An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas
Abstract
:1. Introduction
2. Materials and Methods
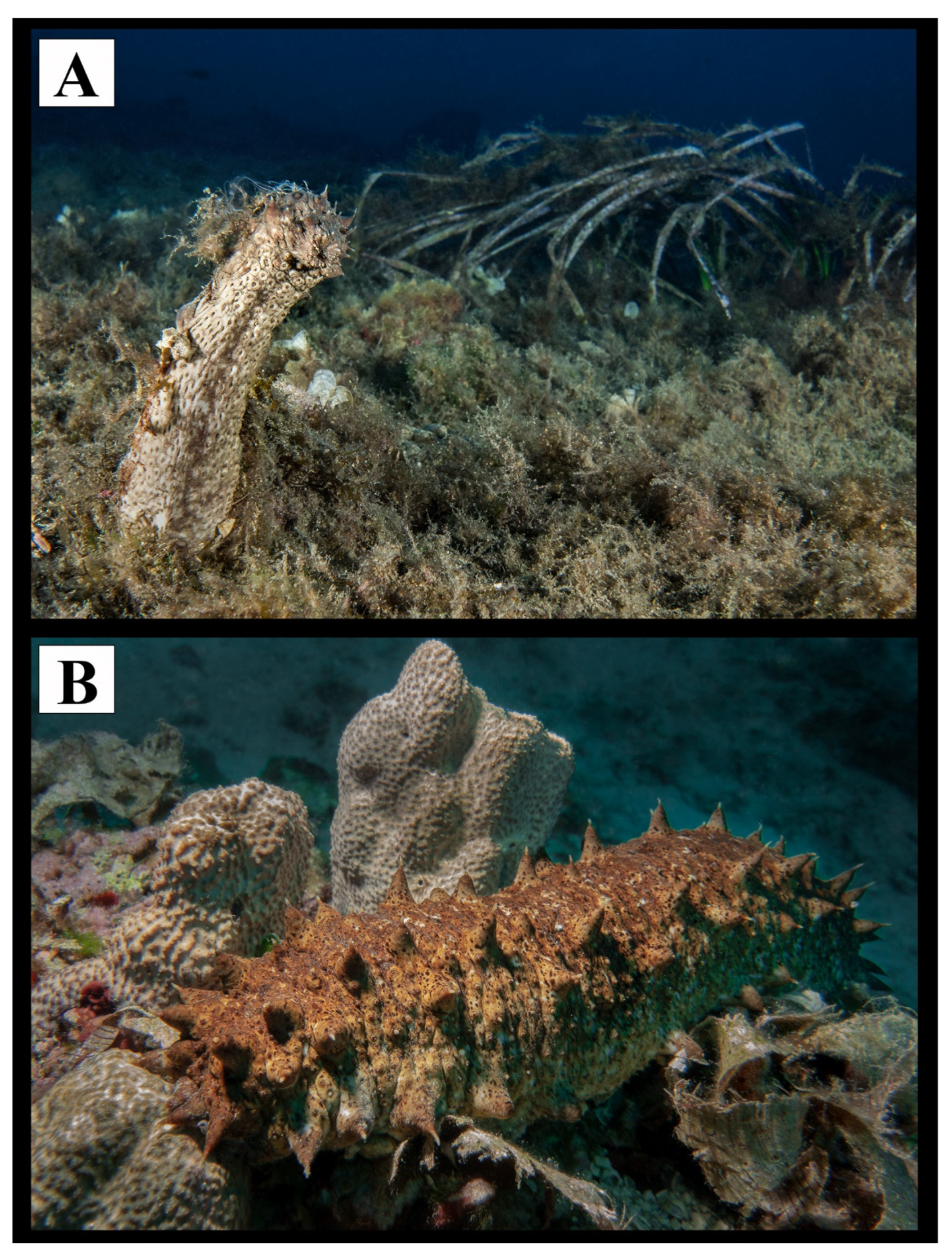
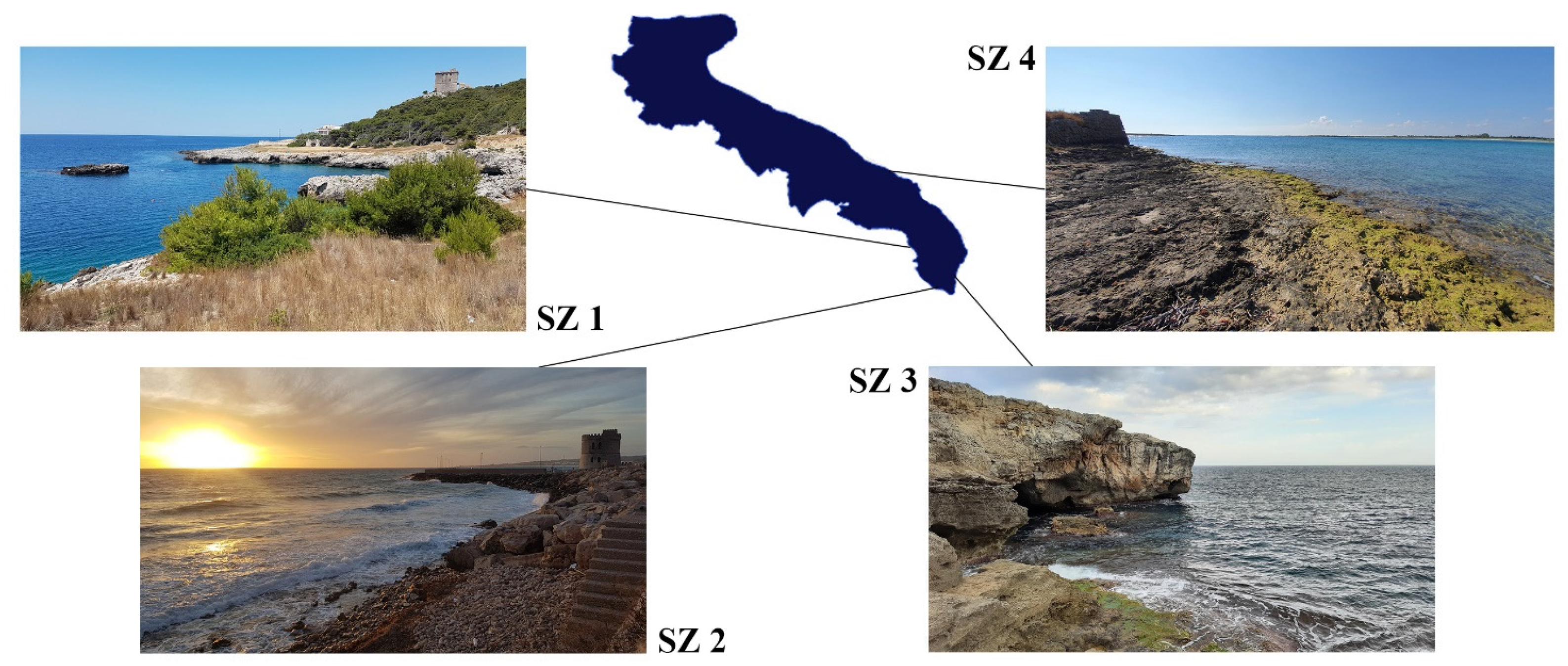
2.1. Sampling Protocol
2.2. Anatomical Dissection
2.3. Microplastic Extraction and Analysis
2.4. Statistical Analysis
3. Results
3.1. Statistical Analysis
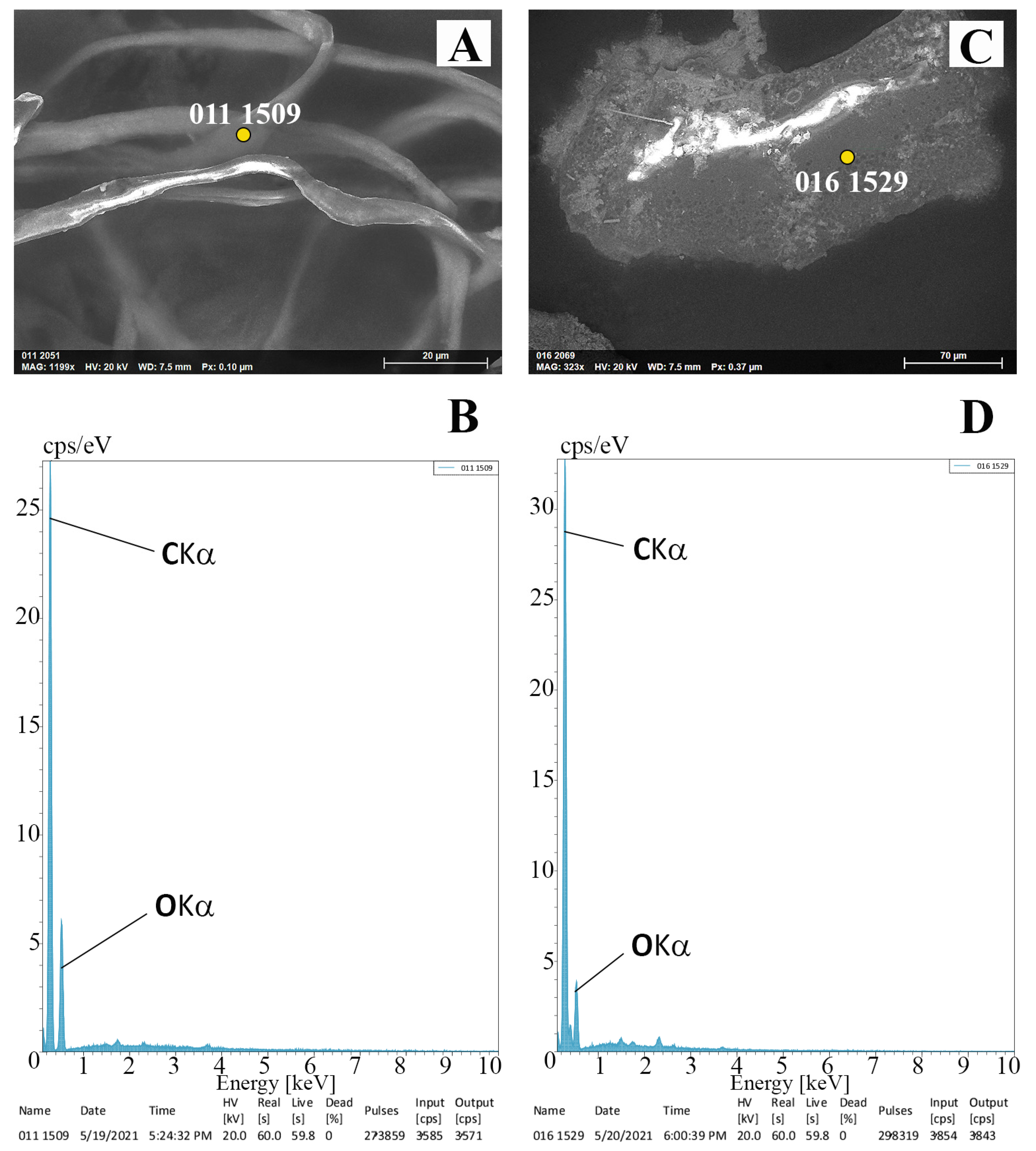
3.2. SEM/EDX Microanalyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Simon, L.; Grelaud, M.; i Orellana, J.G.; Ziveri, P. Microplastic particles in the Ebro Delta, Spain: Occurrence, composition and sources. In MICRO 2018. Fate and Impact of Microplastics: Knowledge, Actions and Solutions; MSFS-RBLZ: Lanzarote, Spain, 2018; p. 136. [Google Scholar]
- Dehaut, A.; Cassone, A.-L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef] [PubMed]
- do Sul, J.A.I.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; Van Der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Poll. 2014, 185, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. Mar. Anthropog. Litter 2015, 117–140. [Google Scholar] [CrossRef]
- Balzan, M.V.H.; Hausson, A.E.R.; Aroua, N.; Baldy, V.; Bou Dagher, M.; Branquinho, C.; Dutay, J.-C.; El Bour, M.; Médail, F.; Mojtahid, M.; et al. Ecosystems. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future; First Medi-terranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; 151p. [Google Scholar]
- Kalimeris, A.; Kassis, D. Sea surface circulation variability in the Ionian-Adriatic Seas. Prog. Oceanogr. 2020, 189, 102454. