Polystyrene Plastic Particles Result in Adverse Outcomes for Hyalella azteca When Exposed at Elevated Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Source and Acclimation
2.2. Particle Source, Particle–Food Preparation, and Concentration Determination
2.3. Exposure and Water Physicochemical Parameters
2.4. Growth: Total Length, Capsule Length, and Dry Weight
2.5. Locomotor Behavior Assay
2.6. Internalization
2.6.1. Group (A): Imaging in 2D
2.6.2. Group (B): Imaging in 3D
2.7. Statistics
3. Results
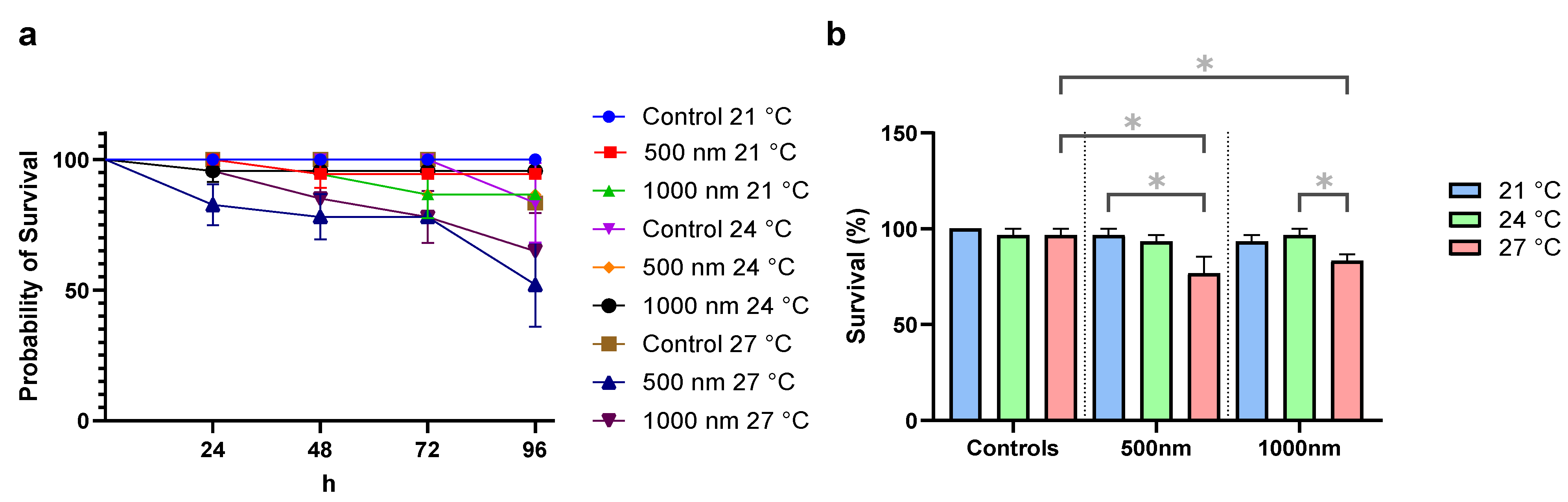
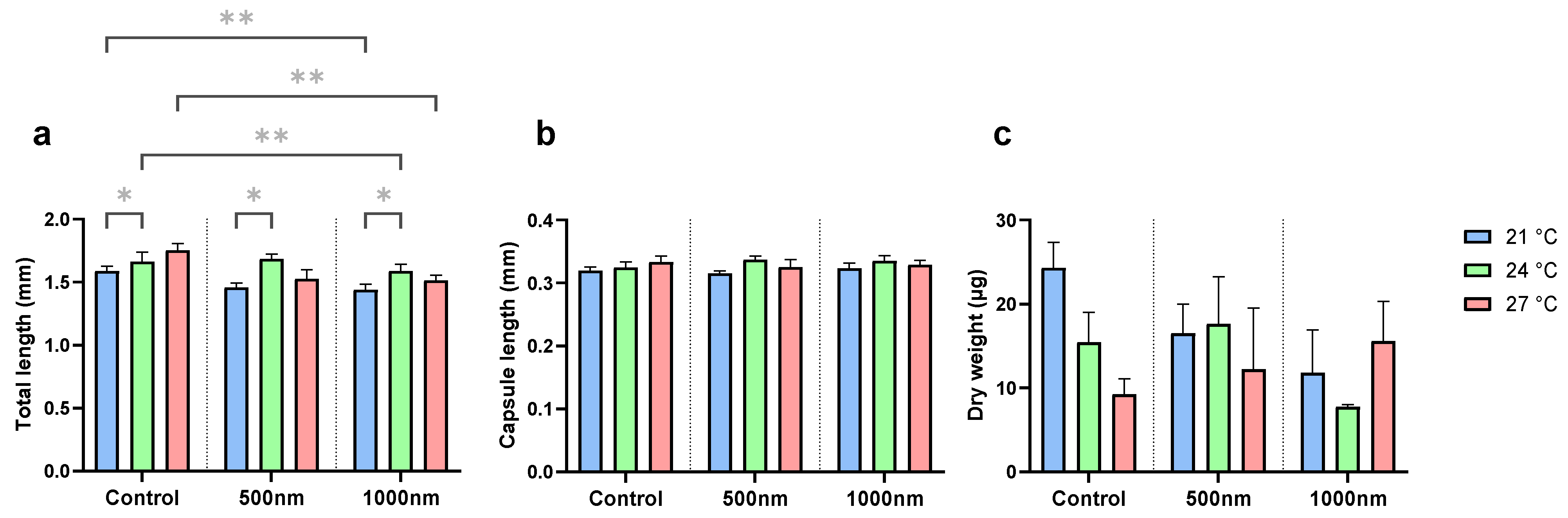
3.1. Survival and Growth
3.2. Locomotor Behavior Assay
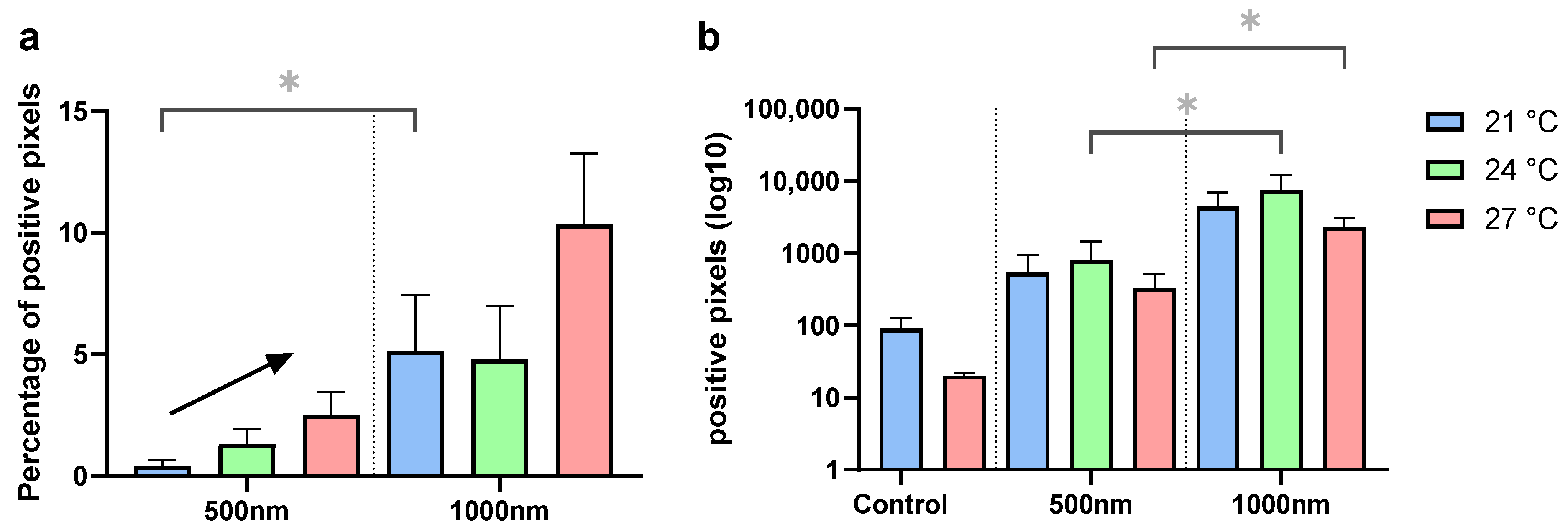
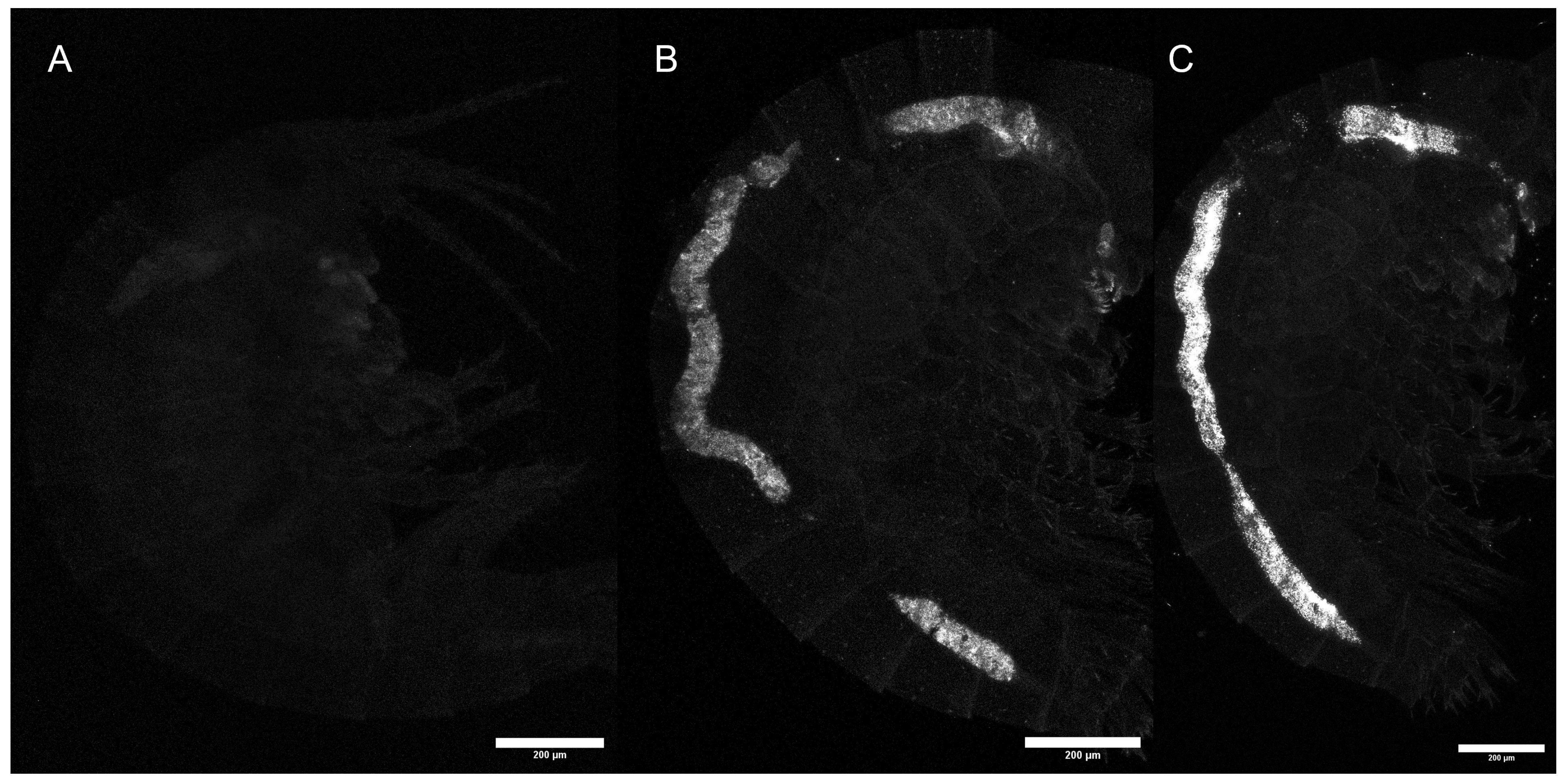
3.3. Internalization
3.3.1. Group (A)
3.3.2. Group (B)
3.4. Correlations between Uptake, Particle Size, and Tested Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baekeland, L.H. The Synthesis, Constitution, and Uses of Bakelite. Ind. Eng. Chem. 1909, 1, 149–161. [Google Scholar] [CrossRef]
- OECD Global Plastics Outlook. Global Plastics Outlook; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. A Methodological Approach of the Current Literature on Microplastic Contamination in Terrestrial Environments: Current Knowledge and Baseline Considerations. Sci. Total Environ. 2020, 730, 139164. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lu, H.; Tian, P.; Xue, Y.; Lu, J.; Tang, M.; Feng, W. Analysis of Microplastics in a Remote Region of the Tibetan Plateau: Implications for Natural Environmental Response to Human Activities. Sci. Total Environ. 2020, 739, 140087. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)Plastics in the Environment—Sources, Fates and Effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Battisti, C.; Fanelli, G.; Gallitelli, L.; Scalici, M. Dunal Plants as Sink for Anthropogenic Marine Litter: The Entrapping Role of Salsola kali L. (1753) in a Mediterranean Remote Beach (Sardinia, Italy). Mar. Pollut. Bull. 2023, 192, 115033. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Fang, J.K.H. Effects of Temperature and Particle Concentration on Aggregation of Nanoplastics in Freshwater and Seawater. Sci. Total Environ. 2022, 817, 152562. [Google Scholar] [CrossRef]
- Felicia Coleman, B.; S Wehle, D.H. Plastic Pollution: A Worldwide Oceanic Problem. Parks 1984, 9, 9–12. [Google Scholar]
- Battisti, C.; Gallitelli, L.; Vanadia, S.; Scalici, M. General Macro-Litter as a Proxy for Fishing Lines, Hooks and Nets Entrapping Beach-Nesting Birds: Implications for Clean-Ups. Mar. Pollut. Bull. 2023, 186, 114502. [Google Scholar] [CrossRef]
- Koltzenburg, S.; Maskos, M.; Nuyken, O. Polymer Chemistry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–584. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a Persistent Marine Pollutant. Annu. Rev. Environ. Resour. 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Environment. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume 58, pp. 51–67. [Google Scholar]
- Liu, F.; Rasmussen, L.A.; Klemmensen, N.D.R.; Zhao, G.; Nielsen, R.; Vianello, A.; Rist, S.; Vollertsen, J. Shapes of Hyperspectral Imaged Microplastics. Environ. Sci. Technol. 2023, 57, 12431–12441. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological Effects of Microplastics on Biota: A Review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size Matters More than Shape: Ingestion of Primary and Secondary Microplastics by Small Predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Doyle, D.; Sundh, H.; Almroth, B.C. Microplastic Exposure in Aquatic Invertebrates Can Cause Significant Negative Effects Compared to Natural Particles—A Meta-Analysis. Environ. Pollut. 2022, 315, 120434. [Google Scholar] [CrossRef] [PubMed]
