Planktonic Copepoda along the Confinement Gradient of the Taranto Sea System (Southern Italy) after Lockdown of Human Activities Due to the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
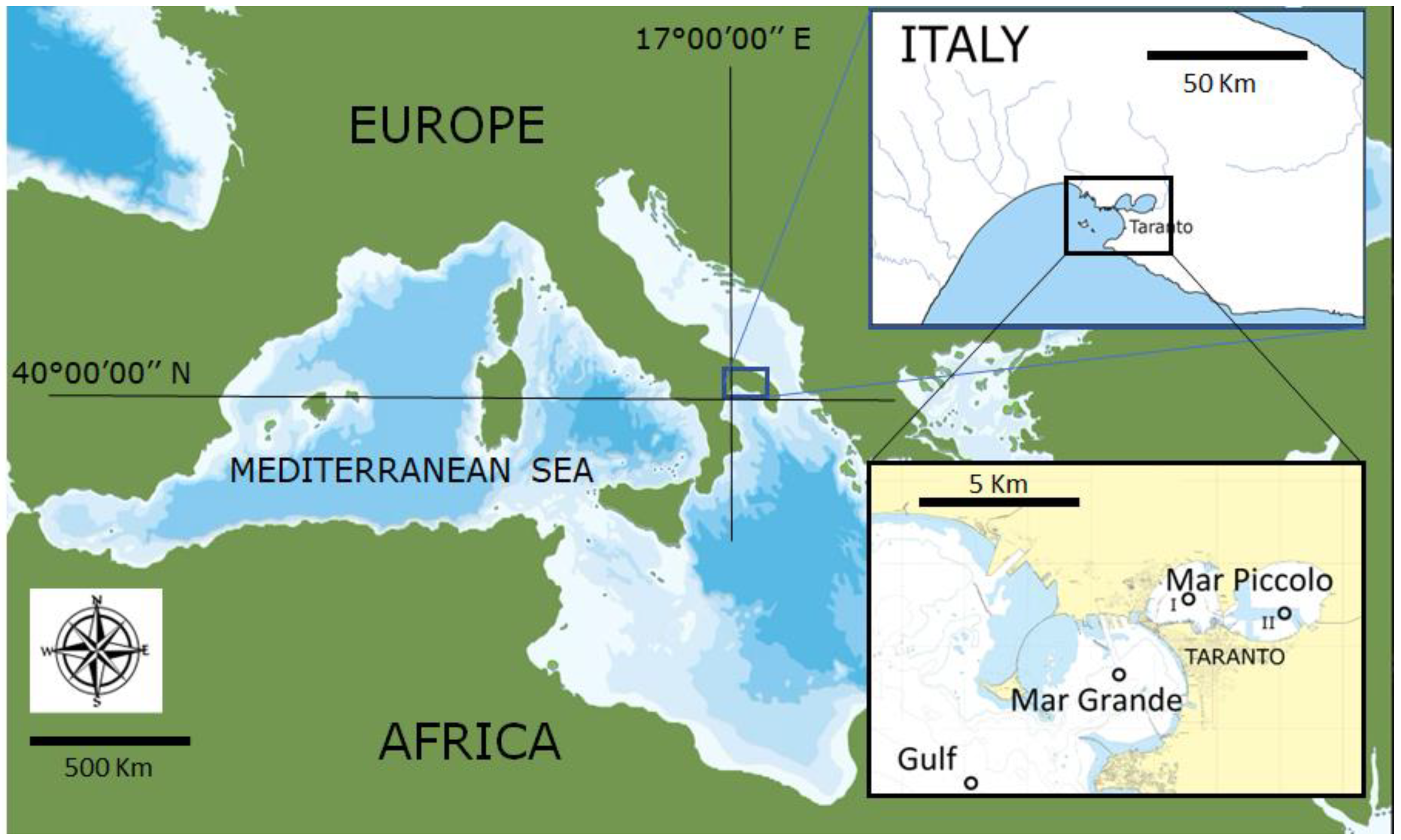
2.2. Field Work
2.3. Laboratory Work
2.4. Comparison with Historical Collections
2.5. Statistical Analysis
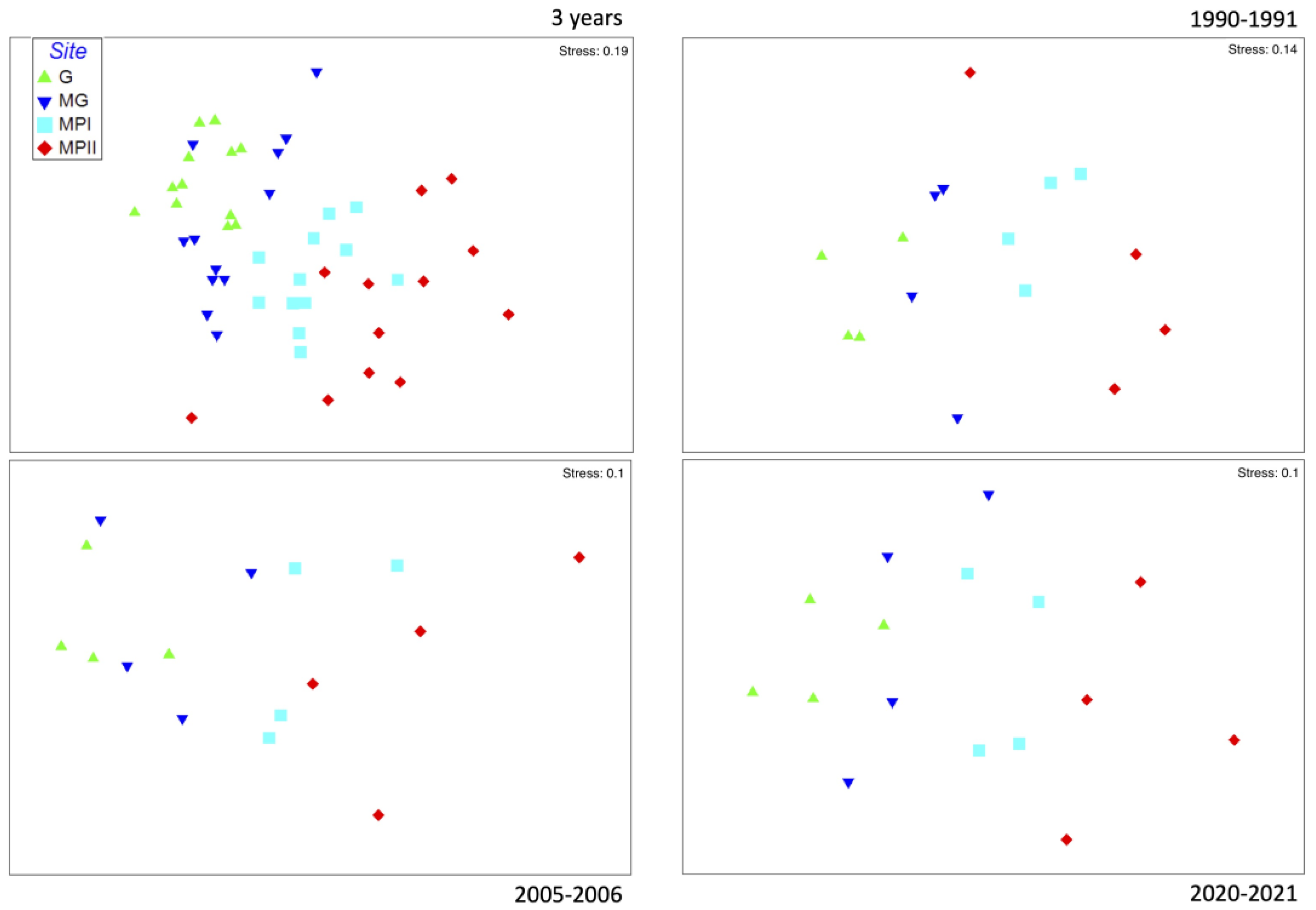
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belmonte, G.; Vaglio, I.; Rubino, F.; Alabiso, G. Zooplankton composition along the confinement gradient of the Taranto Sea System (Ionian Sea, south-eastern Italy). J. Mar. Syst. 2013, 128, 222–238. [Google Scholar] [CrossRef]
- Rice, E.; Dam, H.G.; Stewart, G. Impact of climate change on estuarine zooplankton: Surface water warming in Long Island Sound is associated with changes in copepod size and community structure. Estuaries Coasts 2015, 35, 13–23. [Google Scholar] [CrossRef]
- Guelorget, O.; Perthuisot, J.P. Paralic ecosystem. Biological organization and functioning. Vie Milieu 1992, 42, 215–251. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. Annu. Rev. 2019, 57, 1–88. [Google Scholar]
- Philippi, T.; Sen, J. Hedging one’s evolutionary bets, revisited. Trends Ecol. Evol. 1989, 4, 41–44. [Google Scholar] [CrossRef]
