catalytic activity of TiO2/carbon nanotubes composite catalyst for degradation of chlorpyrifos. J. Phys. Chem. Solids 2019, 129, 180–187. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Ghosh, R.; Dam, S.; Baskey, M. Madhuca longifolia plant mediated green synthesis of cupric oxide nanoparticles: A promising environmentally sustainable material for wastewater treatment and efficient antibacterial agent. J. Photochem. Photobiol. 2018, 189, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Guya, N.; Cakar, S.; Ozacar, M. Comparison of palladium/zinc oxide photocatalysts prepared by different palladium doping methods for congo red degradation. J. Colloid Interface Sci. 2016, 466, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Bishoge, O.K.; Zhang, L.; Suntu, S.L.; Jin, H.; Zewde, A.A.; Qi, Z. Remediation of water and wastewater by using engineered nanomaterials: A review. J. Environ. Sci. Health A 2018, 53, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Berekaa, M.M. Nanotechnology in wastewater treatment; influence of nanomaterials on microbial systems. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 713–726. [Google Scholar] [CrossRef]
- Serrà, A.; Grau, S.; Gimbert-Suriñach, C.; Sort, J.; Nogués, J.; Vallés, E. Magnetically Actuated mesoporous nanowires for enhanced heterogeneous catalysis. Appl. Catal. B Environ. 2017, 217, 81–91. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.B.; Kiran, H.; Iqbal, T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: A review. Environ. Sci. Pollut. Res. 2019, 26, 10515–10528. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Shahrezaei, F.; Farsi, M. Zinc sulfide quantum dots as powerful and efficient nanophotocatalysts for the removal of industrial pollutant. J. Mater. Sci. Mater. Electron. 2016, 27, 9297–9305. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Zhang, Y.; Zhang, L.; Jiao, T. Recent developments in functional nanocomposite photocatalysts for wastewater treatment: A review. Adv. Sustain. Syst. 2022, 6, 2200106. [Google Scholar] [CrossRef]
- Montemagno, C.; Schmidt, J.; Tozzi, S. Biomimetic Membranes. U.S. Patent No. 20040049230, 11 March 2004. [Google Scholar]
- Yu, L.; Ruan, S.; Xu, X.; Zou, R.; Hu, J. Review One-Dimensional nanomaterial-Assembled macroscopic membranes for water treatment. Nano Today 2017, 17, 79–95. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-Based nanosorbents for the removal of heavy-Metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Fuwad, A.; Ryu, H.; Malmstadt, N.; Kim, S.M.; Jeon, T.J. Biomimetic membranes as potential tools for water purification: Preceding and future avenues. Desalination 2019, 458, 97–115. [Google Scholar] [CrossRef]
- Rodovalho, F.L.; Capistrano, G.; Gomes, J.A.; Sodre, F.F.; Chaker, J.A.; Campos, A.F.C.; Bakuzis, A.F.; Sousa, A.H. Elaboration of magneto-Thermally recyclable nanosorbents for remote removal of toluene in contaminated water using magnetic hyperthermia. Chem. Eng. J. 2016, 15, 725–732. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Mussel-Inspired synthesis of magnetic polydopamine–Chitosan nanoparticles as bio sorbent for dyes and metals removal. J. Taiwan Inst. Chem. E 2016, 61, 292–298. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Matis, K.A. Review Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Sahebi, S.; Sheikhi, M.; Ramavandi, B. A new biomimetic aquaporin thin film composite membrane for forward osmosis: Characterization and performance assessment. Desalin. Water Treat. 2019, 148, 42–50. [Google Scholar] [CrossRef]
- Perez, T.; Pasquini, D.; Lima, A.F.; Rosa, E.V.; Sousa, M.H.; Cerqueira, D.A.; Morais, L.C. Efficient removal of lead ions from water by magnetic nanosorbents based on manganese ferrite nano particles capped with thin layers of modified biopolymers. J. Environ. Chem. Eng. 2019, 7, 802–892. [Google Scholar] [CrossRef]
- Manikam, M.K.; Halim, A.A.; Hanafiah, M.M.; Krishnamoorthy, R.R. Removal of ammonia nitrogen, nitrate, phosphorus and COD from sewage wastewater using palm oil boiler ash composite adsorbent. Desal. Water Treat. 2019, 149, 23–30. [Google Scholar] [CrossRef]
