Effects of Climate Change on the Habitat Suitability and Distribution of Endemic Freshwater Fish Species in Semi-Arid Central Anatolian Ecoregion in Türkiye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
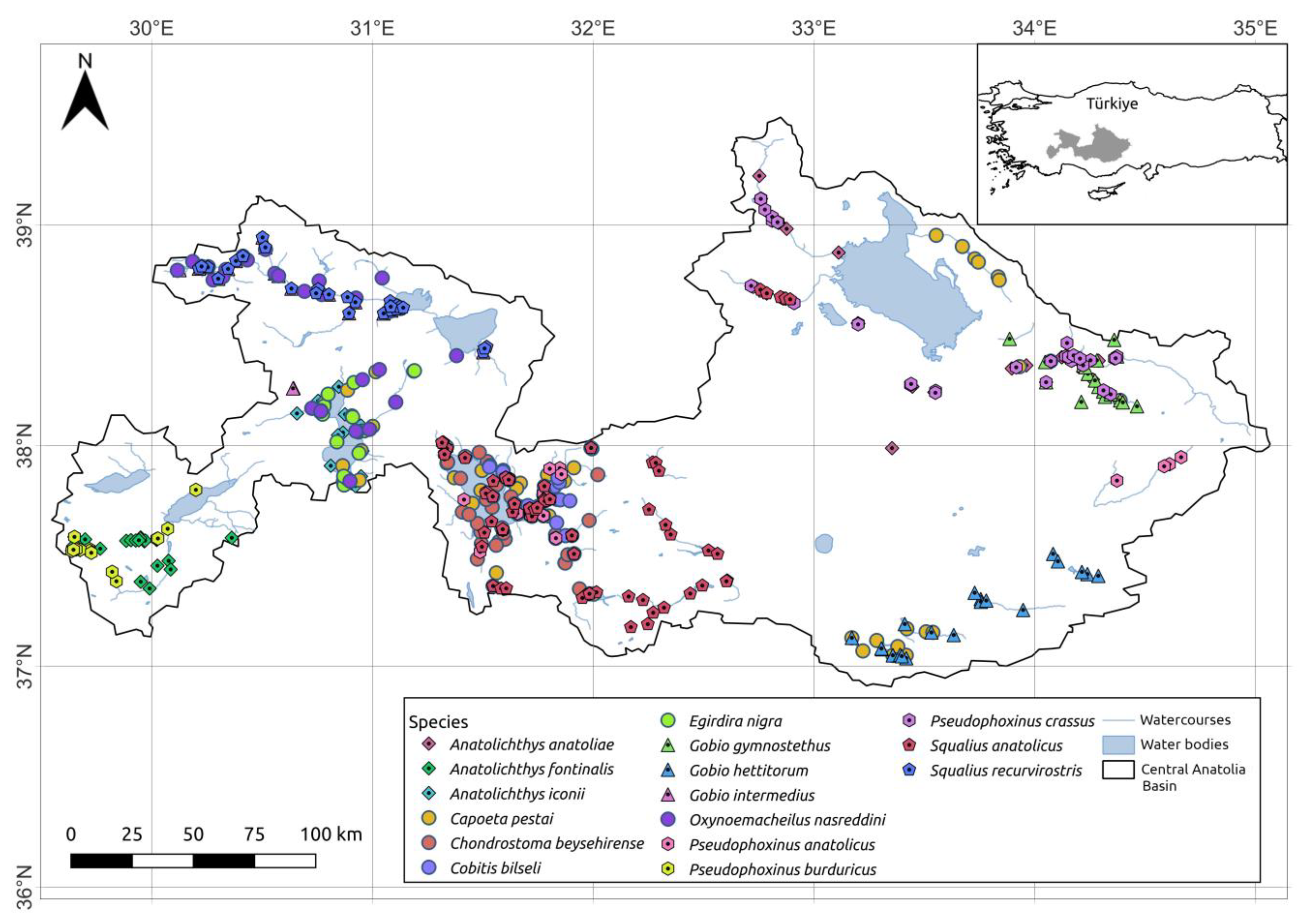
2.2. Fish Presence Data
2.3. Hydro-Climate Data
2.4. Environmental Data
2.5. Species Distribution Modelling
3. Results
3.1. Model Performance
3.2. Importance of Climate Variables
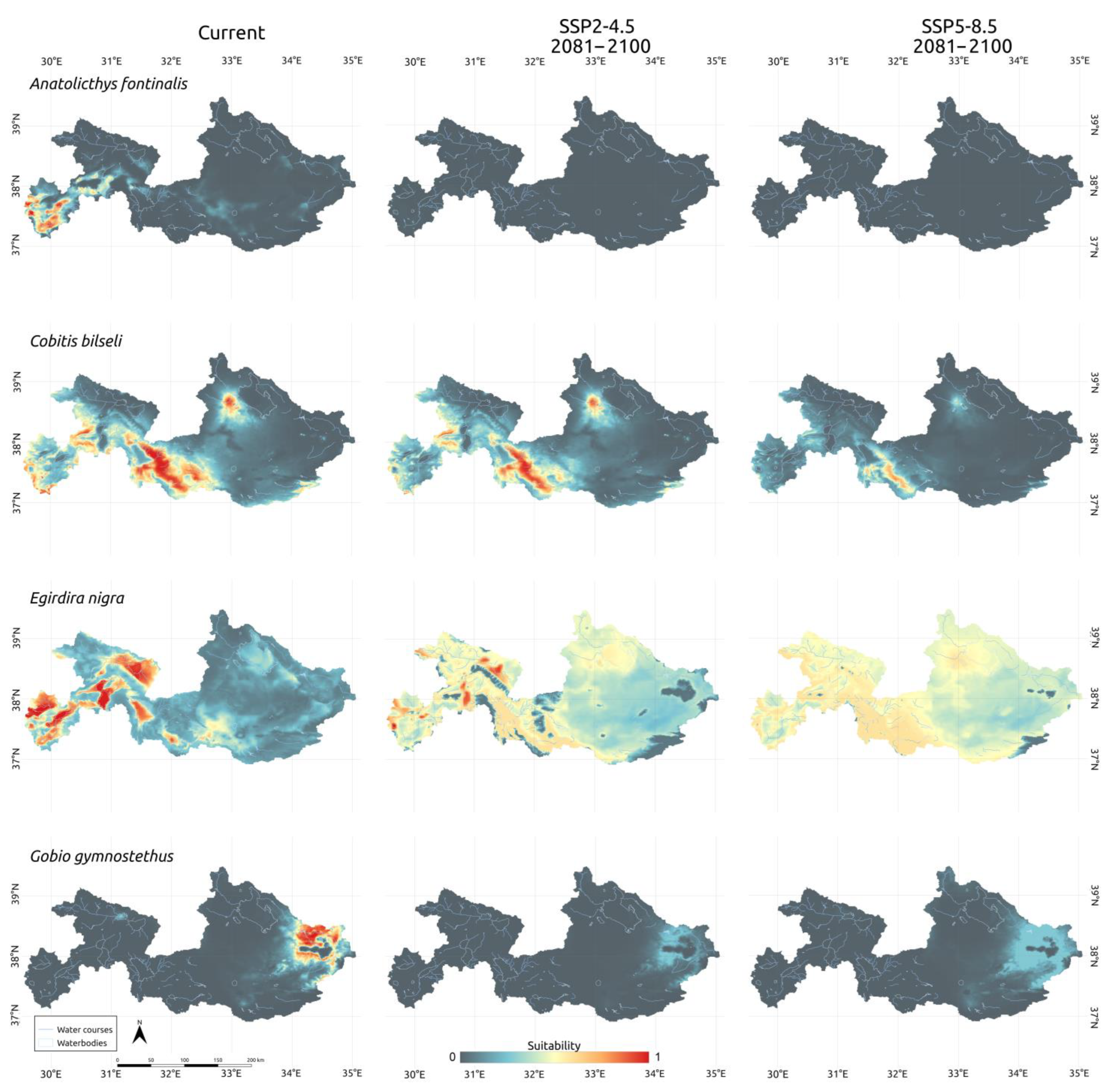
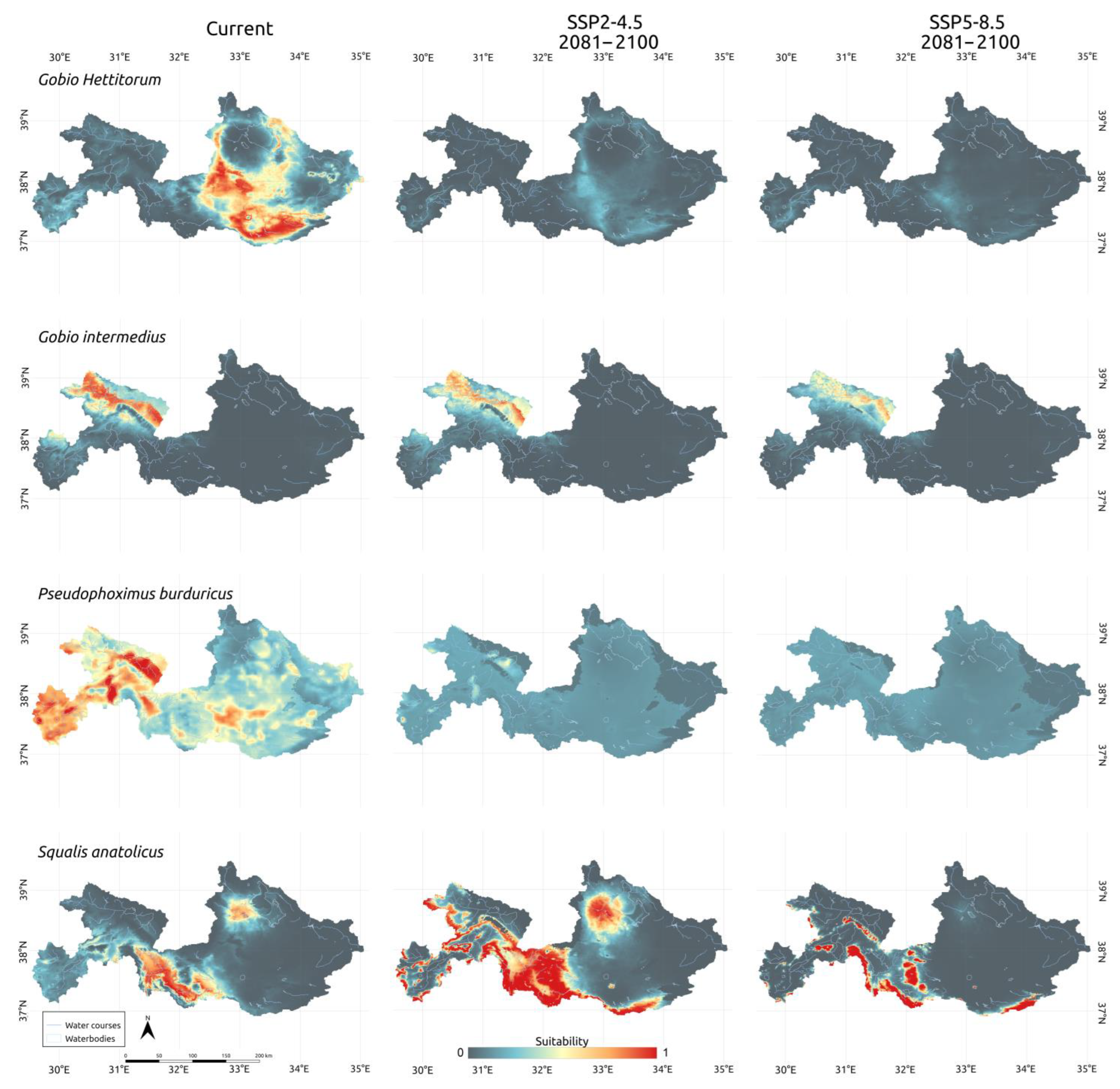
3.3. Climatic Scenarios
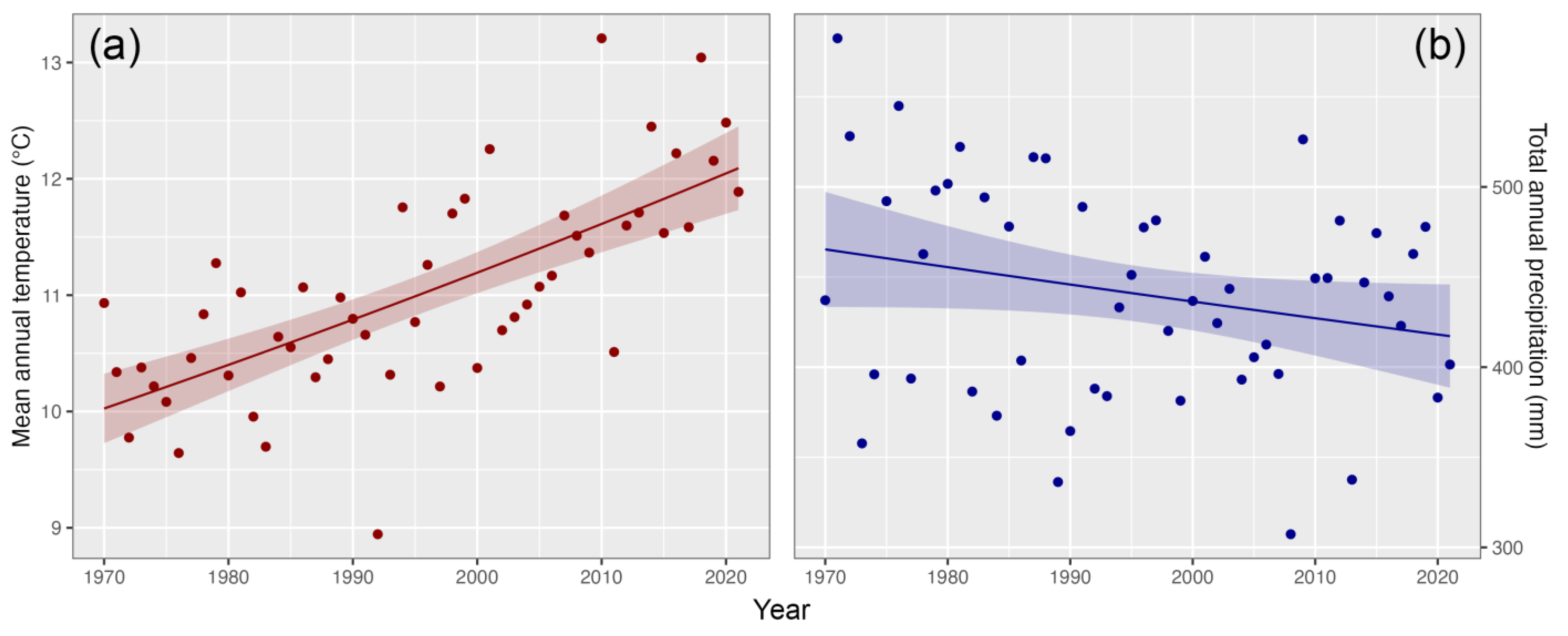
3.4. Hydro-Climate Trends
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nel, J.L.; Roux, D.J.; Abell, R.; Ashton, P.J.; Cowling, R.M.; Higgins, J.V.; Thieme, M.; Viers, J.H. Progress and Challenges in Freshwater Conservation Planning. Aquat. Conserv. 2009, 19, 474–485. [Google Scholar] [CrossRef]
- Döll, P.; Zhang, J. Impact of Climate Change on Freshwater Ecosystems: A Global-Scale Analysis of Ecologically Relevant River Flow Alterations. Hydrol. Earth Syst. Sci. 2010, 14, 783–799. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.N. Species Richness, Habitable Volume, and Species Densities in Freshwater, the Sea, and on Land. Front. Biogeogr. 2012, 4, 105–116. [Google Scholar] [CrossRef]