- Kerfoot, W.C.; Weider, L.J. Experimental paleoecology (resurrection ecology): Chasing Van Valen’s Red Queen hypothesis. Limnol. Oceanogr. 2004, 49 Pt 2, 1300–1316. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Bilal, M.; Ishaq, A.; Din Dar, M.U.; Kumar, R.; Bhat, R.M.; Sher, F. Impact of COVID-related lockdowns on environmental and climate change scenarios. Environ. Res. 2021, 195, 110839. [Google Scholar] [CrossRef]
- Braga, F.; Ciani, D.; Colella, S.; Organelli, E.; Pitarch, J.; Brando, V.E.; Bresciani, M.; Concha, J.A.; Giardino, C.; Scarpa, G.M.; et al. COVID-19 lockdown effects on a coastal marine environment: Disentangling perception versus reality. Sci. Total Environ. 2022, 817, 153002. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, G.; Fanelli, G.; Gravili, C.; Rubino, F. Composition, distribution and seasonality of zooplankton in Taranto seas (Ionian sea, Italy). Biol. Mar. Mediterr. 2001, 8, 352–362. [Google Scholar]
- Alabiso, G.; Cannalire, M.; Ghionda, D.; Milillo, M.; Leone, G.; Caciorna, O. Particulate matter and chemical-physical conditions of an inner sea: The Mar Piccolo in Taranto. A new statistical approach. Mar. Chem. 1997, 58, 373–388. [Google Scholar] [CrossRef]
- Alabiso, G.; Giacomini, M.; Milillo, M.; Ricci, P. The Taranto sea system: 8 years of chemical–physical measurements. Biol. Mar. Mediterr. 2005, 12, 369–373. [Google Scholar]