- Charee, S.W.; Aravinthan, V.; Erdei, L.; Raj, W.S. Use of macadamia nut shell residues as magnetic nanosorbents. Int. Biodeter. Biodegr. 2017, 124, 276–287. [Google Scholar]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and emerging adsorbent technologies for wastewater treatment: Trends, limitations, and environmental implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Wang, J. Micromotors for environmental applications: A review. Environ. Sci. Nano 2018, 5, 1530–1544. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Pumera, M. Chemical energy powered nano/micro/macromotors and the environment. Chem. A Eur. J. 2015, 21, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Simmchen, J.; Jänis, J. Correction: Light-Driven micro-and nanomotors for environmental remediation. Environ. Sci. Nano 2017, 4, 2235. [Google Scholar] [CrossRef]
- Pacheco, M.; López, M.Á.; Jurado-Sánchez, B.; Escarpa, A. Self-Propelled micromachines for analytical sensing: A critical review. Anal. Bioanal. Chem. 2019, 411, 6561–6573. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Wang, Z.; Tian, F.; You, J.A.; Xu, S. A review of fast bubble-Driven micromotors powered by biocompatible fuel: Low-Concentration fuel, bioactive fluid and enzyme. Micromachines 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, J.; Serrà, A.; Tierno, P.; Alcobé, X.; Vallés, E. Magnetic propulsion of recyclable catalytic nanocleaners for pollutant degradation. ACS Appl. Mater. Interfaces 2017, 9, 23859–23868. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Pumera, M. Multifunctional and self-Propelled spherical Janus nano/micromotors: Recent advances. Nanoscale 2018, 10, 16398–16415. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sánchez, B.; Sattayasamitsathit, S.; Gao, W.; Santos, L.; Fedorak, Y.; Singh, V.V.; Orozco, J.; Galarnyk, M.; Wang, J. Self-propelled activated carbon janus micromotors for efficient water purification. Small 2015, 11, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Seah, T.H.; Zhao, G.; Pumera, M. Surfactant Capsules Propel Interfacial Oil Droplets: An Environmental Cleanup Strategy. ChemPlusChem 2013, 78, 395–397. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Dong, R.; Wang, J. Catalytic Iridium-Based Janus Micromotors Powered by Ultralow Levels of Chemical Fuels. J. Am. Chem. Soc. 2014, 136, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, P.; Snow, B.; Santos, R.M.; Chiang, Y.W. Micro-structured copper and nickel metal foams for wastewater disinfection: Proof-of-concept and scale-up. Process Saf. Environ. Prot. 2020, 142, 191–202. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, H.; Li, J.; Song, H.; Wang, S.; Zhang, Z.; Chen, S. Green light–triggered antimicrobial cotton fabric for wastewater disinfection. Mater. Today Phys. 2020, 15, 100254. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, Z.; Zeng, G.; Lai, C.; Duan, A.; Xiao, R.; Huang, D.; Fu, Y.; Yi, H.; Li, B.; et al. Cooperative catalytic performance of bimetallic Ni-Au nanocatalyst for highly efficient hydrogenation of nitroaromatics and corresponding mechanism insight. Appl. Catal. B Environ. 2019, 259, 118035. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Zhong, H.; Wang, Z.; Liu, Z.; Cheng, M.; Liu, G.; Yang, X.; Liu, S. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction? J. Hazard. Mater. 2017, 322, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Pathogen Control in Drinking Water. In Microbiology of Waterborne Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 537–569. [Google Scholar]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ou, T.; Ding, S.; Fang, C.; Xu, Z.; Chu, W. Disinfection by-products as environmental contaminants of emerging concern: A review on their occurrence, fate and removal in the urban water cycle. Crit. Rev. Environ. Sci. Technol. 2023, 53, 19–46. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Vervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Elmi, F.; Alinezhad, H.; Moulana, Z.; Salehian, F.; Tavakkoli, S.M.; Asgharpour, F.; Fallah, H.; Elmi, M.M. The use of antibacterial activity of ZnO nanoparticles in the treatment of municipal wastewater. Water Sci. Technol. 2014, 70, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.H.D.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.C.; Sharma, S.K.; Kennedy, M. Development of iron oxide nanoparticle adsorbents for arsenic and fluoride removal. Desalin. Water Treat. 2017, 67, 187–195. [Google Scholar]
