Abstract
Agricultural practices have a significant impact on stream water quality in rural landscapes; however, there is still little empirical evidence of how different types of cultivation alter the hydrochemistry of running water. Thus, the current study explored the spatial dynamics of selected ion concentrations and their land cover dependence in lowland agricultural catchments. From November 2021 to October 2022, water samples were collected from 30 sites located across small tributaries of the rivers Bzura, Pilica, and Radomka for chemical analysis of their NO3, NO2, NH4, Ca, Mg, K, Na, As, Ba, Sr, and V concentrations. The results indicated a clear spatial heterogeneity of water quality, related to lithology and dominant land cover evaluated with the CORINE Land Cover 2018 dataset. Overall, sites representing agricultural land promoted increased concentrations of major and trace elements, while those with pepper cultivation were additionally contaminated with NO3 and NO2. The correlation performance for nitrogen compounds was the highest for narrower buffer zones, which was not documented for major and trace elements, which were linked more strongly with land cover at larger scales. Such new insights into the water quality dynamics of lowland agricultural catchments, being a simultaneous reflection of lithology, agricultural practices, and several municipal impacts, have significant implications for appropriate water management in rural landscapes.
Keywords:
water quality; agriculture; pepper; orchards; CORINE Land Cover 2018; catchment; nitrate pollution 1. Introduction
Water pollution due to various human impacts is considered a serious challenge faced by state governments in recent years [1,2]. It was documented that one of the most significant sources of such degradation is area-based water contamination from agricultural practices, such as plant cultivation, livestock production, and rural wastewater inflows [3,4,5]. In terms of agricultural practices, nitrogen pollution was documented to be a crucial problem [6], which, among others, leads to excessive eutrophication of lakes and seas [7,8]. Such a process, rapidly increasing after 1950 [9], is observed in the case of the Baltic Sea [10]. It was documented that more than 1/3 of the total nitrogen input transported from Polish watercourses to the Baltic Sea comes from agriculture, while slightly more than half of the nitrogen transport to the Baltic Sea is moved in the form of nitrates [7]. In consequence, excessive concentrations of phytoplankton have been found, along with oxygen deficiency in the bottom zone of the sea [11,12], causing negative effects and exerting spatially heterogeneous effects on the sea ecosystem [11].
In order to reduce agricultural nitrate pollution, the Nitrates Directive [13] was enacted to oblige EU Member States to designate vulnerable areas and to regularly monitor surface water as well as groundwater in these areas. The effects of its introduction have been generally positive, although largely dependent on the actions of member states. For example, in the Netherlands, following the implementation of the Nitrates Directive, nitrates in shallow groundwater were reduced by more than 50% [14], while for rivers located in Denmark the value was approximately 30% [15]. Unfortunately, studies conducted in other European catchments have not shown a similar decreasing trend; in some regions, such as in Northern Italy, the trend was the opposite [16], so future works are strongly recommended.
Eutrophication of freshwater ecosystems is also driven by other biogenic compounds, such as nitrites, phosphates, and ammonium ions [17]. Such contaminants, apart from inorganic fertilizers and livestock manure, could originate from infiltration from septic tanks [18,19]. According to [20], there were more than 1.8 million such installations in Poland in 2017, which could be related to the still-inappropriate level of sewage-system users, including only 42% of people in rural areas. Agricultural practices also result in additional potassium, calcium, and magnesium release from fertilizers, especially in the presence of non-balanced fertilization, in terms of inadequate component ratios [21] as well as the application of fertilizers at inappropriate times, particularly at the end of autumn or winter [22]. Organic fertilizers in the form of animal manures and biosolids may contain potentially toxic elements, such as arsenic, chromium, lead, mercury, nickel, and vanadium [23,24]. Such contaminants are also broadly found in pesticides [25], used for controlling weeds, bacteria, fungi, and insect infestations [26].
There is a broad spectrum of studies concerning the impact of land use/land cover on stream water quality, which use various analytical, statistical, and methodical approaches [27,28]. In recent years, the application of remote-sensing techniques and datasets can be very useful in such investigations, as some of the parameters can be measured directly from the air with the use of satellites, planes, and unmanned aerial vehicles (UAVs) [29]. Their undoubted advantage is fast, spatially extensive, and often cost-effective water quality assessment; for example, chlorophyll-a concentrations, turbidity, and trace metals can be assessed quite well with the use of hyperspectral data [30,31]. However, the most broad applications of remote-sensing techniques can be found in terms of land cover classifications, which are used for simple comparison of water quality within the catchment’s land use area [32], calculation of land use type contributions in buffer zones [33], and landscape metric computing [34,35]. These investigations have used datasets from authorial classifications of land cover [36], as well as broadly available and free datasets, such as CORINE Land Cover and Sentinel-2 Global Land Cover [37]. Such studies have focused mainly on comparing catchments with different land use types, e.g., forested, agricultural, and urbanized [38,39,40]. In turn, there is little evidence from investigations where different types of cultivations in the catchment area were linked with water quality parameters, which could be identified as an important knowledge gap.
To yield some new findings, this study focused on the hydrochemical characterization of lowland agricultural catchments in central Poland. The specific objectives were to explore the spatial dynamics of selected ion concentrations (a); assess the effects of the type of cultivation on their concentrations (b); and examine the impacts of different spatial scales on the relationships between ion concentrations and land cover (c).
2. Study Area
Investigations were carried out in catchments distributed across the Southern Masovia Hills [41], which could be considered as a lowland landscape with altitudes of approximately 150–200 m a.s.l. Superficial deposits in the study area are dominated by fluvioglacial sands, gravels, and glacial clays from the Riss (Odra and Warta) glaciation, which form ground and terminal moraines, eskers, and kames. In the western parts of the study area, there are locally exposed Jurassic rocks, such as sandstones and chalcedonites [42]. According to the Köppen–Geiger classification, the southern Masovia Hills have a warm-summer humid continental climate (Cfb), characterized by dry winters and humid and warm summers [43]. In consequence, the hydrological regime of streams can be considered nival, with high flows during early spring in March and April and low flows during summer and early autumn in July, August, and September [44]. According to state environmental monitoring data from 2016–2021 [45], the status of the majority of surface water bodies in the study area was qualified as bad, mainly due to excessive polycyclic aromatic hydrocarbon (benzo(a)pyrene, benzo(ghi)perylene) concentrations and some biological elements (mainly benthic invertebrates and fishes).
The land cover of the area in question is generally heterogeneous and specific. In the northeastern part of the region near Grójec, Mogielnica, and Biała Rawska, the dominance of orchards is evident, with mainly apple, sweet cherry, cherry, and pear tree cultivation [46,47]. In turn, the southeastern part around Przytyk and Potworów displays pepper cultivars under foil tunnels [46]. The western part is characterized by the highest contribution of forested area (the Spała Forests), with a domination of Scots pine (Pinus sylvestris L.), common oak (Quercus robur L.), and—especially in river valleys—black alder (Alnus glutinosa (L.) Gaertn.). Rural settlements, such as towns and villages, are relatively small and usually do not exceed 500–1000 inhabitants.
3. Materials and Methods
3.1. Field and Laboratory Investigations
Water quality measurements were conducted in 30 small streams—tributaries of the rivers Bzura (“B” sites), Pilica (“P” sites), and Radomka (“R” sites) (Figure 1 and Figure 2). Site selection used a purposive sampling scheme, based primarily on the land use of the upstream catchment area and its maximum variability in terms of different cultivation. In addition, site localization precluded direct anthropogenic modifications, such as point wastewater discharges, dams, and runoffs from motorways, which are documented to directly impact water quality [48,49,50,51]. It is worth noting that all sampling sites were spatially independent, while their upstream catchment areas ranged from 1.5 to 61.0 km2 (Appendix A).
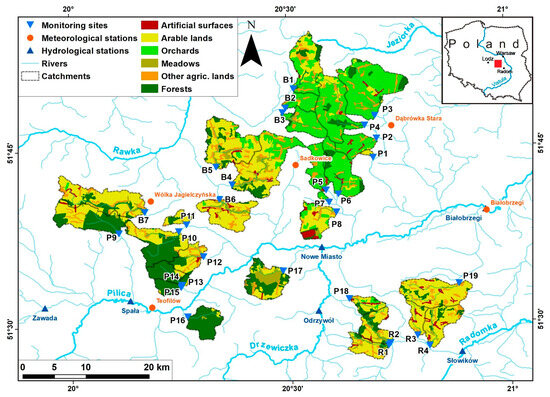
Figure 1.
The localization of investigated sites and catchment land cover. Abbreviations of sites as in Appendix A. Own elaboration based on a digital hydrographic map of Poland and CLC 2018.

Figure 2.
Monitoring sites on the Mogielanka River (a), Eastern Liciążna Stream (b), Cetenka River (c), and Pierzchnianka River (d), as well as examples of the different landscapes with orchards (e) and foil tunnels with pepper cultivation (f).
Measurements were carried out once a month during the hydrological year 2022, from November 2021 to October 2022. Electrical conductivity [µS/cm] was monitored in situ with the use of a portable Hanna Hi 991300 m, with an accuracy of ±2% µS/cm. Water samples were simultaneously collected in 0.35 L polyethylene bottles and immediately transported at 4 °C to the laboratory to conduct the measurements. After the filtration of samples with the use of membrane filters, biogenic compounds (NO3, NO2, NH4, PO4) were determined with the use of an LF 300 photometer and dedicated Testoval reagents (adopting the sulfanilic acid, indophenol blue, and molybdenum blue methods). Quality control of the photometric measurements was conducted using Hach standard buffers; for approximately 10% of random samples, additional control measurements were conducted. The deviations did not exceed 5% in any control samples. Major elements and trace elements (As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, Sr, V, Zn) were determined with a ICP-OES PerkinElmer Optima 5300 DV; during the measurements, appropriate manufacturer standards were used for controlling the relationships between emission intensity and ion concentrations. Due to concentrations being below the detection limit (0.001 mg/L) in the case of most of the trace elements (in more than 50% of samples), only NO3, NO2, NH4, Ca, Mg, K, Na, As, Ba, Sr, and V ions were analyzed in terms of their spatial heterogeneity. In fact, this set of water quality parameters could reflect the effects of lithology (the chemical composition of sedimentary rocks and soils) and varied types of agricultural practices [52,53]. Some of them, such as NH4, Na, and K, can also act as indicators of sewage pollution [54,55].