- Vatova, A. La produttività delle acque del Mar Grande e del Mar Piccolo di Taranto (1962–1969). Boll. Pesca Piscic. Idrobiol. 1972, 27, 81–103. [Google Scholar]
- Strusi, A.; Pastore, M. Osservazioni idrografiche nel Mar Grande e nel Mar Piccolo di Taranto. Campagna 1970–1971. Oebalia 1975, 1, 1–64. [Google Scholar]
- Uye, S.-I. Replacement of large copepods by small ones with eutrophication of embayments. Causes and consequences. Hydrobiologia 1994, 292, 513–519. [Google Scholar] [CrossRef]
- Boltovskoy, D. South Atlantic Zooplankton; Backhuys Publishers: Leiden, The Netherlands, 1999; Volume 2, pp. 869–1098. [Google Scholar]
- Vives, F.; Shmeleva, A.A. Crustacea, Copepodos Marinos I. Calanoida. Fauna Iber. 2007, 29, 1153. [Google Scholar]
- Belmonte, G. Acartiidae Sars G.O., 1903; ICES Identification Leaflet 194 for Plankton, Report; International Council for the Exploration of the Sea: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of biodiversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131. [Google Scholar] [CrossRef]
- Razouls, C.; Desreumaux, N.; Kouenberg, J.; de Bovée, F. Biodiversity of Marine Planktonic Copepods (Morphology, Geographical Distribution and Biological Data). Sorbonne University, CNRS, 2005–2023. Available online: http://copepodes.obs-banyuls.fr/en (accessed on 27 May 2023).
