Occurrence, Fate, and Mass Balance Analysis of Organophosphate Flame Retardants in a Municipal Wastewater Treatment Plant in Hunan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Study Plant and Sample Collection
2.3. Sample Preparation
2.4. QA/QC
2.5. Data Analysis
2.6. Ecological Risk Assessment
3. Results and Discussion
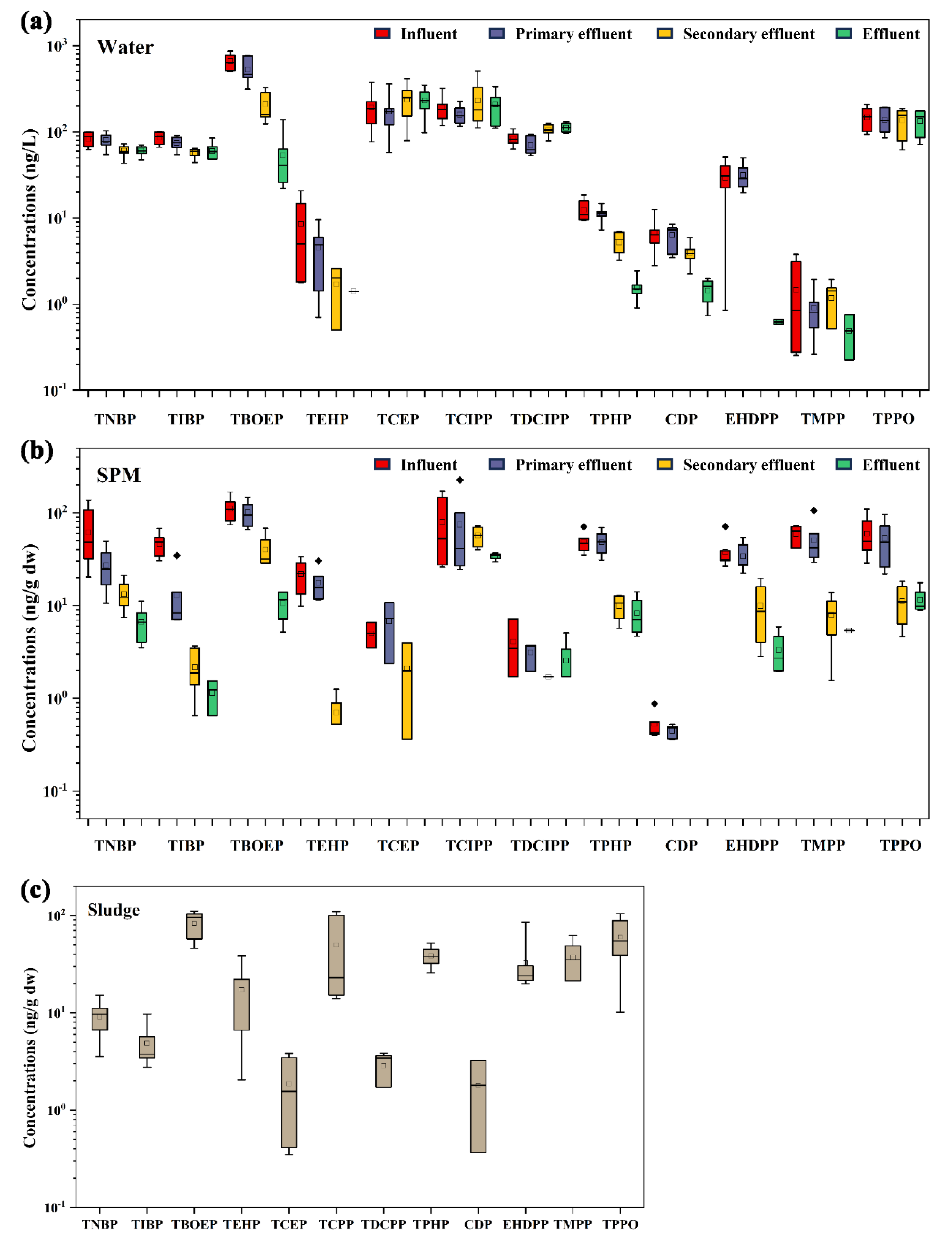
3.1. Occurrence of OPFRs in Wastewater Treatment
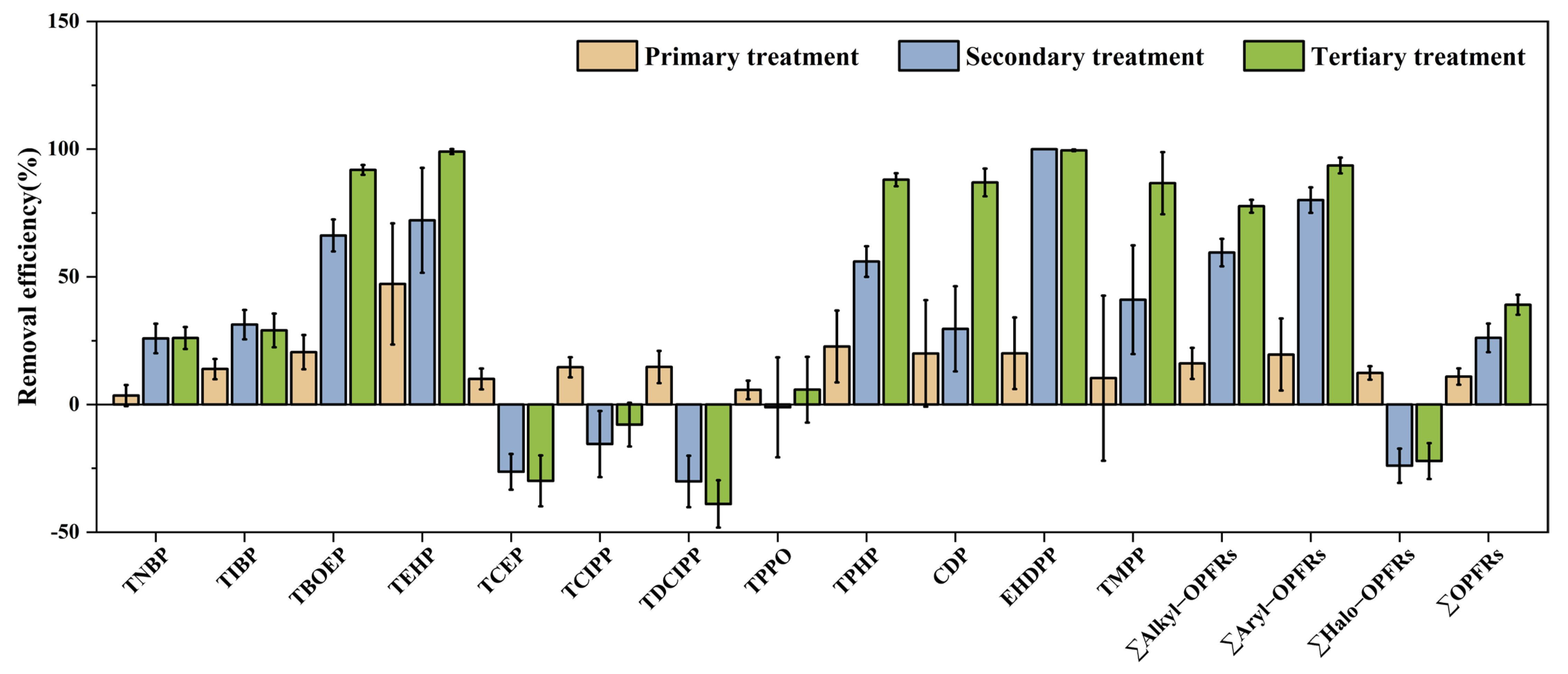
3.2. Distributions and Removal Efficiencies of OPFRs
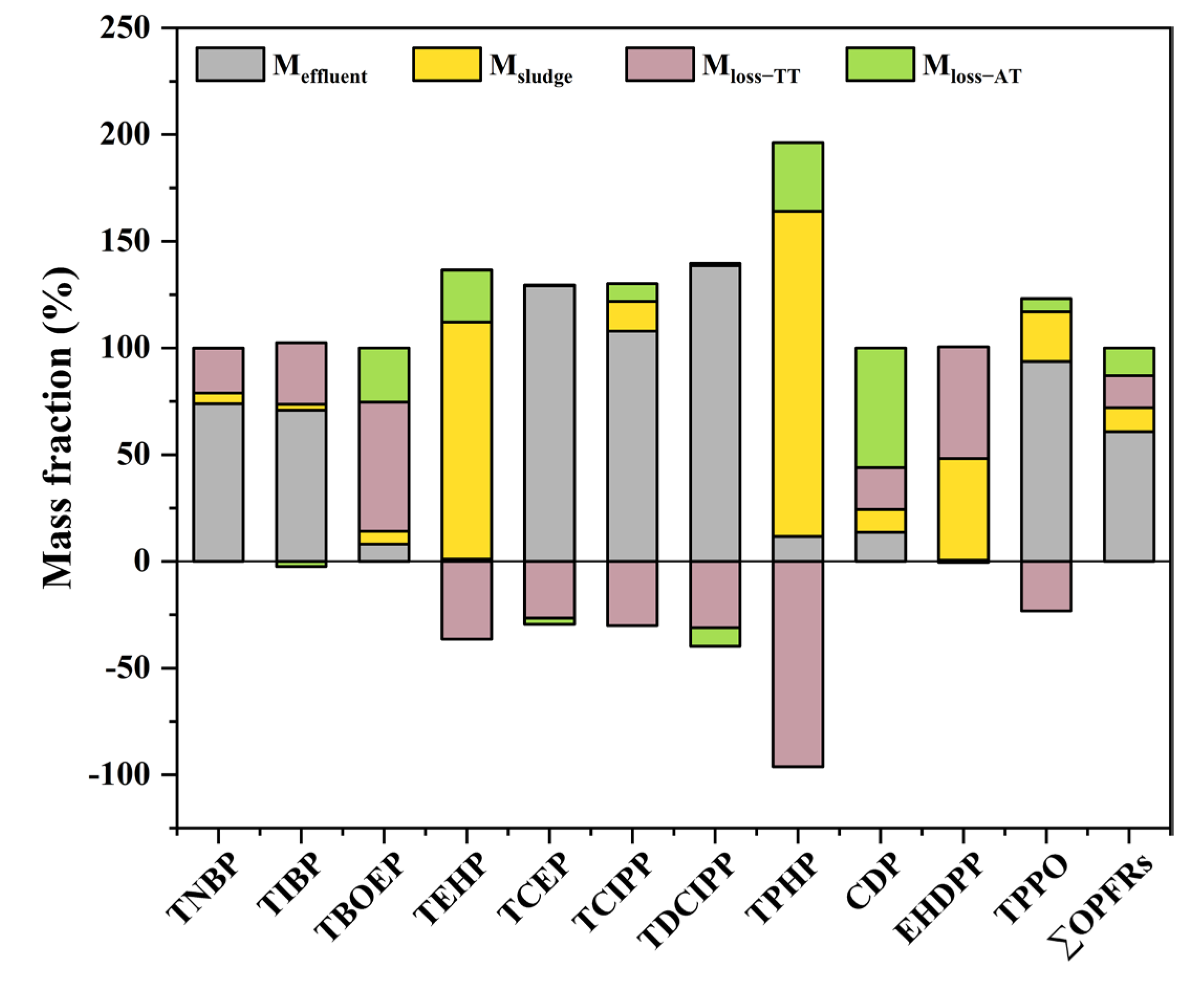
3.3. Mass Balance Analysis
3.4. Mass Loadings and Environmental Emissions
3.5. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Fu, J.; Fu, K.; Hu, B.; Zhou, W.; Fu, Y.; Gu, L.; Zhang, Q.; Zhang, A.; Fu, J.; Jiang, G. Source identification of organophosphate esters through the profiles in proglacial and ocean sediments from Ny-Ålesund, the Arctic. Environ. Sci. Technol. 2023, 57, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Sarvajayakesavalu, S.; Han, Y.; Zhang, H.; Gao, J.; Li, X.; Ma, M. Occurrence, distribution and risk assessment of organophosphate esters (OPEs) in water sources from Northeast to Southeast China. Environ. Pollut. 2022, 307, 119461. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, Y.; Wang, S.; Liu, X. Occurrence, spatiotemporal variation, and ecological risks of organophosphate esters in the water and sediment of the middle and lower streams of the Yellow River and its important tributaries. J. Hazard. Mater. 2023, 443, 130153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Li, H.; Song, Y.; Yang, Z.; Cui, Y. Occurrence of organophosphorus flame retardants in Xiangjiang River: Spatiotemporal variations, potential affecting factors, and source apportionment. Chemosphere 2024, 355, 141822. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wan, Y.; Wang, Y.; He, Z.; Xu, S.; Xia, W. Occurrence, spatial variation, seasonal difference, and ecological risk assessment of organophosphate esters in the Yangtze River, China: From the upper to lower reaches. Sci. Total Environ. 2022, 851, 158021. