Abundance and Characterization of Anthropogenic Microlitter in Effluent from Three Wastewater Treatment Plants in Gran Canaria (Canary Islands, Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Sampling
- PRET: This WWTP, one of the largest on the island, comprises a large pumping station that lifts WW. The samples were collected before the pumping stage, subsequent to the pretreatment steps, which include a 6 mm coarse + 3 mm fine screening followed by desanding and degreasing. The effluent is representative of the discharges that may occur occasionally through excessive storm flow, particularly when the sewerage system is at risk of being overwhelmed, such as during heavy rainfall or emergency situations (e.g., sewer blockages or equipment failures at wastewater treatment works). In such scenarios, the flow from the unitary sewage system that cannot be pumped undergoes pretreatment before being discharged into the sea through the submarine outfall;
- AS: This WWTP is based on AS technology and has several treatment steps, briefly: screening, grit removal, desanding–degreasing, primary settling, aeration/activated sludge, lamellar settling, and chlorination. The treated effluent, with an annual mean flow of 345 m3/h, is representative of biological AS treatment, the predominant technology on the islands. Samples were collected after disinfection treatment, specifically in the outlet chamber just before discharge into the submarine outfall;
- MBR: This WWTP operates on MBR systems. In recent years, due to its high treatment efficiencies, this technology has experienced significant growth, particularly in situations where discharge takes place in sensitive coastal areas or when its effluent is designated for irrigation. Given that the treated water from the sampled plant, with an annual mean flow of 230 m3/h, is primarily intended for irrigation, the effluent samples were collected just before reaching the storage tank. Any effluent not used for irrigation is directed through a submarine outfall.
2.2. Analytical Procedure for Wastewater Samples
2.3. Analytical Procedure of Microlitter
2.4. Chemical (Polymer) Composition of Microlitter Particles
2.5. Statistics
3. Results and Discussion
3.1. Occurrence and Distribution of Microlitter Particles in Wastewater Effluent Samples
3.1.1. Microlitter Particle Occurrence
3.1.2. Microlitter Particle Sizes and Morphology Distribution
3.1.3. Influencing Factors and Seasonal Variation
3.2. Analysis of Microplastic Polymer Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Guidance on Monitoring of Marine Litter in European Seas. A guidance Document within the Common Implementation Strategy for the Marine. Strategy Framework Directive MSFD Technical Subgroup on Marine Litter. 2013. Available online: https://mcc.jrc.ec.europa.eu/documents/201702074014.pdf (accessed on 1 July 2023).
- UNE-CN ISO/TR 21960:2020; Plastics-Environmental Aspects-State of Knowledge and Methodologies. International Organization for Standardization: Geneva, Switzerland, 2020.
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 1 and 2). 2015–2016. Available online: http://www.gesamp.org/publications (accessed on 1 July 2023).
- Arthur, C.; Bamford, H.; Baker, J. The occurrence, effects and fate of small plastic debris in the oceans. In Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 3 September 2008; pp. 9–11. [Google Scholar]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Galgani, F.; Maes, T.; Thompson, R.C. Microplastics in seawater: Recommendations from the marine strategy framework directive implementation process. Front. Mar. Sci. 2016, 3, 219. [Google Scholar] [CrossRef]
- Oliveira, M.; Almeida, M. The why and how of micro(nano)plastic research. TrAC Trends Anal. Chem. 2019, 114, 196–201. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro-and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Gola, D.; Tyagi, P.K.; Arya, A.; Chauhan, N.; Agarwal, M.; Singh, S.K.; Gola, S. The impact of microplastics on marine environment: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100552. [Google Scholar] [CrossRef]
- Steer, M.; Thompson, R.C.; Steer, M.; Thompson, R.C. Plastics and Microplastics: Impacts in the Marine Environment. In Mare Plasticum—The Plastic Sea; Streit-Bianchi, M., Cimadevila, M., Trettnak, W., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Perspectives on micro(nano)plastics in the marine environment: Biological and societal considerations. Water 2020, 12, 3208. [Google Scholar] [CrossRef]