- Janse, J.H.; Kuiper, J.J.; Weijters, M.J.; Westerbeek, E.P.; Jeuken, M.H.J.L.; Bakkenes, M.; Alkemade, R.; Mooij, W.M.; Verhoeven, J.T.A. GLOBIO-Aquatic, a Global Model of Human Impact on the Biodiversity of Inland Aquatic Ecosystems. Environ. Sci. Policy 2015, 48, 99–114. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Nõges, T.; Nõges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological Impacts of Global Warming and Water Abstraction on Lakes and Reservoirs Due to Changes in Water Level and Related Changes in Salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- IPCC. IPCC. IPCC Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; IPCC: Geneva, Switzerland, 2021; ISBN 978-92-9169-158-6. [Google Scholar]
- Munday, P.L.; Jarrold, M.D.; Nagelkerken, I. Ecological Effects of Elevated CO2 on Marine and Freshwater Fishes: From Individual to Community Effects. Fish Physiol. 2019, 37, 323–368. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Hannah, L. Climate Change Biology; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Yousefi, M.; Jouladeh-Roudbar, A.; Kafash, A. Using Endemic Freshwater Fishes as Proxies of Their Ecosystems to Identify High Priority Rivers for Conservation under Climate Change. Ecol. Indic. 2020, 112, 106137. [Google Scholar] [CrossRef]
- Muths, E.; Chambert, T.; Schmidt, B.R.; Miller, D.A.W.; Hossack, B.R.; Joly, P.; Grolet, O.; Green, D.M.; Pilliod, D.S.; Cheylan, M.; et al. Heterogeneous Responses of Temperate-Zone Amphibian Populations to Climate Change Complicates Conservation Planning. Sci. Rep. 2017, 7, 17102. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Oberdorff, T.; Cornu, J.