- Belmonte, G. Calanoida (Crustacea: Copepoda) from the Italian fauna. A Review. Eur. Zool. J. 2018, 85, 274–290. [Google Scholar] [CrossRef]
- Belmonte, G.; Bianchi, C.N. Zooplankton structure and distribution in a brackish water basin. Oebalia 1992, 18, 1–15. [Google Scholar]
- Acri, F.; Bernardi Aubry, F.; Berton, A.; Bianchi, F.; Boldrin, A.; Camatti, E.; Comaschi, A.; Rabitti, S.; Socal, G. Plankton communities and nutrients in the Venice Lagoon: Comparison between current and old data. J. Mar. Syst. 2004, 51, 321–329. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Ribera d’Alcalà, M. Recurrent patterns in zooplankton structure and succession in a variable coastal environment. ICES J. Mar. Sci. 1995, 52, 679691. [Google Scholar] [CrossRef]
- Kurt, T.; Polat, S. Zooplankton abundance, biomass, and size structure in the coastal waters of the northeastern Mediterranean Sea. Turk. J. Zool. 2015, 39, 2. [Google Scholar] [CrossRef]
- Annabi-Trabelsi, N.; Guermazi, W.; Leignel, V.; Al-Enezi, Y.; Karam, Q.; Ali, M.; Ayadi, H.; Belmonte, G. Effects of Eutrophication on Plankton Abundance and Composition in the Gulf of Gabès (Mediterranean Sea, Tunisia). Water 2022, 14, 2230. [Google Scholar] [CrossRef]
- Camatti, E.; Acri, F.; De Lazzari, A.; Nurra, N.; Pansera, M.; Schroeder, A.; Bergamasco, A. Natural or anthropogenic variability? A long-term pattern of the zooplankton communities in an ever-changing transitional ecosystem. Frontiers 2023, 10, 1176829. [Google Scholar] [CrossRef]
- Rubino, F.; Belmonte, G. Habitat Shift for Plankton: The Living Side of Benthic-Pelagic Coupling in the Mar Piccolo of Taranto (Southern Italy, Ionian Sea). Water 2021, 13, 3619. [Google Scholar] [CrossRef]
- Belmonte, G.; Potenza, D. Biogeography of the family Acartiidae (Calanoida) in the Ponto-Mediterranean Province. Hydrobiologia 2001, 453/454, 171–176. [Google Scholar] [CrossRef]
- Lučić, D.; Mozetič, P.; Francé, J.; Lučić, P.; Lipej, L. Additional record of the non-indigenous copepod Pseudodiaptomus marinus (Sato, 1913) in the Adriatic Sea. Acta Adriat. 2015, 56, 275–282. [Google Scholar]
- Gravili, C.; Belmonte, G.; Cecere, E.; Denitto, F.; Giangrande, A.; Guidetti, P.; Longo, C.; Mastrototaro, F.; Moscatello, S.; Petrocelli, A.; et al. Non indigenous species along the Apulian coast, Italy. Chem. Ecol. 2010, 26, 121–142. [Google Scholar] [CrossRef]
| 1990–1991 | 2005–2006 | 2020–2021 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUM (Jul.) | AUT (Oct.) | WIN (Jan.) | SPR (Apr.) | Average 1990–1991 | SUM (Jul.) | AUT (Oct.) | WIN (Jan.) | SPR (Apr.) | Average 2005–2006 | SUM (Jul.) | AUT (Oct.) | WIN (Jan.) | SPR (Apr.) | Average 2020–2021 | ||
| Station G | lat. 40°25′08″ N, long. 17°10′45″ E | |||||||||||||||
| Water column, 50 m | ||||||||||||||||
| Sample vol., m3 | 3.3 | 3.5 | 3.6 | 3.4 | 3.45 | 4.40 | 4.10 | 4.70 | 4.30 | 4.38 | 3.90 | 4.30 | 4.50 | 4.00 | 4.18 | |