- Zhong, L.S.; Hu, J.S.; Cao, A.M.; Liu, Q.; Song, W.G.; Wan, L.J. 3D flowerlike ceria micro/nanocomposite structure and its application for water treatment and CO removal. Chem. Mater. 2007, 19, 1648–1655. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Thomas, S.; Pasquini, D.; Leu, S.-Y.; Gopakumar, W.P. (Eds.) Chapter 17—Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes from Wastewater. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–472. [Google Scholar]
- Daniel, S.; Malathi, S.; Balasubramanian, S.; Sivakumar, M.; Sironmani, T.A. Multifunctional silver, copper and zero valent iron metallic nanoparticles for wastewater treatment. In Application of Nanotechnology in Water; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 435–457. [Google Scholar]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr (VI) removal. J. Mater. Sci. 2014, 49, 4236–4245. [Google Scholar] [CrossRef]
- Ngomsik, A.-F.; Bee, A.; Talbot, D.; Cote, G. Magnetic solid-liquid extraction of Eu (III), La (III), Ni (II) and Co (II) with maghemite nanoparticles. Sep. Purif. Technol. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, A.L.K.; Tao, Y.; Shan, D.; Ting, K.E.; Yin, X.J. Synthesis and characterization of iron oxide nanoparticles and applications in the removal of heavy metals from industrial wastewater. Int. J. Photoenergy 2012, 2012, 608298. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Warner, C.L.; Addleman, R.S.; Cinson, A.D.; Droubay, T.C.; Engelhard, M.H.; Nash, M.A.; Yantasee, W.; Warner, M.G. High-performance, superparamagnetic, nanoparticle-based heavy metal sorbents for removal of contaminants from natural waters. ChemSusChem 2010, 3, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sawafta, R.; Shahwan, T. A comparative study of the removal of methylene blue by iron nanoparticles from water and water ethanol solutions. J. Mol. Liq. 2019, 273, 274–281. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Liu, Z.; Xiong, Y. Preparation and application of stability enhanced magnetic nanoparticles for rapid removal of Cr(VI). Chem. Eng. J. 2011, 175, 222–227. [Google Scholar] [CrossRef]
- Chang, Y.; Zeng, H.C. Controlled synthesis and self-assembly of single-crystalline CuO Nanorods and Nanoribbons. Cryst. Growth Des. 2004, 4, 397–402. [Google Scholar] [CrossRef]
- Singh, K.K.; Senapati, K.K.; Sarma, K.C. Synthesis of superparamagnetic Fe3O4 nanoparticles coated with green tea polyphenols and their use for removal of dye pollutant from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 2214–2221. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chu, W. Heterogeneous catalytic ozonation of phenacetin in water using magnetic spinel ferrite as catalyst: Comparison of surface property and efficiency. J. Mol. Catal. A Chem. 2015, 396, 164–173. [Google Scholar] [CrossRef]
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Martinez, M.; Silley, P. Antimicrobial Drug Resistance. In Comparative and Veterinary Pharmacology; Springer: Hoboken, NJ, USA, 2010; pp. 227–264. [Google Scholar]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Wu, Z.; Yue, X.; Yuan, S.; Lu, H.; Liang, B. Silver oxide as superb and stable Photocatalyst under visible and nearinfrared light irradiation and its Photocatalytic mechanism. Ind. Eng. Chem. Res. 2015, 54, 832–841. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Haq, S.; Rehman, W.; Waseem, M.; Shoukat, S.; Rehman, M. Photocatalytic and antibacterial activities of paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 45045. [Google Scholar] [CrossRef]
- Kumar, V.S.; Nagaraja, B.M.; Shashikala, V.; Padmasri, A.H.; Madhavendra, S.S.; Raju, B.D.; Rao, K.S.R. Highly efficient Ag/C catalyst prepared by electro-chemical deposition method in controlling microorganisms in water. J. Mol. Catal. A Chem. 2004, 223, 313–319. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, V.; Rajendran, K.; Sen, S. Antimicrobial activity of biosynthesized silver oxide nanoparticles. J. Pure Appl. Microbiol. 2014, 4, 3263–3268. [Google Scholar]
- Ganguly, K.; Dutta, S.D.; Patel, D.K.; Lim, K.-T. Silver nanoparticles for wastewater treatment. In Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 385–401. [Google Scholar]