-F.; Beauchard, O.; Brosse, S.; Dürr, H.H.; Grenouillet, G.; Leprieur, F.; Tisseuil, C.; Zaiss, R.; et al. A Scenario for Impacts of Water Availability Loss Due to Climate Change on Riverine Fish Extinction Rates. J. Appl. Ecol. 2013, 50, 1105–1115. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the World’s Freshwater Ecosystems: Physical, Chemical, and Biological Changes. Annu. Rev. Env. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Dudgeon, D. Asian River Fishes in the Anthropocene: Threats and Conservation Challenges in an Era of Rapid Environmental Change. J. Fish Biol. 2011, 79, 1487–1524. [Google Scholar] [CrossRef] [PubMed]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish Conservation in Freshwater and Marine Realms: Status, Threats and Management. Aquat. Conserv. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Climatic Vulnerability of the World’s Freshwater and Marine Fishes. Nat. Clim. Chang. 2017, 7, 718–722. [Google Scholar] [CrossRef]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P.; et al. Impacts of Climate Warming on the Long-Term Dynamics of Key Fish Species in 24 European Lakes. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of Global Warming to the World’s Freshwater Fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Cianfrani, C.; Broennimann, O.; Loy, A.; Guisan, A. More than Range Exposure: Global Otter Vulnerability to Climate Change. Biol. Conserv. 2018, 221, 103–113. [Google Scholar] [CrossRef]