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Oliveira Santos, I.; Umbuzeiro, G.d.A.; Fagnani, E. Occurrence and risk assessment of organophosphate esters in urban rivers from Piracicaba watershed (Brazil). Environ. Sci. Pollut. Res. 2021, 28, 59244–59255. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.-Y.; Lin, H.; Qin, X.; Ruan, Y.; Leung, K.M.; Zeng, E.Y.; Lam, P.K. Insights into the atmospheric persistence, transformation, and health implications of organophosphate esters in urban ambient air. Environ. Sci. Technol. 2022, 56, 12003–12013. [Google Scholar] [CrossRef] [PubMed]
- Suhring, R.; Diamond, M.L.; Scheringer, M.; Wong, F.; Pucko, M.; Stern, G.; Burt, A.; Hung, H.; Fellin, P.; Li, H. Organophosphate esters in Canadian Arctic air: Occurrence, levels and trends. Environ. Sci. Technol. 2016, 50, 7409–7415. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Sellström, U.; De Wit, C.A. Organohalogenated flame retardants and organophosphate esters in office air and dust from Sweden. Environ. Sci. Technol. 2019, 53, 2124–2133. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Zhong, G.; Li, J.; Zhang, G.; Covaci, A. Occurrence and fate of organophosphate ester flame retardants and plasticizers in indoor air and dust of Nepal: Implication for human exposure. Environ. Pollut. 2017, 229, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Wu, C.; Liu, J.; Cai, Y. Occurrence, distribution and risk of organophosphate esters in urban road dust in Beijing, China. Environ. Pollut. 2018, 241, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kim, U.-J.; Kannan, K. Occurrence and distribution of organophosphate esters in sediment from northern Chinese coastal waters. Sci. Total Environ. 2020, 704, 135328. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wen, J.; Zeng, F.; Li, S.; Zhou, X.; Zeng, Z. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere 2017, 175, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.; Li, W.; Zhu, H.; Wang, L.; Sun, H.; Kannan, K. A nationwide survey of 19 organophosphate esters in soils from China: Spatial distribution and hazard assessment. Sci. Total Environ. 2019, 671, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sapozhnikova, Y.; Nuñez, A. Analysis and occurrence of organophosphate esters in meats and fish consumed in the United States. J. Agric. Food Chem. 2019, 67, 12652–12662. [Google Scholar] [CrossRef] [PubMed]