- Matthews, R.W. Photo-oxidation of organic material in aqueous suspensions of titanium dioxide. Water Res. 1986, 20, 569–578. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Guo, M.; Xu, F.; Wang, P.; Du, Y.; Na, P. One-pot synthesis of Mn-doped TiO2 grown on graphene and the mechanism for removal of Cr (VI) and Cr (III). J. Hazard. Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, T.; Shinozaki, K.; Tsuruta, K.; Hirano, K. Photo-electrochemical degradation of some chlorinated organic compounds on n-TiO2 electrode. Chemosphere 2008, 73, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Wang, T.; Li, Y.; Wang, X.; Du, X. Phenyl-functionalization of titanium dioxide-nanosheets coating fabricated on a titanium wire for selective solid-phase microextraction of polycyclic aromatic hydrocarbons from environment water samples. Talanta 2015, 144, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, S.J.; Venkateswaran, P.; Jang, J.S.; Kim, H.; Kim, J.G. Anion co-doped Titania for solar photocatalytic degradation of dyes. Carbon Lett. 2008, 9, 131–136. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.T.; Yun, S.T.; Kim, S.O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Hsieh, C.-T.; Juang, R.-S. Substituent effects on photodegradation of phenols in binary mixtures by hybrid H2O2 and TiO2 suspensions under UV irradiation. J. Taiwan Inst. Chem. Eng. 2016, 62, 68–75. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium. dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, H.; Liu, Y. Synergistic effects of F and Fe in co-doped TiO2 nanoparticles. J. Nanoparticle Res. 2016, 18, 60. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, C.; Jung, K.-W.; Jung, D. Structure related Photocatalytic properties of TiO2. Bull. Korean Chem. Soc. 2009, 30, 402–404. [Google Scholar]
- Liou, W.; Chang, H.-H. Bactericidal effects and mechanisms of visible lightresponsive titanium dioxide Photocatalysts on pathogenic Bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of Photocatalytic degradation of the Bacterial Wall membrane at the TiO2 Interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Memb. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Amini, M.; Rahimpour, A.; Jahanshahi, M. Forward osmosis application of modified TiO2-polyamide thin film nanocomposite membranes. Desalin. Water Treat. 2016, 57, 14013–14023. [Google Scholar] [CrossRef]
- Youssef, A.M.; Malhat, F.M. Selective removal of heavy metals from drinking water using titanium dioxide nanowire. Macromol. Symp. 2014, 337, 96–101. [Google Scholar] [CrossRef]
- Engates, K.; Shipley, H. Adsorption of Pb, cd, cu, Zn, and Ni to titanium dioxide nanoparticles: Effect of particle size, solid concentration, and exhaustion. Environ. Sci. Pollut. Res. Int. 2011, 18, 386–395. [Google Scholar] [CrossRef]
- Silva, A.M.; Silva, C.G.; Dražić, G.; Faria, J.L. Ce-doped TiO2 for photocatalytic degradation of chlorophenol. Catal. Today 2009, 144, 13–18. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Baruah, S.; Pal, S.K.; Dutta, J. Nanostructured Zinc Oxide for Water Treatment. Nanosci. Nanotechnol. 2012, 2, 90–102. [Google Scholar] [CrossRef]
- Gondal, A.M.; Dastageer, M.A.; Khalil, A.; Hayat, K.; Yamani, Z.H. Nanostructured ZnO synthesis and its application for effective disinfection of Escherichia coli microorganism in water. J. Nanopart. Res. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N.B. Preparation and antibacterial activity of chitosan-based nanocomposites containing bentonite-supported silver and zinc oxide nanoparticles for water disinfection. Appl. Clay Sci. 2015, 114, 330–339. [Google Scholar] [CrossRef]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater. Sci. Eng. C 2016, 58, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Dimapilis, E.A.S.; Hsu, C.; Mendoza, R.M.O.; Lu, M. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.; Mousa, M.; Hussein, A.; Hammouti, B.; Hadda, T.B.; Warad, I. Copper (II)-oxide nanostructures: Synhesis, characterizations and their applications—Review. J. Mater. Environ. Sci. 2013, 4, 792–797. [Google Scholar]
- Khare, P.; Sharma, A.; Verma, N. Synthesis of phenolic precursor-based porous carbon beads in situ dispersed with copper–silver bimetal nanoparticles for antibacterial applications. J. Colloid Interface Sci. 2014, 418, 216–224. [Google Scholar] [CrossRef]