3.2. Land Cover Evaluation
Land cover in the upstream catchment area of the monitoring sites was assessed on the basis of the CORINE Land Cover 2018 (CLC 2018) product. This vector dataset of 100 m spatial resolution is based predominantly on the visual interpretation of Landsat satellite imagery [56] and is considered as a reference source, as it covers European spatial and temporal variability. It is used in a lot of studies, such as of forestry changes and urban activities [57,58].
For the purposes of the study, selected land cover classes—artificial surfaces (sum of the classes 1.1, 1.2, 1.3, and 1.4), arable lands (Class 2.1.1), fruit trees and berry plantations (henceforth called “orchards”, Class 2.2.2), and forests (all subclasses of Class 3)—were distinguished with the use of vector-processing tools. Peatbogs, marshes, meadows, and water bodies were omitted from the analysis because of their sporadic occurrence in the catchment areas. Although both CLC 2018 classifications distinguish the various types of forests (coniferous, broadleaf, and mixed), they were joined together as one class “forests”. The percentages of land use in the investigated catchments were computed in ArcMap 10.7.1.
3.3. Statistical Analysis
Initially, all sampling sites were objectively divided according to the dominant type of land cover in the upstream catchment area from the CLC 2018 dataset, similar to [59]. The percentage contributions of artificial surfaces (sum of the classes 1.1, 1.2, 1.3, and 1.4), arable lands (Class 2.1.1), orchards (Class 2.2.2), pastures (Class 2.3.1), and forests (all subclasses of Class 3) were a basis for such a separation using hierarchical cluster analysis. For this purpose, the Ward agglomeration method was applied, while the Euclidean distance was set as a measure of the similarity between the land cover of catchments, represented by sampling sites. The optimal number of clusters was separated with the use of the qj index [60]; however, an additional separation of one cluster was made, as the aggregated clusters should be subjected to a logical interpretation based on the author’s intuition and good knowledge of the research objects [61]. Then, the spatial variability of the measured water contaminants was compared across all sampling sites and divided into groups distinguished using the Ward agglomeration method. In this way, the distribution of EC values, as well as those of NO3, NO2, NH4, Ca, Mg, K, Na, As, Ba, Sr, and V concentrations, was documented on standard box-and-whisker charts, presenting mean, median, interquartile range, and outlier values, similar to [59]. To check if there were statistically significant differences (p < 0.05) between the concentrations of measured water quality parameters in the distinguished groups, the Kruskal–Wallis non-parametric test was selected due to it working with non-normally distributed data. Additionally, the post-hoc Dunn test was used for multiple comparisons for such groups. To provide the spatial context of the water contaminants heterogeneity, annual mean concentrations of contaminants calculated from measurement values are presented graphically on the maps using circles with sequential color and scaling.
To evaluate the relationships between ion concentrations, principal component analysis (PCA) was applied, a method broadly used in recent water quality studies [62,63]. The parameters selected for PCA included EC and mean concentrations of NO3, NO2, NH4, Ca, Mg, K, Na, As, Ba, Sr, and V ions. Initially, variables were tested to see if they met the assumptions of factor analysis. The normality of the distributions of all of the variables was checked with the use of the Shapiro–Wilk test, and the results indicated that some of them did not have a normal distribution (p < 0.05). They were transformed using a logarithmic function (Log10) after [64], and their distribution became satisfactory (p > 0.05). In addition, Bartlett’s test of sphericity was significant (p < 0.001), which indicates that variables are related to each other and can be reduced to a smaller number of components, while the Kaiser–Meyer–Olkin test result (0.738) suggested that the sampling was adequate to a middling level [65]. After final standardization with the use of the common z-score formula, PCA was performed with a correlation matrix on the basis of a 12-variable dataset. The Kaiser empirical criterion was chosen to select significant components, so eigenvalues under 1.0 were removed from further interpretation. Component loadings equal to or greater than 0.50 were considered significant correlations, similar to in [66].
Finally, correlation analysis was applied to evaluate the performance of land cover metrics, calculated for several spatial scales, in explaining ion concentration heterogeneity. The Spearman rank correlation coefficient was used for this purpose, which is favorable in the case of a small sample size and the lack of a normal distribution of data, instead of the Pearson correlation coefficient. Correlation analysis was performed to link mean EC values, as well as mean concentrations of NO3, NO2, NH4, Ca, Mg, K, Na, As, Ba, Sr, and V ions and land cover metrics, calculated for the total catchment area and buffer zones of 50, 100, 250, 500, and 1000 m widths, similar to in [53] and [34]. A statistically significant threshold for the correlation was assumed at p = 0.05, while the analysis (together with statistical testing and PCA) was performed using Statistica 13.5 software and is presented in the form of a heat map.
Meteorological background during the investigated period was provided using mean monthly air-temperature values and monthly precipitation sums from the Dąbrówka Stara meteorological station, operated by the Institute of Meteorology and Water Management, National Research Institute (IMWM-NRI). Such a station, although located in the north-eastern area, is the only one across the investigated area where systematic air temperature measurements are conducted. In the case of precipitation, the available reference period covers the years 2003–2021, while for air temperature the reference period is 2007–2021. Hydrological data, used for streamflow variability evaluation, were acquired from IMWM-NRI gauging stations on the rivers Pilica (station Nowe Miasto), Radomka (Rogożek), Drzewiczka (Odrzywół), and Rawka (Kęszyce).
4. Results
4.1. Hydrometeorological Background
The investigated period, covering the hydrological year 2022, was slightly warmer and drier than the reference period 2007–2021 (Figure 3). Mean air temperature on the Dąbrówka Stara meteorological station reached 9.0 °C, which was 0.2 °C higher than the mean value for the multi-year period 2007–2021. Air temperature followed a typical seasonal pattern, with the maximum monthly mean value occurring in August (20.2 °C) and the minimum in December (−1.6 °C). The sum of precipitation reached 596.1 mm, which accounted for 95.7% of the average sum of precipitation calculated for the reference period (622.7 mm). The sums of precipitation varied significantly in certain months; the highest precipitation sum was noted in July at 115 mm, while the lowest was documented in March (1.1 mm). However, the winter and summer half-years were characterized by similar precipitation sums in comparison with the multi-year period, representing 95.1 and 96.1% of the average sum from 2003–2021, respectively. Streamflow patterns observed across hydrological stations located on the Pilica, Radomka, Drzewiczka, and Rawka rivers were similar and reflected meteorological conditions (Figure 3). High streamflow peaks were observed in winter and spring as a result of snowmelt, while, from June to September, low flows appeared due to intensive evapotranspiration. Thus, water quality sampling took place in different hydrometeorological conditions, which makes the obtained results representative.
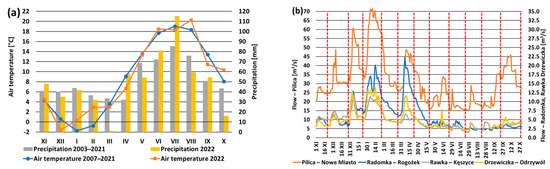
Figure 3.
Mean monthly air temperature and precipitation at the Dąbrówka Stara meteorological station in the years 2007–2021 (a) and mean daily streamflow rate in Nowe Miasto (Pilica River), Rogożek (Radomka River), Kęszyce (Rawka River), and Odrzywół (Drzewiczka River) (b) during the investigated period. Red vertical dashed lines indicate sampling dates (right graph). Based on the data from IMWM-NRI.
4.2. Land Cover Classification
Different contributions of CLC 2018 classes were the basis for site separation in terms of the most similar land cover structures in their upstream catchment areas. The cluster analysis method made it possible to distinguish three main groups of sites (Figure 4). Those with evident domination of orchards (more than 65% contribution to the total area) were linked into the first cluster (O). All sites with more than 50% forests in their upstream catchment areas were grouped into the second cluster (F); the only exception was P17 (the Kiełcznica River), where forested areas accounted for 42.9% while 24.1% of the upstream catchment area was occupied by pastures. Thirdly, the most extensive cluster was distinguished mainly by sites with more than 50% arable land in total in the upstream catchment area. The B1 and B4 sites did not meet these criteria, as the slightly lower contributions of arable lands (35.3% and 49.0%, respectively) were compensated by greater participation of orchards (38.5% and 19.7%, respectively). Nevertheless, due to the specific cultivations in some catchments (Figure 5) (foil tunnels with pepper) expected to influence water quality, arable sites were divided into two smaller groups—arable land (catchments B1, B4–B7, P1, P8, P11, and P12) and pepper sites (P18, P19, and R1–R4).
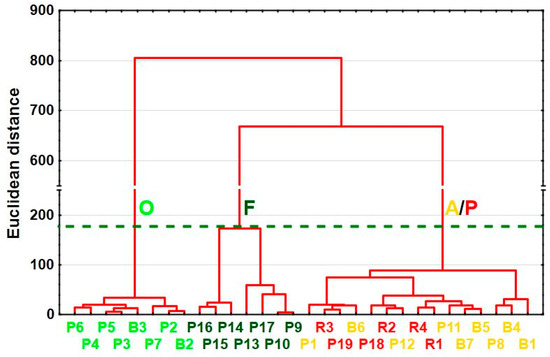
Figure 4.
Clustering results using the Ward method for CLC 2018 classification. Sites, representing different configurations of land use, are distinguished by color. Horizontal green dashed line indicates optimal number of clusters. Abbreviations: O—orchard catchments (light green), F—forested catchments (dark green), A—arable land catchments (yellow), P—pepper catchments (red).
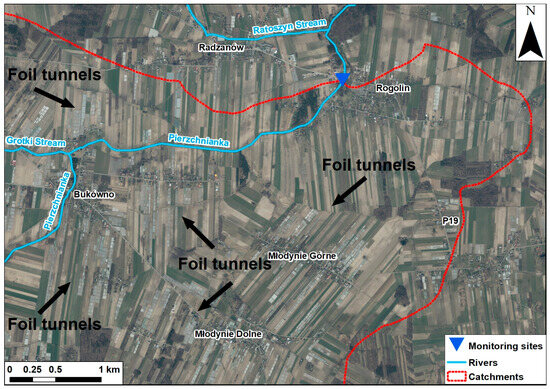
Figure 5.