- Pantelaki, I.; Voutsa, D. Occurrence, analysis and risk assessment of organophosphate esters (OPEs) in biota: A review. Mar. Pollut. Bull. 2020, 160, 111547. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Z.; He, L.; Cao, S.; Song, H.; Yu, Z.; Sheng, G.; Fu, J. The occurrence and removal of organophosphate ester flame retardants/plasticizers in a municipal wastewater treatment plant in the Pearl River Delta, China. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1291–1297. [Google Scholar] [CrossRef]
- Lian, M.; Lin, C.; Li, Y.; Hao, X.; Wang, A.; He, M.; Liu, X.; Ouyang, W. Distribution, partitioning, and health risk assessment of organophosphate esters in a major tributary of middle Yangtze River using Monte Carlo simulation. Water Res. 2022, 219, 118559. [Google Scholar] [CrossRef]
- Kim, U.-J.; Oh, J.K.; Kannan, K. Occurrence, removal, and environmental emission of organophosphate flame retardants/plasticizers in a wastewater treatment plant in New York State. Environ. Sci. Technol. 2017, 51, 7872–7880. [Google Scholar] [CrossRef]
- Iqbal, M.; Syed, J.H.; Katsoyiannis, A.; Malik, R.N.; Farooqi, A.; Butt, A.; Li, J.; Zhang, G.; Cincinelli, A.; Jones, K.C. Legacy and emerging flame retardants (FRs) in the freshwater ecosystem: A review. Environ. Res. 2017, 152, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Luo, Y.; Song, J.; Li, S.; Lin, S.; Xiong, Y.; Fang, S.; Tang, J. Pollution characteristics and emissions of typical organophosphate esters of a wastewater treatment plant. Environ. Sci. Pollut. Res. 2022, 29, 25892–25901. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Ramos, D.D.; Dantas, R.F.; Junior, A.M.; Lacorte, S.; Sans, C.; Esplugas, S. Can activated sludge treatments and advanced oxidation processes remove organophosphorus flame retardants? Environ. Res. 2016, 144, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Yang, P.; Zhao, J.; Zhang, H. Comparison of wastewater treatment processes on the removal efficiency of organophosphate esters. Water Sci. Technol. 2016, 74, 1602–1609. [Google Scholar] [CrossRef]