- Biefel, F.; Geist, J.; Connon, R.; Pollution, B.H.-E. Interactive Effects between Water Temperature, Microparticle Compositions, and Fiber Types on the Marine Keystone Species Americamysis Bahia; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Redondo-Hasselerharm, P.E.; Falahudin, D.; Peeters, E.T.H.M.; Koelmans, A.A. Microplastic Effect Thresholds for Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol. 2018, 52, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Magester, S.; Barcelona, A.; Colomer, J.; Serra, T. Vertical Distribution of Microplastics in Water Bodies Causes Sublethal Effects and Changes in Daphnia Magna Swimming Behaviour. Ecotoxicol. Environ. Saf. 2021, 228, 113001. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, K.; Schwarz, A.; Gomez-Mestre, I.; Meyer, R.; Domscheit, M.; Bartels, F.; Schaeffer, S.M.; Engelkes, K. Life in Plastic, It’s Not Fantastic: Sublethal Effects of Polyethylene Microplastics Ingestion throughout Amphibian Metamorphosis. Sci. Total Environ. 2023, 885, 163779. [Google Scholar] [CrossRef] [PubMed]
- Greven, A.C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Harper, B.; Brander, S.; Harper, S. Toxicity of Micro and Nano Tire Particles and Leachate for Model Freshwater Organisms. J. Hazard. Mater. 2022, 429, 128319. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally Relevant Concentrations of Polyethylene Microplastics Negatively Impact the Survival, Growth and Emergence of Sediment-Dwelling Invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; Van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef] [PubMed]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of Nano- and Microplastics for Freshwater Ecosystems: A Critical Review. TrAC Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Keerthika, K.; Padmavathy, P.; Rani, V.; Jeyashakila, R.; Aanand, S.; Kutty, R.; Tamilselvan, R.; Subash, P. Microplastics Accumulation in Pelagic and Benthic Species along the Thoothukudi Coast, South Tamil Nadu, India. Mar. Pollut. Bull. 2023, 189, 114735. [Google Scholar] [CrossRef]
- Zavala-Alarcón, F.L.; Huchin-Mian, J.P.; González-Muñoz, M.D.P.; Kozak, E.R. In Situ Microplastic Ingestion by Neritic Zooplankton of the Central Mexican Pacific. Environ. Pollut. 2023, 319, 120994. [Google Scholar] [CrossRef] [PubMed]
- Mcilwraith, H.K.; Kim, J.; Helm, P.; Bhavsar, S.P.; Metzger, J.S.; Rochman, C.M. Evidence of Microplastic Translocation in Wild-Caught Fish and Implications for Microplastic Accumulation Dynamics in Food Webs. ACS Publ. 2021, 55, 12372–12382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, X.; Xu, X.; Takai, Y.; Ogawa, H.; Shimasaki, Y.; Oshima, Y. Uptake and Depuration Kinetics of Microplastics with Different Polymer Types and Particle Sizes in Japanese Medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2021, 212, 112007. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Feminella, J.W.; Power, M.E. Biotic Interactions in Freshwater Benthic Habitats. J. N. Am. Benthol. Soc. 2010, 29, 220–244. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Freshwater Sediment: A Review on Methods, Occurrence, and Sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.L.R.; Nipper, M. Sediment Toxicity Tests Using the Burrowing Amphipod Tiburonella Viscana (Amphipoda: Platyischnopidae). Ecotoxicol. Environ. Saf. 2007, 66, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Amphipoda. