Abstract
Oilfield brine is the largest byproduct stream generated during the extraction of crude oil and natural gas and may be considered a resource for the production of potable water and valuable raw materials. The high salinity of such waters limits the application of typical membrane-based techniques. In most oilfields, waste cold energy from the process of the low-temperature separation of natural gas is available and may be used as a source of cold for the freezing desalination (FD) of brine. As a result of the FD process, two streams are obtained: partially desalinated water and concentrated brine. The partially desalinated water may be suitable for non-potable applications or as a feed for membrane desalination. The concentrated brine from the FD could be used as a feed for the recovery of selected chemicals. This paper focuses on verifying the above-described concept of the freezing desalination of oilfield brine on a laboratory scale. The brine from a Polish oilfield located in the Carpathian Foredeep was used as a feed. Four freezing–thawing stages were applied to obtain low-salinity water, which subsequently was treated by reverse osmosis. The obtained permeate meets the criteria recommended for irrigation and livestock watering. The concentrated brine enriched with iodine (48 mg/L) and lithium (14 mg/L) was subjected to recovery tests. Ion exchange resin Diaion NSA100 allowed us to recover 58% of iodine. Lithium recovery using Mn- and Ti-based sorbents varies from 52 to 93%.
1. Introduction
Crude oil and natural gas are extremely valuable commodities in the modern world, the demand for which is constantly growing. Oil is the primary raw material of the petrochemical industry, natural gas is used in many industrial processes, and both are among the most important energy raw materials [1,2]. The high demand for fossil fuels implies their increased extraction, which in turn leads to the formation of a significant volume of wastewater. The extraction of crude oil and natural gas from onshore and offshore wellbores is accompanied by the generation of large amounts of oilfield brine also known as oilfield-produced water (in the oil industry, both terms are often used interchangeably). Depending on the stage or method of hydrocarbon extraction, the brine can consist of different components. In the case of primary oil recovery (where crude oil is transported to the surface due to the difference in pressure between the reservoir and the wellbore), the oilfield brine consists of natural formation water mixed with hydrocarbons. However, if secondary (e.g., waterflooding) or tertiary (e.g., chemical injection) oil recovery processes are utilized, or in the case of hydraulic fracturing, the brine also contains water or fluids previously pumped under high pressures into the well [2,3,4,5,6]. The volume of the oilfield brine produced is not constant and depends, among other, on the location of the field, formation type, extraction technology, and wellbore operation time (the older the well, the more produced water is formed) [2,4]. It is estimated that the daily global production of brine from oil and gas fields is approximately 250 million barrels (i.e., approximately 39.8 million m3), and shows an upward trend [2,3]. The United States of America, one of the largest oil and gas producers, generated 25.86 billion barrels of brine (the volume for 26 states) in 2021, both from onshore (98.4%) and offshore (1.6%) wells [5]. To quantify the volume of brine generated in relation to the volume of oil and gas obtained, the parameters called the WOR (water-to-oil ratio = barrel of produced water/barrel of oil) and WGR (water-to-gas ratio = barrel of produced water/million cubic feet of gas) are used, respectively. The average global WOR value is approximately 3:1–4:1, although the value may vary significantly from one oilfield to another (0.4:1–36:1). In general, the WOR increases with the age of wellbore and is forecast to reach 12:1 in the near future, and therefore, the amount of oilfield-produced water will drastically increase, which will be a serious ecological problem and will require appropriate management [4,5,7]. The WGR parameter can also have values from a wide range (e.g., 0.1:1–15.4:1 m3 of produced water/1000 m3 of gas) and increases with the field operation time [7].
Oilfield brine is characterized by a complex composition, which is highly variable (Table 1) and contingent primarily on the geographical location of the reservoir, the structure, depth, and age of the reservoir, the type of geological formation, the type and composition of the extracted hydrocarbons, and the extraction method [1,3,8,9,10]. Produced water contains chemicals naturally occurring in the reservoir (e.g., hydrocarbons, inorganic salts, metals, radionuclides, and dissolved gases such as carbon dioxide and hydrogen sulfide) and those introduced during the extraction process (e.g., organic and inorganic acids, corrosion inhibitors, scale inhibitors, friction reducers, gelling agents, crosslinkers, biocides, gel beakers, and surfactants) [1,11,12]. The most important physicochemical parameters characterizing the brine from different oil and gas fields are presented in Table 1.

Table 1.
Ranges of the most important physicochemical parameters of brine from different oil and gas fields [3,4,7,13,14,15,16].
As can be seen, produced water has high electrical conductivity, high salinity caused by a high concentration of sodium, potassium, calcium, magnesium, and chloride (the salinity of brine is usually higher than seawater, which is 35 g/L [1]), a high content of total suspended solids (TSS) and total dissolved solids (TDS), and an acid-to-alkaline reaction. The content of the organic compounds expressed as a chemical oxygen demand (COD) and total organic carbon (TOC) is also high. BTEX (benzene, toluene, ethylbenzene, and xylene), phenol, polycyclic aromatic hydrocarbons (PAH) as well as oil and grease are also important components of the brine and their concentrations can be quite high. The concentration of heavy metals can range from a few μg/L to hundreds of mg/L.
The huge amount of oilfield brine produced requires a proper management strategy, taking into account the volume and composition of the brine, management costs, and local legislation [14,17]. In the USA, the primary method of produced water management is its reuse in the oil and gas industry (approximately 95.7%) through reinjection into the reservoir for enhanced oil recovery (EOR) or disposal. Other methods include discharge into water bodies and the land surface provided that the required water quality standards are met (approximately 1.2%), evaporation under the influence of sunlight (approximately 0.2%), sale between oil and gas operators and for domestic use (approximately 1.2%), and beneficial reuse after appropriate treatment, e.g., for the preparation of drilling and fracturing fluids, for irrigating fields, or removing dust from roads (approximately 1.4%) [5]. However, reinjection into the well is not always feasible and it may also have an adverse effect on the environment, causing induced seismic activity and groundwater contamination [1]. Therefore, the idea of using brine as a source of valuable raw materials (especially lithium) [14,18,19] and potable and non-potable water is becoming more and more popular [7,8,17]. The production of potable water from oilfield brine will both ensure freshwater supplies as well as minimize the volume of the concentrate reinjected into disposal wells. The recovery of water would be particularly beneficial in areas where it is deficient [7,19]. Due to the fact that produced water has a complex composition and contains many substances harmful to the environment and toxic to humans, its purification is not easy and requires the use of a multi-stage technology (pretreatment, main treatment, and post-treatment); however, the specific treatment method applied depends on the desired quality of the water [3,4,15]. The primary treatment involves the elimination of oil, grease, and suspended solids and can be achieved using, for example, a hydrocyclone, a gravity separator, and sedimentation, coagulation/flocculation, or dissolved air flotation techniques. The purpose of the secondary treatment is to remove dissolved components, i.e., dissolved organic compounds (BTEX, phenols, or PAHs) and dissolved inorganic salts (Na+, K+, Mg2+, Ca2+ and other cations, and inorganic anions). Treatment methods used in this step include adsorption, biological methods, and desalination by membrane processes (e.g., reverse osmosis (RO) and electrodialysis) or thermal processes (evaporation) [3,12,15,20]. There are numerous review papers discussing available desalination methods for this kind of feed [21,22,23]. In general, both membrane-based desalination and thermal processes are technically feasible to handle the produced water. All evaporation technologies are energy-consuming and usually preferred in regions with a hot climate like the Middle East and North Africa. More than 2/3 of all desalination plants apply different membrane systems. Reverse osmosis is the prevailing technology with a share of 69% of the installed capacity in the world [24]. The RO has many advantages, including low energy consumption (for seawater, it uses 2 kWh/m3–6 kWh/m3 of product water), technological maturity, low chemical use for feed pretreatment, and being easy to scale and operate [25]. The main limitation of RO is the salinity of the feed. The driving force for the RO system is the osmotic pressure and, for example, for seawater with TDS of 35,000 mg/L, the osmotic pressure will reach about 24 bars [24]. An increasing operational pressure will increase the cost of maintaining and driving the pumps, as well as reduce the durability of membranes due to excessive fouling [25,26,27]. The production of permeate (freshwater) will be up to 50% for feed brine with a TDS content of <70,000 mg/L and only up to 10% for feed brine with a TDS content of 85,000 mg/L [22]. For many oilfield-produced waters the content of dissolved salts exceeds 100,000 mg/L and their use in RO systems is limited. To overcome this problem, many hybrid configurations were proposed [10]. The tertiary treatment (in order to obtain potable water) consists of a further reduction of contaminants and is carried out using, e.g., advanced oxidation processes and disinfection with ozone, chlorine compounds, or UV radiation [3,15,20].