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Bernardi, G.; Russo, G.F. Plastic litter transfer from sediments towards marine trophic webs: A case study on holothurians. Mar. Pollut. Bull. 2018, 135, 376–385. [Google Scholar] [CrossRef]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, M.; Pietroletti, M.; Monakhova, Y.B. FTIR spectroscopy supported by statistical techniques for the structural characterization of plastic debris in the marine environment: Application to monitoring studies. Mar. Pollut. Bull. 2016, 106, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Analyt. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm− 1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef]
- Gniadek, M.; Dąbrowska, A. The marine nano-and microplastics characterisation by SEM-EDX: The potential of the method in comparison with various physical and chemical approaches. Mar. Pollut. Bull. 2019, 148, 210–216. [Google Scholar] [CrossRef]
- Furfaro, G.; D’Elia, M.; Mariano, S.; Trainito, E.; Solca, M.; Piraino, S.; Belmonte, G. SEM/EDX analysis of stomach contents of a sea slug snacking on a polluted seafloor reveal microplastics as a component of its diet. Sci. Rep. 2022, 12, 10244. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242, 1557–1565. [Google Scholar] [CrossRef]
- Denuncio, P.; Bastida, R.; Dassis, M.; Giardino, G.; Gerpe, M.; Rodríguez, D. Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar. Pollut. Bull. 2011, 62, 1836–1841. [Google Scholar] [CrossRef]
- Laist, D.W. Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine Debris: Sources, Impacts, and Solutions; Springer: Berlin/Heidelberg, Germany, 1997; pp. 99–139. [Google Scholar]
- Lazar, B.; Gračan, R. Ingestion of marine debris by loggerhead sea turtles, Caretta caretta, in the Adriatic Sea. Mar. Pollut. Bull. 2011, 62, 43–47. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.-L.; Heubeck, M.; Jensen, J.-K.; Le Guillou, G. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Sheavly, S.; Register, K. Marine debris & plastics: Environmental concerns, sources, impacts and solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar]