Catchment represented by the P19 site with intensive pepper cultivation in foil tunnels, visible only on the aerial image. Own elaboration based on the Public Geoportal of Poland.
4.3. Spatial Variability of the Water Quality Parameters
Clear spatial variability in the selected water contaminants was observed in the investigated lowland catchments during the hydrological year 2022, as indicated by statistical analysis of the measurement data. The distribution of the concentrations, divided into agricultural and forest catchments, shows the impact of land cover on the concentrations of selected ions (Figure 6). The most distinctive group was sites representing forested catchments, such as the rivers Gać (P9), Luboczanka (P10), and Olszówka (P14), as well as the Ceteń Stream (P16), where the lowest mean and maximum concentration values were recorded, as well as the lowest EC (Figure 6). This was particularly visible in the case of the NO3 ion, the concentrations of which did not exceed 5 mg/L, as well as for major (Ca, Mg, K, Na) and trace elements (As, Ba, Sr, V).
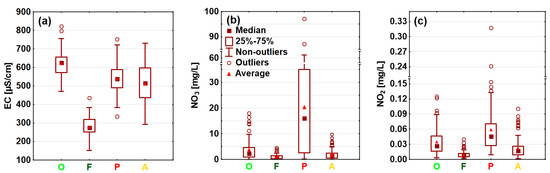
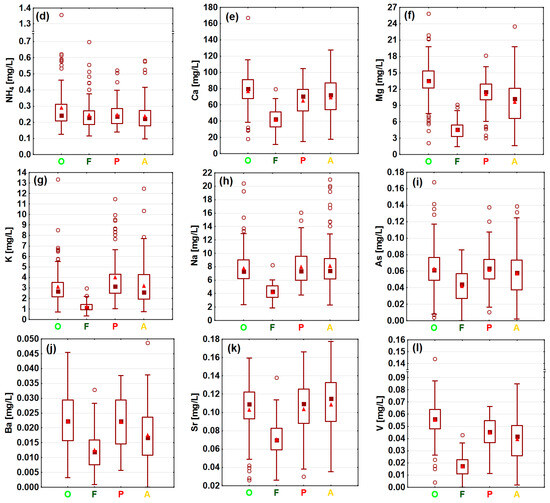
Figure 6.
Measured values and concentrations of (a) EC, (b) NO3, (c) NO2, (d) NH4, (e) Ca, (f) Mg, (g) K, (h) Na, (i) As, (j) Ba, (k) Sr, and (l) V in certain types of sites, grouped by land cover.
Agricultural areas, which represent sites with high participation of orchards, pepper cultivation, and arable land in their upstream catchment areas, exhibited different patterns of ion concentrations (Figure 6). Overall, most of the water quality parameters reached higher values in such sites, as documented by the distribution of measured concentrations, especially of macroelements, which mean concentrations were usually several dozen mg/L higher than in forested sites. Differences between the concentrations in forested and agricultural areas were statistically significant, as indicated by the Kruskal–Wallis test for most of the measured parameters (p < 0.05) (Appendix B). Only NH4 concentrations, which were generally uniform (Figure 6) and did not follow a clear spatial pattern across arable, orchard, pepper, and forested catchments (Figure 7), did not exhibit significant differences (p > 0.05). Within the group of agricultural sites, notably smaller variability in ion concentrations was documented (Figure 6). As highlighted by the post-hoc Dunn statistical test (p > 0.05), arable lands, orchards, and pepper catchments were not significantly differentiated in terms of K, Na, As, or Sr concentrations. However, in the case of EC values and Mg and V concentrations, only arable lands and pepper sites did not vary significantly; in orchard catchments, relatively higher EC values, as well as Mg and V concentrations, were noted, as indicated by the value distribution (Figure 6). In terms of Ba concentrations, only orchard and pepper sites were significantly similar (p > 0.05). In the case of Ca the situation was completely different—only orchard and pepper sites were significantly different. A completely outstanding pattern was found in the case of biogenic compounds, such as NO3 and NO2 ions; these were significantly different across all agricultural lands, as streams draining catchments with pepper cultivation exhibited greatly increased NO3 and NO2 concentrations (Figure 6 and Figure 7). For example, in the Pierzchnianka River and Kozieniec Stream, mean concentrations of a major biogenic compound—NO3—reached 30 mg/L. In contrast, arable lands, represented by sites such as the Krzemionka and Rylka rivers, exhibited the lowest nitrogen contamination of all of the agricultural sites (Figure 6 and Figure 7).
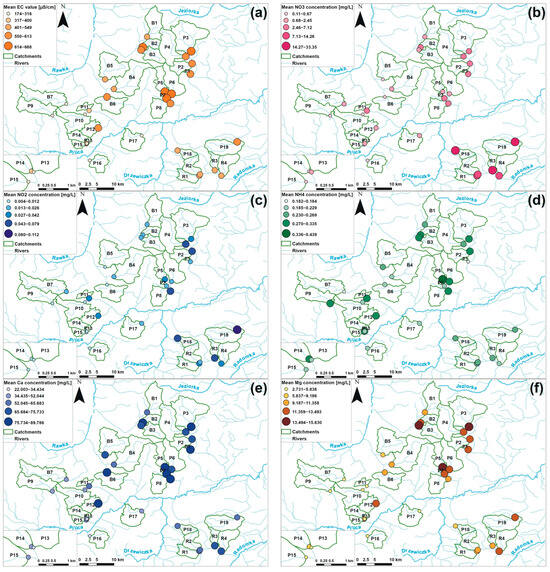
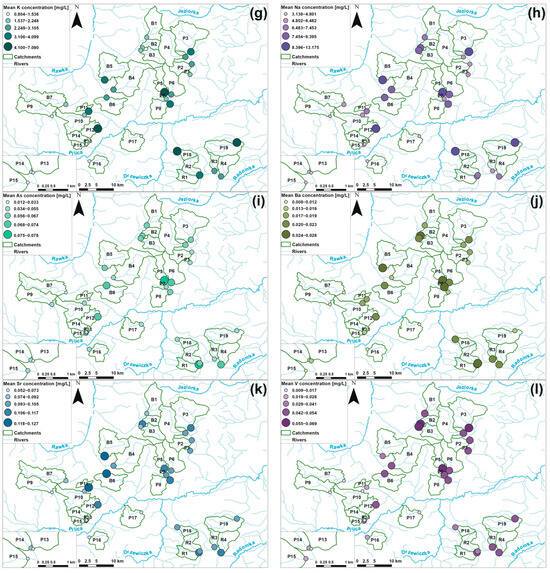
Figure 7.
Spatial variability of the mean values of EC (a) and the concentrations of NO3 (b), NO2 (c), NH4 (d), Ca (e), Mg (f), K (g), Na (h), As (i), Ba (j), Sr (k), and V (l) in the investigated monitoring sites during the hydrological year 2022.
Additional insights into the relationships between certain water quality parameters, as well as into their spatial heterogeneity, were provided by PCA. The spatial variability of ion concentrations across streams was represented by two independent components (factors), the cumulative explained variance of which was 74.1% (Table 1). The first principal component (PC1) explained 62.8% of the variability in the dataset. As indicated by the loadings of the first principal component (PC1), EC, Ca, Mg, K, Na, As, Ba, Sr, and V were negatively correlated on a very high to moderate level (Figure 8a). The second principal component (PC2) explained 13.4% of the variability in the dataset and was associated with biogenic compounds, such as NO3 and NO2 (Figure 8a). It was related negatively to NO3 and NO2 (Cload = −0.83 and −0.73, respectively). Projection of cases on the factor plane defined by PC1 and PC2 indicated a clear distinction between sampling sites, representing differences in the dominant land cover of the upstream catchment areas (Figure 8b). The first PC exhibited a transition from agricultural to forested sites, reflected by decreasing concentrations of major and trace elements and, in consequence, a decrease in EC. The second PC was primarily linked to NO3 and NO2 concentrations, which increased from forest catchments to reach their maximum values in the case of pepper sites, particularly the Kozieniec Stream (P18) and the Pierzchnianka River (P19). It is worth noting that while the pepper and forest catchments were grouped relatively closely, the catchments representing arable land had a greater spread of points on the projection plane, which indicate that the highest variability in ion concentrations was inside this group (Figure 8b).

Table 1.
Component loadings (Cload), eigenvalues, and explained variance obtained in the PCA analysis.
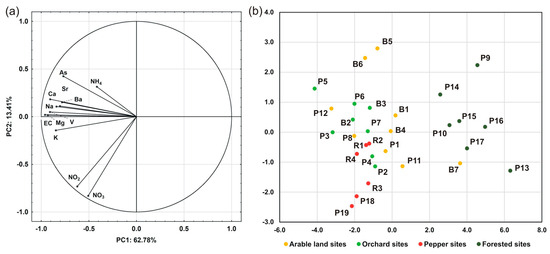
Figure 8.
Component loadings associated with all measured water quality parameters (a) and a projection of certain cases (sites) on the factor plane (b) in the PCA analysis. Note the distinction of sites in terms of their dominant land cover.
4.4. Relationships between Land Use and Water Quality
The overall pattern of Spearman correlation coefficients, characterizing the relationships between the concentrations of selected ions and land cover from the CLC 2018 dataset in the upstream catchment areas representing sampling sites, is presented in Table 2.

Table 2.
Spearman rank correlation coefficients linking land cover metrics from CLC 2018 and the mean concentrations of the measured water quality parameters in lowland streams during the study period. Abbreviations: 50, 100, 250, 500, and 1000—buffer zone widths in meters, TCA—total catchment area. Bold type indicates a statistically significant correlation at p = 0.05.