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Péry, A.R.R.; Dargelos, S.; Quéau, H.; Garric, J. Preparatory Work to Propose Water-Only Tests with the Amphipod Hyalella Azteca: Comparison with Sediment Toxicity Tests. Bull. Environ. Contam. Toxicol. 2005, 75, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Javidmehr, A.; Kass, P.H.; Deanovic, L.A.; Connon, R.E.; Werner, I. 10-Day Survival of Hyalella Azteca as a Function of Water Quality Parameters. Ecotoxicol. Environ. Saf. 2015, 115, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Growth, Development and Reproduction of Hyalella azteca (Saussure, 1858) in Laboratory Culture on JSTOR. Available online: https://www.jstor.org/stable/20106425 (accessed on 1 May 2024).
- Khan, F.R.; Halle, L.L.; Palmqvist, A. Acute and Long-Term Toxicity of Micronized Car Tire Wear Particles to Hyalella Azteca. Aquat. Toxicol. 2019, 213, 105216. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2017, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An Overview on Biodegradation of Polystyrene and Modified Polystyrene: The Microbial Approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Queiroz, L.G.; Achiles Prado, C.C.; de Melo, E.C.; de Moraes, B.R.; Ando, R.A.; de Paiva, T.C.B.; Pompeo, M.M. Expressive Biofragmentation of Polystyrene Microplastics by the Amphipod Hyalella Azteca. SSRN Electron. J. 2022. [CrossRef]
- Rani-Borges, B.; Queiroz, L.G.; Prado, C.C.A.; de Melo, E.C.; de Moraes, B.R.; Ando, R.A.; de Paiva, T.C.B.; Pompêo, M. Exposure of the Amphipod Hyalella Azteca to Microplastics. A Study on Subtoxic Responses and Particle Biofragmentation. Aquat. Toxicol. 2023, 258, 106516. [Google Scholar] [CrossRef]
- Verslycke, T.; Janssen, C.R. Effects of a Changing Abiotic Environment on the Energy Metabolism in the Estuarine Mysid Shrimp Neomysis Integer (Crustacea: Mysidacea). J. Exp. Mar. Biol. Ecol. 2002, 279, 61–72. [Google Scholar] [CrossRef]
- Reyes, B.A.; Pendergast, J.S.; Yamazaki, S. Mammalian Peripheral Circadian Oscillators Are Temperature Compensated. J. Biol. Rhythm. 2008, 23, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zheng, Y.; Dai, C.; Duan, H.; Gao, M.; Ali, M.R.; Sui, L. Effect of Polystyrene Microplastics and Temperature on Growth, Intestinal Histology and Immune Responses of Brine Shrimp Artemia Franciscana. J. Oceanol. Limnol. 2021, 39, 979–988. [Google Scholar] [CrossRef]
- Na, J.; Song, J.; Jung, J. Elevated Temperature Enhanced Lethal and Sublethal Acute Toxicity of Polyethylene Microplastic Fragments in Daphnia Magna. Environ. Toxicol. Pharmacol. 2023, 102, 104212. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.C.; Hartz, K.E.H.; Fulton, C.A.; Lydy, M.J.; Brander, S.M.; Hung, T.-C.; Fangue, N.A.; Connon, R.E. Exposure to Permethrin or Chlorpyrifos Causes Differential Dose- and Time-Dependent Behavioral Effects at Early Larval Stages of an Endangered Teleost Species. Endanger. Species Res. 2021, 44, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Mauduit, F.; Amer, N.R.; Biefel, F.; Hladik, M.L.; Connon, R.E.; Brander, S.M. Salinity Changes the Dynamics of Pyrethroid Toxicity in Terms of Behavioral Effects on Newly Hatched Delta Smelt Larvae. Toxics 2021, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Hasenbein, S.; Lawler, S.P.; Geist, J.; Connon, R.E. The Use of Growth and Behavioral Endpoints to Assess the Effects of Pesticide Mixtures upon Aquatic Organisms. Ecotoxicology 2015, 24, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Kristofco, L.A.; Cruz, L.C.; Haddad, S.P.; Behra, M.L.; Chambliss, C.K.; Brooks, B.W. Age Matters: Developmental Stage of Danio Rerio Larvae Influences Photomotor Response Thresholds to Diazinion or Diphenhydramine. Aquat. Toxicol. 2016, 170, 344–354. [Google Scholar] [CrossRef]