In the recent scientific literature, there has been a trend to consider the recovery of many raw materials from low-concentrated feeds such as seawater, desalination brines, oil- and gas-produced waters, geothermal aquifers, and acid mine drainage [28]. Based on these considerations, it can be concluded that there are huge reserves of this type of raw material, but the main obstacle is the unacceptably high cost of pre-concentration processes. One of the methods to overcome this limitation is to combine the recovery of valuable raw materials with the production of usable water. Such a scenario has recently been proposed in the literature for the recovery of valuable raw materials from seawater desalination concentrate [29]. A similar approach can be employed for oilfield brine, which typically has a richer composition than seawater. Oilfield brine is considered a source of valuable raw materials such as lithium, iodine, strontium, and boron [16,30,31,32,33], and in the future, it may also be considered a source of cesium and rubidium [34,35]. Moreover, the brine can be a source of important high-volume products, such as potassium chloride and magnesium chloride, used in fertilizers [31]. Some of the elements contained in the brine are currently considered critical in different regions of the world, e.g., in the European Union, boron, lithium, magnesium, and manganese are considered strategic raw materials, while strontium and barium are considered critical raw materials [36]. At present, two technologies for the recovery of valuable materials from oilfield brine are the most advanced. The first is the commercial production of iodine, especially in the Japan [16], and the second is lithium recovery plant construction projects in the USA [14]. In Japan, iodine is recovered on a commercial scale from gas-field brines, among others, in the Minami–Kanto gas field (the iodine concentration in brine is 93–130 mg/L), the Niigata gas field (the iodine concentration in brine is 40–67 mg/L), the Nakajo oil and gas field (the iodine concentration in brine is approximately 85 mg/L), and the Sadowara gas field (the iodine concentration in brine is approximately 80 mg/L) [16]. Japan accounts for about 30% of global iodine production from natural gas brine and is the second-largest producer of iodine. Two types of technology are used: the blowing-out method and the anion-exchange resin method.
The development of the lithium-ion battery sector stimulates the economy to look for new sources of lithium, including oilfield brines. More than a dozen laboratory and pilot projects are currently underway to develop lithium recovery technology, as well as two lithium recovery plant construction projects. The Lanxess plant in El Dorado, Arkansas, assumes an annual production of 20,900 tonnes of lithium carbonate, while the Southwest Arkansas project targets an annual production of 30,000 tonnes of battery-grade lithium hydroxide. The average concentration of lithium in the feed oilfield brines was reported to be equal to 168 mg/L. A direct lithium extraction process (based on the application of lithium-ion sieves (LISs)) will be employed [14]. To the best of our knowledge, there are no commercial-scale facilities producing other raw materials (Sr, B, Cs, Rb, Mg, or K) from oilfield brines, but many scientific efforts are focused on the development of technology for the recovery of such elements as rubidium, cesium [35], strontium [37], and boron [38,39] from oilfield brines. It may be concluded that the best solution for oilfield brine treatment is to combine the process of recovering usable water from pretreated brine and the subsequent recovery of valuable materials from concentrated post-process brine. Taking into account that the concentration of dissolved solids in oilfield-produced water is higher than in seawater, the direct application of RO for water recovery will be limited (by osmotic pressure) for brine containing approximately 100 g/L of salts. Therefore, for many oilfield brines, other methods should be employed. Due to the high cost of distillation methods, there is a gap where alternative methods may be applied.
Recently, there has been a growing interest in the application of freezing methods in the desalination of seawater. Freezing desalination is an elegant approach where a natural phenomenon of the formation of freshwater ice above a salty solution is used. Thermodynamically, the freezing process is much more favorable than water vaporization (the latent heat is 330 kJ/kg and 2256 kJ/kg, respectively) [40]. At low temperatures, some corrosion and scaling problems are less intense [41,42]. Kalista et al. [43] estimate that the cost of water production using the FD technique is only USD 0.34/m3 compared to USD 0.75/m3 for reverse osmosis, USD 0.96/m3 for multi-stage flash distillation and USD 0.86/m3 for multiple-effect distillation. The main limitation for the wide industrial application of the FD is access to sources of cold energy. The regasification of LNG is reported as a promising source of cold, where about 1024 kJ/kg-LNG is available in the form of physical exergy [44]. This energy may be used for a variety of applications, which was well summarized by Akashah et al. [45]. A techno-economic analysis of Ong and Chen [46] shows that an optimized direct-contact type of seawater FD process is able to produce 1.6 kg/s of desalinated water from 7.8 kg/s of seawater with an electric power consumption of 1.66 kW.
At gas and oilfields, the cold energy is produced during the pretreatment of natural gas. Water and C3+ hydrocarbons present in the gas may condensate during transportation via pipelines, causing severe operational problems such as corrosion, unpredictable pressure changes, and hydrate formation [47]. To control the dew point (the highest temperature at a given pressure at which the liquid can form), different refrigeration processes may be applied including mechanical refrigeration, Joule–Thomson (JT) valve refrigeration, and cryogenic refrigeration by a turboexpander [48]. JT throttling expansion is most often employed due to the high well-head pressure, low operating costs, and simplicity [49]. Depending on the refrigeration method, the achieved dew point of the gas stream varies from −8.4 to −24.9 °C [48], which means that the cold gas stream is available at the oilfield (in the surface facility). Usually, this cold is simply wasted because natural gas must be heated up to the proper temperature before being injected into the supply network [50]. Therefore, we suggest using the waste cold generated during gas purification for the freezing desalination of produced water. This seems to be a natural coupling of two treatment process realized at the same oilfield. In order to better understand the proposed concept, a preliminary flow diagram is provided in Appendix A. A typical freezing desalination process includes four basic apparatus: a freezer, a washer, a melter, and a heat removal system [51]. At such an early stage of the work, it is not possible to indicate specific design solutions, including the type of heat exchanger or the method of separation of ice crystals, but the general guidelines are as follows:
- (1)
- The process should be carried out indirectly—the gas must not mix with the brine because commercial natural gas must not contain traces of water.
- (2)
- The process should be continuous or semi-continuous; the batch mode applied in this study may not be easily scalable.
- (3)
- In order to optimize heat flow, both streams (gas and water) should be contacted in countercurrent.