- Ryan, P.G.; Moore, C.J.; Van Franeker, J.A.; Moloney, C.L. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.; Clegg, I.; Bass, C. Marine Debris: A Global Picture of the Impact on Animal Welfare and of Animal-Focused Solutions; WSPA International: London, UK, 2012. [Google Scholar]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Sayogo, B.; Patria, M.; Takarina, N. The density of microplastic in sea cucumber (Holothuria sp.) and sediment at Tidung Besar and Bira Besar island, Jakarta. J. Phys. Conf. Ser. 2020, 1524, 012064. [Google Scholar] [CrossRef]
- Navarro, P.; García-Sanz, S.; Barrio, J.; Tuya, F. Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar. Biol. 2013, 160, 2957–2966. [Google Scholar] [CrossRef]
- Graham, E.R.; Thompson, J.T. Deposit-and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Chen, Y.; Zhao, C.; Liu, L.; Gu, Y.; Ren, Y.; Xia, B. Adverse effects of dietary virgin (nano) microplastics on growth performance, immune response, and resistance to ammonia stress and pathogen challenge in juvenile sea cucumber Apostichopus japonicus (Selenka). J. Hazard. Mater. 2022, 423, 127038. [Google Scholar] [CrossRef]
- Rabeh, I.; Telahigue, K.; Bejaoui, S.; Hajji, T.; Chouba, L.; EL Cafsi, M.h.; Soudani, N. Effects of mercury graded doses on redox status, metallothionein levels and genotoxicity in the intestine of sea cucumber Holothuria forskali. Chem. Ecol. 2019, 35, 204–218. [Google Scholar] [CrossRef]