- Evans, H.L. Neurotoxicity Expressed in Naturally Occurring Behavior. In Neurobehavioral Toxicity: Analysis and Interpretation; CRC Press: Boca Raton, FL, USA, 1994; pp. 111–135. [Google Scholar]
- Bridges, C.M. Tadpole Swimming Performance and Activity Affected by Acute Exposure to Sublethal Levels of Carbaryl. Environ. Toxicol. Chem. 1997, 16, 1935–1939. [Google Scholar] [CrossRef]
- Grue, C.; Gardner, S.; Gibert, P.L.; Dell Omo, G. Behavioural Ecotoxicology; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Jacob, H.; Besson, M.; Swarzenski, P.W.; Lecchini, D.; Metian, M. Effects of Virgin Micro- and Nanoplastics on Fish: Trends, Meta-Analysis, and Perspectives. Environ. Sci. Technol. 2020, 54, 4733–4745. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Zheng, H.; Wang, M.; Fu, Y.; Luo, X.; Li, F.; Wang, Z. Polystyrene Microplastics Impaired the Feeding and Swimming Behavior of Mysid Shrimp Neomysis Japonica. Mar. Pollut. Bull. 2020, 150, 110660. [Google Scholar] [CrossRef]
- Britt, E. Erickson Getting a Grip on Microplastics’ Risks. CEN Glob. Enterp. 2022, 100, 20–25. [Google Scholar] [CrossRef]
- Qiao, R.; Mortimer, M.; Richter, J.; Rani-Borges, B.; Yu, Z.; Heinlaan, M.; Lin, S.; Ivask, A. Hazard of Polystyrene Micro-and Nanospheres to Selected Aquatic and Terrestrial Organisms. Sci. Total Environ. 2022, 853, 158560. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; US EPA: Washington, DC, USA, 2002; p. EPA-821-R-02-012. [Google Scholar]
- US EPA. Ecological Effects Test Guidelines OPPTS 850.1020 Gammarid Acute Toxicity Test. Available online: https://scholar.google.com/scholar_lookup?title=Ecological%20Effects%20Test%20Guidelines%20OPPTS%20850.1020%20Gammarid%20Acute%20Toxicity%20Test&publication_year=1996&author=USEPA (accessed on 25 February 2024).
- US EPA. Methods for Measuring the Toxicity and Bioaccumulation of Sediment-Associated Contaminantswith Freshwater Invertebrates; Duluth, M.N., Verschueren, K., Eds.; US EPA: Washington, DC, USA, 2000; Volume 58, pp. 255–267. [Google Scholar]
- Götz, A.; Imhof, H.K.; Geist, J.; Beggel, S. Moving Toward Standardized Toxicity Testing Procedures with Particulates by Dietary Exposure of Gammarids. Environ. Toxicol. Chem. 2021, 40, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Götz, A.; Beggel, S.; Geist, J. Dietary Exposure to Four Sizes of Spherical Polystyrene, Polylactide and Silica Nanoparticles Does Not Affect Mortality, Behaviour, Feeding and Energy Assimilation of Gammarus Roeseli. Ecotoxicol. Environ. Saf. 2022, 238, 113581. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying Ecological Risks of Aquatic Micro- and Nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef]