- Allaker, R.P.; Memarzadeh, K. Nanoparticles and the control of oral infections. Int. J. Antimicrob. Agents 2014, 43, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Chen, Y.; Li, M.; Liu, K.; Li, X. Influence of copper nanoparticles on the physical-chemical properties of activated sludge. PLoS ONE 2014, 9, e0092871. [Google Scholar] [CrossRef] [PubMed]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, T.; Xu, N.; Zhang, Y.; Wang, S. Enzyme-linked immunosorbent assay and colloidal gold immunoassay for ochratoxin A: Investigation of analytical conditions and sample matrix on assay performance. Anal. Bioanal. Chem. 2007, 289, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.B.O.; Kim, K.Y.; Chung, D.H. Development and validation of a gold nanoparticle immunochromatographic assay (ICG) for the detection of zearalenone. J. Agric. Food Chem. 2009, 57, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Darbha, G.K.; Singh, A.K.; Rai, U.S.; Yu, E.; Yu, H.; Ray, P.C. Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 8038–8043. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wang, F.; Liu, X. One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar] [CrossRef]
- Li, J. Electrocatalytic oxidation of nitrite at gold nanoparticle-polypyrrole nanowire modified glassy carbon electrode. Chin. J. Chem. 2009, 27, 2373–2378. [Google Scholar] [CrossRef]
- Daniel, W.L.; Han, M.S.; Lee, J.S.; Mirkin, C.A. Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.S.R.; Singh, A.K.; Senapati, D.; Yu, H.; Ray, P.C. Gold nanoparticle-based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef]
- Rassaei, L.; Sillanpää, M.; Marken, F. Carbon nanoparticle–chitosan thin film electrodes: Physisorption versus Chemisorption. Electrochim. Acta 2008, 53, 5732–5738. [Google Scholar] [CrossRef]
- Hennebel, T.; De Corte, S.; Vanhaecke, L.; Vanherck, K.; Forrez, I.; De Gusseme, B.; Verhagen, P.; Verbeken, K.; Van der Bruggen, B.; Vankelecom, I.; et al. Removal of diatrizoate with catalytically active membranes incorporating microbially produced palladium nanoparticles. Water Res. 2010, 44, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Benner, J.; Clauwaert, P.; Vanhaecke, L.; Aelterman, P.; Boon, N.; Verstraete, W. Dehalogenation of environmental pollutants in microbial electrolysis cells with biogenic palladium nanoparticles. Biotechnol. Lett. 2011, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- De Gusseme, B.; Hennebel, T.; Vanhaecke, L.; Soetaert, M.; Desloover, J.; Wille, K.; Verbeken, K.; Verstraete, W.; Boon, N. Biogenic palladium enhances diatrizoate removal from hospital wastewater in a microbial electrolysis cell. Environ. Sci. Technol. 2011, 45, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Forrez, I.; Carballa, M.; Fink, G.; Wick, A.; Hennebel, T.; Vanhaecke, L.; Ternes, T.; Boon, N.; Verstraete, W. Biogenic metals for the oxidative and reductive removal of pharmaceuticals, biocides and iodinated contrast media in a polishing membrane bioreactor. Water Res. 2011, 45, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yong, K. Morphology-controlled synthesis of highly adsorptive tungsten oxide nanostructures and their application to water treatment. J. Mater. Chem. 2010, 20, 10146–10151. [Google Scholar] [CrossRef]
- Wang, X.; Xu, D.; Chen, L.; Tan, X.; Zhou, X.; Ren, A.; Chen, C. Sorption and complexation of Eu (III) on alumina: Effects of pH, ionic strength, humic acid and chelating resin on kinetic dissociation study. Appl. Radiat. Isot. 2006, 64, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interface Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Pakniat, M.; Mahmoudi, Z.; Hajati, S.; Sahraei, R.; Daneshfar, A. Synthesis of nickel sulfide nanoparticles loaded on activated carbon as a novel adsorbent for the competitive removal of methylene blue and safranin-O. Spectrochim. Acta A 2014, 123, 402–409. [Google Scholar] [CrossRef]
- Sudhasree, S.; Shakila Banu, A.; Brindha, P.; Kurian, G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Zhang, G.; Zhao, L. Room-temperature synthesis of Ni nanoparticles as the absorbent used for sewage treatment. Adv. Mater. Sci. Eng. 2015, 2015, 973648. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 0080426824. [Google Scholar]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of carbon nanotubes for piezoresistive-sensing applications: A review. J. Sci. Adv. Mater. Devices 2021, 7, 100416. [Google Scholar] [CrossRef]