| Temp. °C | surface | 25.10 | 21.10 | 14.20 | 14.80 | 18.80 | 25.20 | 26.10 | 14.80 | 17.00 | 20.78 | 26.10 | 26.60 | 14.20 | 13.10 | 20.00 |
| bottom | 17.50 | 15.50 | 14.00 | 13.90 | 15.23 | 21.10 | 22.10 | 14.80 | 15.20 | 18.30 | 19.20 | 22.30 | 13.50 | 14.00 | 17.25 | |
| Oxygen % | surface | 74.90 | 87.20 | 86.00 | 83.90 | 83.00 | 100.10 | 98.30 | 92.30 | 100.80 | 97.88 | 91.60 | 102.50 | 75.50 | 92.70 | 90.58 |
| bottom | 90.20 | 90.00 | 83.10 | 82.00 | 86.33 | 115.40 | 111.50 | 101.90 | 110.10 | 109.73 | 126.20 | 112.70 | 82.70 | 96.70 | 104.58 | |
| Sal. ‰ | surface | 38.50 | 38.50 | 38.00 | 38.30 | 38.33 | 37.20 | 37.50 | 38.70 | 38.40 | 37.95 | 37.20 | 37.30 | 38.80 | 38.20 | 37.88 |
| bottom | 38.60 | 38.50 | 38.30 | 38.50 | 38.48 | 37.90 | 37.60 | 38.80 | 38.90 | 38.30 | 37.90 | 37.80 | 38.90 | 38.80 | 38.35 | |
| Station MG | lat. 40°27′35″ N, long. 17°13′28″ E | |||||||||||||||
| Water column, 25 m | ||||||||||||||||
| Sample vol., m3 | 3.30 | 3.30 | 3.00 | 3.10 | 3.18 | 2.80 | 3.10 | 2.60 | 2.80 | 2.83 | 3.10 | 3.00 | 3.50 | 2.60 | 3.05 | |
| Temp. °C | surface | 25.00 | 20.30 | 13.10 | 14.90 | 18.33 | 27.30 | 19.40 | 13.60 | 14.80 | 18.78 | 26.70 | 26.80 | 12.10 | 13.30 | 19.73 |
| bottom | 23.00 | 15.10 | 12.80 | 14.00 | 16.23 | 22.40 | 19.20 | 14.20 | 14.60 | 17.60 | 23.80 | 25.70 | 12.90 | 14.00 | 19.10 | |
| Oxygen % | surface | 86.70 | 82.00 | 84.40 | 85.00 | 84.53 | 110.50 | 102.70 | 100.00 | 103.00 | 104.05 | 95.20 | 97.00 | 88.80 | 67.80 | 87.20 |
| bottom | 89.30 | 88.60 | 87.90 | 86.70 | 88.13 | 101.90 | 92.70 | 102.20 | 104.50 | 100.33 | 108.10 | 100.20 | 92.40 | 79.40 | 95.03 | |
| Sal. ‰ | surface | 39.00 | 37.80 | 37.80 | 38.20 | 38.20 | 38.30 | 38.00 | 37.80 | 37.40 | 37.88 | 37.20 | 37.40 | 38.10 | 38.10 | 37.70 |
| bottom | 38.60 | 38.60 | 38.00 | 38.50 | 38.43 | 37.70 | 38.50 | 38.60 | 38.30 | 38.28 | 37.70 | 37.80 | 38.80 | 38.70 | 38.25 | |
| Station MP I | lat. 40°29′24″ N, long. 17°15′54″ E | |||||||||||||||
| Water column, 12 m | ||||||||||||||||
| Sample vol., m3 | 1.30 | 1.50 | 1.10 | 1.20 | 1.28 | 1.60 | 1.40 | 1.70 | 1.50 | 1.55 | 1.20 | 2.10 | 3.00 | 1.60 | 1.98 | |
| Temp. °C | surface | 25.60 | 21.10 | 11.90 | 15.20 | 18.45 | 28.10 | 19.40 | 12.40 | 14.80 | 18.68 | 27.30 | 27.50 | 10.60 | 13.00 | 19.60 |
| bottom | 24.00 | 21.80 | 13.20 | 14.50 | 18.38 | 26.00 | 19.80 | 13.90 | 14.80 | 18.63 | 25.40 | 26.60 | 12.10 | 13.40 | 19.38 | |
| Oxygen % | surface | 90.00 | 84.30 | 90.30 | 90.10 | 88.68 | 97.70 | 99.10 | 99.70 | 103.90 | 100.10 | 98.40 | 101.70 | 86.30 | 74.30 | 90.18 |
| bottom | 80.10 | 86.80 | 89.00 | 88.00 | 85.98 | 84.00 | 88.20 | 97.80 | 109.70 | 94.93 | 104.20 | 104.80 | 94.30 | 87.00 | 97.58 | |
| Sal. ‰ | surface | 37.60 | 37.70 | 36.30 | 36.50 | 37.03 | 37.10 | 37.20 | 36.10 | 35.60 | 36.50 | 35.80 | 35.80 | 36.70 | 36.60 | 36.23 |