- Lasee, S.; Mauricio, J.; Thompson, W.A.; Karnjanapiboonwong, A.; Kasumba, J.; Subbiah, S.; Morse, A.N.; Anderson, T.A. Microplastics in a Freshwater Environment Receiving Treated Wastewater Effluent. Integr. Environ. Assess. Manag. 2017, 13, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of Microplastics in Samples of Zooplankton from Portuguese Coastal Waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian Seas: A Hot Spot of Pelagic Microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Schönemann, A.M. Currently Monitored Microplastics Pose Negligible Ecological Risk to the Global Ocean. Sci. Rep. 2020, 10, 22281. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton Response to Polystyrene Microplastics: Perspective from an Entire Growth Period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, J.; Zhang, M.; Jiang, W. Aggregation and Deposition Kinetics of Polystyrene Microplastics and Nanoplastics in Aquatic Environment. Bull. Environ. Contam. Toxicol. 2021, 107, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, F.M.; Lasenby, D.C. Seasonal Trends in the Head Capsule Length and Body Length/Weight Relationships of Two Amphipod Species. Crustaceana 1998, 71, 399–410. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Hutton, S.J.; Dickens, J.M.; Pedersen, E.I.; Harper, S.L.; Brander, S.M. Natural and Synthetic Microfibers Alter Growth and Behavior in Early Life Stages of Estuarine Organisms. Front. Mar. Sci. 2023, 9, 991650. [Google Scholar] [CrossRef]
- Siddiqui, S.; Dickens, J.M.; Cunningham, B.E.; Hutton, S.J.; Pedersen, E.I.; Harper, B.; Harper, S.; Brander, S.M. Internalization, Reduced Growth, and Behavioral Effects Following Exposure to Micro and Nano Tire Particles in Two Estuarine Indicator Species. Chemosphere 2022, 296, 133934. [Google Scholar] [CrossRef]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella Azteca to Acute and Chronic Microplastic Exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Mattsson, K.; Adolfsson, K.; Ekvall, M.T.; Borgström, M.T.; Linse, S.; Hansson, L.A.; Cedervall, T.; Prinz, C.N. Translocation of 40 Nm Diameter Nanowires through the Intestinal Epithelium of Daphnia Magna. Nanotoxicology 2016, 10, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Peijnenburg, W.J.G.M.; Li, R.; Yang, J.; Zhou, Q. Confocal Measurement of Microplastics Uptake by Plants. MethodsX 2020, 7, 100750. [Google Scholar] [CrossRef]
- Ma, C.; Li, L.; Chen, Q.; Lee, J.S.; Gong, J.; Shi, H. Application of Internal Persistent Fluorescent Fibers in Tracking Microplastics in Vivo Processes in Aquatic Organisms. J. Hazard. Mater. 2021, 401, 123336. [Google Scholar] [CrossRef] [PubMed]
- Kuehr, S.; Kaegi, R.; Maletzki, D.; Schlechtriem, C. Testing the Bioaccumulation Potential of Manufactured Nanomaterials in the Freshwater Amphipod Hyalella Azteca. Chemosphere 2021, 263, 127961. [Google Scholar] [CrossRef] [PubMed]
- Kuehr, S.; Diehle, N.; Kaegi, R.; Schlechtriem, C. Ingestion of Bivalve Droppings by Benthic Invertebrates May Lead to the Transfer of Nanomaterials in the Aquatic Food Chain. Environ. Sci. Eur. 2021, 33, 35. [Google Scholar] [CrossRef]
- Kuehr, S.; Windisch, H.; Schlechtriem, C.; Leon, G.; Gasparini, G.; Gimeno, S. Are Fragrance Encapsulates Taken Up by Aquatic and Terrestrial Invertebrate Species? Environ. Toxicol. Chem. 2022, 41, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.; Cheung, Y.B.; Parmar, M.K. Survival Analysis: A Practical Approach, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006; pp. 1–266. [Google Scholar] [CrossRef]