Generally, the presence of artificial surfaces in upstream catchment areas was positively related to concentrations of NO3, NO2, K, Na, and Sr at all spatial scales, with the strongest relationships found for K (0.75). Furthermore, significant relationships (p < 0.05) were calculated at some spatial scales for Ca, Mg, As, and Ba, but with notably lower performance. Only the concentrations of vanadium were not linked with artificial surfaces in upstream catchment areas. A similar pattern was exhibited by the arable land class, but for this land cover type a slightly lower number of statistically significant correlations was noted (Table 2). Arable lands clearly promoted increased mean NO3 and NO2 concentrations at the sampling sites, as indicated by positive, statistically significant correlation values at all spatial scales (p < 0.05). An analogous effect was observed in the case of K and Sr concentrations; however, the relationships were weaker and appeared only for selected buffer zones. The impact of arable lands on the concentrations of other major and trace elements was not documented (Table 2). Finally, positive, significant relationships with ion concentrations were also found in the case of orchards; the presence of this type of cultivation in buffer zones and upstream catchment areas was related to major elements (Ca, Mg, and Na) as well as trace elements (Ba and V). For biogenic compounds, the relationships, even if positive, were not significant. A notably different pattern was found for forested areas (both coniferous and deciduous), the effect of which was usually negative and led to a decrease in their concentrations (Table 2). Statistically significant correlations (p < 0.05) were documented for all measured ions with the exception of NH4; the best performance was calculated for Ca, K, and Sr ions.
The performance of the correlation analysis was also dependent on the spatial scale, e.g., the width of the buffer zone. Overall, in the case of biogenic compounds, such as NO3 and NO2, slightly better performance was documented for narrower buffer zones of 50, 100, and 250 m widths, both for arable lands and forests. The influence of artificial surfaces did not exhibit such a pattern. The correlation values were the highest for the 1000-m-wide buffer zones; however, the differences in performance were generally insignificant (Table 2). The opposite pattern was observed for other contaminants, such as major and trace elements. For Ca, Mg, K, and Na, as well as for As, Ba, Sr, and V, the correlation performance usually increased with the width of the buffer zone, reaching its maximum values at 500 or 1000 m scales. In the case of NH4 ions, there was no spatial tendency, as none of the land cover metrics were linked significantly with their concentration (Table 2).
5. Discussion
Spatial variability in selected macro and trace elements was explored in streams representing lowland agricultural catchments with similar hydrological and climatological properties. Although numerous studies characterizing the impact of land cover on water quality have recently appeared [67,68], the novelty of the present investigation is the inclusion of specific types of agricultural practices, such as fruit tree plantations and pepper cultivations, creating unique landscapes in rural areas.
5.1. Spatial Variability Evaluation
As expected, the type of dominant land cover in upstream catchment areas has a significant impact on water quality at monitoring sites over the year. Overall, sites representing agricultural catchments promote higher concentrations of measured ions in comparison with forested ones, which was previously reported in the literature [67,69]. Cultivated lands in the area in question were generally developed on sediments and soils naturally enriched in Ca and Mg, such as clays and postglacial tills [70]; in those deposits, As, Sr, and V concentrations are also higher than on fluvioglacial sands and sandstones [71,72,73], where forested areas dominate. However, apart from the chemical compositions of sedimentary rocks across the catchment areas, increased concentrations of major and trace elements across agricultural sites could also be related to agricultural activity, such as intensive fertilization, liming, and pesticides [74,75,76,77,78]; the last was particularly evidenced in orchard catchments, in terms of the highest As and V concentrations, where a lot of pesticides are applied to control numerous insects, fungi, and diseases [79]. In addition, trace elements, such as As and V, could be components of commercial phosphate fertilizers [23,73]. This overall pattern was revealed by PCA; all agricultural sites were spread relatively closely on the first principal component, which, similarly to in [80], could be related to lithology (chemical composition of sediments) and anthropogenic activity. The last could be seen, especially, within sites representing intensive fruit tree and pepper cultivation, which exhibited similar, increased Ba concentrations; this element was previously expected to be an indicator of such nonpoint agricultural sources of ions [81]. In consequence, the cooperation and mutual interpenetration of geological (lithology) and anthropogenic factors influencing water hydrochemistry is present in such agricultural catchments.
A specific, interesting pattern of ion concentrations was exhibited by sites representing pepper cultivation in foil tunnels, which was highlighted mainly by a second PC, related to the inputs of nitrogen fertilizers. Sites with such cultivation promoted increased concentrations of NO3, which, during the cold half-year, regularly exceeded 30–40 mg/L, while maximum values even reached 97.1 mg/L at site P18 (Kozieniec Stream). Documented nitrate contamination is also relatively high in comparison with concentrations presented by [82,83] for other agricultural regions of Poland. The elevated concentrations of NO3 in the current study should be strictly related to the intensive fertilization of pepper cultivation. According to [84,85], the demand of peppers is approximately 200–300 kg/ha of N; in contrast, fruit trees in full fruiting require only around 50 kg/ha of N [84,86]. Moreover, the inadequate ratio of nitrogen to potassium in fertilizers is expected to be a second reason for contamination; the appropriate ratio on agricultural lands should be between 1:1.2 and 1:1.5 [21], but in the case of the pepper areas the ratio of nitrogen to potassium was estimated at 1:0.4–1:0.7. This could result in limited potential for nitrogen uptake by plants and, consequently, leaching of nitrates from the soil to groundwater and streams. In contrast to the sites representing pepper cultivation, those draining arable lands were the most variable in terms of impact on water quality. In fact, arable lands could be managed differently in terms of fertilization and pesticide use according to the type of cultivation (e.g., wheat, barley, corn, vegetables) and its specific requirements [84].
The opposite impact was found in the case of forested areas, which were characterized by lower concentrations of all measured ions at the annual timescale, varying significantly from other types of catchments. Generally, it was confirmed that woodlands, both coniferous and deciduous, could accelerate purification effects on stream water quality, as reported previously by [87,88]. Forests provide an accumulation of nitrogen, phosphorus, and metallic elements [89], mainly due to tree uptake [90] and the presence of litter layer- and organic-rich soils, supporting complex and differentiated soil microbial communities [91]. However, it must be emphasized that several factors influence the purification effects of forests, such as management practices [92], tree species composition [93], and the spatial configuration of woodlands [94], which could be also a reason behind the relatively high variability of forested sites in terms of water quality.
Finally, a clear spatial tendency was not documented for NH4, the concentrations of which were not significantly different despite differences in land cover in upstream catchment areas; an analogous pattern was previously documented by [95] using the example of springs in western Germany. In fact, NH4 concentrations reflect contamination from various forms of human activity across the investigated catchments, such as inflows from sewage discharges, livestock production, the fruit-processing industry, and landfills [96]. Thus, the presence of rural and urban settlements contributes to increased NH4 concentrations [35,97], which could be accelerated by the lack of a sewage network in such areas [20]. The slightly lower factor loading for K concentrations in the PCA suggests that this ion could also reflect sewage contamination. Potassium, considered the “neglected nutrient” [98], is broadly used in fertilizers; however, it has a different migration path in comparison with nitrates, as K is washed less deep into the soil profile, because it is better absorbed by clay minerals [98]. In this way, the case of the Bystrzyca River [99] suggests that the K concentration resulted from pollution with domestic sewage rather than from the use of fertilizers, which might be exhibited in the investigated catchments.
5.2. The Role of Spatial Scale for Water Quality
Studies focusing on land cover effects on water quality have been conducted at several spatial scales, mainly to evaluate different predictors of water contaminants for management purposes [94]. Catchment scale [100], buffer zones [101], circular buffers [28], and distance-weighted approaches [27] have been adopted in numerous studies, which have modeled the spatial variability of biogenic compounds, oxygen indices, and trace metals [53,94]. There are no unified conclusions about the most informative spatial scale, as in some studies the whole catchment is the key to understanding some of the water quality parameters [102,103]. There is also broad evidence that land use in the close proximity of streams may be the most important in terms of explaining water quality [37,104]. As suggested previously, the comparison of such results is difficult due to the heterogeneous land cover datasets used, different periods of interest, and factors such as topography and relief [33,105]. Nevertheless, in the current study, some interesting tendencies and patterns were noted on the basis of the CLC 2018 dataset. Generally, in the case of major (Ca, Mg, K, Na) and trace elements (As, Ba, Sr, and V), the best performance in explaining spatial variability was noted for wider buffer zones, particularly those 500 m and 1000 m wide. In turn, NO3 and NO2 concentrations were slightly better explained for narrower buffer zones (50, 100, and 250 m wide); however, higher correlation performance was also documented for artificial areas in the case of wider buffer zones. This corresponds with PCA and suggests that the concentrations of major elements and trace metals have lithological and anthropogenic origins, facilitated by a larger absolute area of orchards, forests, and artificial areas in catchments. In turn, the better correlation performance for nitrogen compounds in narrower buffer zones could be related to the high mobility of nitrate ions [106] and, simultaneously, their preferable uptake by vegetation even at scales of dozens of meters [107]. Additionally, buffer zones with natural vegetation in sites representing pepper cultivation are generally narrower in comparison with orchard areas. This is due to the unsuitability of wetlands for the cultivation of fruit trees [108], as well as the lack of cattle farming [109] in orchard-dominated catchments. Thus, buffer zones in pepper catchments do not provide an appropriate geochemical barrier, in contrast to orchards, where forests or agriculturally unused grasslands hinder the migration of nitrates into streams. Finally, it is worth noting that a clear distinction in predictors was observed for major and trace elements, suggesting their dominant sources. Orchards significantly increased Ca, Mg, Ba, and V concentrations, while artificial surfaces were better correlated with K and Na, confirming that such ions may be associated with urbanization and domestic activity. Different patternwas documented for NH4, being a primary indicator of stream pollution [110] with no statistically significant relationship with land cover type. It must be emphasized that the impact of various land cover types on water quality is differentiated seasonally, which is broadly documented to mainly be the result of hydrometeorological conditions and vegetation cycles [111].
5.3. Recommendations and Limitations
Water quality evaluation seems to remain an important research issue, as the enrichment of lotic ecosystems in nutrients can lead to eutrophication and limited water suitability for drinking purposes [112]. Simultaneous with low flows and increased water temperature, nutrient enrichment can lead to unexpected ecological disasters, such as in the case of the Odra River in 2022, where toxins released by the haptophyte golden algae, Prymnesium parvum, resulted in mass mortality among fish, bivalves, and water snails [113]. Thus, such water quality studies are particularly significant in the initial parts of stream systems, which are greatly vulnerable in terms of hydrochemical alteration and therefore crucial in appropriate water management [114].