- Tortonese, E.; Vadon, C. Oursins et Holothuries. In Fiches FAO D’identification des Espèces Pour les Besoins de la Pêche—Mediterranee et Mer Noire; Fischer, W., Bouchon, M.L., Scneider, M., Eds.; FAO Publication: Rome, Italy, 1987; pp. 734–760. [Google Scholar]
- Massin, C.J.; Jangoux, M. Observations écologiques sur Holothuria tubulosa, H. poli et H. forskali (Echinodermata-Holothuroidea) et comportement alimentaire de H. tubulosa. Cah. Biol. Mar. 1976, 17, 45–59. [Google Scholar]
- Iwalaye, O.A.; Moodley, G.K.; Robertson-Andersson, D.V. The possible routes of microplastics uptake in sea cucumber Holothuria cinerascens (Brandt, 1835). Environ. Pollut. 2020, 264, 114644. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Goodwin, D.S. Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the North Pacific Subtropical Gyre. PeerJ 2013, 1, e184. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall International Editions: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Etcheverry, M.; Ferreira, M.L.; Capiati, N.J.; Pegoretti, A.; Barbosa, S.E. Strengthening of polypropylene–glass fiber interface by direct metallocenic polymerization of propylene onto the fibers. Compos. A Appl. Sci. Manuf. 2008, 39, 1915–1923. [Google Scholar] [CrossRef]
- Ivanič, A.; Kravanja, G.; Kidess, W.; Rudolf, R.; Lubej, S. The influences of moisture on the mechanical, morphological and thermogravimetric properties of mineral wool made from basalt glass fibers. Materials 2020, 13, 2392. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.; Al-Salloum, Y.; Almusallam, T. Performance of glass fiber reinforced plastic bars as a reinforcing material for concrete structures. Compos. B Eng. 2000, 31, 555–567. [Google Scholar] [CrossRef]