- Vega-Garcia, D.; Brito-Parada, P.R.; Cilliers, J.J. Optimising Small Hydrocyclone Design Using 3D Printing and CFD Simulations. Chem. Eng. J. 2018, 350, 653–659. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia Magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.E.; Assidqi, K.; Zamani, N.P.; Appel, D.; Perschke, M.; Huhn, M.; Lenz, M. Suspended Micro-Sized PVC Particles Impair the Performance and Decrease Survival in the Asian Green Mussel Perna Viridis. Mar. Pollut. Bull. 2016, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, N.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Liu, H.P.; Xu, Z. Microplastics Have a More Profound Impact than Elevated Temperatures on the Predatory Performance, Digestion and Energy Metabolism of an Amazonian Cichlid. Aquat. Toxicol. 2018, 195, 67–76. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, Z.; Yang, L.; Zhou, W.; Qin, Z.; Zhang, H. Size-Dependent Toxicological Effects of Polystyrene Microplastics in the Shrimp Litopenaeus Vannamei Using a Histomorphology, Microbiome, and Metabolic Approach. Environ. Pollut. 2023, 316, 120635. [Google Scholar] [CrossRef] [PubMed]
- Blarer, P.; Burkhardt-Holm, P. Microplastics Affect Assimilation Efficiency in the Freshwater Amphipod Gammarus Fossarum. Environ. Sci. Pollut. Res. 2016, 23, 23522–23532. [Google Scholar] [CrossRef]
- Straub, S.; Hirsch, P.E.; Burkhardt-Holm, P. Biodegradable and Petroleum-Based Microplastics Do Not Differ in Their Ingestion and Excretion but in Their Biological Effects in a Freshwater Invertebrate Gammarus Fossarum. Int. J. Environ. Res. Public Health 2017, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Kratina, P.; Watts, T.J.; Green, D.S.; Kordas, R.L.; O’Gorman, E.J. Interactive Effects of Warming and Microplastics on Metabolism but Not Feeding Rates of a Key Freshwater Detritivore. Environ. Pollut. 2019, 255, 113259. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.; Chatterjee, S.; Kamphuis, B.; Segers-Nolten, I.M.J.; Claessens, M.M.A.E.; Blum, C. Nanoplastic Sizes and Numbers: Quantification by Single Particle Tracking. Environ. Sci. Nano 2021, 8, 723–730. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of Size and Temperature on Metabolic Rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Ohlberger, J. Climate Warming and Ectotherm Body Size—From Individual Physiology to Community Ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Rall, B.C.; Brose, U.; Hartvig, M.; Kalinkat, G.; Schwarzmüller, F.; Vucic-Pestic, O.; Petchey, O.L. Universal Temperature and Body-Mass Scaling of Feeding Rates. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, E.H.; Scherrey, P.M. Digestive Anatomy of Halella Azteca (Crustacea, Amphipoda). J. Morphol. 1983, 175, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.; Wang, J.; Li, J.; Liu, S.; Tu, J.; Chen, Y.; Zong, Y.; Zhang, P.; Wang, Z.; et al. Influence of Microplastics on the Growth and the Intestinal Microbiota Composition of Brine Shrimp. Front. Microbiol. 2021, 12, 717272. [Google Scholar] [CrossRef] [PubMed]
- van Pomeren, M.; Brun, N.R.; Peijnenburg, W.J.G.M.; Vijver, M.G. Exploring Uptake and Biodistribution of Polystyrene (Nano)Particles in Zebrafish Embryos at Different Developmental Stages. Aquat. Toxicol. 2017, 190, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, T.; Monopoli, M.P.; Kim, J.; Salvati, A.; Dawson, K.A. Elution of Labile Fluorescent Dye from Nanoparticles during Biological Use. PLoS ONE 2011, 6, 25556. [Google Scholar] [CrossRef] [PubMed]
- Schür, C.; Rist, S.; Baun, A.; Mayer, P.; Hartmann, N.B.; Wagner, M. When Fluorescence Is Not a Particle: The Tissue Translocation of Microplastics in Daphnia Magna Seems an Artifact. Environ. Toxicol. Chem. 2019, 38, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of Toxic Chemicals with Microplastics: A Critical Review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Chernikova, T.N.; Yakimov, M.M.; Golyshin, P.N.; Timmis, K.N. Chaperonins Govern Growth of Escherichia Coli at Low Temperatures. Nat. Biotechnol. 2003, 21, 1267. [Google Scholar] [CrossRef]