The current study revealed significant stream pollution by nitrates in catchments with intensive pepper cultivation. However, an analogous situation might also be observed in similar catchments with the cultivation of vegetables, which have relatively low nutrient use efficiency compared with arable crops [115]. Our recommendation to reduce nitrate pollution in such areas might be to build denitrification barriers to absorb leached nitrogen compounds. Such a barrier is an organic layer (containing carbon) mixed with soil, which achieves the reduction of nitrate to gaseous nitrogen by denitrifying bacteria with limited oxygen and abundant organic carbon [116]. In many cases, the reduction in nitrate concentration using barriers is over 50%, and studies by [117,118] show that this method can also be useful for the removal of pollutants from domestic wastewater. Another possibility is the use of highly effective buffer zones, which are strips of grass or woodlands between arable land and streams. [119] found that the application of such ‘saturated buffers’ using plants such as ryegrass, fescue, bluegrass, and timothy allowed nitrate–nitrogen concentrations to be reduced from 15 mg N-NO3/L to less than 1.5 mg N-NO3/L. The high effectiveness of such zones was also noted in a study by [120], in which nitrate concentrations exceeding 100 mg/L were reduced by 99% with a zone width of 45 m. Finally, an interesting pattern to reduce the nitrate contamination of streams could be the use of crop rotation with leguminous plants, which are known for their major role in nutrient capture in soils [121]. It was proven that crop rotation with leguminous plants generally produces lower nitrogen runoff to groundwater and further streams than monocultures [122,123]. Such solutions require careful overall consideration and attention by managers on a local scale, as agriculture nonpoint sources remain a significant problem in such areas. It is worth noting that the investigated period (hydrological year 2022) was extremely unstable in Europe, mainly due to the ongoing EU–Russia gas war, as well as EU embargos on fertilizers from Belarus and Russia, which resulted in significant increases in fertilizer prices [124]. This in consequence could have led to a decrease in fertilizer use and thus slightly less nitrogen pollution than during previous years. Economic motives have been a catalyst for reducing nitrate pollution, as evidenced previously across post-socialist countries in the 1990s, e.g., in Poland [125], Slovakia [126], and the Czech Republic [127].
Finally, the obtained results also have practical significance in terms of future investigations and approaches related to land cover–water quality interaction.Land use/land cover data derived from aerial or satellite data sources should take into account different types of agricultural practices due to their different impacts, as was confirmed for the example of orchards, pepper, and arable lands sites. Many studies have separated out only the class of agricultural land/crops [39,53,62], which is, by its nature, a great simplification if the cultivated areas represent significantly different crops in terms of nutrient demand and agricultural practices.
Author Contributions
Conceptualization, K.S. and M.Ł.; methodology, K.S. and M.Ł.; software, K.S. and M.Ł.; validation, K.S. and M.Ł.; formal analysis, K.S. and M.Ł.; investigation, K.S., M.K. and M.Ł.; resources, K.S., M.K. and M.Ł.; data curation, K.S. and M.Ł.; writing—original draft preparation, K.S. and M.Ł.; writing—review and editing, K.S. and M.Ł.; visualization, K.S. and M.Ł.; supervision, K.S. and M.Ł.; project administration, M.Ł.; funding acquisition, M.Ł. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the University of Warsaw, grant number BOB-661-453/2021, and by the Faculty of Geography and Regional Studies, grant number SWIB 53/2021 and SWIB 90/2023.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to express their gratitude to Wiktoria Malinowska and Katarzyna Sosnowska for the laboratory assistance. The authors also thank the two anonymous reviewers for their constructive comments.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Detailed characteristics of catchments in terms of selected land cover types’ contributions. Abbreviations: A—catchment area [km2], AS—artificial surfaces [%], AL—arable lands [%], O—orchards [%], M—meadows [%], TAL—total agricultural lands [%], F—forests [%].
Table A1.
Detailed characteristics of catchments in terms of selected land cover types’ contributions. Abbreviations: A—catchment area [km2], AS—artificial surfaces [%], AL—arable lands [%], O—orchards [%], M—meadows [%], TAL—total agricultural lands [%], F—forests [%].
| Site | River (Alternative Name in Brackets) | Surface Water Body (Name and European Code) | Geographical Coordinates (WGS 84) | A [km2] | Land Use/Land Cover Type Share—Data from CLC 2018 [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AS | AL | O | M | TAL | F | |||||
| B1 | Białka | Białka PLRW200010272669 | 51.834625 N 20.514992 E | 34.9 | 0.5 | 35.3 | 38.5 | 0 | 85.8 | 13.6 |
| B2 | Stanisławów Stream | 51.807655 N 20.495808 E | 12.1 | 0 | 2.2 | 83.8 | 0 | 97.7 | 2.3 | |
| B3 | Chodnów Stream | 51.801074 N 20.488062 E | 20.2 | 0 | 2.9 | 77.5 | 7 | 96.1 | 3.9 | |
| B4 | Rylka | Rylka PLRW200010272649 | 51.700008 N 20.370354 E | 57.2 | 1.3 | 49 | 19.7 | 9.8 | 89.2 | 9.5 |
| B5 | Regnów Stream (Ossowice-Regnów Canal) | 51.725096 N 20.333412 E | 47.7 | 1.2 | 59 | 7.9 | 4.9 | 84.7 | 14.1 | |
| B6 | Strzałki Stream (Grabice Canal) | 51.679680 N 20.340846 E | 30.5 | 5.3 | 71.2 | 0.9 | 12.8 | 93.6 | 1.1 | |
| B7 | Krzemionka | Rawka to Krzemionka PLRW2000102726199 | 51.663103 N 20.168514 E | 45 | 1.9 | 65.7 | 0.9 | 2.8 | 80.8 | 17.3 |
| P1 | Dańków Stream | Mogielanka PLRW200010254929 | 51.735027 N 20.694095 E | 1.5 | 0 | 77.3 | 3.8 | 0 | 88.8 | 11.2 |
| P2 | Huta Błędowska Stream | 51.762066 N 20.702355 E | 16.4 | 5.5 | 2 | 84.6 | 0 | 94.5 | 0 | |
| P3 | Ciechlin Stream (Machnatka) | 51.794843 N 20.700564 E | 61 | 1.3 | 3.8 | 76.7 | 1.6 | 90.5 | 8.2 | |
| P4 | Mogielanka | 51.781233 N 20.676230 E | 44.5 | 0.6 | 10.9 | 67.5 | 2.6 | 90.1 | 9.3 | |
| P5 | Trębaczew Stream | Gostomka PLRW2000102549149 | 51.690339 N 20.584836 E | 17.4 | 2.9 | 0 | 78.1 | 1.9 | 90.9 | 6.2 |
| P6 | Gostomka (Żelazna) | 51.684113 N 20.612259 E | 33.5 | 1.4 | 2.9 | 73.7 | 0 | 84 | 14.6 | |
| P7 | Władysławów Stream | 51.673083 N 20.591273 E | 1.7 | 0 | 15.5 | 84.5 | 0 | 100 | 0 | |
| P8 | Rosocha Stream | 51.658828 N 20.607247 E | 23.4 | 13.9 | 50.5 | 19 | 2.6 | 80.8 | 5.3 | |
| P9 | Gać | Gać PLRW200010254729 | 51.632941 N 20.109243 E | 36.7 | 1.3 | 38.2 | 0 | 0.7 | 43.5 | 55.2 |
| P10 | Luboczanka | Luboczanka (Lubocz) PLRW200010254769 | 51.634699 N 20.246401 E | 36.5 | 0.2 | 37.7 | 0.2 | 1.2 | 44.3 | 55.5 |
| P11 | Kanice Nowe Stream | 51.644287 N 20.264033 E | 6.8 | 0.4 | 72.7 | 0 | 0 | 78.9 | 20.7 | |
| P12 | Rzeczyca | 51.598556 N 20.301362 E | 16.7 | 2.4 | 54.1 | 0 | 5.9 | 76.3 | 21.3 | |
| P13 | Liciążna Stream (E) | Olszówka PLRW2000102547569 | 51.558735 N 20.252704 E | 1.8 | 0 | 19.5 | 0 | 0 | 19.5 | 80.5 |
| P14 | Olszówka (Struga) | 51.558061 N 20.251921 E | 13.5 | 0 | 8.2 | 0 | 0 | 11.6 | 88.4 | |
| P15 | Liciążna Stream (W) | 51.555406 N 20.250376 E | 7.3 | 0 | 2 | 0 | 0 | 5.1 | 94.9 | |
| P16 | Ceteń Stream (Cetenka) | Ceteń Stream PLRW2000102547529 | 51.513607 N 20.263300 E | 19.8 | 0 | 3.4 | 0 | 1.4 | 4.8 | 95.2 |
| P17 | Kiełcznica | Kiełcznica PLRW200015254792 | 51.576481 N 20.484243 E | 30.2 | 0.7 | 26.3 | 0 | 24.1 | 56.4 | 42.9 |
| P18 | Kozieniec Stream | Kozieniec Stream PLRW200010254889 | 51.535054 N 20.633020 E | 10.5 | 7.9 | 52.1 | 12.8 | 4.8 | 72.2 | 19.9 |
| P19 | Pierzchnianka | Pierzchnianka PLRW200010254949 | 51.554206 N 20.884437 E | 39.4 | 4.3 | 74.5 | 0 | 0.9 | 88 | 7.7 |
| R1 | Przystałowice Duże Stream | Wiązownica PLRW200010252499 | 51.468265 N 20.721298 E | 15.2 | 2.7 | 58.5 | 0 | 16.1 | 87.8 | 9.5 |
| R2 | Potworów Stream | 51.471326 N 20.724903 E | 19.6 | 3.3 | 51.6 | 0 | 12.6 | 73.7 | 23 | |
| R3 | Grabowska Wola Stream | 51.481252 N 20.787154 E | 10.4 | 8.9 | 80.3 | 0 | 3.2 | 88 | 3.1 | |
| R4 | Wrzeszczów Stream | 51.466403 N 20.814512 E | 24.4 | 5.9 | 65.8 | 0 | 8.2 | 81.8 | 12.3 | |
Appendix B

Table A2.
Detailed Kruskal–Wallis test statistics and Dunn post-hoc multiple comparisons between types of agricultural cultivation. Green fields indicate statistically significant differences at p < 0.05. Abbreviations: O—orchard sites, F—forested sites, P—pepper sites, and A—arable land sites.