In this work, we propose a combination of reverse osmosis and freezing desalination as a hybrid process for the production of potable water and a concentrate for the recovery of chemical elements. Moderately saline oilfield brine with a TDS content of <100,000 mg/L was used as a feed. Freezing desalination was utilized as the first stage to produce partially desalinated water (a TDS content of <20,000 mg/L) suitable as a feed for a reverse osmosis system. The concentrate obtained from cryoconcentration was applied as a feed for the recovery of valuable elements (lithium and iodine). So far, this type of process combination has not been tested or even considered theoretically. The aim of the presented study was to verify the proposed concept on a laboratory scale using a real sample of oilfield brine. Particular attention was paid to mass exchange during freezing desalination and reverse osmosis. The chemical composition (concentration profiles of selected elements) of important process streams was determined.
2. Materials and Methods
2.1. Materials
2.1.1. Oilfield Brine
The depleted hydrocarbon reservoirs with high potential for producing reservoir water in Poland are located in the south of the country, in the Carpathian Foredeep. The Carpathian Foredeep is asymmetric and filled mainly with clastic Miocene sediments up to 3 km thick [52]. Based on available data and our own measurements as part of the CompLithium project [53], the authors sampled about 50 L of brine from a selected reservoir located in the eastern part of the Carpathian Foredeep.
2.1.2. Chemicals and Other Materials
Polypropylene wool (WuSORB OP-20) for oil sorption was purchased from HAPPY END PL Sp. z o.o. (Cieszyn, Poland). Polypropylene bag filters with filtration degrees of 10 µm and 5 µm were delivered by Techniczne24 (Stalowa Wola, Poland). SAX 13 coagulant (sodium aluminate solution) was supplied by Antidotum Łódz Company (Łódz, Poland). Ion exchange resin Diaion NSA100 was provided by Alfa Aesar (Ward Hill, MA, USA). Lithium-selective adsorbents (LIS3, LIS4, LIS10, LIS11, and LIS12) were prepared according to the procedure described in [54]. Low-cost Aquafilter TFC-75F (Aquafilter Europe Sp. z o.o., Łódź, Poland) membrane was used in reverse osmosis experiments. The 30% solution of H2O2, dichloromethane, and NaOH were analytical-grade and were delivered by Pol Aura (Morąg, Poland). Standard solutions for AAS and ICP-MS produced by PerkinElmer (Waltham, MA, USA) and Agilent (Wilmington, DE, USA) were used for preparation of standards in AAS and ICP-MS measurements. Demineralized water from the Millipore system (Merck KGaA, Darmstadt, Germany) (>18 MΩ) was used to dilute samples to a level suitable for AAS and ICP-MS measurements.
2.2. Analytical Methods
Selected physical and chemical properties of the oilfield brine used were studied according to the ASTM [55] and PN-EN ISO [56] standards. Table 2 summarizes the applied research methodology.

Table 2.
Research methods/apparatus used to analyze selected physicochemical properties of the brine used.
The concentrations of Ca, Fe, K, Li, Mg, and Na were determined by flow injection atomic absorption spectrometry (FI-AAS). Each analysis was repeated five times, and the mean value was taken as the result. Initially, the concentration was determined using the calibration curve method. To deal with matrix effect, the samples were spiked with a solution containing the standard of the element being determined and the final concentration was calculated based on the increase in analytical signal.
The concentrations of Al, Ag, As, B, Ba, Br, Cd, Co, Cr, Cs, Cu, I, Mn, Mo, Ni, Pb, Rb, Se, Sr, and Zn were determined using inductively coupled plasma mass spectrometry (ICP-MS). The following parameters of nebulizer, ion source, and interface were used during the analyses: Nebulizer Gas Flow [NEB] = 0.90, Auxiliary Gas Flow = 1.40, Plasma Gas Flow = 15.00, Lens Voltage = 7, ICP RF Power = 1100, Analog Stage Voltage = −1850, Pulse Stage Voltage = 800, Quadrupole Rod Offset Std [QRO] = 0.00, Cell Rod Offset Std [CRO] = −10.00, Discriminator Threshold = 70.00, and Cell Path Voltage Std [CPV] = −15.00. The acquisition time for single element was 1500 ms and the flushing time between samples was 2 min. The calibration curve was prepared in the range of 1–10 ppb and the analyzed samples were diluted to the appropriate level. As with AAS measurements, to cope with matrix effect, the samples were spiked with a solution containing the standard of determined element and the final concentration was calculated on the basis of the increase in analytical signal.
2.3. Brine Processing
The brine processing experiment was performed on a 20 L sample of collected brine. The method of processing is presented in Figure 1. Firstly, the brine was subjected to pretreatment (filtration 1, 2, and coagulation), and in the next step, freezing desalination was carried out. Partially desalinated water was subjected to reverse osmosis. The concentrated brine formed in the freezing–thawing process was a feed for the recovery of iodine and lithium.
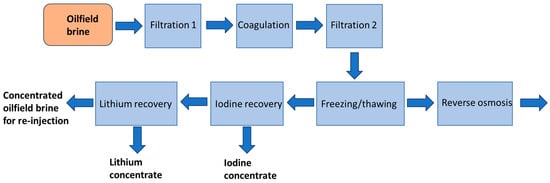
Figure 1.
Scheme of brine processing.
2.3.1. Brine Pretreatment
The raw brine was characterized by high turbidity and a thin layer of oil was visible on the top. The basic pretreatment was applied according to the following procedure: 20 L of brine was filtered through a 10 µm filter bag filled with a 1 cm layer of hydrophobic non-woven polypropylene. Then, commercial coagulant SAX 13 at a concentration of 200 mg/L was added to the obtained filtrate. The treated water was allowed to settle for 1 h and gently separated from the sludge via decantation and filtration through a 5 µm filter bag (Techniczne24, Stalowa Wola, Poland). The brine prepared in this way was used in further experiments.
2.3.2. Freezing Desalination
The aim of the experiment was to study freezing desalination under laboratory conditions using a small amount of brine, as described in Figure 2. Changes in the composition of the streams were examined using AAS and ICP-MS methods. Four stages of the freezing–thawing process were carried out. The collected intermediate streams were combined and applied in the next freezing–thawing stage. Freezing and thawing were performed slowly because we wanted to obtain equilibrium data.
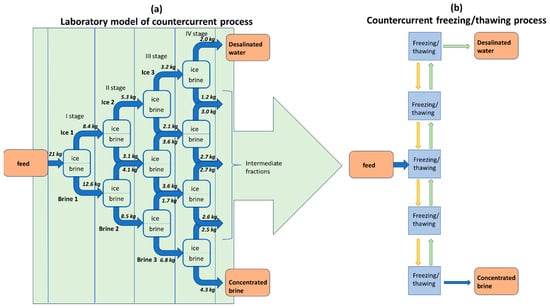
Figure 2.
Scheme of freezing desalination experiment (a) as a model of the countercurrent process (b). Samples of ice 1, 2, and 3 and brine 1, 2, and 3 as well as concentrated brine and desalinated water were collected and analyzed.