- Saborowski, R.; Korez, Š.; Riesbeck, S.; Weidung, M.; Bickmeyer, U.; Gutow, L. Shrimp and Microplastics: A Case Study with the Atlantic Ditch Shrimp Palaemon Varians. Ecotoxicol. Environ. Saf. 2022, 234, 113394. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Feeding Type and Development Drive the Ingestion of Microplastics by Freshwater Invertebrates. Sci. Rep. 2017, 7, 17006. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.M.; Moreira-Santos, M.; Ribeiro, R. Active and Passive Spatial Avoidance by Aquatic Organisms from Environmental Stressors: A Complementary Perspective and a Critical Review. Environ. Int. 2016, 92–93, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative Investigation of the Mechanisms of Microplastics and Nanoplastics toward Zebrafish Larvae Locomotor Activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene Microplastics Alter the Behavior, Energy Reserve and Nutritional Composition of Marine Jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Laist, D.W. Overview of the Biological Effects of Lost and Discarded Plastic Debris in the Marine Environment. Mar. Pollut. Bull. 1987, 18, 319–326. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-Term Exposure with High Concentrations of Pristine Microplastic Particles Leads to Immobilisation of Daphnia Magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic Ingestion Decreases Energy Reserves in Marine Worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin Microplastics Cause Toxicity and Modulate the Impacts of Phenanthrene on Biomarker Responses in African Catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Ogonowski, M.; Gerdes, Z.; Gorokhova, E. What We Know and What We Think We Know about Microplastic Effects—A Critical Perspective. Curr. Opin. Environ. Sci. Health 2018, 1, 41–46. [Google Scholar] [CrossRef]
- Anggraini, R.R.; Risjani, Y.; Yanuhar, U. Plastic Litter as Pollutant in the Aquatic Environment: A Mini-Review. J. Ilm. Perikan. Dan Kelaut. 2020, 12, 167–180. [Google Scholar] [CrossRef]
- Queiroz, L.G.; Rani-Borges, B.; Prado, C.C.A.; de Moraes, B.R.; Ando, R.A.; de Paiva, T.C.B.; Pompêo, M. Realistic Environmental Exposure to Secondary PET Microplastics Induces Biochemical Responses in Freshwater Amphipod Hyalella Azteca. Chem. Ecol. 2023, 39, 288–301. [Google Scholar] [CrossRef]
| Treatment | Temperature | Cycle | Thigmotaxis | TDM (mm) | Velocity (mm/s) | Cruising Mean | Moving Mean | Acceleration Mean (mm/s2) | Meander Min (deg/mm) | Zone Alternation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | 21 °C | Dark | Mean | 0.17 | 648.17 | 16,200.01 | 0.16 | 0.09 | 32.48 | 548,707.01 | 41.74 |
| SEM | 0.04 | 77.73 | 1942.64 | 0.01 | 0.00 | 14.92 | 154,155.45 | 6.40 | |||
| Light | Mean | 0.14 | 665.36 | 16,629.49 | 0.19 | 0.09 | 62.44 | 447,422.19 | 35.65 | ||
| SEM | 0.03 | 80.66 | 2016.02 | 0.02 | 0.00 | 22.39 | 72,998.12 | 5.83 | |||
| 24 °C | Dark | Mean | 0.20 | 501.83 | 12,526.44 | 0.15 | 0.09 | 71.83 | 41,3407.62 | 56.02 | |
| SEM | 0.04 | 61.83 | 1543.29 | 0.01 | 0.00 | 18.13 | 67,852.27 | 7.44 | |||
| Light | Mean | 0.20 | 528.67 | 13,196.59 | 0.15 | 0.08 | 26.67 | 568,502.63 | 58.46 | ||
| SEM | 0.04 | 55.91 | 1395.66 | 0.01 | 0.00 | 9.78 | 130,905.13 | 7.16 | |||
| 27 °C | Dark | Mean | 0.33 | 447.96 | 11,172.90 | 0.17 | 0.10 | 56.47 | 510,822.34 | 77.50 | |
| SEM | 0.05 | 84.81 | 2115.36 | 0.01 | 0.01 | 20.02 | 269,753.60 | 10.82 | |||
| Light | Mean | 0.28 | 495.26 | 12,352.93 | 0.19 | 0.09 | 75.39 | 775,993.28 | 74.13 | ||