- Moaseri, E.; Karimi, M.; Maghrebi, M.; Baniadam, M. Fabrication of multi-walled carbon nanotube–carbon fiber hybrid material via electrophoretic deposition followed by pyrolysis process. Compos. Part A Appl. Sci. Manuf. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.; Annuar, M.S.M.; Samsudin, E.M.B.; Bagheri, S. Covalent functionalization schemes for tailoring solubility of multi-walled carbon nanotubes in water and acetone solvents. Sci. Adv. Mater. 2015, 7, 2726–2737. [Google Scholar] [CrossRef]
- Obaidullah, I. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar]
- Kalra, A.; Garde, S.; Hummer, G. Osmotic water transport through carbon nanotube membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, P.; Guan, C.; Song, X.; Zhang, Q.; Leong, S.S.J.; Tang, C.; Xu, R. Hollow fiber membrane decorated with Ag/MWNTs: Toward effective water disinfection and biofouling control. ACS Nano 2011, 5, 10033–10040. [Google Scholar] [CrossRef]
- Akha, N.Z.; Salehi, S.; Anbia, M. Removal of arsenic by metal organic framework/chitosan/carbon nanocomposites: Modeling, optimization, and adsorption studies. Int. J. Biol. Macromol. 2022, 208, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, Y.; Huang, X.; Tang, S.; Chen, H.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem. Eng. J. 2022, 450, 138264. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; ArancibiaBulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Gu, C.; Zhang, C. Water disinfection processes change the cytotoxicity of C60 fullerene: Reactions at the nano-bio interface. Water Res. 2019, 163, 114867. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lee, J.Y.; Park, K.H.; Kim, W.; Lee, J.H.; Lee, J.H. Photosensitized Production of Singlet Oxygen via C60 Fullerene Covalently Attached to Functionalized Silica-coated Stainless-Steel Mesh: Remote Bacterial and Viral Inactivation. Appl. Catal. B Environ. 2020, 270, 118862. [Google Scholar] [CrossRef]
- Jani, M.; Arcos-Pareja, J.A.; Ni, M. Engineered Zero-Dimensional Fullerene/Carbon Dots-Polymer Based Nanocomposite Membranes for Wastewater Treatment. Molecules 2020, 25, 4934. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Scrivens, W.A.; Tour, J.M.; Creek, K.E.; Pirisi, L. Synthesis of 14C-Labeled C60, Its Suspension in Water, and Its Uptake by Human Keratinocytes. J. Am. Chem. Soc. 1994, 116, 4517–4518. [Google Scholar] [CrossRef]
- Moussa, F.; Trivin, F.; Céolin, R.; Hadchouel, M. Fullerene Science and Technology Early effects of C 60 Administration in Swiss Mice: A Preliminary Account for in vivo C60 Toxicity. Fuller. Sci. Technol. 1996, 4, 21–29. [Google Scholar] [CrossRef]
- Adam, A.M.; Saad, H.A.; Atta, A.A.; Alawsat, M.; Hegab, M.S.; Altalhi, T.A.; Refat, M.S. An environmentally friendly method for removing Hg (II), Pb (II), Cd (II) and Sn (II) heavy metals from wastewater using novel metal–carbon-based composites. Crystals 2021, 11, 882. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and Graphene. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Jayakaran, P.; Nirmala, G.S.; Govindarajan, L. Qualitative and Quantitative Analysis of Graphene-Based Adsorbents in Wastewater Treatment. Int. J. Chem. Eng. 2019, 2019, 9872502. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of graphene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. Nano 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Mohanta, D.; Mahanta, A.; Mishra, S.R.; Jasimuddin, S.; Ahmaruzzaman, M. Novel SnO2@ZIF-8/gC3N4 nanohybrids for excellent electrochemical performance towards sensing of p-nitrophenol. Environ. Res. 2021, 197, 111077. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.S.; Qiu, F.X.; Rong, J.; Yan, J.; Zhao, H.; Zhu, X.L.; Yang, D.Y. Synthesis of porous g-C3N4/La and enhanced photocatalytic activity for the degradation of phenol under visible light irradiation. J. Solid State Chem. 2015, 230, 126–134. [Google Scholar] [CrossRef]