- Kavad, B.; Pandey, A.; Tadavi, M.; Jakharia, H. A review paper on effects of drilling on glass fiber reinforced plastic. Proc. Technol. 2014, 14, 457–464. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Efficacy of novel Al–Zr impregnated cellulose adsorbent prepared using microwave irradiation for the facile defluoridation of water. J. Environ. Chem. Eng. 2013, 1, 1325–1335. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abd El-Aziz, E.; Abou Elmaaty, T.M.; Ramadan, S.M. Multifunctional cellulose-containing fabrics using modified finishing formulations. RSC Adv. 2017, 7, 33219–33230. [Google Scholar] [CrossRef]
- Bahsis, L.; El Ayouchia, H.B.; Anane, H.; Benhamou, K.; Kaddami, H.; Julve, M.; Stiriba, S.-E. Cellulose-copper as bio-supported recyclable catalyst for the clickable azide-alkyne [3+ 2] cycloaddition reaction in water. Int. J. Biol. Macromol. 2018, 119, 849–856. [Google Scholar] [CrossRef]
- Taylor, P.D.; Vinn, O.; Kudryavtsev, A.; Schopf, J.W. Raman spectroscopic study of the mineral composition of cirratulid tubes (Annelida, Polychaeta). J. Struct. Biol. 2010, 171, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanna, B.; Jayaraju, N.; Prasad, T.L.; Sreenivasulu, G.; Nagalakshmi, K.; Kumar, M.P.; Madakka, M. Data on Molluscan Shells in parts of Nellore Coast, southeast coast of India. Data Brief 2018, 16, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a multi-technology system. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Van, H.T.; Sy, H.L.; Nguyen, T.M.L.; Nguyen, D.K. Application of Mussell-derived biosorbent to remove NH 4+ from aqueous solution: Equilibrium and Kinetics. SN Appl. Sci. 2021, 3, 496. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Yu, X.; Ladewig, S.; Bao, S.; Toline, C.A.; Whitmire, S.; Chow, A.T. Occurrence and distribution of microplastics at selected coastal sites along the southeastern United States. Sci. Total Environ. 