- da Costa, J.P.; Chamkha, M.; Ksibi, M.; Sayadi, S. Effects of microplastics’ physical and chemical properties on aquatic organisms: State-of-the-art and future research trends. TrAC 2023, 166, 117192. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Coyle, R.; Hardiman, G.; O’Driscoll, K. Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud. Chem. Environ. Eng. 2020, 2, 100010. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Schmidt, C.; Kumar, R.; Yang, S.; Büttner, O. Microplastic particle emission from wastewater treatment plant effluents into river networks in Germany: Loads, spatial patterns of concentrations and potential toxicity. Sci. Total Environ. 2020, 737, 139544. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.; Hurley, R.; Nizzetto, L.; Rico, A.; Vighi, M. Spatio-temporal distribution of microplastics in a Mediterranean river catchment: The importance of wastewater as an environmental pathway. J. Hazard. Mater. 2021, 420, 126481. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Rauert, C.; Thomas, K.V. Mass quantification of microplastic at wastewater treatment plants by pyrolysis-gas chromatography–mass spectrometry. Sci. Total Environ. 2023, 856, 159251. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic Pollution Is Widely Detected in US Municipal Wastewater Treatment Plants Effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hüffer, T.; Kl¨ockner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester textiles as a source of microplastics from households: A mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef]
- de Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brou- ta-Agnesa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of Microplastic Release Caused by Textile Washing Processes of Synthetic Fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.Z.; Al Kendi, R.; Thiemann, T. The Effect of Wastewater Treatment Plants on Retainment of Plastic Microparticles to Enhance Water Quality—A Review. J. Environ. Prot. 2021, 12, 161–195. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics?-Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, M.; Chiavola, A.; Di Marcantonio, C.; Boni, M.R. Presence and fate of microplastics in the water sources: Focus on the role of wastewater and drinking water treatment plants. J. Water Process Eng. 2021, 40, 101787. [Google Scholar] [CrossRef]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Fiore, M.; Zuccarello, P.; Conti, G.O.; Grasso, A.; Nicolosi, I.; Copat, C.; Ferrante, M. Efficiency of wastewater treatment plants (WWTPs) for microplastic removal: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8014. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; López-Castellanos, J.; Olmos, S.; Rojo, D. Characterization and removal efficiencies of microplastics discharged from sewage treatment plants in Southeast Spain. Water Res. 2023, 244, 120479. [Google Scholar] [CrossRef]
- Hajji, S.; Ben-Haddad, M.; Abelouah, M.R.; De-la-Torre, G.E.; Alla, A.A. Occurrence, characteristics, and removal of microplastics in wastewater treatment plants located on the Moroccan Atlantic: The case of Agadir metropolis. Sci. Total Environ. 2023, 862, 160815. [Google Scholar] [CrossRef]
- Flores-Munguía, E.J.; Rosas-Acevedo, J.L.; Ramírez-Hernández, A.; Aparicio-Saguilan, A.; Brito-Carmona, R.M.; Violante-González, J. Release of Microplastics from Urban Wastewater Treatment Plants to Aquatic Ecosystems in Acapulco, Mexico. Water 2023, 15, 3643. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 164, 19–40. [Google Scholar]
- Real Decreto 1365/2018, de 2 de noviembre, por el que se aprueban las estrategias marinas. In B.O.E.; Ministerio para la Transición Ecológica, Gobierno de España: Madrid, Spain, 2018; Volume 279, pp. 112104–112115.
- Amendments Adopted by the European Parliament on 5 October 2023 on the Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast). Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2023-0355_EN.html (accessed on 24 October 2023).
- Real Decreto-ley 11/1995, de 28 de Diciembre, por el Que se Establecen las Normas Aplicables al Tratamiento de las Aguas Residuales Urbanas. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-1995-27963 (accessed on 1 July 2023).
- Carr, S.; Liu, J.; Tesoro, A. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174e182. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN 872:2006; Calidad del Agua. Determinación de los Sólidos en Suspensión. Método de Filtración por Filtro de Fibra de Vidrio. Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2006.