- Liang, K.; Liu, J. Understanding the distribution, degradation and fate of organophosphate esters in an advanced municipal sewage treatment plant based on mass flow and mass balance analysis. Sci. Total Environ. 2016, 544, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Pantelaki, I.; Voutsa, D. Occurrence and removal of organophosphate esters in municipal wastewater treatment plants in Thessaloniki, Greece. Environ. Res. 2022, 214, 113908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Zhu, F.; Li, C.; Xu, Y.; Qing, D.; Wang, J. The influence of an upgrade on the reduction of organophosphate flame retardants in a wastewater treatment plant. Chemosphere 2020, 256, 126895. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, J.; Zhang, B.; Chen, L.; Wei, S.; Pan, B. Unveiling the Occurrence and Potential Ecological Risks of Organophosphate Esters in Municipal Wastewater Treatment Plants across China. Environ. Sci. Technol. 2023, 57, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Wang, J.; Wang, B.; Xin, M.; Lin, C.; Gu, X.; He, M.; Liu, X.; Ouyang, W. Spatiotemporal variations and the ecological risks of organophosphate esters in Laizhou Bay waters between 2019 and 2021: Implying the impacts of the COVID-19 pandemic. Water Res. 2023, 233, 119783. [Google Scholar] [CrossRef]
- Rodríguez, I.; Calvo, F.; Quintana, J.; Rubi, E.; Rodil, R.; Cela, R. Suitability of solid-phase microextraction for the determination of organophosphate flame retardants and plasticizers in water samples. J. Chromatogr. A 2006, 1108, 158–165. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Reemtsma, T. Liquid chromatography−tandem mass spectrometry determination of nonionic organophosphorus flame retardants and plasticizers in wastewater samples. Anal. Chem. 2005, 77, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Yuan, Y.; He, H.; Liang, K.; Zhang, H.; Zhao, J. Occurrence, distribution, and potential affecting factors of organophosphate flame retardants in sewage sludge of wastewater treatment plants in Henan Province, Central China. Chemosphere 2016, 152, 245–251. [Google Scholar] [CrossRef]