The pretreated brine (21 kg) was cooled to approximately −10 °C within 24 h in the first stage. Ice (described as Ice 1; 8.4 kg) was separated from the brine (described as Brine 1; 12.6 kg). Ice 1 was thawed and subsequently cooled to approximately −10 °C during 24 h. Then, the mixture was left at room temperature to partially thaw. Two fractions were obtained from Ice 1: Ice 2 (5.3 kg) and brine (3.1 kg). Brine 1 was cooled to approximately −15 °C for 24 h and subsequently separated into brine (Brine 2; 8.5 kg) and ice (4.1 kg). The intermediate brine and the ice from second stage were combined, thawed, and cooled down to a temperature of approximately −10 °C. Similar operations were performed at the third and fourth freezing stages. The cooling time was the same for all cases (24 h), the main difference was the final temperature during the cooling fractions: Brine 2 was cooled to −17 °C, Brine 3 to −20 °C. Ice 2 and 3 were cooled to −10 °C and, in the next step, partially thawed—during thawing, the conductivity was controlled and based on this information a decision was made regarding when to stop thawing and collect the fractions. Finally, 2 kg of desalinated water, 4.3 kg of concentrated brine and 14.7 kg of intermediate fractions were collected.
In each case, cooling was carried out using a cryostat as a source of cold. The freezing process was conducted periodically. The brine container was slowly stirred using a mechanical stirrer and cooled to the expected temperature. The container was equipped with a bottom drain that allowed the brine to be separated from the ice.
2.3.3. Reverse Osmosis
Reverse osmosis was performed using low-cost Aquafilter TFC-75F membrane (Aquafilter Europe Sp. z o.o., Łódź, Poland) and a small laboratory set-up. The process was carried out at a constant transmembrane pressure of 5 bars. The permeate to reject volume ratio was maintained at 5/1. The process was conducted at room temperature. The permeate flow was approximately 5 mL/min.
2.3.4. Recovery of Iodine and Lithium
Static tests of the sorption of iodine and lithium were carried out using the samples of concentrated brine. Diaion NSA100 resin was used to recover iodine from the concentrated brine. For this purpose, the method described in the literature was adopted [57]. Here, 1 mL of 30% H2O2 was added to a sample of 1 L of concentrated brine and mixed for 30 min, then 10 mL of resin was added and mixed for 3 h at room temperature. The concentration of iodine prior and after the sorption test was determined using ICP-MS method.
Manganese and titanium oxide-based lithium ion selective sieves tested in our previous work [54] were used for lithium adsorption from concentrated brine. For this, 100 mL of concentrated brine was contacted with 0.2 g of sorbent, and 100 µL of 3 M NaOH was added to correct pH. The mixture was stirred for 8 h until equilibrium was achieved. Lithium concentration was determined by AAS method before and after sorption.
3. Results and Discussion
3.1. Brine Pretreatment
Brine pretreatment is aimed at reducing the content of dispersed hydrocarbons and suspended solids. Organics compounds during freezing can concentrate in the desalinated water and hinder its quality. Particulate matter may disturb the growth of ice crystals. Figure 3a shows the appearance of brine samples after each treatment stage. The basic water quality indicators for raw and treated brine are summarized in Table 3.

Figure 3.
(a) Appearance of raw brine (vial 1), brine after mechanical filtration (vial 2), and brine after coagulation and sedimentation (vial 3); (b) Inside of the 10 µm filter bag after filtering 20 L of brine.

Table 3.
Brine characteristics before and after pretreatment.
In typical desalination units based on reverse osmosis or membrane distillation, the feed requires excessive pretreatment to remove all potential fouling compounds [58,59]. To obtain high-quality products, the pretreatment must involve chemicals (coagulants and antiscalants) and ultrafiltration [60,61]. The applied pretreatment allowed a significant reduction in the content of suspended solids via mechanical filtration. The main load of suspended solids was captured by the filter bag, which formed an even layer on its surface (Figure 3b). The small layer of hydrophobic non-woven polypropylene in the filter bag effectively reduced the oil content to 32 mg/L. The silica content was reduced at a relatively low dose of coagulant (200 mg/L). The slight increase in the pH was related to the addition of SAX 13 which is sodium aluminate. The two-stage pretreatment removed 99.5% of the TSS and 99.9% of oil and grease. Chromatograms for raw brine and brine after the first and second filtration are presented in the Appendix B. The obtained water does not meet the quality criteria recommended by membrane manufacturers [62], but the freezing desalination method does not require the intensive pretreatment of brine [40]. In fact, freezing should enhance the separation of contaminants (organic compounds form a thin layer on the top of ice while heavier solids sink to the bottom).
3.2. Freezing Desalination
It is commonly known that the eutectic point appears in the phase diagrams of binary systems for NaCl-H2O, KCl-H2O, MgCl2-H2O, and CaCl2-H2O. If such a two-component solution with a salt concentration below the eutectic point is cooled very slowly, the pure ice should crystallize and the brine should concentrate. However, in practice, it is difficult to achieve a state that will be close to the equilibrium. One of the main problems is the trapping of slurry droplets in the freezing ice, especially when high ice recovery is required [63]. Therefore, the obtained ice is most often contaminated with brine, which we also observed. Due to this fact, a continuous countercurrent process was proposed; according to the literature data, contaminated ice is contacted with desalinated water in a countercurrent washer [51]. Since conducting a continuous process on a laboratory scale using a small sample is complicated, we decided to carry out a batch multistage process. The main goal of the freezing experiment was to obtain data regarding the distribution of individual elements at subsequent stages of the process; therefore, in our opinion, this approach is acceptable. As a result of the experiment, we obtained information on the distribution of individual elements during subsequent stages of desalination, which is summarized in Table 4.

Table 4.
Changes in the composition of brine and ice at different stages of the process.
The data presented in Table 4 confirm the ion rejection phenomenon during the freezing of aqueous solutions. The concentration of almost all elements in desalinated water (ice after melting) is lower than in the feed brine. The only exception was molybdenum, which was concentrated in ice fractions. There are several reasons for this behavior. The ice crystal lattice has small dimensions and the incorporation of any ions is hindered. The solubility of most salts decreases with temperature [64] and some precipitated crystals may sink to the bottom (accumulate in the lower layer). Molecular dynamics simulations performed by Luo et al. [65] revealed that the hydration energy of Na+ and Cl− with water (as a liquid) is stronger than that with ice. To assess the partitioning of each element between the ice phase and the solute, the rejection ratio (RR) and the enrichment ratio (ER) may by calculated as follows [66]:
where Cif is the concentration of the i-th element in feed brine, Cii is its concentration in the ice (desalinated water), and Cic is its concentration in the concentrated brine.
For most elements (except Mo) the rejection ratio is about 95%; only Co, Zn, and Cd show some affinity towards ice. Freezing desalination is effective in removal, especially of heavy metals. Melak et al. [67] obtained up to 97% removal of Cr(VI) from water with the FD. Wijewardena [68] achieved about 99% reduction in the concentration of Cr(VI), Ni, Co, and Pd in ice in single-stage freezing. The highest enrichment rates were obtained in the following order: Al (201%) > I > Ca > Mg > Li > Sr > Na (146%). These elements have the highest tendency to remain in the solute which implicates that freezing desalination may be a suitable method of obtaining their concentrates. Among them, lithium and iodine have the highest potential to be economically recovered and their recovery is tested in further parts of this research.