2018, 613, 298–305. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Grelaud, M.; Franci, M.; Ziveri, P. Are research methods shaping our understanding of microplastic pollution? A literature review on the seawater and sediment bodies of the Mediterranean Sea. Environ. Pollut. 2022, 292, 118275. [Google Scholar] [CrossRef]
- Suaria, G.; Aliani, S. Floating debris in the Mediterranean Sea. Mar. Pollut. Bull. 2014, 86, 494–504. [Google Scholar] [CrossRef]
- Thiel, M.; Hinojosa, I.; Miranda, L.; Pantoja, J.; Rivadeneira, M.; Vásquez, N. Anthropogenic marine debris in the coastal environment: A multi-year comparison between coastal waters and local shores. Mar. Pollut. Bull. 2013, 71, 307–316. [Google Scholar] [CrossRef]
- Christina Araújo, M.; Costa, M. An analysis of the riverine contribution to the solid wastes contamination of an isolated beach at the Brazilian Northeast. Manag. Environ. Qual. 2007, 18, 6–12. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Rech, S.; Macaya-Caquilpán, V.; Pantoja, J.; Rivadeneira, M.; Madariaga, D.J.; Thiel, M. Rivers as a source of marine litter–a study from the SE Pacific. Mar. Pollut. Bull. 2014, 82, 66–75. [Google Scholar] [CrossRef]
- Hanke, G.; Galgani, F.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Van Franeker, J.A.; Vlachogianni, T.; et al. Guidance on Monitoring of Marine Litter in European Seas; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Inter. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A. Microplastics in the marine environment. Mar. Poll. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Clark, J.; Lewis, C.; Halsband, C.; Galloway, T.S. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 2016, 50, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628, 740–747. [Google Scholar] [CrossRef]