| bottom | 38.30 | 38.10 | 37.60 | 38.10 | 38.03 | 38.60 | 37.9 | 37.10 | 38.40 | 38.03 | 37.40 | 37.30 | 38.40 | 38.20 | 37.83 | |
| Station MP II | lat. 40°29′28″ N, long. 17°18′00″ E | |||||||||||||||
| Water column, 9 m | ||||||||||||||||
| Sample vol., m3 | 1.40 | 1.20 | 1.00 | 1.10 | 1.18 | 1.30 | 1.10 | 1.20 | 1.40 | 1.25 | 1.10 | 0.40 | 1.20 | 1.50 | 1.05 | |
| Temp. °C | surface | 25.90 | 20.30 | 10.20 | 15.30 | 17.93 | 28.90 | 21.00 | 11.20 | 15.00 | 19.03 | 27.90 | 27.50 | 10.00 | 12.60 | 19.50 |
| bottom | 23.40 | 20.20 | 11.00 | 15.10 | 17.43 | 27.10 | 21.40 | 12.50 | 14.70 | 18.93 | 26,20 | 26.80 | 11.00 | 13.60 | 19.40 | |
| Oxygen % | surface | 88.60 | 87.00 | 87.10 | 87.60 | 87.58 | 101.00 | 99.80 | 117.60 | 106.20 | 106.15 | 99,20 | 95.20 | 91.00 | 78.30 | 90.93 |
| bottom | 84.50 | 82.70 | 88.00 | 88.00 | 85.80 | 91.30 | 96.10 | 107.20 | 110.30 | 101.23 | 104.60 | 90.30 | 97.00 | 90.00 | 95.48 | |
| Sal. ‰ | surface | 37.70 | 37.50 | 36.00 | 36.50 | 36.93 | 37.00 | 37.20 | 35.90 | 36.10 | 36.55 | 35.80 | 36.10 | 35.90 | 36.30 | 36.03 |
| bottom | 38.20 | 37.60 | 37.10 | 37.50 | 37.60 | 37.90 | 37.60 | 37.90 | 37.90 | 37.83 | 36.70 | 37.00 | 36.50 | 37.30 | 36.88 | |
| Total, Times | Total, Stations | Total, Seasons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAXON | T1 | T2 | T3 | G | MG | MPI | MPII | SUM | AUT | WIN | SPR |
| Acartia adriatica | 0.06 | 0.17 | 0.17 | 0.08 | |||||||
| Acartia clausi | 305.16 | 100.10 | 84.95 | 164.27 | 412.18 | 222.27 | 395.24 | 164.27 | 599.08 | 356.31 | 834.36 |
| Acartia discaudata var. medit | 0.35 | 2.72 | 0.38 | 1.29 | 6.49 | 0.92 | 1.29 | 7.00 | 4.54 | 0.70 | |
| Acartia italica | 48.00 | 21.80 | 12.84 | 83.17 | 56.36 | 63.73 | 116.19 | 86.58 | 127.79 | ||
| Acartia margalefi | 0.19 | 3.19 | 0.10 | 0.51 | 2.75 | 1.02 | 4.87 | 0.51 | 2.89 | 5.27 | 5.26 |
| Acartia negligens | 0.20 | 1.24 | 1.51 | 4.16 | 1.71 | 4.16 | 3.73 | 3.67 | 0.27 | ||
| Acartia tonsa | 0.25 | 0.28 | 0.06 | 0.62 | 0.34 | ||||||
| Paracartia latisetosa | 81.38 | 6.64 | 5.91 | 71.33 | 138.97 | 31.70 | 80.90 | 154.02 | 140.20 | ||
| Pteriacartia josephinae | 2.76 | 1.19 | 1.18 | 0.62 | 11.00 | 0.62 | 14.15 | 1.58 | 3.69 | ||
| Augaptilus sp. | 0.11 | 0.21 | 0.11 | 1.01 | 1.01 | 0.15 | 0.28 | 0.30 | |||
| Haloptilus longicornis | 0.20 | 0.48 | 0.48 | 0.27 | |||||||
| Calanopia sp. | 0.04 | 0.10 | 0.10 | 0.05 | |||||||
| Calanus helgolandicus | 6.94 | 12.42 | 6.08 | 12.42 | 6.08 | 9.25 | |||||
| Mesocalanus sp. | 0.05 | 0.14 | 0.00 | 0.14 | 0.07 | ||||||
| Nannocalanus minor | 0.25 | 1.21 | 0.59 | 4.01 | 0.67 | 4.01 | 1.46 | 2.40 | 0.33 | ||
| Neocalanus sp. | 0.03 | 0.29 | 0.08 | 0.38 | 0.08 | 0.77 | 0.43 | ||||
| Candacia undet. | 1.18 | 1.49 | 1.30 | 8.27 | 0.58 | 8.27 | 2.88 | 2.53 | 1.80 | ||
| Paracandacia sp. | 0.24 | 0.63 | 0.63 | 0.32 | |||||||