- Clesceri, L.; Grenberg, A.; Trussell, R.; Franson, M. Standard Methods for the Examination of Water and Wastewater, 17th ed.; Editions Diaz de Santos, Spain; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- ECHA. Restriction proposal for intentionally added microplastics in the EU—annex XV—intentionally added microplastics. In European Chemicals Agency; ECHA/NR/19/28; ECHA: Helsinki, Finland, 2019. [Google Scholar]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Microplastics’ analysis in water: Easy handling of samples by a new Thermal Extraction Desorption-Gas Chromatography-Mass Spectrometry (TED-GC/MS) methodology. Talanta 2023, 253, 123829. [Google Scholar] [CrossRef] [PubMed]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; Van Loosdrecht, M.C.M. Sieving wastewater–Cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ho, D.; Santoro, D.; Torfs, E.; Doucet, J.; Vanrolleghem, P.A.; Nakhla, G. Experimental assessment and validation of quantification methods for cellulose content in municipal wastewater and sludge. Environ. Sci. Pollut. Res. 2018, 25, 16743–16753. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Bahreini, G.; Ho, D.; Sridhar, G.; Gupta, M.; Wessels, C.; Marcelis, P.; Elbeshbishy, E.; Rosso, D.; Santoro, D.; et al. Fate of cellulose in primary and secondary treatment at municipal water resource recovery facilities. Water Environ. Res. 2019, 91, 1479–1489. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Marine Litter: A Global Challenge; UNEP: Nairobi, Kenya, 2009; 232p. [Google Scholar]
- Egea-Corbacho, A.; Martín-García, A.P.; Franco, A.A.; Albendín, G.; Arellano, J.M.; Rodríguez, R.; Quiroga, J.M.; Coello, M.D. A method to remove cellulose from rich organic samples to analyse microplastics. J. Clean. Prod. 2022, 334, 130248. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Pavithra, N.; Méndez-Herrera, P.F.; López-López, J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Sci. Total Environ. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Freeman, S.; Booth, A.M.; Sabbah, I.; Tiller, R.; Dierking, J.; Klun, K.; Rotter, A.; Ben-David, E.; Javidpour, J.; Angel, D.L. Between source and sea: The role of wastewater treatment in reducing marine microplastics. J. Environ. Manag. 2020, 266, 110642. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Microplastic pollution in wastewater treatment plants in the city of Cádiz: Abundance, removal efficiency and presence in receiving water body. Sci. Total Environ. 2021, 776, 145795. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Egea-Corbacho, A.; Martín-García, A.P.; Franco, A.A.; Quiroga, J.M.; Andreasen, R.R.; Jørgensen, M.K.; Christensen, M.L. Occurrence, identification and removal of microplastics in a Wastewater Treatment Plant compared to an advanced MBR technology: Full-scale pilot plant. J. Environ. Chem. Eng. 2023, 11, 109644. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2016; p. 315. [Google Scholar]
- Kong, S.; Lv, X.; Peng, D.; Chen, M. A new test method for biodegradability of plastics in sediment. Environ. Technol. Innov. 2021, 21, 101217. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Wang, X.; Hou, F.; Li, P.; van der Hoek, J.P.; Liu, G. Assessing the Mass Concentration of Microplastics and Nanoplastics in Wastewater Treatment Plants by Pyrolysis Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 3114–3123. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. J. Chem. Eng. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Roscher, L.; Halbach, M.; Nguyen, M.T.; Hebeler, M.; Luschtinetz, F.; Scholz-Böttcher, B.M.; Primpke, S.; Gerdts, G. Microplastics in two German wastewater treatment plants: Year-long effluent analysis with FTIR and Py-GC/MS. Sci. Total Environ. 2022, 817, 152619. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Goedecke, C.; Eisentraut, P.; Altmann, K.; Elert, A.M.; Bannick, C.G.; Ricking, M.; Obermaier, N.; Barthel, A.-K.; Schmitt, T.; Jekel, M.; et al. Development of a Routine Screening Method for the Microplastic Mass Content in a Wastewater Treatment Plant Effluent. Front. Environ. Chem. 2022, 3, 844633. [Google Scholar] [CrossRef]
- Franco, A.A.; Iglesias-Arroyo, D.; Egea-Corbacho, Á.; Martín-García, A.P.; Quiroga, J.M.; Coello, M.D. Influence of tourism on microplastic contamination at wastewater treatment plants in the coastal municipality of Chiclana de la Frontera. Sci. Total Environ. 2023, 900, 165573. [Google Scholar] [CrossRef] [PubMed]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Plastics Europe, 2022. Plastics-the Facts. October 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 4 July 2023).