- Marklund, A.; Andersson, B.; Haglund, P. Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ. Sci. Technol. 2005, 39, 7423–7429. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Q.; Liu, J.; Liu, S.; Liu, C.; Deng, Q.; Zeng, X.; Yu, Z. Occurrence of organophosphate esters and their diesters degradation products in industrial wastewater treatment plants in China: Implication for the usage and potential degradation during production processing. Environ. Pollut. 2019, 250, 559–566. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Q.; Zhang, R.; Xing, L. Organophosphate esters in rural wastewater along the Yangtze River Basin: Occurrence, removal efficiency and environmental implications. J. Environ. Manag. 2023, 345, 118830. [Google Scholar] [CrossRef]
- Su, G.; Letcher, R.J.; Yu, H. Organophosphate flame retardants and plasticizers in aqueous solution: pH-dependent hydrolysis, kinetics, and pathways. Environ. Sci. Technol. 2016, 50, 8103–8111. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Multi-residue analytical method for the determination of emerging pollutants in water by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Cristale, J.; Dantas, R.F.; De Luca, A.; Sans, C.; Esplugas, S.; Lacorte, S. Role of oxygen and DOM in sunlight induced photodegradation of organophosphorous flame retardants in river water. J. Hazard. Mater. 2017, 323, 242–249. [Google Scholar] [CrossRef]
- O’Brien, J.W.; Thai, P.K.; Brandsma, S.H.; Leonards, P.E.; Ort, C.; Mueller, J.F. Wastewater analysis of Census day samples to investigate per capita input of organophosphorus flame retardants and plasticizers into wastewater. Chemosphere 2015, 138, 328–334. [Google Scholar] [CrossRef]
- Xing, R.; Zhang, P.; Zheng, N.; Ji, H.; Shi, R.; Ge, L.; Ma, H. Organophosphate esters in the seawater of the Bohai Sea: Environmental occurrence, sources and ecological risks. Mar. Pollut. Bull. 2023, 191, 114883. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.; Rila, J.; Traas, T.; Posthuma-Doodeman, C.; Posthumus, R. Environmental Risk Limits for Several Phosphate Esters, with Possible Application as Flame Retardant; RIVM Rapport 601501024; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2006. [Google Scholar]