- Marcer, A.; Sáez, L.; Molowny-Horas, R.; Pons, X.; Pino, J. Using Species Distribution Modelling to Disentangle Realised versus Potential Distributions for Rare Species Conservation. Biol. Conserv. 2013, 166, 221–230. [Google Scholar] [CrossRef]
- Gama, M.; Crespo, D.; Dolbeth, M.; Anastácio, P.M. Ensemble Forecasting of Corbicula Fluminea Worldwide Distribution: Projections of the Impact of Climate Change. Aquat. Conserv. 2017, 27, 675–684. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Pitman, A.J. Why Is the Choice of Future Climate Scenarios for Species Distribution Modelling Important? Ecol. Lett. 2008, 11, 1135–1146. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Lek, S.; Lim, P.; Grenouillet, G. Climate Change Hastens the Turnover of Stream Fish Assemblages. Glob. Chang. Biol. 2008, 14, 2232–2248. [Google Scholar] [CrossRef]
- Kuru, M.; Yerli, S.V.; Mangıt, F.; Ünlü, E.; Alp, A. Fish Biodiversity in Inland Waters of Turkey. J. Acad. Doc. Fish. Aquac. 2014, 1, 93–120. [Google Scholar]
- Çiçek, E.; Sungur, S.; Fricke, R. Freshwater Lampreys and Fishes of Turkey; A Revised and Updated Annotated Checklist 2020. Zootaxa 2020, 4809, 241–270. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.G.; Coad, B.W.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- WRI World Resources Institute Aqueduct. Available online: http://www.aqueduct.wri.org (accessed on 4 February 2023).