- Van Do, M.; Le, T.X.T.; Vu, N.D.; Dang, T.T. Distribution and occurrence of microplastics in wastewater treatment plants. Environ. Technol. Innov. 2022, 26, 102286. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, S. Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes 2021, 9, 617. [Google Scholar] [CrossRef]
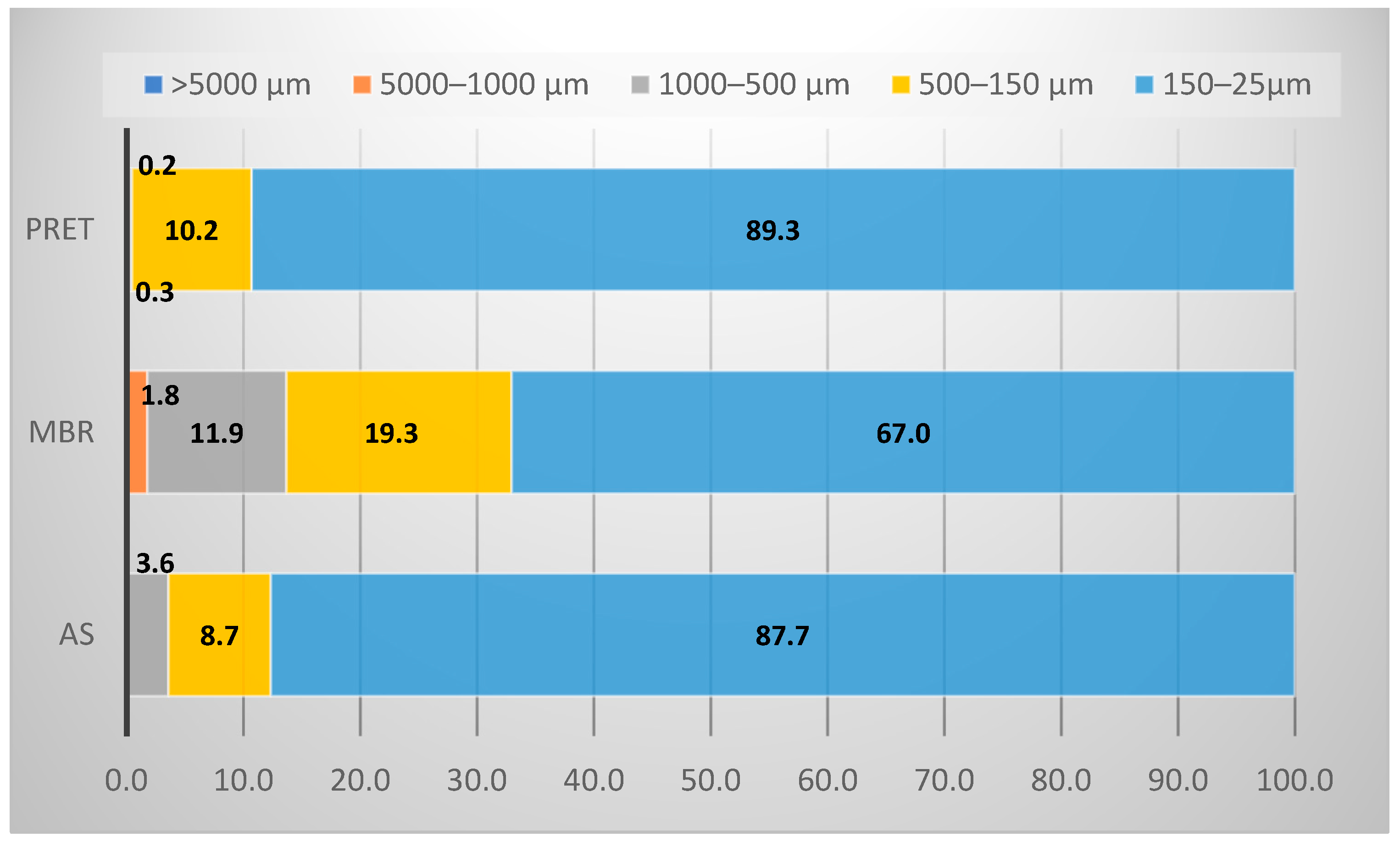


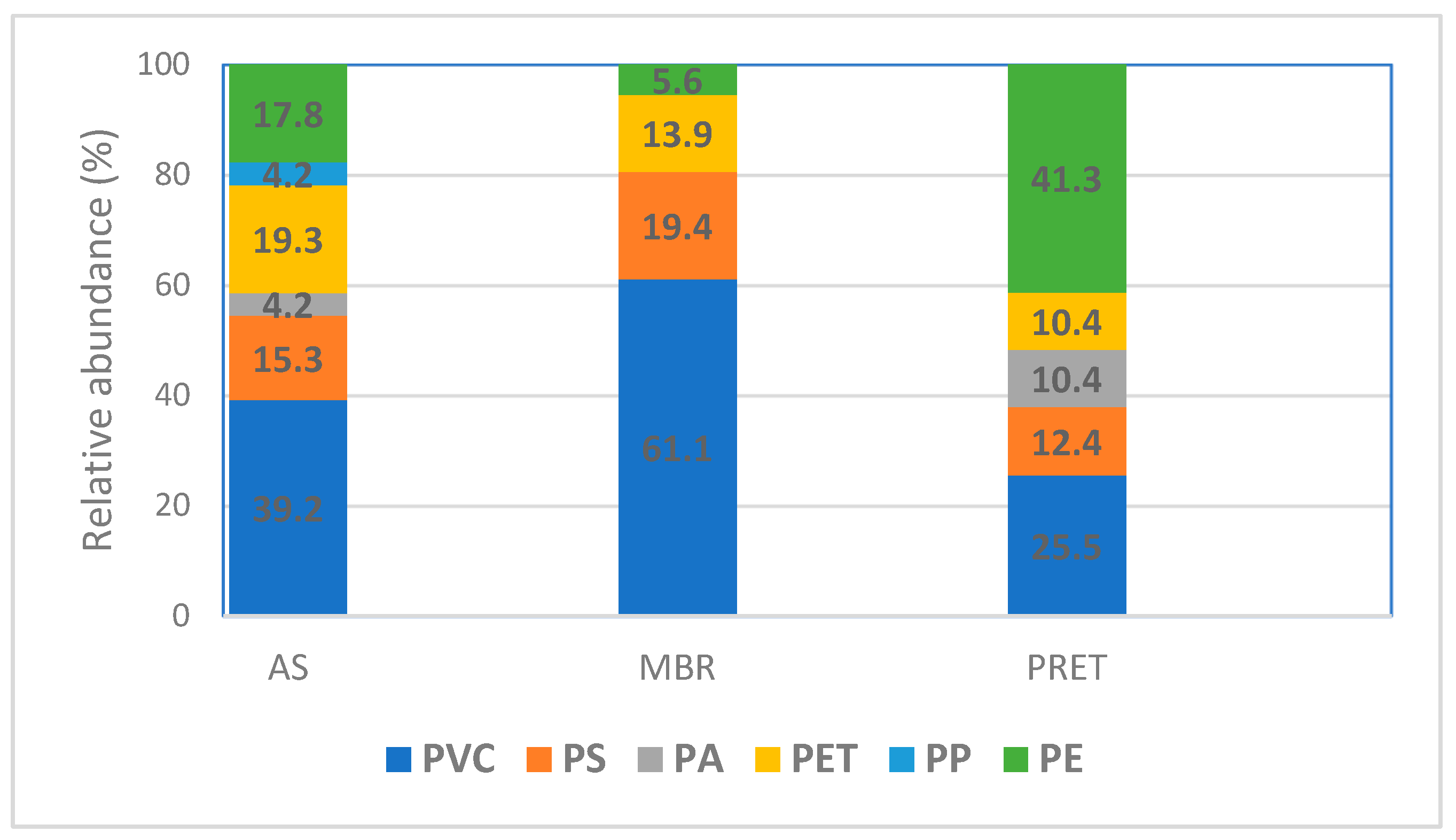
| Label | Wastewater Source | Size (p.e.) 1 | Main Treatment |
|---|---|---|---|
| PRET 2 | Household | - | Pumping station; coarse and fine screening + degritting + degreasing |
| AS 3 | Household + industry (10%) | 171,600 | Pretreatment + settling + activated sludge + chlorination |
| MBR 4 | Household + industry (25%) | 50,000 | Membrane bioreactor + chlorination |
| PRET (MP/L) | AS (MP/L) | MBR (MP/L) | |
|---|---|---|---|
| Jun-22 | 7813 | 50.87 | 8.00 |
| Jul-22 | 7817 | 10.14 | 4.60 |
| Aug-22 | 3967 | 8.90 | 3.20 |