- Atwood, E.C.; Falcieri, F.M.; Piehl, S.; Bochow, M.; Matthies, M.; Franke, J.; Carniel, S.; Sclavo, M.; Laforsch, C.; Siegert, F. Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: Comparing remote sensing and hydrodynamic modelling with in situ sample collections. Mar. Pollut. Bull. 2019, 138, 561–574. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Irigoien, X.; Duarte, C.M. Plastic Accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean; GESAMP Reports and Studies, No. 99; Kershaw, P.J., Turra, A., Galgani, F., Eds.; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; p. 130. Available online: http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean (accessed on 19 October 2022).
- Mansui, J.; Darmon, G.; Ballerini, T.; Van Canneyt, O.; Ourmieres, Y.; Miaud, C. Predicting marine litter accumulation patterns in the Mediterranean basin: Spatio-temporal variability and comparison with empirical data. Prog. Oceanogr. 2020, 182, 102268. [Google Scholar] [CrossRef]
- Zbyszewski, M.; Corcoran, P.L.; Hockin, A. Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J. Great Lakes Res. 2014, 40, 288–299. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603, 616–626. [Google Scholar] [CrossRef]
- Lusher, A.; Welden, N.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. In Analysis of Nanoplastics and Microplastics in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–148. [Google Scholar]