- Li, Y.P.; Wu, S.L.; Huang, L.Y.; Xu, H.; Zhang, R.X.; Qu, M.L.; Gao, Q.; Li, H.M. g-C3N4 modified Bi2O3 composites with enhanced visible-light photocatalytic activity. J. Phys. Chem. Solids 2015, 76, 112–119. [Google Scholar] [CrossRef]
- He, Y.M.; Cai, J.; Li, T.T.; Wu, Y.; Lin, H.J.; Zhao, L.H.; Luo, M.F. Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem. Eng. J. 2013, 215–216, 721–730. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zhang, W.D. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. Appl. Surf. Sci. 2014, 301, 428–435. [Google Scholar] [CrossRef]
- Thondavada, N.; Chokkareddy, R.; Naidu, N.V.; Redhi, G.G. Environmental science and engineering applications of polymer and nanocellulose-based nanocomposites. In Composites for Environmental Engineering; Wiley-Scrivener: Salem, MA, USA, 2019; pp. 135–178. [Google Scholar]
- Tesh, S.J.; Scott, T.B. Nanocomposites for water remediation: A review. Adv. Mater. 2014, 26, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Niasari, M.S. Ship-in-a-bottle synthesis, characterization and catalytic oxidation of styrene by host (nanopores of zeolite-Y)/guest ([bis(2-hydroxyanil) acetylacetonato manganese (III)]) nanocomposite materials (HGNM). Microporous Mesoporous Mater. 2006, 95, 248–256. [Google Scholar] [CrossRef]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Green ZnO nanorod material for dye degradation and detoxifcation of pharmaceutical wastes in water. J. Environ. Chem. Eng. 2020, 8, 104295. [Google Scholar] [CrossRef]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Highly stable, reusable, and MW-assisted prepared ZnO nanorods for wastewater decontamination: Precursors ratios efect and insights on matrix and pollutants mineralization. J. Environ. Chem. Eng. 2021, 9, 104630. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Chen, F. Photocatalytic degradation of MB by novel and environmental ZnO/Bi2WO6-CC hierarchical heterostructures. Mater. Charact. 2022, 189, 111961. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Mohammadi, M.; Maleki, A.; Jafari, A.; Shahmoradi, B.; Rezaee, R.; Safari, M.; Daraei, H.; Giahi, O.; Yetilmezsoy, K.; et al. Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in aqueous solution using Mn-doped ZnO/graphene nanocomposite under LED radiation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 923–934. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, Q.; Du, J.; Yin, P.; Yi, J.; Liu, Y.; Wu, X.; Zhang, Z. A Z-scheme CuO–ZnO–ZnS–CuS quaternary nanocomposite for solar-light-driven photocatalytic performance. Curr. Appl. Phys. 2022, 39, 113–121. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@ GO and MOF@ CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; Islam, M.T.; Turley, R.S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J.A.; Curry, M.L.; Lopez, J.; et al. Sustainable synthesis and remarkable adsorption capacity of MOF/graphene oxide and MOF/CNT based hybrid nanocomposites for the removal of Bisphenol A from water. Sci. Total Environ. 2019, 673, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Xiao, Y.; Tong, M.; Huang, H.; Liu, D.; Wang, L.; Zhong, C. Synthesis of CNT@ MIL-68 (Al) composites with improved adsorption capacity for phenol in aqueous solution. Chem. Eng. J. 2015, 275, 134–141. [Google Scholar] [CrossRef]
- Ajabshir, S.Z.; Morassaei, M.S.; Niasari, M.S. Facile fabrication of Dy2Sn2O7-SnO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. J. Colloid Interface Sci. 2017, 497, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: Potential Reinforcement in Composites. Natural Polymers: Volume 2: Nanocomposites; Royal Society of Chemistry: Cambridge, UK, 2012; Volume 2, pp. 1–32. [Google Scholar]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication; Membrane Fabrication: Principles, Optimization and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Maphutha, S.; Moothi, K.; Meyyappan, M.; Iyuke, S.E. A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing wastewater. Sci. Rep. 2013, 3, 1509. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Berber, M.R. Current advances of polymer composites for water treatment and desalination. J. Chem. 2020, 2020, 7608423. [Google Scholar] [CrossRef]