Table A2.
Detailed Kruskal–Wallis test statistics and Dunn post-hoc multiple comparisons between types of agricultural cultivation. Green fields indicate statistically significant differences at p < 0.05. Abbreviations: O—orchard sites, F—forested sites, P—pepper sites, and A—arable land sites.
| Parameter | H | Χ2 | df | Median | p-Level | Catchment Groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | F | P | A | |||||||
| EC | 223.92 | 165.06 | 3 | 531.50 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| NO3 | 115.56 | 76.45 | 3 | 1.62 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| NO2 | 141.09 | 98.89 | 3 | 0.02 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| NH4 | 9.75 | 6.82 | 3 | 0.23 | 0.078 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| Ca | 107.98 | 107.23 | 3 | 66.85 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| Mg | 183.50 | 134.81 | 3 | 10.40 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| K | 157.00 | 113.40 | 3 | 2.38 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| Na | 129.93 | 105.31 | 3 | 6.68 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| As | 36.27 | 26.55 | 3 | 0.06 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| Ba | 64.27 | 49.62 | 3 | 0.02 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| Sr | 83.05 | 81.08 | 3 | 0.10 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
| V | 167.57 | 124.63 | 3 | 0.04 | 0.00 | O | ||||
| F | ||||||||||
| P | ||||||||||
| A | ||||||||||
References
- Afroz, R.; Masud, M.M.; Akhtar, R.; Duasa, J.B. Water pollution: Challenges and future direction for water resource management policies in Malaysia. Environ. Urban. ASIA 2014, 5, 63–81. [Google Scholar] [CrossRef]
- Han, D.; Currell, M.; Cao, G. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Campling, P.; Joris, I.; Calliera, M.; Capri, E.; Marchis, A.; Kuczynska, A.; Vereijken, T.; Majewska, Z.; Belmans, E.; Borremans, L.; et al. A multiactor, participatory approach to identify policy and technical barriers to better farming practicies that protect our drinking wáter sources. Sci. Total Environ. 2021, 755, 142971. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A. Sources and Leaching of Nitrate Contamination in Groundwater. Curr. Sci. 2020, 118, 883–891. [Google Scholar] [CrossRef]
- Preisner, M.; Smol, M.; Szołdrowska, D. Trends, insights and effects of the Urban Wastewater Treatment Directive (91/271/EEC) implementation in the light of the Polish coastal zone eutrophication. Environ. Manag. 2021, 67, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S.; Kelly, M.G.; Várbíró, G.; Borics, G.; Erős, T.; Hellsten, S.; Kolada, A.; Lukács, B.A.; Solheim, A.L.; López, J.P.; et al. Estimating nutrient thresholds for eutrophication management: Novel insights from understudied lake types. Sci. Total Environ. 2022, 827, 154242. [Google Scholar] [CrossRef]
- Andersen, J.H.; Carstensen, J.; Conley, D.J.; Dromph, K.; Fleming-Lehtinen, V.; Gustafsson, B.G.; Josefson, A.B.; Norkko, A.; Villnäs, A.; Murray, C. Long-Term Temporal and Spatial Trends in Eutrophication Status of the Baltic Sea. Biol. Rev. 2017, 92, 135–149. [Google Scholar] [CrossRef]
- Murray, C.J.; Muller-Karulis, B.; Carstensen, J.; Conley, D.J.; Gustafsson, B.G.; Andersen, J.H. Past, Present and Future Eutrophication Status of the Baltic Sea. Front. Mar. Sci. 2019, 6, 2. [Google Scholar] [CrossRef]
- Rönnberg, C.; Bonsdorff, E. Baltic Sea eutrophication: Area-specific ecological consequences. Hydrobiologia 2004, 514, 227–241. [Google Scholar] [CrossRef]
- Savchuk, O.P. Large-Scale Nutrient Dynamics in the Baltic Sea, 1970–2016. Front. Mar. Sci. 2018, 5, 5. [Google Scholar] [CrossRef]
- EUR-Lex Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Off. J. Eur. Communities 1991, 375, 1–8. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31991L0676:EN:HTML (accessed on 19 November 2023).
- Van Grinsven, H.J.M.; Tiktak, A.; Rougoor, C.W. Evaluation of the Dutch implementation of the nitrates directive, the water framework directive and the national emission ceilings directive. NJAS-Wagening. J. Life Sci. 2016, 78, 69–84. [Google Scholar] [CrossRef]
- Smith, R.J.N.; Glegg, G.A.; Parkinson, R.; Richards, J.P. Evaluating the implementation of the Nitrates Directive in Denmark and England using an actor-orientated approach. Eur. Environ. 2007, 17, 124–144. [Google Scholar] [CrossRef]
- Musacchio, A.; Re, V.; Mas-Pla, J.; Sacchi, E. EU Nitrates Directive, from theory to practice: Environmental effectiveness and influence of regional governance on its performance. Ambio 2020, 49, 504–516. [Google Scholar] [CrossRef]
- Soro, M.-P.; N’goran, K.M.; Ouattara, A.A.; Yao, K.M.; Kouassi, N.L.B.; Diaco, T. Nitrogen and phosphorus spatio-temporal distribution and fluxes intensifying eutrophication in three tropical rivers of Côte d’Ivoire (West Africa). Mar. Pollut. Bull. 2023, 186, 114391. [Google Scholar] [CrossRef]
- Richards, S.; Paterson, E.; Withers, P.J.; Stutter, M. Septic tank discharges as multi-pollutant hotspots in catchments. Sci. Total Environ. 2016, 542, 854–863. [Google Scholar] [CrossRef]
- Pouye, A.; Cissé Faye, S.; Diédhiou, M.; Gaye, C.B.; Taylor, R.G. Nitrate contamination of urban groundwater and heavy rainfall: Observations from Dakar, Senegal. Vadose Zone J. 2023, 22, e20239. [Google Scholar] [CrossRef]
- Piasecki, A. Water and Sewage Management Issues in Rural Poland. Water 2019, 11, 625. [Google Scholar] [CrossRef]
- Ławniczak, A.E.; Zbierska, J.; Nowak, B.; Achtenberg, K.; Grześkowiak, A.; Kanas, K. Impact of agriculture and land use on nitrate contamination in groundwater and running waters in central-west Poland. Environ. Monit. Assess. 2016, 188, 172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Hatano, R.; Zhao, Y.; Woli, K.P.; Kuramochi, K.; Shimizu, M.; Hayakawa, A. Factors controlling nitrogen and dissolved organic carbon exports across timescales in two watersheds with different land uses. Hydrol. Process. 2014, 28, 5105–5121. [Google Scholar] [CrossRef]
- Mortvedt, J.J. Heavy metal contaminants in inorganic and organic fertilizers. Fert. Res. 1995, 43, 55–61. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ. Pollut. 1996, 92, 19–25. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Staponites, L.R.; Barták, V.; Bílý, M.; Simon, O.P. Performance of landscape composition metrics for predicting water quality in headwater catchments. Sci. Rep. 2019, 9, 14405. [Google Scholar] [CrossRef]
- Gelsey, K.; Chang, H.; Ramirez, D. Effects of landscape characteristics, anthropogenic factors, and seasonality on water quality in Portland, Oregon. Environ. Monit. Assess. 2023, 195, 219. [Google Scholar] [CrossRef]
- Yang, H.; Kong, J.; Hu, H.; Du, Y.; Gao, M.; Chen, F. A Review of Remote Sensing for Water Quality Retrieval: Progress and Challenges. Remote Sens. 2022, 14, 1770. [Google Scholar] [CrossRef]
- Hajigholizadeh, M.; Moncada, A.; Kent, S.; Melesse, A.M. Land–Lake Linkage and Remote Sensing Application in Water Quality Monitoring in Lake Okeechobee, Florida, USA. Land 2021, 10, 147. [Google Scholar] [CrossRef]
- Niu, C.; Tan, K.; Jia, X.; Wang, X. Deep learning based regression for optically inactive inland water quality parameter esti-mation using airborne hyperspectral imagery. Environ. Pollut. 2021, 286, 117534. [Google Scholar] [CrossRef]
- Matysik, M.; Absalon, D.; Ruman, M. Surface water quality in relation to land cover in agricultural catchments (Liswarta river basin case study). Pol. J. Environ. Stud. 2015, 24, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wagner, P.D.; Fohrer, N. Effects of land cover, topography, and soil on stream water quality at multiple spatial and seasonal scales in a German lowland catchment. Ecol. Indic. 2021, 120, 106940. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Zhong, J.; Li, C. Spatial Scale Effects of the Variable Relationships between Landscape Pattern and Water Quality: Example from an Agricultural Karst River Basin, Southwestern China. Agric. Ecosyst. Environ. 2020, 300, 106999. [Google Scholar] [CrossRef]
- Pak, H.; Chuah, C.; Yong, E.; Snyder, S. Effects of land use configuration, seasonality and point source on water quality in a tropical watershed: A case study of the Johor River Basin. Sci. Total Environ. 2021, 780, 146661. [Google Scholar] [CrossRef] [PubMed]
- Tahiru, A.A.; Doke, D.A.; Baatuuwie, B.N. Effect of land use and land cover changes on water quality in the Nawuni Catchment of the White Volta Basin, Northern Region, Ghana. Appl. Water Sci. 2020, 10, 198. [Google Scholar] [CrossRef]
- Łaszewski, M.; Fedorczyk, M.; Gołaszewska, S.; Kieliszek, Z.; Maciejewska, P.; Miksa, J.; Zacharkiewicz, W. Land Cover Effects on Selected Nutrient Compounds in Small Lowland Agricultural Catchments. Land 2021, 10, 182. [Google Scholar] [CrossRef]
- Poor, C.J.; McDonnell, J.J. The effects of land use on stream nitrate dynamics. J. Hydrol. 2007, 332, 54–68. [Google Scholar] [CrossRef]
- Żelazny, M.; Siwek, J. Determinants of Seasonal Changes in Streamwater Chemistry in Small Catchments with Different Land Use: Case Study from Poland’s Carpathian Foothills. Pol. J. Environ. Stud. 2012, 21, 791–804. [Google Scholar]
- Camara, M.; Jamil, N.R.; Bin Abdullah, A.F. Impact of Land Uses on Water Quality in Malaysia: A Review. Ecol. Process. 2019, 8, 10. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Makowska, A. Mapa Geologiczna Polski 1:200,000, Arkusz: Skierniewice; Państwowy Instytut Geologiczny: Warsaw, Poland, 1970. Available online: https://bazadata.pgi.gov.pl/data/mgp200/mapy/edycja1/mgp200A49-edycja1.jpg (accessed on 19 November 2023).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Piniewski, M. Classification of natural flow regimes in Poland. River Res. Appl. 2017, 33, 1205–1218. [Google Scholar] [CrossRef]
- Główny Inspektorat Ochrony Środowiska (Chief Inspectorate of Environmental Protection). Ocena Stanu Jednolitych Części wód rzek i Zbiorników Zaporowych w Latach 2016–2021 na Podstawie Monitoringu. Available online: https://wody.gios.gov.pl/pjwp/api/publications/media/782 (accessed on 19 November 2023).