| Centropages kroyeri | 29.69 | 5.90 | 5.33 | 2.81 | 64.81 | 32.10 | 2.75 | 2.81 | 77.13 | 41.70 | 42.34 |
| Centropages hamatus | 0.20 | 0.27 | 0.54 | ||||||||
| Centropages ponticus | 33.69 | 0.42 | 23.93 | 1.38 | 63.90 | 51.18 | 6.40 | 1.38 | 121.77 | 53.92 | 52.49 |
| Centropages typicus | 11.43 | 5.51 | 8.92 | 24.47 | 28.96 | 0.53 | 2.75 | 24.47 | 40.92 | 8.26 | 21.32 |
| Centropages violaceus | 1.53 | 24.30 | 0.19 | 52.17 | 11.50 | 5.00 | 0.19 | 52.17 | 43.90 | 7.04 | |
| Isias clavipes | 57.46 | 175.08 | 162.92 | 55.97 | 622.27 | 117.03 | 42.06 | 55.97 | 1029.05 | 375.71 | 120.71 |
| Clausocalanus arcuicornis | 12.76 | 4.69 | 0.99 | 22.01 | 24.91 | 0.92 | 22.01 | 26.52 | 7.17 | 17.55 | |
| Clausocalanus furcatus | 19.26 | 3.32 | 36.81 | 36.14 | 71.90 | 1.25 | 36.14 | 121.02 | 50.51 | 26.03 | |
| Clausocalanus joboei | 4.91 | 6.26 | 18.17 | 3.03 | 0.25 | 18.17 | 11.38 | 2.15 | 7.27 | ||
| Clausocalanus paululus | 3.26 | 2.08 | 9.89 | 1.58 | 9.89 | 4.82 | 4.56 | ||||
| Calocalanus sp. | 15.20 | 45.27 | 5.84 | 113.55 | 28.58 | 1.89 | 25.00 | 113.55 | 36.70 | 66.47 | 46.62 |
| Paracalanus spp. | 85.09 | 1001.82 | 286.98 | 83.14 | 2378.33 | 634.82 | 179.94 | 83.14 | 3023.43 | 2003.70 | 350.83 |
| Parvocalanus sp. | 0.06 | 0.08 | 0.16 | 0.11 | 0.16 | 0.11 | 0.11 | 0.19 | |||
| Pseudocalanus sp. | 0.40 | 1.00 | 1.90 | 1.67 | 0.17 | 1.90 | 1.67 | 1.50 | 0.54 | ||
| Ctenocalanus vanus | 1.29 | 4.83 | 15.15 | 28.90 | 7.54 | 0.08 | 28.90 | 27.78 | 7.49 | 2.39 | |
| Diaixis sp. | 0.41 | 1.74 | 4.51 | 1.23 | 4.51 | 1.23 | 2.33 | 0.54 | |||
| Eucalanus sp. | 11.53 | 0.11 | 0.78 | 3.29 | 23.78 | 5.00 | 3.29 | 25.53 | 5.14 | 15.37 | |
| Pareuchaeta sp. | 0.26 | 2.41 | 6.77 | 0.33 | 6.77 | 0.33 | 3.21 | 0.34 | |||
| Heterorhabdus papilliger | 0.56 | 1.45 | 0.05 | 1.45 | 0.05 | 0.75 | |||||
| Lucicutia sp. | 0.69 | 0.48 | 1.83 | 4.72 | 0.74 | 4.72 | 3.39 | 1.18 | 2.20 | ||
| Macandrewella sp. | 0.31 | 0.62 | 0.38 | 1.29 | 0.38 | 2.12 | 0.83 | 0.42 | |||
| Scolecithricella sp. | 0.38 | 1.02 | 0.00 | 1.02 | 0.51 | ||||||
| Scolecithrix sp. | 0.38 | 0.66 | 1.80 | 0.11 | 1.80 | 0.99 | 0.17 | 0.54 | |||
| Mecynocera clausi | 0.77 | 5.93 | 1.21 | 15.12 | 3.86 | 0.17 | 0.33 | 15.12 | 5.52 | 9.35 | 1.47 |
| Pleuromamma gracilis | 0.10 | 0.32 | 1.37 | 1.37 | 0.43 | 0.13 | |||||
| Pleuromamma xiphias | 0.00 | 0.16 | 0.70 | 1.35 | 1.35 | 0.94 | 0.42 | ||||
| Metridia sp. | 0.61 | 0.18 | 0.19 | 2.12 | 2.12 | 0.26 | 0.24 | 0.81 | |||
| Anomalocera patersoni | 0.06 | 0.17 | 0.17 | 0.08 | |||||||
| Pontella sp. | 0.14 | 2.35 | 3.00 | 0.33 | 0.17 | 3.00 | 3.47 | 0.35 | |||
| Pseudodiaptomus sp. | 1.00 | 1.33 | 2.65 | 1.33 | |||||||
| Temora longicornis | 4.31 | 1.01 | 1.83 | 9.67 | 1.34 | 1.83 | 11.29 | 8.16 | |||
| Temora stylifera | 11.11 | 3.83 | 39.57 | 39.22 | 47.34 | 5.22 | 39.22 | 108.47 | 54.58 | 14.94 | |
| Temora turbinata | 1.73 | 2.88 | 1.75 | 2.88 | 1.75 | 2.31 | |||||