- Hu, M.Z.; Engtrakul, C.; Bischoff, B.L.; Lu, M.; Alemseghed, M. Surface-Engineered inorganic nanoporous membranes for vapor and pervaporative separations of water–ethanol mixtures. Membranes 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, L.; Jia, N.; Wang, R.; Liu, L.; Gao, C. Polyphenol-metal manipulated nanohybridization of CNT membranes with FeOOH nanorods for high-flux, antifouling and self-cleaning oil/water separation. J. Membr. Sci. 2020, 600, 117857. [Google Scholar] [CrossRef]
- Ebert, K.; Fritsch, D.; Koll, J.; Tjahjawiguna, C. Influence of inorganic fillers on the compaction behavior of porous polymer-based membranes. J. Membr. Sci. 2004, 233, 71–78. [Google Scholar] [CrossRef]
- Haider, M.S.; Shao, G.N.; Imran, S.M.; Park, S.S.; Abbas, N.; Tahir, M.S.; Hussain, M.; Bae, W.; Kim, H.T. Aminated polyethersulfone-silver nanoparticles (AgNPs-APES) composite membranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. C 2016, 62, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kumari, P.; Pandey, S.; Narayan, T. Removal of chromium (VI) using poly (methylacrylate) functionalized guar gum. Bioresour. Technol. 2009, 100, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.Y.; Chen, D.H. Fast adsorption of methylene blue on polyacrylic acidbound iron oxide magnetic nanoparticles. Dye Pigment. 2004, 61, 93–98. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Xu, X.; Zhang, X.; Xue, Y.; Mi, J.; Mo, Z.; Fan, Y.; Hu, L.; Yang, X. Porous boron nitride with a high surface area: Hydrogen storage and water treatment. Nanotechnology 2013, 24, 155603. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-L.; Lun, N.; Qi, Y.-X.; Zhu, H.-L.; Han, F.-D.; Yin, L.-W.; Fan, R.-H.; Bai, Y.-J.; Bi, J.-Q. Simple synthesis of mesoporous boron nitride with strong cathodoluminescence emission. J. Solid State Chem. 2011, 184, 859–862. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Samba, J.; Heck, K.; Huang, X.; Lee, J.; Metz, J.; Bhati, M.; Fortner, J.; Li, Q.; et al. Surface hydrophobicity of boron nitride promotes PFOA photocatalytic degradation. Chem. Eng. J. 2024, 483, 149134. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, P.; Borthwick, A.G.L.; Chen, H.; Ni, J. Adsorption mechanisms of thallium(I) and thallium (III) by titanate nanotubes: Ion-exchange and coprecipitation. J. Colloid Interface Sci. 2014, 423, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cai, Y.; Yang, G.; Liu, Y.; Zeng, G.; Zhou, Y.; Li, S.; Wang, J.; Zhang, S.; Fang, Y.; et al. Cobalt nanoparticles-embedded magnetic carbon ordered mesoporous for highly effective adsorption of rhodamine B. Appl. Surf. Sci. 2014, 314, 746–753. [Google Scholar] [CrossRef]
- Liu, F.; Wen, L.-X.; Li, Z.; Sun, H.; Yu, W.; Chen, J. Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater. Res. Bull. 2006, 41, 2268–2275. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. Synthesis of magnetite particles for enhanced environmental performance: Comparative analysis of three schemes and their applications for phosphorus recovery from high-strength wastewater. Mater. Chem. Phys. 2024, 317, 129136. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; Alrawashdeh, A.I.; Etiwi, M.T.; Alnawaiseh, A.; Shahid, M.K.; Masad, M.H.M.; Oran, S.O.; Alshahateet, S.F. Silver Nanoparticles (Ag-NPs) Embedded in Zeolite Framework: A Comprehensive Study on Bromide Removal from Water, Including Characterization, Antibacterial Properties, and Adsorption Mechanisms. Desalin. Water Treat. 2024, 317, 100139. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, J.; Mishra, A.; Pandey, M.; Thakur, R.R.; Chand, S.; Rout, P.R.; Shahid, M.K. Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water 2024, 16, 1481. https://doi.org/10.3390/w16111481
Tripathy J, Mishra A, Pandey M, Thakur RR, Chand S, Rout PR, Shahid MK. Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water. 2024; 16(11):1481. https://doi.org/10.3390/w16111481
Chicago/Turabian StyleTripathy, Jasaswini, Akanshya Mishra, Mayank Pandey, Rakesh Ranjan Thakur, Sasmita Chand, Prangya Ranjan Rout, and Muhammad Kashif Shahid. 2024. "Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review" Water 16, no. 11: 1481. https://doi.org/10.3390/w16111481
APA StyleTripathy, J., Mishra, A., Pandey, M., Thakur, R. R., Chand, S., Rout, P. R., & Shahid, M. K. (2024). Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water, 16(11), 1481. https://doi.org/10.3390/w16111481