- Kulikowski, R. Ogrodnictwo w Polsce. Rozmieszczenie, struktura upraw i rola w produkcji rolniczej. Przegląd Geogr. 2007, 79, 79–98. [Google Scholar]
- Wójcik, M.; Traczyk, A. Changes in the Spatial Organisation of Fruit Growing at the Beginning of the 21St Century: The Case of Grójec Poviat (Mazovia Voivodeship, Poland). Quaest. Geogr. 2017, 36, 71–84. [Google Scholar] [CrossRef][Green Version]
- Lenart-Boroń, A.; Wolanin, A.; Jelonkiewicz, E.; Żelazny, M. The effect of anthropogenic pressure shown by microbiological and chemical water quality indicators on the main rivers of Podhale, southern Poland. Environ. Sci. Pollut. Res. 2017, 24, 12938–12948. [Google Scholar] [CrossRef]
- Jandová, V.; Bucková, M.; Hegrová, J.; Dostál, I.; Huzlík, J.; Effenberger, K.; Ličbinský, R. The Relationship among Precipitation, Application of Salt in Winter Road Maintenance and the Quality of Waterways and Soil around Motorway. Water 2020, 12, 2206. [Google Scholar] [CrossRef]
- Lachhab, A.; Trent, M.M.; Motsko, J. Multimetric approach in the effects of small impoundments on stream water quality: Case study of Faylor and Walker Lakes on Middle Creek, Snyder County, PA. Water Environ. J. 2021, 35, 1007–1017. [Google Scholar] [CrossRef]
- Stępniewski, K.; Łaszewski, M. Spatial and Seasonal Dynamics of Inorganic Nitrogen and Phosphorous Compounds in an Orchard-Dominated Catchment with Anthropogenic Impacts. Sustainability 2021, 13, 11337. [Google Scholar] [CrossRef]
- Moss, B. Water Pollution by Agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, Z.; Zhang, H.; Xie, H.; Cao, Y. Effects of land use types on dissolved trace metal concentrations in the Le’an River Basin, China. Environ. Monit. Assess. 2017, 189, 633. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.; Lewis, G.P.; Sargent, K.A. Influence of wastewater-treatment effluent on concentrations and fluxes of solutes in the Bush River, South Carolina, during extreme drought conditions. Environ. Geosci. 2004, 11, 28–41. [Google Scholar] [CrossRef]
- Figueroa-Nieves, D.; McDowell, W.H.; Potter, J.D.; Martínez, G.; Ortiz-Zayas, J.R. Effects of sewage effluents on water quality in tropical streams. J. Environ. Qual. 2014, 43, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Rosina, K.; Batista e Silva, F.; Vizcaino, P.; Herrera, M.M.; Freire, S.; Schiavina, M. Increasing the detail of European land use/cover data by combining heterogeneous data sets. Int. J. Digit. Earth 2018, 13, 602–626. [Google Scholar] [CrossRef]
- Popovici, E.A.; Bălteanu, D.; Kucsicsa, G. Assessment of changes in land-use and land-cover pattern in Romania using CORINE Land Cover Database. Carpathian J. Earth Environ. Sci. 2013, 8, 195–208. [Google Scholar]
- Śleszyński, P.; Gibas, P.; Sudra, P. The Problem of Mismatch between the CORINE Land Cover Data Classification and the Development of Settlement in Poland. Remote Sens. 2020, 12, 2253. [Google Scholar] [CrossRef]
- Kändler, M.; Blechinger, K.; Seidler, C.; Pavlů, V.; Šanda, M.; Dostál, T.; Krása, J.; Vitvar, T.; Štich, M. Impact of land use on water quality in the upper Nisa catchment in the Czech Republic and in Germany. Sci. Total Environ. 2017, 586, 1316–1325. [Google Scholar] [CrossRef]
- Gutry-Korycka, M.; Woronko, D.; Suchożebrski, J. Uwarunkowanie regionalne maksymalnych prawdopodobnych przepływów rzek polskich. Prace Stud. Geogr. 2009, 43, 25–48. [Google Scholar]
- Żelazny, M. Czasowo-Przestrzenna Zmienność cech Fizykochemicznych wód Tatrzańskiego Parku Narodowego; Instytut Geografii i Gospodarki Przestrzennej Uniwersytetu Jagiellońskiego: Kraków, Poland, 2012. [Google Scholar]
- Miranda, L.S.; Deilami, K.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Influence of land use class and configuration on water-sediment partitioning of heavy metals. Sci. Total Environ. 2022, 804, 150116. [Google Scholar] [CrossRef]
- Havlíková, P.; Mrkva, L.; Chuman, T.; Janský, B. Surface water quality in the rural catchment of the Šlapanka River, Czechia: Change over time. Environ. Earth Sci. 2023, 82, 379. [Google Scholar] [CrossRef]
- Mori, G.B.; Paula, F.R.; Ferraz, S.F.B.; Camargo, A.F.M.; Martinelli, L.F. Influence of landscape properties on stream water quality in agricultural catchments in Southeastern Brazil. Ann. Limnol.-Inter. J. Lim. 2015, 51, 11–21. [Google Scholar] [CrossRef]
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Żelazny, M.; Rajwa-Kuligiewicz, A.; Bojarczuk, A.; Pęksa, Ł. Water temperature fluctuation patterns in surface waters of the Tatra Mts., Poland. J. Hydrol. 2018, 564, 824–835. [Google Scholar] [CrossRef]
- de Mello, K.D.; Valente, R.A.; Randhir, T.O.; Vettorazzi, C.A. Impacts of tropical forest cover on water quality in agricultural watersheds in southeastern Brazil. Ecol. Indic. 2018, 93, 1293–1301. [Google Scholar] [CrossRef]
- Peng, S.; Li, S. Scale relationship between landscape pattern and water quality in different pollution source areas: A case study of the Fuxian Lake watershed, China. Ecol. Indic. 2021, 121, 107136. [Google Scholar] [CrossRef]
- White, S.A.; Santos, I.R.; Hessey, S. Nitrate loads in sub-tropical headwater streams driven by intensive horticulture. Environ. Pollut. 2018, 243, 1036–1046. [Google Scholar] [CrossRef]
- Sowiński, P. Variability of the content of macroelements in soils of a young glacial river valley-A geochemical landscape approach. J. Elem. 2016, 21, 1343–1358. [Google Scholar] [CrossRef]
- Cappuyns, V.; Slabbinck, E. Occurrence of Vanadium in Belgian and European Alluvial Soils. Appl. Environ. Soil Sci. 2012, 2012, 979501. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D. Arsenic in Groundwater and the Environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 279–310. [Google Scholar] [CrossRef]
- Musgrove, M. The occurrence and distribution of strontium in U.S. groundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Traaen, T.S.; Frogner, T.; Hindar, A.; Kleiven, E.; Lande, A.; Wright, R.F. Whole-catchment liming at Tjønnstrond, Norway: An 11-year record. Water Air Soil Pollut. 1997, 94, 163–180. [Google Scholar] [CrossRef]
- Orzepowski, W.; Pulikowski, K. Magnesium, calcium, potassium and sodium content in groundwater and surface water in arable lands in the commune of Kąty Wrocławskie. J. Elementol. 2008, 13, 605–614. [Google Scholar]
- Hamilton, P.A.; Helsel, D.R. Effects of agriculture on ground-water quality in five regions of the United States. Groundwater 2010, 33, 217–226. [Google Scholar] [CrossRef]
- Simon, L. Potentially harmful elements in agricultural soils. In PHEs, Environment and Human Health: Potentially Harmful Elements in the Environment and the Impact on Human Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 85–150. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Q.; Wang, S.; Wang, J.; Li, Z.; Liu, X. Chemical behaviours of Arsenium, Chromium, Mercury, Lead, and Strontium in aqueous system. E3S Web Conf. 2021, 290, 01022. [Google Scholar] [CrossRef]
- Simon, S.; Brun, L.; Guinaudeau, J.; Sauphanor, B. Pesticide use in current and innovative apple orchard systems. Agron. Sustain. Dev. 2011, 31, 541–555. [Google Scholar] [CrossRef]
- Wieczorek, K.; Turek, A.; Wolf, W.M. Combined Effect of Climate and Anthropopressure on River Water Quality. Int. J. Environ. Res. Public Health 2023, 20, 3032. [Google Scholar] [CrossRef]
- Ahlgren, J.; Djodjic, F.; Wallin, M. Barium as a potential indicator of phosphorus in agricultural runoff. J. Environ. Qual. 2012, 41, 208–216. [Google Scholar] [CrossRef]
- Kuczyńska, A.; Jarnuszewski, G.; Nowakowska, M.; Wexler, S.K.; Wiśniowski, Z.; Burczyk, P.; Durkowski, T.; Woźnicka, M. Identifying causes of poor water quality in a Polish agricultural catchment for designing effective and targeted mitigation measures. Sci. Total Environ. 2021, 765, 144125. [Google Scholar] [CrossRef]
- Bawiec, A.; Kajewska-Szkudlarek, J.; Pulikowski, K.; Pawęska, K. Assessment of the Validity of Introducing Nitrate Vulnerable Zones in Large Areas. Sustainability 2022, 14, 6585. [Google Scholar] [CrossRef]
- Ilnicki, P. Polskie Rolnictwo a Ochrona Środowiska; Wyd. AR: Poznań, Poland, 2004. [Google Scholar]
- Anyszka, Z.; Rogowska, M.; Stępowska, A.; Ślusarski, C.; Wrzodak, R. Metodyka Integrowanej Ochrony Papryki; Instytut Ogrodnictwa: Skierniewice, Poland, 2013; Available online: https://www.inhort.pl/files/sor/metodyki_ior/Metodyka_int_ochrony_papryki_doradcy.pdf (accessed on 19 November 2023).