Partially desalinated waters obtained as a result of the freezing process may have applications in the oil industry. A study by Ribeiro et al. [69] shows that produced water from Brazil’s Urucu reservoir could be reused for preparing water-based drilling muds. The presence of NaCl in the reservoir water did not significantly affect the properties of the mud, and the authors recommended limiting the content of calcium ions to 0.73 g/L and magnesium ions to 9.41 g/L. Pretreated brine can also be used in completion, workover, and packer fluids [70], and for pre-washing equipment and tanks at the drilling site. The quality requirements for this type of application are quite low and particulate matter should be removed, but a significant reduction in salinity and hydrocarbon content is not necessary. Pretreated brine is an excellent medium to be applied in EOR methods. Many coreflood experiments [71,72] showed notable oil recovery during so-called low-salinity flooding when the concentration of NaCl was between 0 and 1%. Due to environmental concerns, flowback water and produced brines are reused for the preparation of fracturing fluids instead of fresh water. Water for fracturing fluids should meet the following criteria: a chlorides concentration from 2000 to 40,000 mg/L, Ca < 300 mg/L, Fe < 10 mg/L, Mg < 100 mg/L, and Ba < 2 mg/L [73].
3.3. Reverse Osmosis
Water from freezing desalination does not fully meet the requirements for drinking water for humans, but it can be used in agriculture, especially for livestock watering. To improve the quality of the water, we decided to check if low-pressure (and low-cost) reverse osmosis may be applied to achieve this goal. The composition of the produced permeate is compared with the requirements for water used in agriculture and with potable water in Table 5.
The permeate has a slightly higher salinity than acceptable for potable use, but this could be easily eliminated in the second stage of RO by using this permeate as a feed. In this study, a low-pressure reverse osmosis (LPRO) system was applied with an operating pressure of 5 bar and a slight increase in the salt passage was observed when operating in a constant pressure mode. Similar behavior for the LPRO membrane was reported by Park and Kwon [74]. Energy savings may compensate for the lower efficiency of such a system. The elevated concentrations of Se, Mo, Pb, Cr, and Cd in the permeate exceed the standards for humans, but are still acceptable for animals. The study by Lumami Kapepula et al. [75] shows that commercial RO membranes are sometimes inefficient in removing some heavy metals and there is still a need to develop new membrane materials. The concentration of boron in the water from freezing desalination and the subsequent permeate is similar, about 3 mg/L. There are numerous papers describing challenges in boron removal and in most cases, the boron content is reduced in permeate post-treatment [76,77]. Despite concerns about the boron toxicity, the real-field study by Mendoza-Grimón et al. [78] shows that no phytotoxicity was observed in banana orchards after 30 years of irrigation using water with a boron concentration above 1.0 mg/L. The salinity tolerance of crops differs significantly [79] and the quality of the water used for irrigation should be carefully selected for each case separately.

Table 5.
Water quality requirements for crop irrigation, livestock watering, and drinking water for humans.
Table 5.
Water quality requirements for crop irrigation, livestock watering, and drinking water for humans.
| Parameter | Water for Irrigation [80] | Water for Livestock Watering [80] | Potable Water for Humans [81,82] | Permeate |
|---|---|---|---|---|
| Sodium [mg/L] | <460 | 1000 | 200 | 320 |
| Calcium [mg/L] | <100 | 1000 | 100 | 12 |
| Magnesium [mg/L] | >25 | <250 | - | 1.15 |
| Boron [mg/L] | 0.75–6.0 | 5.0 | 1.5 | 3.1 |
| Lithium [mg/L] | <2.5 | - | - | 0.08 |
| Iron [mg/L] | 5.0–20.0 | - | 0.2 | <0.1 |
| Selenium [mg/L] | 0.02 | 0.1 | 0.05 | 0.12 |
| Molybdenum [mg/L] | 0.01–0.05 | 0.5 | - | 0.12 |
| Barium [mg/L] | 0.001–0.0375 | 10 | 2 | 0.08 |
| Manganese [mg/L] | 0.2–10.0 | 0.05 | 0.05 | 0.03 |
| Aluminum [mg/L] | 5.0–20.0 | 5.0 | 0.05–0.2 | 0.05 |
| Nickel [mg/L] | 0.2–2.0 | 1.0 | 0.02 | 0.002 |
| Lead [mg/L] | 5.0–10.0 | 0.05 | 0.0015 | 0.13 |
| Copper [mg/L] | 0.2–5.0 | 0.5 | 1.3 | 0.002 |
| Cadmium [mg/L] | 0.01–0.05 | 0.05 | 0.005 | 0.03 |
| Cobalt [mg/L] | 0.05–5.0 | 1.0 | - | 0.03 |
| Chromium [mg/L] | 0.1–1.0 | 1.0 | 0.01 | 0.06 |
| Arsenic [mg/L] | 0.1–2.0 | 0.2 | 0.01 | 0.01 |
| Zinc [mg/L] | 2–10 | 24 | 5 | 0.002 |
| Fluoride [mg/L] | 1.0–15.0 | 2.0 | 4 | - |
| Chloride [mg/L] | 525–2450 | 1600–4000 | 250 | - |
| pH | 6.5–8.4 | 5.5–8.5 | 6.5–9.5 | - |
| TDS [mg/L] | 704–4080 | 3000–7000 | 500 | 860 |
| Conductivity [μS/cm] | 1100–5100 | 5000–11,000 | 2500 | <2000 |
3.4. Adsorption of Iodine and Lithium from Concentrated Brine
The efficiency of iodine and lithium recovery under static conditions is presented in Table 6. The iodine concentration in the brine after the freezing process is similar to the concentration of brines currently used for iodine production (e.g., in the Niigata gas field, the brine iodine level is in the range of 40–67 mg/L). Anion exchange resins are the most popular and patented materials for iodine recovery. Herkelmann et al. [83] patented the application of a commercially available resin called Serdolit AWGTM from the SERVA company (Catoosa, OK, USA), proving that iodine may be successfully recovered from a feed of any composition, including a solution containing organic compounds. Venkat et al. [84] patented a two-stage anion exchange method, allowing the recovery of up to 99% of iodides from wastewaters with a very wide range of iodide concentrations (from approximately 0.1 g/L to 100 g/L). In the first stage, the strongly basic anion exchange resin should be applied (including Amberlites IRA 400, IRA-410, IRA-402, IRA-458, and IRA-440c), and in the second stage, a weakly basic anion exchange resin in a free-base form is needed (suitable resins are: Amberlyst A-21 and Amberlites IRA-67, IRA-93, IRA-94, and IRA-35). These resins sorb essentially all anions, but their selectivity may usually be ranked as follows: SO42− > I− >NO3− > Br− > Cl− > OH−. To ensure the high purity of the recovered iodine, a proper sequence of wash solvents is required. The Diaion NSA100 resin applied in this study is a strongly basic gel-type anion exchange resin with trimethyl ammonium functional groups in the chloride form [85], similar to the resins used by Venkat et al. [84]. The adsorption efficiency under static conditions here reaches approximately 60%, which is a promising result, but further optimization is needed to establish the best operating parameters (like pH, temperature, and type and dose of oxidant).

Table 6.
Efficiency of iodine and lithium recovery from concentrated brine and efficiency of lithium recovery from previously tested brine.
The concentration of lithium in the brine after the freezing process increased by over 60% compared to the initial brine. The lithium-selective sorbents selected for this study were previously tested by us using different synthetic brines [54]. Our previous research shows that the composition of brine (the presence of ions other than lithium) significantly affects the sorption capacity and only some Mn- and Ti-based adsorbents are suitable for the recovery of lithium from concentrated brines. Manganese oxide-based materials (LIS10, LIS11, and LIS12) are characterized by a greater affinity for lithium than titanium oxide-based materials (LIS3 and LIS4). The efficiency of lithium recovery on the Mn- and Ti-based sorbents from the obtained concentrate and previously tested brine is summarized in Table 6.