| SEM | 0.05 | 81.29 | 2027.59 | 0.01 | 0.01 | 30.74 | 263,831.07 | 10.38 | |||
| 500 nm | 21 °C | Dark | Mean | ↑ 0.32 | ↓ 394.47 | ↓ 9859.24 | 0.14 | 0.10 | ↑ 81.01 | ↓ 321,832.86 | ↑ 75.52 |
| SEM | 0.05 | 44.41 | 1109.99 | 0.01 | 0.01 | 21.92 | 91,097.54 | 8.93 | |||
| Light | Mean | ↑ 0.33 | 420.62 | 10,512.77 | 0.14 | ↑ 0.10 | 91.31 | ↓ 337,481.7 | ↓ 81.69 | ||
| SEM | 0.05 | 54.43 | 1360.34 | 0.01 | 0.00 | 24.56 | 92,749.78 | 8.74 | |||
| 24 °C | Dark | Mean | ↑ 0.40 | 548.34 | 13,687.72 | 0.15 | 0.09 | 85.23 | ↓ 131,648.31 | ↑ 94.84 | |
| SEM | 0.05 | 88.12 | 2199.78 | 0.01 | 0.01 | 26.31 | 16,606.04 | 8.44 | |||
| Light | Mean | ↑ 0.40 | 606.83 | 15,147.97 | 0.16 | ↓ 0.09 | ↑ 162.32 | ↓ 222,524.87 | ↑ 94.63 | ||
| SEM | 0.05 | 101.73 | 2539.74 | 0.01 | 0.00 | 46.82 | 58,640.33 | 9.38 | |||
| 27 °C | Dark | Mean | 0.27 | ↑ 645.79 | ↑ 16,107.80 | 0.21 | 0.08 | 79.04 | 78,552,518.10 | 59.93 | |
| SEM | 0.04 | 84.73 | 2113.20 | 0.02 | 0.00 | 27.67 | 50,329,114.99 | 6.37 | |||
| Light | Mean | 0.22 | 663.80 | 16,557.51 | 0.20 | 0.08 | 38.31 | 52,073,199.65 | 53.63 | ||
| SEM | 0.04 | 85.59 | 2134.75 | 0.02 | 0.00 | 11.29 | 38,954,708.05 | 6.06 | |||
| 1000 nm | 21 °C | Dark | Mean | 0.26 | ↓ 460.38 | ↓ 11,506.56 | 0.15 | 0.09 | 90.86 | 288,840.77 | 56.74 |
| SEM | 0.05 | 62.18 | 1554.11 | 0.01 | 0.01 | 27.82 | 46,682.69 | 8.55 | |||
| Light | Mean | 0.27 | ↓ 475.73 | ↓ 11,890.01 | 0.14 | 0.09 | 77.79 | ↓ 307,032.33 | 61.56 | ||
| SEM | 0.05 | 64.43 | 1610.24 | 0.01 | 0.01 | 24.39 | 48,446.12 | 8.92 | |||
| 24 °C | Dark | Mean | 0.21 | 642.22 | 16,030.16 | 0.13 | ↓ 0.08 | 55.44 | 368,102.43 | 47.63 | |
| SEM | 0.04 | 79.95 | 1995.47 | 0.01 | 0.00 | 14.53 | 68,938.96 | 7.91 | |||
| Light | Mean | 0.21 | 697.79 | 17,417.42 | 0.14 | 0.08 | 53.18 | ↓ 316,204.54 | 51.54 | ||
| SEM | 0.04 | 88.89 | 2218.80 | 0.01 | 0.00 | 14.26 | 65,979.64 | 7.84 | |||
| 27 °C | Dark | Mean | 0.28 | 487.30 | 12,156.83 | ↓ 0.14 | ↓ 0.08 | 54.77 | 566,070.12 | 58.57 | |
| SEM | 0.05 | 69.85 | 1742.71 | 0.01 | 0.00 | 17.89 | 257,837.42 | 7.38 | |||
| Light | Mean | 0.28 | 508.36 | 12,682.07 | ↓ 0.13 | 0.09 | 57.39 | 332,440.14 | 61.65 | ||
| SEM | 0.05 | 77.09 | 1923.03 | 0.01 | 0.01 | 18.50 | 67,763.56 | 8.43 | |||
| Sign. category effect | Particle treatment | <0.001 | 0.82 | 0.82 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Temperature | 0.47 | 0.34 | 0.34 | <0.001 | 0.02 | 0.03 | 0.04 | 0.28 | |||
| Cycle | 0.49 | 0.47 | 0.47 | 0.81 | 0.60 | 0.48 | 0.59 | 0.96 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biefel, F.; Brander, S.M.; Connon, R.E.; Geist, J. Polystyrene Plastic Particles Result in Adverse Outcomes for Hyalella azteca When Exposed at Elevated Temperatures. Water 2024, 16, 1360. https://doi.org/10.3390/w16101360
Biefel F, Brander SM, Connon RE, Geist J. Polystyrene Plastic Particles Result in Adverse Outcomes for Hyalella azteca When Exposed at Elevated Temperatures. Water. 2024; 16(10):1360. https://doi.org/10.3390/w16101360
Chicago/Turabian StyleBiefel, Felix, Susanne M. Brander, Richard E. Connon, and Juergen Geist. 2024. "Polystyrene Plastic Particles Result in Adverse Outcomes for Hyalella azteca When Exposed at Elevated Temperatures" Water 16, no. 10: 1360. https://doi.org/10.3390/w16101360
APA StyleBiefel, F., Brander, S. M., Connon, R. E., & Geist, J. (2024). Polystyrene Plastic Particles Result in Adverse Outcomes for Hyalella azteca When Exposed at Elevated Temperatures. Water, 16(10), 1360. https://doi.org/10.3390/w16101360