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Effect of Nitrogen Fertilization of Apple Orchard on Soil Mineral Nitrogen Content, Yielding of the Apple Trees and Nutritional Status of Leaves and Fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Clune, J.W.; Crawford, J.K.; Chappell, W.T.; Boyer, E.W. Differential effects of land use on nutrient concentrations in streams of Pennsylvania. Environ. Res. Commun. 2020, 2, 115003. [Google Scholar] [CrossRef]
- Fernandes, A.C.P.; Martins, L.M.D.O.; Fernandes, L.F.S.; Cortes, R.M.V.; Pacheco, F.A.L. Effect of landscape metrics on water quality over three decades: A case study of the Ave River basin, Portugal. WIT Trans. Ecol. Environ. 2020, 242, 39–49. [Google Scholar] [CrossRef]
- Jachniak, E.; Jaguś, A.; Młyniuk, A.; Nycz, B. The quality problems of the dammed water in the mountain forest catchment. J. Ecol. Eng. 2019, 20, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, F.M.; Cañas, R.A.; de la Torre, F.N.; Pascual, M.B.; Castro-Rodríguez, V.; Avila, C. Nitrogen metabolism and biomass production in forest trees. Front. Plant Sci. 2018, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ayele, G.T.; Yu, B.; Bruere, A.; Hamilton, D. Response of streamflow and nutrient loads in a small temperate catchment subject to land use change. Environ. Monit. Assess. 2023, 195, 1418. [Google Scholar] [CrossRef]
- Hughes, A.O.; Quinn, J.M. The effect of forestry management activities on stream water quality within a headwater plantation Pinus radiata forest. For. Ecol. Manag. 2019, 439, 41–54. [Google Scholar] [CrossRef]
- Schulz, H.; Härtling, S.; Stange, C.F. Species-specific differences in nitrogen uptake and utilization by six European tree species. J. Plant Nutr. Soil Sci. 2011, 174, 28–37. [Google Scholar] [CrossRef]
- Song, M.; Jiang, Y.; Liu, Q.; Tian, Y.; Liu, Y.; Xu, X.; Kang, M. Catchment versus Riparian Buffers: Which Land Use Spatial Scales Have the Greatest Ability to Explain Water Quality Changes in a Typical Temperate Watershed? Water 2021, 13, 1758. [Google Scholar] [CrossRef]
- Weber, G.; Kubiniok, J. Spring waters as an indicator of nitrate and pesticide pollution of rural watercourses from nonpoint sources: Results of repeated monitoring campaigns since the early 2000s in the low mountain landscape of Saarland, Germany. Environ. Sci. Eur. 2022, 34, 53. [Google Scholar] [CrossRef]
- Przydatek, G. Multi-indicator analysis of the influence of old municipal landfill sites on the aquatic environment: Case study. Environ. Monit. Assess. 2019, 191, 773. [Google Scholar] [CrossRef]
- Dębska, K.; Rutkowska, B.; Szulc, W.; Gozdowski, D. Changes in Selected Water Quality Parameters in the Utrata River as a Function of Catchment Area Land Use. Water 2021, 13, 2989. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Skowron, P.; Skowrońska, M.; Bronowicka-Mielniczuk, U.; Filipek, T.; Igras, J.; Kowalczyk-Juśko, A.; Krzepiło, A. Anthropogenic sources of potassium in surface water: The case study of the Bystrzyca river catchment, Poland. Agric. Ecosys. Environ. 2018, 265, 454–460. [Google Scholar] [CrossRef]
- Aalipour, M.; Antczak, E.; Dostál, T.; Amiri, B.J. Influences of Landscape Configuration on River Water Quality. Forests 2022, 13, 222. [Google Scholar] [CrossRef]
- de Mello, K.; Valente, R.A.; Ribeiro, M.P.; Randhir, T. Effects of forest cover pattern on water quality of low-order streams in an agricultural landscape in the Pirapora river basin, Brazil. Environ. Monit. Assess. 2022, 194, 189. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Trolle, D.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; Olesen, J.E.; Jeppesen, E. Watershed land use effects on lake water quality in Denmark. Ecol. Appl. 2012, 22, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Y.; Hao, Y.; Lu, J. Identification of the control factors affecting water quality variation at multi-spatial scales in a headwater watershed. Environ. Sci. Pollut. Res. 2021, 28, 11129–11141. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.U.; Khanday, S.A.; Islam, S.T.; Sabha, I. Understanding the spatiotemporal pollution dynamics of highly fragile montane watersheds of Kashmir Himalaya, India. Environ. Pollut. 2021, 286, 117335. [Google Scholar] [CrossRef]
- Ye, L.; Cai, Q.-H.; Liu, R.-Q.; Cao, M. The influence of topography and land use on water quality of Xiangxi River in Three Gorges Reservoir region. Environ. Geol. 2009, 58, 937–942. [Google Scholar] [CrossRef]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Newbold, J.D. Streamside Forest Buffer Width Needed to Protect Stream Water Quality, Habitat, and Organisms: A Literature Review. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 560–584. [Google Scholar] [CrossRef]
- Buler, Z. Przygotowanie gleby oraz zakładanie sadu. In Metodyka Integrowanej Ochrony Jabłoni; Sobiczewski, P., Ed.; Instytut Ogrodnictwa: Skierniewice, Poland, 2013; Available online: http://www.inhort.pl/files/FAPA/sadownictwo/Doradca/JABLON-DORADCA%20II%20K%20A.pdf (accessed on 19 November 2023).
- Agricultural Census. Statistics Poland. 2020. Available online: https://bdl.stat.gov.pl/bdl/dane/podgrup/temat (accessed on 19 November 2023).
- Carey, R.O.; Migliaccio, K.W. Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems: A review. Environ. Manag. 2009, 44, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Lintern, A.; Wbb, J.A.; Ryu, D.; Liu, S.; Bende-Michl, U.; Waters, D.; Leahy, P.; Wilson, P.; Western, A.W. Key factors influencing differences in stream water quality across space. WIREs Water 2018, 5, e1260. [Google Scholar] [CrossRef]
- Sadeq, M.; Moe, C.L.; Attarassi, B.; Cherkaoui, I.; ElAouad, R.; Idrissi, L. Drinking water nitrate and prevalence of methemoglobinemia among infants and children aged 1–7 years in Moroccan areas. Int. J. Hyg. Environ. Health 2008, 211, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Szlauer-Łukaszewska, A.; Ławicki, Ł.; Engel, J.; Drewniak, E.; Ciężak, K.; Marchowski, D. Quantifying a mass mortality event in freshwater wildlife within the Lower Odra River: Insights from a large European river. Sci. Total Environ. 2024, 907, 167898. [Google Scholar] [CrossRef] [PubMed]
- Sługocki, Ł.; Czerniawski, R. Water Quality of the Odra (Oder) River before and during the Ecological Disaster in 2022: A Warning to Water Management. Sustainability 2023, 15, 8594. [Google Scholar] [CrossRef]
- Tei, F.; De Neve, S.; de Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Bednarek, A.; Szklarek, S.; Zalewski, M. Nitrogen pollution removal from areas of intensive farming—Comparison of various denitrification biotechnologies. Ecohydrol. Hydrobiol. 2014, 14, 132–141. [Google Scholar] [CrossRef]
- Robertson, W.D.; Blowes, D.W.; Ptacek, C.J.; Cherry, J.A. Long-term performance of in situ reactive barriers for nitrate remediation. Groundwater 2000, 38, 689–695. [Google Scholar] [CrossRef]
- Schipper, L.A.; Cameron, S.G.; Warneke, S. Nitrate removal from three different effluents using large-scale denitrification beds. Ecol. Eng. 2010, 36, 1552–1557. [Google Scholar] [CrossRef]
- Streeter, M.T.; Schilling, K.E. Quantifying the effectiveness of a saturated buffer to reduce tile NO3-N concentrations in eastern Iowa. Environ. Monit. Assess. 2021, 193, 500. [Google Scholar] [CrossRef]
- Izydorczyk, K.; Michalska-Hejduk, D.; Jarosiewicz, P.; Bydałek, F.; Frątczak, W. Extensive grasslands as an effective measure for nitrate and phosphate reduction from highly polluted subsurface flow—Case studies from Central Poland. Agric. Water Manage. 2018, 203, 240–250. [Google Scholar] [CrossRef]
- Nakhone, L.N.; Tabatabai, M.A. Nitrogen mineralization of leguminous crops in soils. J. Plant Nutr. Soil Sci.-Z. Fur Pflanzenernahr. Und Bodenkd. 2008, 171, 231–241. [Google Scholar] [CrossRef]
- Lötjönen, S.; Ollikainen, M. Does crop rotation with legumes provide an efficient means to reduce nutrient loads and GHG emissions? Rev. Agric. Food Environ. 2017, 98, 283–312. [Google Scholar] [CrossRef]
- Koropeckyj-Cox, L.; Christianson, R.D.; Yuan, Y. Effectiveness of conservation crop rotation for water pollutant reduction from agricultural areas. Trans. ASABE 2021, 64, 691–704. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef]
- Górski, J.; Dragon, K.; Kaczmarek, P. Nitrate pollution in the Warta River (Poland) between 1958 and 2016: Trend and causes. Environ. Sci. Pollut. Res. 2019, 26, 2038–2046. [Google Scholar] [CrossRef]
- Pekárová, P.; Pekár, J. The impact of land use on stream water quality in Slovakia. J. Hydrol. 1996, 180, 333–350. [Google Scholar] [CrossRef]
- Langhammer, J. Water Quality Changes in the Elbe River Basin, Czech Republic, in the Context of the Post-Socialist Economic Transition. GeoJournal 2010, 75, 185–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).