- Yılmaz, G.; Çolak, M.A.; Özgencil, İ.K.; Metin, M.; Korkmaz, M.; Ertuğrul, S.; Soyluer, M.; Bucak, T.; Tavşanoğlu, Ü.N.; Özkan, K.; et al. Decadal Changes in Size, Salinity, Waterbirds, and Fish in Lakes of the Konya Closed Basin, Turkey, Associated with Climate Change and Increasing Water Abstraction for Agriculture. Inland Waters 2021, 11, 538–555. [Google Scholar] [CrossRef]
- Çolak, M.A.; Öztaş, B.; Özgencil, İ.K.; Soyluer, M.; Korkmaz, M.; Ramírez-garcía, A.; Metin, M.; Yılmaz, G.; Ertuğrul, S.; Tavşanoğlu, Ü.N.; et al. Increased Water Abstraction and Climate Change Have Substantial Effect on Morphometry, Salinity, and Biotic Communities in Lakes: Examples from the Semi-Arid Burdur Basin (Turkey). Water 2022, 14, 1241. [Google Scholar] [CrossRef]
- Yagmur, N.; Bilgilioglu, B.B.; Dervisoglu, A.; Musaoglu, N.; Tanik, A. Long and Short-Term Assessment of Surface Area Changes in Saline and Freshwater Lakes via Remote Sensing. Water Environ. J. 2021, 35, 107–122. [Google Scholar] [CrossRef]
- Şenkul, Ç.; Ören, A.; Doğan, U.; Eastwood, W.J. Late Holocene Environmental Changes in the Vicinity of Kültepe (Kayseri), Central Anatolia, Turkey. Quatern. Int. 2018, 486, 107–115. [Google Scholar] [CrossRef]
- Türkeş, M.; Öztaş, T.; Tercan, E.; Erpul, G.; Karagöz, A.; Dengiz, O.; Doğan, O.; Şahin, K.; Avcıoğlu, B. Desertification Vulnerability and Risk Assessment for Turkey via an Analytical Hierarchy Process Model. Land Degrad. Dev. 2020, 31, 205–214. [Google Scholar] [CrossRef]
- Çiçek, E.; Fricke, R.; Sungur, S.; Eagderi, S. Endemic Freshwater Fishes of Turkey. FishTaxa 2018, 3, 1–39. [Google Scholar]
- Kosswig, C. Zoogeography of the Near East. Syst. Biol. 1955, 4, 49–96. [Google Scholar] [CrossRef]
- Meijers, M.J.M.; Brocard, G.Y.; Whitney, D.L.; Mulch, A. Paleoenvironmental Conditions and Drainage Evolution of the Central Anatolian Lake System (Turkey) during Late Miocene to Pliocene Surface Uplift. Geosphere 2020, 16, 490–509. [Google Scholar] [CrossRef]
- Kuzucuoğlu, C. Geomorphological Landscapes in the Konya Plain and Surroundings. In Landscapes and Landforms of Turkey; World Geomorphological Landscapes; Springer: Cham, Switzerland, 2019; pp. 353–368. [Google Scholar]
- Giannetto, D.; Innal, D. Status of Endemic Freshwater Fish Fauna Inhabiting Major Lakes of Turkey under the Threats of Climate Change and Anthropogenic Disturbances: A Review. Water 2021, 13, 1534. [Google Scholar] [CrossRef]
- Boll, T.; Levi, E.E.; Bezirci, G.; Özuluğ, M.; Tavşanoğlu, Ü.N.; Çakıroğlu, A.İ.; Özcan, S.; Brucet, S.; Jeppesen, E.; Beklioğlu, M. Fish Assemblage and Diversity in Lakes of Western and Central Turkey: Role of Geo-Climatic and Other Environmental Variables. Hydrobiologia 2016, 771, 31–44. [Google Scholar] [CrossRef]
- Morrongiello, J.R.; Beatty, S.J.; Bennett, J.C.; Crook, D.A.; Ikedife, D.N.E.N.; Kennard, M.J.; Kerezsy, A.; Lintermans, M.; McNeil, D.G.; Pusey, B.J.; et al. Climate Change and Its Implications for Australia’s Freshwater Fish. Mar. Freshw. Res. 2011, 62, 1082–1098. [Google Scholar] [CrossRef]
- Freyhof, J.; Bergner, L.; Ford, M. Threatened Freshwater Fishes of the Mediterranean Basin Biodiversity Hotspot: Distribution, Extinction Risk and the Impact of Hydropower. Euronatur and Riverwatch; Museum für Naturkunde Berlin (MfN)–Leibniz Institute for Evolution and Biodiversity Science: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Kesici, E.; Kesici, C. The Effects of Interferences in Natural Structure of Lake Egirdir (Isparta) to Ecological Disposition of the Lake. Su Ürün. Derg. 2006, 23, 99–103. [Google Scholar]
- Lane, M.A.; Edwards, J.L. The Global Biodiversity Information Facility (GBIF). In Biodiversity Databases; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-15233-7. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 5 March 2023).
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 5 March 2023).