- Sbrana, A.; Valente, T.; Bianchi, J.; Franceschini, S.; Piermarini, R.; Saccomandi, F.; de Lucia, A.G.; Camedda, A.; Matiddi, M.; Silvestri, C. From inshore to offshore: Distribution of microplastics in three Italian seawaters. Environ. Sci. Pollut. Res. 2023, 30, 21277–21287. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; González, G.P.; Martínez, R.M.G.; Rojas, D.L.S.; Hernando, P.F.; Deudero, S. Assessing microplastic ingestion and occurrence of bisphenols and phthalates in bivalves, fish and holothurians from a Mediterranean marine protected area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic microfiber emissions to land rival those to waterbodies and are growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef]


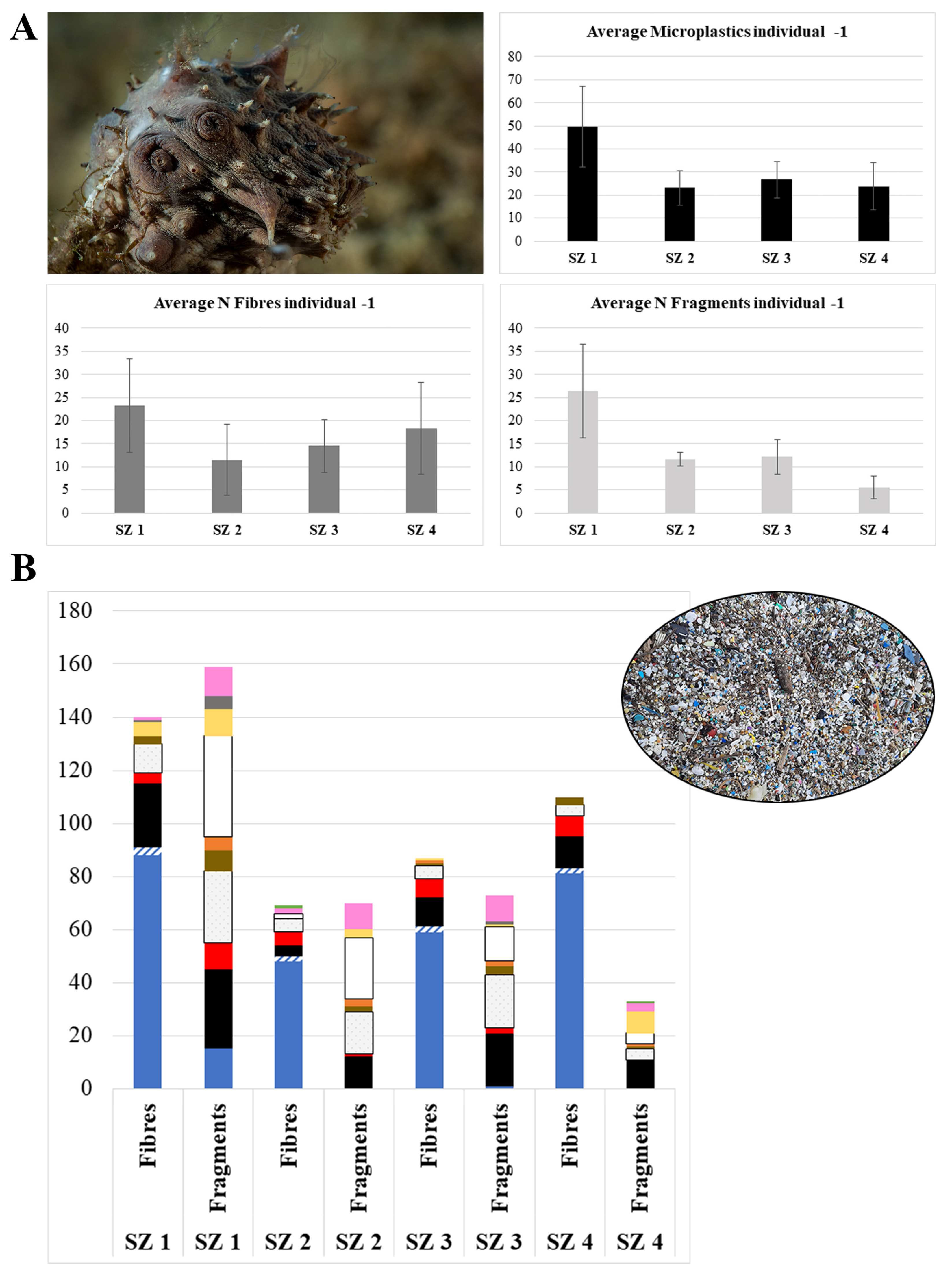
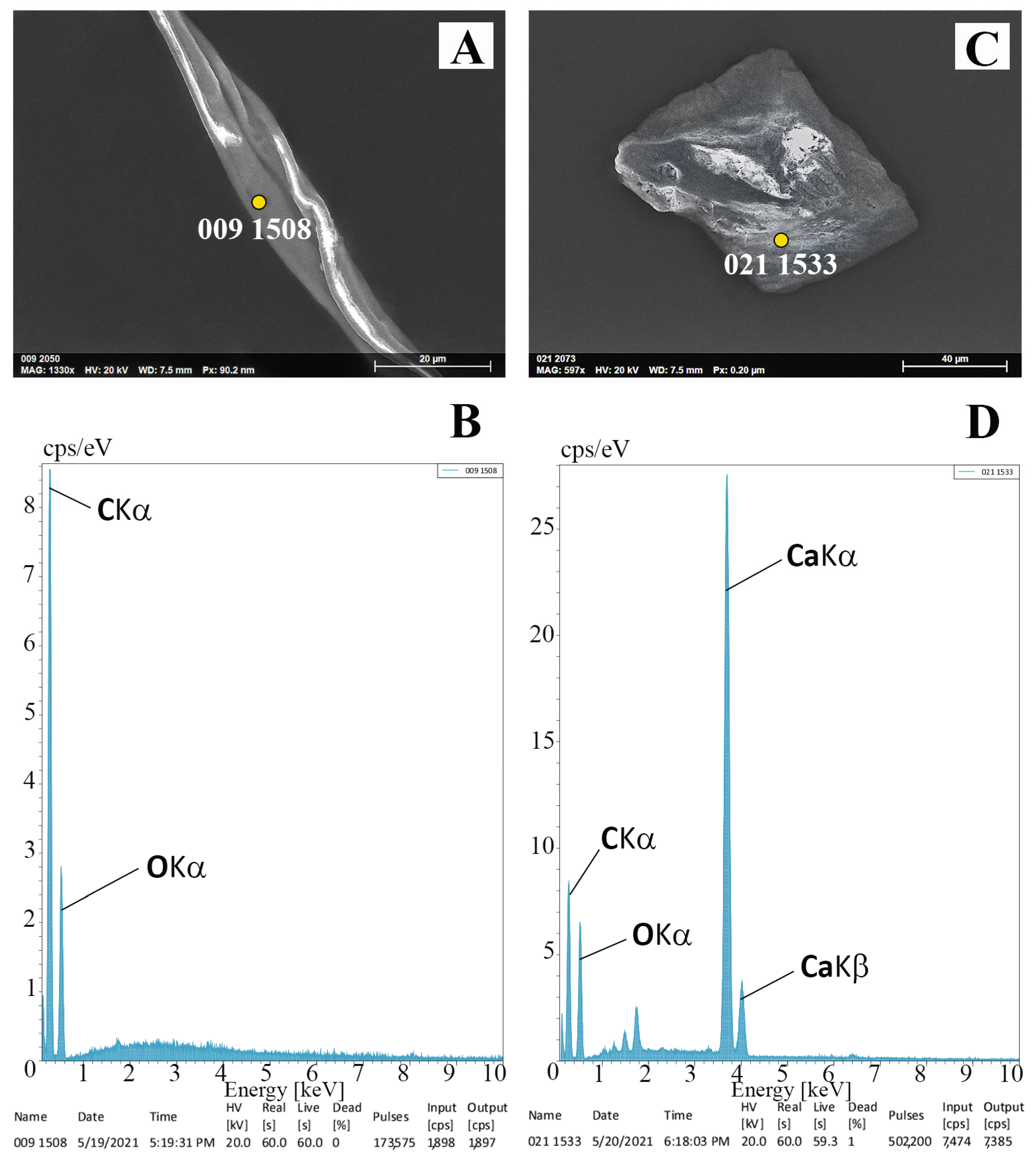
| Station | Average Fibers/Indiv. | Average Fragments/Indiv. | Average MPs/Indiv. |
|---|---|---|---|
| SZ 1 | 23 ± 10 | 27 ± 10 | 50 ± 17 |
| SZ 2 | 12 ± 8 | 12 ± 2 | 23 ± 8 |
| SZ 3 | 15 ± 6 | 12 ± 4 | 27 ± 8 |
| SZ 4 | 18 ± 10 | 6 ± 3 | 24 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martines, A.; Furfaro, G.; Solca, M.; Muzzi, M.; Di Giulio, A.; Rossi, S. An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas. Water 2023, 15, 1597. https://doi.org/10.3390/w15081597
Martines A, Furfaro G, Solca M, Muzzi M, Di Giulio A, Rossi S. An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas. Water. 2023; 15(8):1597. https://doi.org/10.3390/w15081597
Chicago/Turabian StyleMartines, Alessandra, Giulia Furfaro, Michele Solca, Maurizio Muzzi, Andrea Di Giulio, and Sergio Rossi. 2023. "An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas" Water 15, no. 8: 1597. https://doi.org/10.3390/w15081597
APA StyleMartines, A., Furfaro, G., Solca, M., Muzzi, M., Di Giulio, A., & Rossi, S. (2023). An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas. Water, 15(8), 1597. https://doi.org/10.3390/w15081597