| Sep-22 | 15,133 | 58.80 | 13.00 |
| Oct-22 | 2340 | 23.60 | 4.40 |
| Nov-22 | 6086 | 19.20 | 12.00 |
| Jan-23 | 9428 | 48.06 | 13.60 |
| Feb-23 | 7108 | 42.11 | 17.40 |
| Max | 15,133 | 58.80 | 17.40 |
| Min | 2340 | 8.90 | 3.20 |
| Average | 7461.50 | 32.71 | 9.53 |
| SD | 3843.87 | 19.55 | 5.21 |
| Median | 7460.50 | 32.86 | 10.00 |
| IQR | 2663.50 | 31.83 | 8.60 |
| Polymer | MP Average Mass Concentration (µg/L) | ||
|---|---|---|---|
| AS | MBR | PRET | |
| Polyvinyl chloride (PVC) | 13.00 | 8.00 | 43.33 |
| Polystyrene (PS) | 0.67 | 1.00 | 10.00 |
| Polyamide (PA) | 0.33 | <LOQ | 6.67 |
| Polyethylene terephthalate (PET) | 1.00 | 0.67 | 6.67 |
| Polypropylene (PP) | 0.33 | <LOQ | <LOQ |
| Polyethylene (PE) | 1.67 | 0.33 | 56.67 |
| Total ∑ polymer) | 17.00 | 10.00 | 123.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, M.R.; Rodríguez, J.R.B. Abundance and Characterization of Anthropogenic Microlitter in Effluent from Three Wastewater Treatment Plants in Gran Canaria (Canary Islands, Spain). Water 2024, 16, 64. https://doi.org/10.3390/w16010064
Sanz MR, Rodríguez JRB. Abundance and Characterization of Anthropogenic Microlitter in Effluent from Three Wastewater Treatment Plants in Gran Canaria (Canary Islands, Spain). Water. 2024; 16(1):64. https://doi.org/10.3390/w16010064
Chicago/Turabian StyleSanz, Marta Rodrigo, and Juana R. Betancort Rodríguez. 2024. "Abundance and Characterization of Anthropogenic Microlitter in Effluent from Three Wastewater Treatment Plants in Gran Canaria (Canary Islands, Spain)" Water 16, no. 1: 64. https://doi.org/10.3390/w16010064
APA StyleSanz, M. R., & Rodríguez, J. R. B. (2024). Abundance and Characterization of Anthropogenic Microlitter in Effluent from Three Wastewater Treatment Plants in Gran Canaria (Canary Islands, Spain). Water, 16(1), 64. https://doi.org/10.3390/w16010064