- Freyhof, J.; Yoğurtçuoğlu, B. A Proposal for a New Generic Structure of the Killifish Family Aphaniidae, with the Description of Aphaniops teimorii (Teleostei: Cyprinodontiformes). Zootaxa 2020, 4810, 421–451. [Google Scholar] [CrossRef] [PubMed]
- Kelleci, M.; Seçer, B.; ÇiÇek, E.; Sungur, S. Aksaray İli (Türkiye) İhtiyofaunası. Acta Aquat. Turc. 2021, 17, 279–289. [Google Scholar] [CrossRef]
- Kuyumcu, M.; Aksu, İ.; Bektaş, Y. Genetic Analysis of Aphaniidae Hoedeman, 1949 (Teleostei: Cyprinodontiformes) Family in Anatolia. J. Anatol. Environ. Anim. Sci. 2021, 6, 627–634. [Google Scholar] [CrossRef]
- Yoğurtçuoğlu, B.; Kaya, C.; Freyhof, J. Oxynoemacheilus nasreddini, a New Nemacheilid Loach from Central Anatolia (Teleostei: Nemacheilidae). Zootaxa 2021, 4974, 135–150. [Google Scholar] [CrossRef]
- Mangit, F.; Korkmaz, M.; Sü, U.; Yerli, S.V. Actual Status of Eber Lake in Terms of Fish Community Structure. J. Limnol. Fish. Res. 2017, 3, 101–106. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- RCoreTeam R. R Foundation for Statistical Computing, Version 4.1.3; RCoreTeam: Vienna, Austria, 2022.
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A State-of-the-Art Global Reanalysis Dataset for Land Applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 Global Reanalysis. Q. J. R. Meteor. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- ECMWF IFS Documentation CY47R3—Part I: Observations. Available online: https://www.ecmwf.int/en/elibrary/81268-ifs-documentation-cy47r3-part-i-observations (accessed on 15 March 2023).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-87458-6. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modelling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A Protocol for Conducting and Presenting Results of Regression-Type Analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmons, J.; O’neill, B.C.; Fujimori, S.; Tavoni, M. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-0-691-13688-2. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Ondo, I.; Thuiller, W.; Guéguen, M.; Pironon, S. A New R Application for Modelling Species Distribution. In Proceedings of the Species On The Move 2019, Kruger National Park, South Africa, 22–26 July 2019. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting Species Distributions from Small Numbers of Occurrence Records: A Test Case Using Cryptic Geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A Flexible Platform for Reproducible Modeling of Species Niches and Distributions Built for Community Expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the Predictive Performance of Habitat Models Developed Using Logistic Regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Peterson, A.T.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. Ntbox: An r Package with Graphical User Interface for Modelling and Evaluating Multidimensional Ecological Niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 5 March 2023).
- Stewart, B.A.; Ford, B.M.; Benson, J.A. Using Species Distribution Modelling to Identify ‘Coldspots’ for Conservation of Freshwater Fishes under a Changing Climate. Aquat. Conserv. 2022, 32, 576–590. [Google Scholar] [CrossRef]
- Frederico, R.G.; Dias, M.S.; Jézéquel, C.; Tedesco, P.A.; Hugueny, B.; Zuanon, J.; Torrente-Vilara, G.; Ortega, H.; Hidalgo, M.; Martens, K.; et al. The Representativeness of Protected Areas for Amazonian Fish Diversity under Climate Change. Aquat. Conserv: Mar. Freshw. Ecosyst. 2021, 31, 1158–1166. [Google Scholar] [CrossRef]
- Hrbek, T.; Stölting, K.N.; Bardakci, F.; Küçük, F.; Wildekamp, R.H.; Meyer, A. Plate Tectonics and Biogeographical Patterns of the Pseudophoxinus (Pisces: Cypriniformes) Species Complex of Central Anatolia, Turkey. Mol. Phylogenet. Evol. 2004, 32, 297–308. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Fløjgaard, C.; Svenning, J.-C. Climate Change Risks and Conservation Implications for a Threatened Small-Range Mammal Species. PLoS ONE 2010, 5, e10360. [Google Scholar] [CrossRef]