| Agetus sp. | 0.36 | 0.89 | 0.89 | 0.48 | |||||||
| Corycaeus spp. | 0.06 | 11.52 | 10.34 | 3.33 | 1.69 | 0.17 | 10.34 | 23.73 | 12.03 | 0.25 | |
| Farranula rostrata | 5.66 | 5.81 | 7.64 | 24.00 | 15.15 | 1.44 | 0.20 | 24.00 | 32.37 | 11.49 | 7.90 |
| Urocorycaeus sp. | 0.03 | 0.94 | 0.49 | 0.33 | 0.51 | 0.49 | 2.43 | 0.69 | 0.08 | ||
| Oithona nana | 743.97 | 1580.46 | 1864.63 | 590.10 | 2494.86 | 9059.06 | 30,921.56 | 590.10 | 6110.44 | 12,400.89 | 32,033.11 |
| Oithona plumifera | 12.32 | 0.09 | 24.37 | 8.61 | 24.37 | 8.86 | 16.43 | ||||
| Oithona similis | 72.48 | 78.29 | 48.88 | 209.22 | 219.07 | 38.74 | 0.20 | 209.22 | 307.52 | 175.79 | 98.22 |
| Triconia conifera | 0.67 | 1.61 | 0.17 | 1.61 | 0.17 | 0.89 | |||||
| Oncaea spp. | 156.93 | 951.01 | 222.53 | 2323.74 | 850.27 | 100.79 | 45.93 | 2323.74 | 1287.85 | 1409.44 | 303.02 |
| Copilia sp. | 0.07 | 0.03 | 0.07 | 0.36 | 0.36 | 0.09 | 0.13 | 0.09 | |||
| Vettoria sp. | 0.15 | 0.39 | 0.39 | 0.19 | |||||||
| Canuella sp. | 50.19 | 14.47 | 20.56 | 14.55 | 18.63 | 160.49 | 50.28 | 50.35 | 227.41 | ||
| Clytemnestra rostrata | 0.31 | 0.68 | 0.06 | 0.78 | 1.33 | 0.58 | 0.00 | 0.78 | 1.41 | 1.49 | 0.49 |
| Euterpina acutifrons | 466.09 | 381.71 | 447.47 | 249.21 | 1551.61 | 1379.52 | 462.63 | 249.21 | 2316.31 | 2294.45 | 1099.68 |
| Microsetella spp. | 47.36 | 113.87 | 47.98 | 222.62 | 217.17 | 46.91 | 7.21 | 222.62 | 320.38 | 206.35 | 78.53 |
| Macrosetella gracilis | 0.23 | 0.23 | 0.92 | 0.92 | 0.31 | 0.61 | |||||
| Tisbe sp. | 0.31 | 5.47 | 8.12 | 7.29 | 7.29 | 8.54 | |||||
| Monstrilla sp. | 0.58 | 0.16 | 0.03 | 1.08 | 0.60 | 0.05 | 0.03 | 1.30 | 1.37 | 0.27 | |
| Totals | 2330.81 | 4948.85 | 3694.89 | 1493.25 | 3308.62 | 4124.63 | 10,926.87 | 1493.25 | 5692.86 | 7024.08 | 12,072.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte, G.; Denti, G.; Rubino, F. Planktonic Copepoda along the Confinement Gradient of the Taranto Sea System (Southern Italy) after Lockdown of Human Activities Due to the COVID-19 Pandemic. Water 2023, 15, 2449. https://doi.org/10.3390/w15132449
Belmonte G, Denti G, Rubino F. Planktonic Copepoda along the Confinement Gradient of the Taranto Sea System (Southern Italy) after Lockdown of Human Activities Due to the COVID-19 Pandemic. Water. 2023; 15(13):2449. https://doi.org/10.3390/w15132449
Chicago/Turabian StyleBelmonte, Genuario, Giuseppe Denti, and Fernando Rubino. 2023. "Planktonic Copepoda along the Confinement Gradient of the Taranto Sea System (Southern Italy) after Lockdown of Human Activities Due to the COVID-19 Pandemic" Water 15, no. 13: 2449. https://doi.org/10.3390/w15132449
APA StyleBelmonte, G., Denti, G., & Rubino, F. (2023). Planktonic Copepoda along the Confinement Gradient of the Taranto Sea System (Southern Italy) after Lockdown of Human Activities Due to the COVID-19 Pandemic. Water, 15(13), 2449. https://doi.org/10.3390/w15132449