The composition of the concentrate from freezing desalination is more complex than that of the model brine (it contains more mono- and divalent ions), which may hinder the recovery of lithium. The sorption capacity of the LIS10 material towards lithium is approximately 6.5 mg/g and this value is similar to those reported by other researchers. Karshyga et al. [86] examined the recovery of lithium from brine produced from the oil and gas fields of JSC “Mangystaumunaigas” (Kazakhstan). The composition of this brine, which roughly resembles our concentrate, is as follows: lithium 5.9–7.8 mg/L, sodium 25,000–30,000 mg/L, calcium 4000–6000 mg/L, magnesium 1200–2000 mg/L, and chlorides 42,000–60,000 mg/L. The Mn-based sorbent synthesized by Karshyga et al. [86] allowed lithium recovery to be 86.1% after 48 h of contact duration and with a sorption capacity of 3.67 mg/g.
4. Conclusions
A multistage method for the treatment of moderately saline oilfield brine was tested on a laboratory scale. The obtained results show that freezing desalination is an interesting method for removing not only main salts, but also traces of heavy metal salts (especially Ni, Cu, Pb, and Cr). The concentrated brine enriched with iodine (up to 48 mg/L) and lithium (up to 14 mg/L) may be used as a feed for lithium and iodine recovery. Laboratory tests of the sorption of lithium and iodine on lithium-selective sieves and ion exchange resin, respectively, demonstrated that a high degree of recovery may be achieved (58% for iodine and 93% for lithium). The results indicate that the proposed technological concept can be an effective way of recovering valuable raw materials, as well as usable water (for mining, agriculture, and even as a source of potable water), but many details require further consideration. Additional research concerning the optimization of individual processes is necessary and this issue will be the subject of future works.
Author Contributions
Conceptualization, G.R.; methodology, G.R.; validation, G.R., E.K. and M.P.; formal analysis, G.R., E.K. and M.M.; investigation, G.R., E.K. and M.P.; resources, E.K. and M.P.; data curation, E.K.; writing—original draft preparation, G.R., M.M. and E.K.; writing—review and editing, M.M. and G.R.; visualization, M.M., E.K. and G.R.; supervision, G.R.; project administration, E.K.; funding acquisition, E.K. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Center for Research and Development (NCBR) in the frame of Project Contract No. LIDER/34/0174/L-12/20/NCBR/2021 under the LEADER Program.
Data Availability Statement
The original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Mirosław Krośniak (Jagiellonian University Medical College) for assistance with the ICP-MS analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
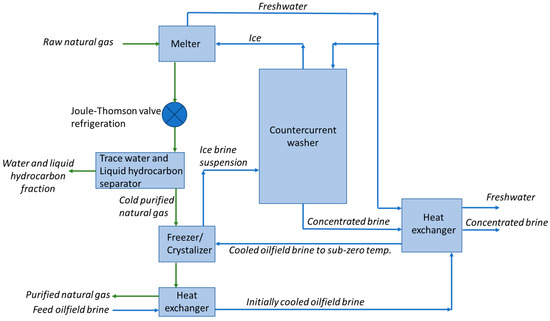
Figure A1.
Preliminary flow diagram for coupling of freezing desalination of oilfield brine with purification of natural gas. The developed concept is based on the continuous freezing desalination process described in [51].
Appendix B
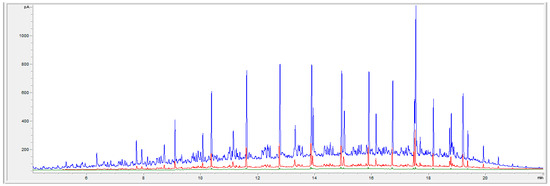
Figure A2.
Chromatograms recorded for raw brine (blue line), brine after filtration 1 (red line), and after coagulation and filtration 2 (green line).
References
- Gangwar, A.; Rawat, S.; Rautela, A.; Yadav, I.; Singh, A.; Kumar, S. Current advances in produced water treatment technologies: A perspective of techno-economic analysis and life cycle assessment. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Patni, H.; Ragunathan, B. Recycling and re-usage of oilfield produced water—A review. Mater. Today Proc. 2023, 77, 307–313. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Ramirez Canon, A.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Kaabi, M.A.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Proc. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- ALL Consulting. U.S. Produced Water Volumes and Management Practices in 2021; Ground Water protection Council: Oklahoma City, OK, USA, 2022; Available online: https://www.gwpc.org/wp-content/uploads/2021/09/2021_Produced_Water_Volumes.pdf (accessed on 20 April 2024).
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and treatment technologies applied for produced water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Echchelh, A.; Hess, T.; Sakrabani, R. Reusing oil and gas produced water for irrigation of food crops in drylands. Agric. Water Manag. 2018, 206, 124–134. [Google Scholar] [CrossRef]
- Miranda, M.A.; Ghosh, A.; Mahmodi, G.; Xie, S.; Shaw, M.; Kim, S.; Krzmarzick, M.J.; Lampert, D.J.; Aichele, C.P. Treatment and recovery of high-value elements from produced water. Water 2022, 14, 880. [Google Scholar] [CrossRef]
- Waly, M.M.; Mickovski, S.B.; Thomson, C. Application of circular economy in oil and gas produced water treatment. Sustainability 2023, 15, 2132. [Google Scholar] [CrossRef]
- Shahrim, A.A.; Abounahia, N.M.; El-Sayed, A.M.A.; Saleem, H.; Zaidi, S.J. An overview on the progress in produced water desalination by membrane-based technology. J. Water Process Eng. 2023, 51, 103479. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, L.; Xu, X.; Wang, H.; Xu, P. Analysis of regulatory framework for produced water management and reuse in major oil- and gas-producing regions in the United States. Water 2022, 14, 2162. [Google Scholar] [CrossRef]
- Nath, F.; Chowdhury, M.O.S.; Rhaman, M.M. Navigating produced water sustainability in the oil and gas sector: A Critical review of reuse challenges, treatment technologies, and prospects ahead. Water 2023, 15, 4088. [Google Scholar] [CrossRef]
- Alomar, T.S.; Hameed, B.H.; Usman, M.; Almomani, F.A.; Ba-abbad, M.M.; Khraisheh, M. Recent advances on the treatment of oil fields produced water by adsorption and advanced oxidation processes. J. Water Process Eng. 2022, 49, 103034. [Google Scholar] [CrossRef]
- Knapik, E.; Rotko, G.; Marszałek, M. Recovery of lithium from oilfield brines—Current achievements and future perspectives: A Mini review. Energies 2023, 16, 6628. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Kaneko, N.; Kaiho, T. Iodine production from natural gas brine. In Iodine Chemistry and Applications; Kaiho, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 231–241. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.A.; Nicot, J.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef]
- Khatoon, R.; Raksasat, R.; Ho, Y.C.; Lim, J.W.; Jumbri, K.; Ho, C.-D.; Chan, Y.J.; Abdelfattah, E.A.; Khoo, K.S. Reviewing advanced treatment of hydrocarbon-contaminated oilfield-produced water with recovery of lithium. Sustainability 2023, 15, 16016. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, P.; Tu, W.; Sun, H.; Li, S.; Zhang, Y. Lithium recovery from oil and gas produced water: Opportunities, challenges, and future outlook. J. Water Process Eng. 2023, 55, 104148. [Google Scholar] [CrossRef]