- Erk’akan, F.; Atalay-Ekmekçi, F.G.; Nalbant, T.T. A Review of the Genus Cobitis in Turkey (Pisces: Ostariophysi: Cobitidae). Hydrobiologia 1999, 403, 13–26. [Google Scholar] [CrossRef]
- Küçük, F. Extinct Endemic Fishes of Turkey: Alburnus akili (Gövce) and Pseudophoxinus handlirschi (Kavinne) (Pisces: Cyprinidae). Turk. J. Fish. Aquat. Sci. 2012, 12, 21. [Google Scholar] [CrossRef]
- Innal, D.; Erk’akan, F. Effects of Exotic and Translocated Fish Species in the Inland Waters of Turkey. Rev. Fish Biol. 2006, 16, 39–50. [Google Scholar] [CrossRef]
- Yoğurtçuoğlu, B.; Uyan, U.; Ekmekçi, F.G. The Influence of Environmental Instability on the Reproductive Strategy of the Critically Endangered Acıgöl Killifish (Aphanius transgrediens). J. Fish Biol. 2020, 97, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Yerli, S.V.; Alp, A.; Yeğen, V.; Uysal, R.; Apaydın Yağcı, M.; Balık, İ. Evaluation of the Ecological and Economical Results of the Introduced Alien Fish Species in Lake Eğirdir, Turkey. Turk. J. Fish. Aquat. Sci. 2013, 13, 795–809. [Google Scholar] [CrossRef] [PubMed]
- İlhan, A. Threatened Fishes of the World: Chondrostoma beysehirense Bogutskaya, 1997 (Cyprinidae). Environ. Biol. Fish. 2009, 86, 483–484. [Google Scholar] [CrossRef]
- Özuluğ, M.; Oǧuz Öztürk, M. Threatened Fishes of the World: Pseudophoxinus anatolicus (Hanko 1924) (Cyprinidae), Central Anatolia, Turkey. Environ. Biol. Fish. 2008, 83, 183–184. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Menon, M.; Woods, T.; Tassone, S.; Reese, J.; Vergamini, M.; Kellogg, E. On the Use of Climate Covariates in Aquatic Species Distribution Models: Are We at Risk of Throwing out the Baby with the Bath Water? Ecography 2018, 41, 695–712. [Google Scholar] [CrossRef]
- Pépino, M.; Rodríguez, M.A.; Magnan, P. Fish Dispersal in Fragmented Landscapes: A Modeling Framework for Quantifying the Permeability of Structural Barriers. Ecol. Appl. 2012, 22, 1435–1445. [Google Scholar] [CrossRef]
- Kates, D.; Dennis, C.; Noatch, M.R.; Suski, C.D. Responses of Native and Invasive Fishes to Carbon Dioxide: Potential for a Nonphysical Barrier to Fish Dispersal. Can. J. Fish. Aquat. Sci. 2012, 69, 1748–1759. [Google Scholar] [CrossRef]
- Maynard, D.S.; Crowther, T.W.; Bradford, M.A. Competitive Network Determines the Direction of the Diversity–Function Relationship. Proc. Natl. Acad. Sci. USA 2017, 114, 11464–11469. [Google Scholar] [CrossRef] [PubMed]
- Lake, P.S.; Bond, N.; Reich, P. Linking Ecological Theory with Stream Restoration. Freshw. Biol. 2007, 52, 597–615. [Google Scholar] [CrossRef]
- Schmidt, H.; Radinger, J.; Teschlade, D.; Stoll, S. The Role of Spatial Units in Modelling Freshwater Fish Distributions: Comparing a Subcatchment and River Network Approach Using MaxEnt. Ecol. Model. 2020, 418, 108937. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Graham, C.H. The Ability of Climate Envelope Models to Predict the Effect of Climate Change on Species Distributions. Glob. Chang. Biol. 2006, 12, 2272–2281. [Google Scholar] [CrossRef]
- Logez, M.; Bady, P.; Pont, D. Modelling the Habitat Requirement of Riverine Fish Species at the European Scale: Sensitivity to Temperature and Precipitation and Associated Uncertainty. Ecol. Freshw. Fish 2012, 21, 266–282. [Google Scholar] [CrossRef]
- Conti, L.; Comte, L.; Hugueny, B.; Grenouillet, G. Drivers of Freshwater Fish Colonisations and Extirpations under Climate Change. Ecography 2015, 38, 510–519. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats Reduce Animal’s Exposure to Climate Extremes. Glob. Chang. Biol. 2014, 20, 495–503. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Gillingham, P.K.; Hill, J.K.; Huntley, B.; Kunin, W.E.; Roy, D.B.; Thomas, C.D. Habitat Microclimates Drive Fine-Scale Variation in Extreme Temperatures. Oikos 2011, 120, 1–8. [Google Scholar] [CrossRef]
- MoEUCC. Climate Adaptation Strategy and Action Plan of Türkiye; Ministery of Environment, Urbanisation and Climate Change: Ankara, Turkey, 2011.