- NORMAN. NORMAN Ecotoxicology Database—Lowest PNECs. 2022. Available online: https://www.norman-network.com/nds/ecotox/lowestPnecsIndex.php (accessed on 1 May 2024).
- Lian, M.; Lin, C.; Wu, T.; Xin, M.; Gu, X.; Lu, S.; Cao, Y.; Wang, B.; Ouyang, W.; Liu, X. Occurrence, spatiotemporal distribution, and ecological risks of organophosphate esters in the water of the Yellow River to the Laizhou Bay, Bohai Sea. Sci. Total Environ. 2021, 787, 147528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Union Risk Assessment Report: Tris (2-chloroethyl) Phosphate, TCEP; European Commission: Luxembourg, 2009.
- Shi, Y.; Gao, L.; Li, W.; Wang, Y.; Liu, J.; Cai, Y. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ. Pollut. 2016, 209, 1–10. [Google Scholar] [CrossRef]
- Green, N.; Schlabach, M.; Bakke, T.; Brevik, E.; Dye, C.; Herzke, D.; Huber, S.; Plosz, B.; Remberger, M.; Schøyen, M. Screening of Selected Metals and New Organic Contaminants 2007. Phosphorus Flame Retardents, Polyfluorinated Organic Compounds, Nitro-PAHs, Silver, Platinum and Sucralose in Air, Wastewater Treatment Falcilities, and Freshwater and Marine Recipients; Norsk Institutt for Vannforskning: Oslo, Norway, 2008. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, Y.; Li, H.; Yang, Z. Occurrence, Fate, and Mass Balance Analysis of Organophosphate Flame Retardants in a Municipal Wastewater Treatment Plant in Hunan Province, China. Water 2024, 16, 1462. https://doi.org/10.3390/w16111462
Liu Y, Song Y, Li H, Yang Z. Occurrence, Fate, and Mass Balance Analysis of Organophosphate Flame Retardants in a Municipal Wastewater Treatment Plant in Hunan Province, China. Water. 2024; 16(11):1462. https://doi.org/10.3390/w16111462
Chicago/Turabian StyleLiu, Yang, Yang Song, Haipu Li, and Zhaoguang Yang. 2024. "Occurrence, Fate, and Mass Balance Analysis of Organophosphate Flame Retardants in a Municipal Wastewater Treatment Plant in Hunan Province, China" Water 16, no. 11: 1462. https://doi.org/10.3390/w16111462
APA StyleLiu, Y., Song, Y., Li, H., & Yang, Z. (2024). Occurrence, Fate, and Mass Balance Analysis of Organophosphate Flame Retardants in a Municipal Wastewater Treatment Plant in Hunan Province, China. Water, 16(11), 1462. https://doi.org/10.3390/w16111462