- Cooper, C.M.; McCall, J.; Stokes, S.C.; McKay, C.; Bentley, M.J.; Rosenblum, J.S.; Blewett, T.A.; Huang, Z.; Miara, A.; Talmadge, M.; et al. Oil and gas produced water reuse: Opportunities, treatment needs, and challenges. ACS EST Eng. 2022, 2, 347–366. [Google Scholar] [CrossRef]
- Shah, K.M.; Billinge, I.H.; Chen, X.; Fan, H.; Huang, Y.; Winton, R.K.; Yip, N.Y. Drivers, challenges, and emerging technologies for desalination of high-salinity brines: A critical review. Desalination 2022, 538, 115827. [Google Scholar] [CrossRef]
- Bello, A.S.; Zouari, N.; Da’ana, D.A.; Hahladakis, J.N.; Al-Ghouti, M.A. An overview of brine management: Emerging desalination technologies, life cycle assessment, and metal recovery methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef]
- Feria-Díaz, J.J.; Correa-Mahecha, F.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Barrera-Rojas, J. Recent desalination technologies by hybridization and integration with reverse osmosis: A Review. Water 2021, 13, 1369. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Alsarayreh, A.A.; Al-Obaidi, M.A.; Farag, S.K.; Patel, R.; Mujtaba, I.M. Performance evaluation of a medium-scale industrial reverse osmosis brackish water desalination plant with different brands of membranes. A simulation study. Desalination 2021, 503, 114927. [Google Scholar] [CrossRef]
- Sener, S.E.C.; Thomas, V.M.; Hogan, D.E.; Maier, R.M.; Carbajales-Dale, M.; Barton, M.D.; Karanfil, T.; Crittenden, J.C.; Amy, G.L. Recovery of critical metals from aqueous sources. ACS Sustain. Chem. Eng. 2021, 9, 11616. [Google Scholar] [CrossRef]
- Sharkh, B.A.; Al-Amoudi, A.A.; Farooque, M.; Fellows, C.M.; Ihm, S.; Lee, S.; Li, S.; Voutchkov, N. Seawater desalination concentrate—A new frontier for sustainable mining of valuable minerals. npj Clean. Water 2022, 5, 9. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Haon, T.A.; Lienhard, J.H. Lithium recovery from oil and gas produced water: A need for a growing energy industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Xiao, F. Characterization and treatment of Bakken oilfield produced water as a potential source of value-added elements. Sci. Total Environ. 2021, 770, 145283. [Google Scholar] [CrossRef] [PubMed]
- Voronov, A.; Vinograd, N. Industrially valuable components in oilfield waters of Russia. In Thermal and Mineral Waters Origin, Properties and Applications; Balderer, W., Porowski, A., Idris, H., LaMoreaux, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 111–118. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B. Water accompanying hydrocarbon deposits as a potential source of iodine, lithium and strontium. Min. Res. Manag. 2016, 32, 31–44. (In Polish) [Google Scholar] [CrossRef]
- Sharma, K.S.; Truong, D.Q.; Guo, J.; An, K.A.; Naidu, G.; Deka, B.J. Recovery of rubidium from brine sources utilizing diverse separation technologies. Desalination 2023, 556, 116578. [Google Scholar] [CrossRef]
- Fang, D.; Lu, M.; Wang, Y.; Ma, L.; Li, K.; Liu, H.; Zhang, H.; Shi, G.; Wu, Z.; Ye, X. Extraction of rubidium and cesium from oilfield brine by the two-step adsorption–flotation method. Min. Eng. 2023, 201, 108161. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the EU 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 20 April 2024).
- Schaller, J.; Headley, T.; Prigent, S.; Breuer, R. Potential mining of lithium, beryllium and strontium from oilfield wastewater after enrichment in constructed wetlands and ponds. Sci. Total Environ. 2014, 493, 910. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, R.; Ho, T.-C.; Kutty, S.R.B.M.; Jumbri, K.; Thant, M.M.M.; Han, D.S. Lithium and boron recovery from oil field produced water: A mini review. In Proceedings of the International Conference on Emerging Smart Cities (ICESC2022), Kuching, Malaysia, 1–2 December 2022. [Google Scholar] [CrossRef]
- Lu, M.; Shao, L.; Yang, Y.; Li, P. Simultaneous recovery of lithium and boron from brine by the collaborative adsorption of lithium-ion sieves and boron chelating resins. Ind. Eng. Chem. Res. 2023, 62, 1508. [Google Scholar] [CrossRef]
- Najim, A. A review of advances in freeze desalination and future prospects. NPJ Clean. Water 2022, 5, 15. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, M.; Chen, X.D. Freezing-melting process and desalination: I. review of the state-of-the-art. Sep. Purif. Rev. 2006, 35, 59–96. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Kalista, B.; Shin, H.; Cho, J.; Jang, A. Current development and future prospect review of freeze desalination. Desalination 2018, 447, 167–181. [Google Scholar] [CrossRef]
- Yadav, S.; Banerjee, R.; Seethamraju, S. Thermodynamic analysis of LNG regasification process. Chem. Eng. Trans. 2022, 94, 919–924. [Google Scholar] [CrossRef]
- Noor Akashah, M.H.; Mohammad Rozali, N.E.; Mahadzir, S.; Liew, P.Y. Utilization of cold energy from LNG regasification process: A review of current trends. Processes 2023, 11, 517. [Google Scholar] [CrossRef]
- Ong, C.W.; Chen, C.-L. Technical and economic evaluation of seawater freezing desalination using liquefied natural gas. Energy 2019, 181, 429–439. [Google Scholar] [CrossRef]
- Karimi, A.; Abdi, M.A. Selective dehydration of high-pressure natural gas using supersonic nozzles. Chem. Eng. Process. Process Intensif. 2009, 48, 560–568. [Google Scholar] [CrossRef]
- Shamsi, M.; Farokhi, S.; Pourghafari, M.; Bayat, A. Tuning the natural gas dew point by Joule-Thomson and Mechanical Refrigeration processes: A comparative energy and exergy analysis. J. Pet. Sci. Eng. 2022, 212, 110270. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Naderifar, A.; Farhadi, F.; Pazuki, G. Thermodynamic analysis and process optimization of a natural gas liquid recovery unit based on the Joule—Thomson process. J. Nat. Gas. Sci. Eng. 2021, 96, 104265. [Google Scholar] [CrossRef]
- Kidnay, A.J.; Parrish, W.R.; McCartney, D.G. Fundamentals of Natural Gas Processing, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Williams, P.M.; Ahmad, M.; Connolly, B.S.; Oatley-Radcliffe, D.L. Technology for freeze concentration in the desalination industry. Desalination 2015, 356, 314. [Google Scholar] [CrossRef]
- Chmielowska, A.; Sowiżdżał, A.; Tomaszewska, B. Prospects of using hydrocarbon deposits from the autochthonous miocene formation (Eastern Carpathian Foredeep, Poland) for geothermal purposes. Energies 2021, 14, 3102. [Google Scholar] [CrossRef]
- CompLithium. Complex Technology for Lithium and Potable Water Recovery from Produced Waters. Available online: https://complithium.agh.edu.pl/ (accessed on 20 April 2024).
- Knapik, E.; Rotko, G.; Marszałek, M.; Piotrowski, M. Comparative study on lithium recovery with ion-selective adsorbents and extractants: Results of multi-stage screening test with the use of brine simulated solutions with increasing complexity. Energies 2023, 16, 3149. [Google Scholar] [CrossRef]
- ASTM D5907-18; Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water. ASTM International: West Conshohocken, PA, USA, 2018.