- Ergönül, M.B.; Breine, J.; Atasağun, S. Length-Weight Relationships of Two Threatened Species Endemic to Turkey: Gobio insuyanus Ladiges and Gobio microlepidotus Battalgil. Fish. Aquat. Life 2019, 27, 118–121. [Google Scholar] [CrossRef]
- Freyhof, J.; Özuluğ, M.; Saç, G. Neotype Designation of Aphanius iconii, First Reviser Action to Stabilise the Usage of A. fontinalis and A. meridionalis and Comments on the Family Group Names of Fishes Placed in Cyprinodontidae (Teleostei: Cyprinodontiformes). Zootaxa 2017, 4294, 573–585. [Google Scholar] [CrossRef]
- Yegen, V.; Sari, H.M. Endemik Eğirdir Yağ Balığı (Pseudophoxinus egridiri) Populasyonunun Büyüme Özellikleri. J. Limnol. Fish. Res. 2021, 7, 241–249. [Google Scholar] [CrossRef]
- Seçer, B.; Sungur, S.; Çiçek, E.; Ceylan, M. Freshwater Fish Fauna of Niğde. J. Limnol. Fish. Res. 2020. [Google Scholar] [CrossRef]
- Çiçek, E. Seminemacheilus dursunavsari, a New Nemachelid Species (Teleostei: Nemacheilidae) from Turkey. Iran. J. Ichthyol. 2020, 7, 68–77. [Google Scholar]
- Yoğurtçuoğlu, B.; Kaya, C.; Geiger, M.F.; Freyhof, J. Revision of the Genus Seminemacheilus, with the Description of Three New Species (Teleostei: Nemacheilidae). Zootaxa 2020, 4802, 477–501. [Google Scholar] [CrossRef]
- Ekmekçi, F.G. The Effects of High Salinity on the Production of Capoeta tinca in a Naturally Contaminated River. Turk. J. Zool. 2002, 26, 265–270. [Google Scholar]
- İlhan, A.; Balık, S.; Sarı, H.M. Orta ve Batı Anadolu Endemik Içsu Balıklarının Günümüzdeki Dağılımları ve Koruma Statüleri. J. Fish. Aquat. Sci. 2014, 29, 9–34. [Google Scholar] [CrossRef]

| Species | AUC Values |
|---|---|
| Anatolichthys anatoliae (Leidenfrost 1912) | 0.88 |
| Anatolichthys fontinalis (Akşiray, 1948) | 0.98 |
| Anatolichthys iconii (Akşiray, 1948) | 0.98 |
| Capoeta pestai (Pietschmann, 1933) | 0.85 |
| Chondrostoma beysehirense Bogutskaya, 1997 | 0.93 |
| Cobitis bilseli Battalgil, 1942 | 0.96 |
| Egirdira nigra (Kosswig & Geldiay, 1952) | 0.97 |
| Gobio gymnostethus Ladiges, 1960 | 0.97 |
| Gobio hettitorum Ladiges, 1960 | 0.94 |
| Gobio intermedius Battalgil, 1944 | 0.96 |
| Oxynoemacheilus nasreddini Yoğurtçuoğlu, Kaya & Freyhof, 2021 | 0.94 |
| Pseudophoxinus anatolicus (Hankó, 1925) | 0.88 |
| Pseudophoxinus burduricus Küçük, Gülle, Güçlü, Çiftçi & Erdoğan, 2013 | 0.95 |
| Pseudophoxinus crassus (Ladiges, 1960) | 0.89 |
| Squalius anatolicus (Bogutskaya, 1997) | 0.91 |
| Squalius recurvirostris Özuluğ & Freyhof, 2011 | 0.97 |
| Species | Conservation Status * | Range Size Change (%) | |||
|---|---|---|---|---|---|
| SSP2-4.5 2041–2060 | SSP2-4.5 2081–2100 | SSP5-8.5 2041–2060 | SSP5-8.5 2081–2100 | ||
| Anatolichthys anatoliae | NT | 214 | 216 | 226 | 239 |
| Anatolichthys fontinalis | NE | −100 | −100 | −100 | −100 |
| Anatolichthys iconii | NE | 1386 | 1521 | 1468 | 1172 |
| Capoeta pestai | CR | 150 | 175 | 162 | 189 |
| Chondrostoma beysehirense | EN | 138 | 123 | 107 | 47 |
| Cobitis bilseli | EN | −21 | −48 | −47 | −100 |
| Egirdira nigra | EN | 7.8 | −76 | −70 | −100 |
| Gobio gymnostethus | CR | −100 | −100 | −100 | −100 |
| Gobio hettitorum | CR | −100 | −100 | −100 | −100 |
| Gobio intermedius | EN | 6.1 | −30 | 17 | −70 |
| Oxynoemacheilus nasreddini | NE | 106 | 91 | 97 | 75 |
| Pseudophoxinus anatolicus | EN | 155 | 164 | 162 | 172 |
| Pseudophoxinus burduricus | EN | −97 | −100 | −100 | −100 |
| Pseudophoxinus crassus | EN | 118 | 132 | 130 | 146 |
| Squalius anatolicus | LC | 251 | 162 | 177 | −26 |
| Squalius recurvirostris | VU | 49 | 22 | 60 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, M.; Mangıt, F.; Dumlupınar, İ.; Çolak, M.A.; Akpınar, M.B.; Koru, M.; Pacheco, J.P.; Ramírez-García, A.; Yılmaz, G.; Amorim, C.A.; et al. Effects of Climate Change on the Habitat Suitability and Distribution of Endemic Freshwater Fish Species in Semi-Arid Central Anatolian Ecoregion in Türkiye. Water 2023, 15, 1619. https://doi.org/10.3390/w15081619
Korkmaz M, Mangıt F, Dumlupınar İ, Çolak MA, Akpınar MB, Koru M, Pacheco JP, Ramírez-García A, Yılmaz G, Amorim CA, et al. Effects of Climate Change on the Habitat Suitability and Distribution of Endemic Freshwater Fish Species in Semi-Arid Central Anatolian Ecoregion in Türkiye. Water. 2023; 15(8):1619. https://doi.org/10.3390/w15081619
Chicago/Turabian StyleKorkmaz, Mustafa, Fatih Mangıt, İlayda Dumlupınar, Mehmet Arda Çolak, Mustafa Berkay Akpınar, Meltem Koru, Juan Pablo Pacheco, Arely Ramírez-García, Gültekin Yılmaz, Cihelio Alves Amorim, and et al. 2023. "Effects of Climate Change on the Habitat Suitability and Distribution of Endemic Freshwater Fish Species in Semi-Arid Central Anatolian Ecoregion in Türkiye" Water 15, no. 8: 1619. https://doi.org/10.3390/w15081619