- PN-EN ISO 9377-2:2003; Water Quality—Determination of Hydrocarbon Oil Index—Part 2: Method Using Solvent Extraction and Gas Chromatography. PKN: Warsaw, Poland, 2013.
- Yu, X.; Cui, W.; Zhang, F.; Guo, Y.; Deng, T. Removal of iodine from the salt water used for caustic soda production by ion-exchange resin adsorption. Desalination 2019, 458, 76. [Google Scholar] [CrossRef]
- Abushawish, A.; Bouaziz, I.; Almanassra, I.W.; AL-Rajabi, M.M.; Jaber, L.; Khalil, A.K.A.; Takriff, M.S.; Laoui, T.; Shanableh, A.; Atieh, M.A.; et al. Desalination pretreatment technologies: Current status and future developments. Water 2023, 15, 1572. [Google Scholar] [CrossRef]
- Wang, J.; Sim, L.N.; Ho, J.S.; Nakano, K.; Kinoshita, Y.; Sekiguchi, K.; Chong, T.H. Evaluation of ceramics adsorption filter as a pretreatment for seawater reverse-osmosis desalination. Membranes 2022, 12, 1209. [Google Scholar] [CrossRef]
- Altmann, T.; Rousseva, A.; Vrouwenvelder, J.; Shaw, M.; Das, R. Effectiveness of ceramic ultrafiltration as pretreatment for seawater reverse osmosis. Desalination 2023, 564, 116781. [Google Scholar] [CrossRef]
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Pilot-scale study on the treatment of basal aquifer water using ultrafiltration, reverse osmosis and evaporation/crystallization to achieve zero-liquid discharge. J. Environ. Manag. 2016, 165, 213–223. [Google Scholar] [CrossRef]
- DuPont Tech Manual Excerpt. Water Chemistry and Pretreatment. Feedwater Type and Analysis. 2022. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-NF-FilmTec-Feedwater-Type-Analysis-Manual-Exc-45-D01545-en.pdf (accessed on 20 April 2024).
- Barma, M.C.; Peng, Z.; Moghtaderi, B.; Doroodchi, E. Freeze desalination of drops of saline solutions. Desalination 2021, 517, 115265. [Google Scholar] [CrossRef]
- Vrbka, L.; Jungwirth, P. Brine rejection from freezing salt solutions: A molecular dynamics study. Phys. Rev. Lett. 2005, 95, 148501. [Google Scholar] [CrossRef]
- Luo, S.; Jin, Y.; Tao, R.; Li, H.; Li, C.; Wang, J.; Li, Z. Molecular understanding of ion rejection in the freezing of aqueous solutions. Phys. Chem. Chem. Phys. 2021, 23, 13292–13299. [Google Scholar] [CrossRef]
- Badawy, S.M. Experimental and kinetic modeling study of multistage freezing-melting process and salt rejection of seawater. Cold Reg. Sci. Technol. 2022, 194, 103457. [Google Scholar] [CrossRef]
- Melak, F.; Ambelu, A.; Laing, G.D.; Alemayehu, E. Freeze desalination as point of use water treatment technology: A case of chromium (VI) removal from water. Proceedings 2018, 2, 173. [Google Scholar] [CrossRef]
- Wijewardena, D. Toxic and Precious Metals Removal from Wastewater Using Freeze Concentration and Electrodeionization; Lakehead University: Thunder Bay, ON, Canada, 2018. [Google Scholar]
- Ribeiro, L.S.; Dantas, T.N.C.; Dantas Neto, A.A.; Melo, K.C.; Moura, M.C.P.A.; Aum, P.T.P. The use of produced water in water-based drilling fluids: Influence of calcium and magnesium concentrations. Braz. J. Pet. Gas. 2016, 10, 233–245. [Google Scholar] [CrossRef][Green Version]
- Caenn, R.; Darley, H.C.H.; Gray, G. Completion, workover, packer, and reservoir drilling fluids. In Composition and Properties of Drilling and Completion Fluids, 7th ed.; Gulf Professional Publishing: Waltham, MA, USA, 2017; pp. 461–519. [Google Scholar] [CrossRef]
- Chaabi, O.; Al Kobaisi, M.; Haroun, M. Quantifying the low salinity waterflooding effect. Energies 2021, 14, 1979. [Google Scholar] [CrossRef]
- Aljuboori, F.A.; Lee, J.H.; Elraies, K.A.; Stephen, K.D. Using low salinity waterflooding to improve oil recovery in naturally fractured reservoirs. Appl. Sci. 2020, 10, 4211. [Google Scholar] [CrossRef]
- Stewart, D. Beneficial reuse of produced and flowback water. In Proceedings of the US EPA Technical Workshop on Analytical Chemical Methods for Hydraulic Fracturing, Research Triangle Park, NC, USA, 25 February 2013. [Google Scholar]
- Park, H.-G.; Kwon, Y.-N. Long-term stability of low-pressure reverse osmosis (RO) membrane operation—A pilot scale study. Water 2018, 10, 93. [Google Scholar] [CrossRef]
- Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.-D.; Van Der Bruggen, B.; Luis, P. Evaluation of commercial reverse osmosis and nanofiltration membranes for the removal of heavy metals from surface water in the Democratic Republic of Congo. Clean. Technol. 2022, 4, 1300–1316. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Chen, P.; Li, K.; Zhou, Q.; Ye, M.; Zhang, L.; Lu, Y. Advances in technologies for boron removal from water: A comprehensive review. Int. J. Environ. Res. Public Health 2022, 19, 10671. [Google Scholar] [CrossRef] [PubMed]
- Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Rahman, R.A.; Ismail, A.F.; Illias, R.M.; Othman, M.H.D.; Rahman, M.A.; Bilad, M.R.; Naseer, M.N. Adsorptive Membrane for Boron Removal: Challenges and Future Prospects. Membranes 2022, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Grimón, V.; Fernández-Vera, J.R.; Hernández-Moreno, J.M.; Palacios-Díaz, M.d.P. Sustainable irrigation using non-conventional resources: What has happened after 30 years regarding boron phytotoxicity? Water 2019, 11, 1952. [Google Scholar] [CrossRef]
- Hernández, J.A. Salinity tolerance in plants: Trends and perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed]
- Dolan, F.; Cath, T.; Hogue, T. Assessing the feasibility of using produced water for irrigation in Colorado. Sci. Total Environ. 2018, 640–641, 619–628. [Google Scholar] [CrossRef]
- The National Primary Drinking Water Regulations (NPDWR). U.S. Environmental Protection Agency. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 20 April 2024).
- Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 20 April 2024).
- Herkelmann, R.; Rudolph, W.; Seffer, D. Method of Recovering Iodine. U.S. Patent No. US00535661A, 18 October 1994. [Google Scholar]
- Venkat, E.; Magliette, R.J.; McKinney, D.; Michaels, A.S. Recovery of Iodide from Chemical Process Wastewater. U.S. Patent No. US006379556B1, 30 April 2002. [Google Scholar]
- DIAIONTM NSA100. Product Data Sheet No. 01-04-A-3506. Available online: https://www.diaion.com/en/products/ion_exchange_resins/strongly_basic_anion/data_sheet_sa/pdf/nsa100.pdf (accessed on 12 May 2024).
- Karshyga, Z.; Yersaiynova, A.; Yessengaziyev, A.; Orynbayev, B.; Kvyatkovskaya, M.; Silachyov, I. Synthesis of manganese oxide sorbent for the extraction of lithium from hydromineral raw materials. Materials 2023, 16, 7548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).