Recent Advances in 1,4-Dioxane Removal Technologies for Water and Wastewater Treatment
Abstract
:1. Introduction
| Property | Value | Structure |
|---|---|---|
| Molecular weight | 88.11 g mol−1 | 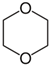 |
| Density (25 °C) | 1.033 g cm−3 | |
| Boiling point | 101.1 °C | |
| Water solubility | Miscible | |
| Henry’s law constant at 25 °C | 4.80 × 10−6 atm-m3 mol−1 | |
| Octanol-water partition coefficient (log Kow) | −0.27 | |
| Organic carbon partition coefficient (log Koc) | 1.23 |
2. Material and Methods
3. Physical-Chemical Processes for 1,4-Dioxane Removal
3.1. 1,4-Dioxane Removal by Adsorption
| Adsorbent | qmax (mg-1,4-Dioxane g-Adsorbent−1) | Desorption | Reference |
|---|---|---|---|
| Norit 1240 GAC | 59.56 | 50% desorption when rising with ammonia mineral salt medium | [24] |
| Sawdust GAC | 0.410 | Not tested | [25] |
| ZSM-5 zeolite | 22.44 to 107.36 | Not tested | [27] |
| Titanium silicate (TS-1) | 85.1 | 60% desorption when rising with mineral salt medium | [26] |
| Thiol-functionalized titanium silicate (TS-SH) | 112 | Quick desorption with 1 M HNO3 | [28] |
| Sulfonic acid functionalized titanium silicate (TS-SO3H) | 164 | Quick desorption with 1 M HNO3 | [28] |
| AmbersorbTM 560 polymer | ~200 | Not tested | [29] |
| Resorcinarene cavitand polymers | N/A | Not tested | [30] |
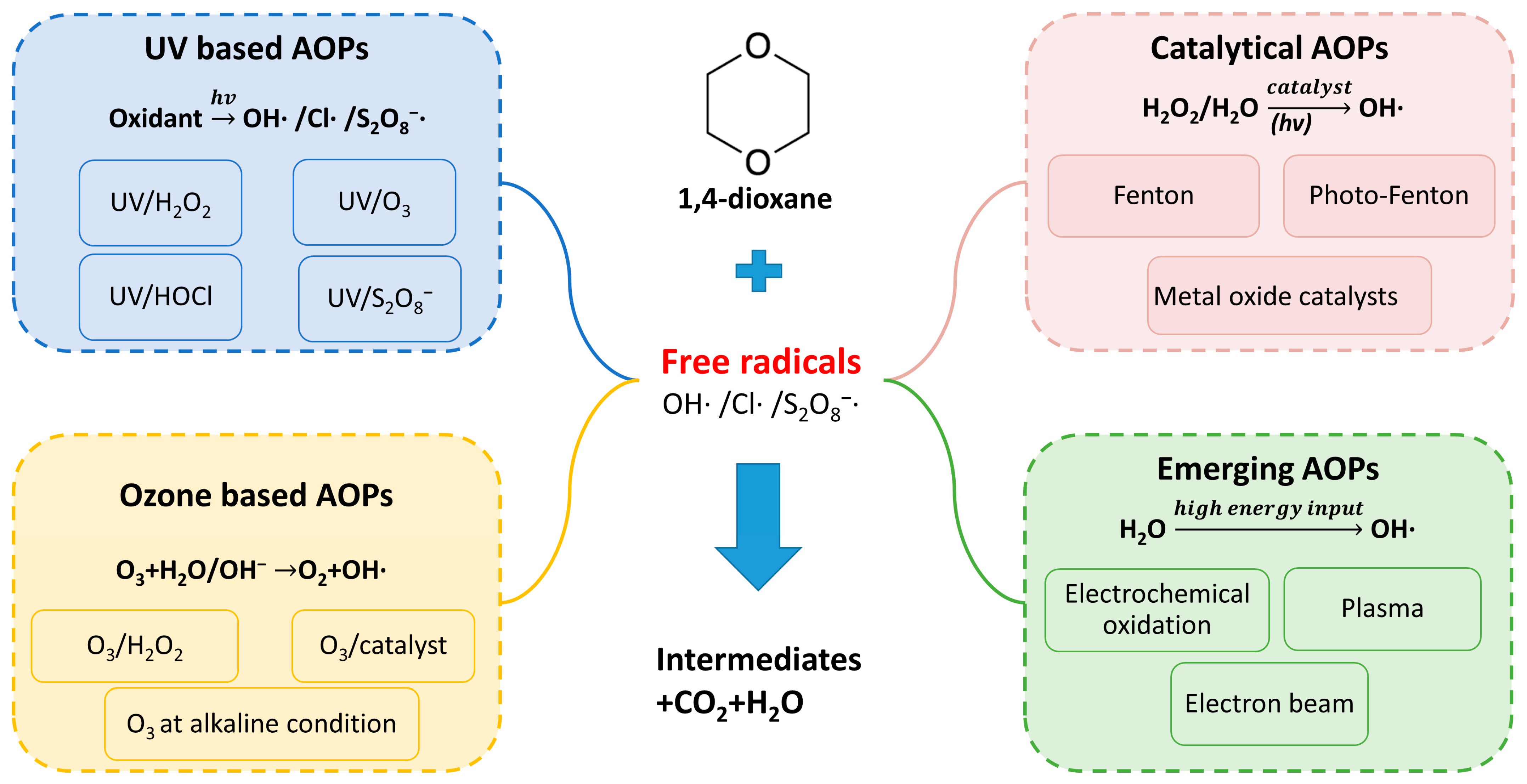
3.2. 1,4-Dioxane Removal by Advanced Oxidation Processes
4. Biological Treatment for 1,4-Dioxane Removal
4.1. Microbiology of 1,4-Dioxane Degrading Pure Strains and Microbial Communities
4.1.1. Aerobic 1,4-Dioxane Biodegradation
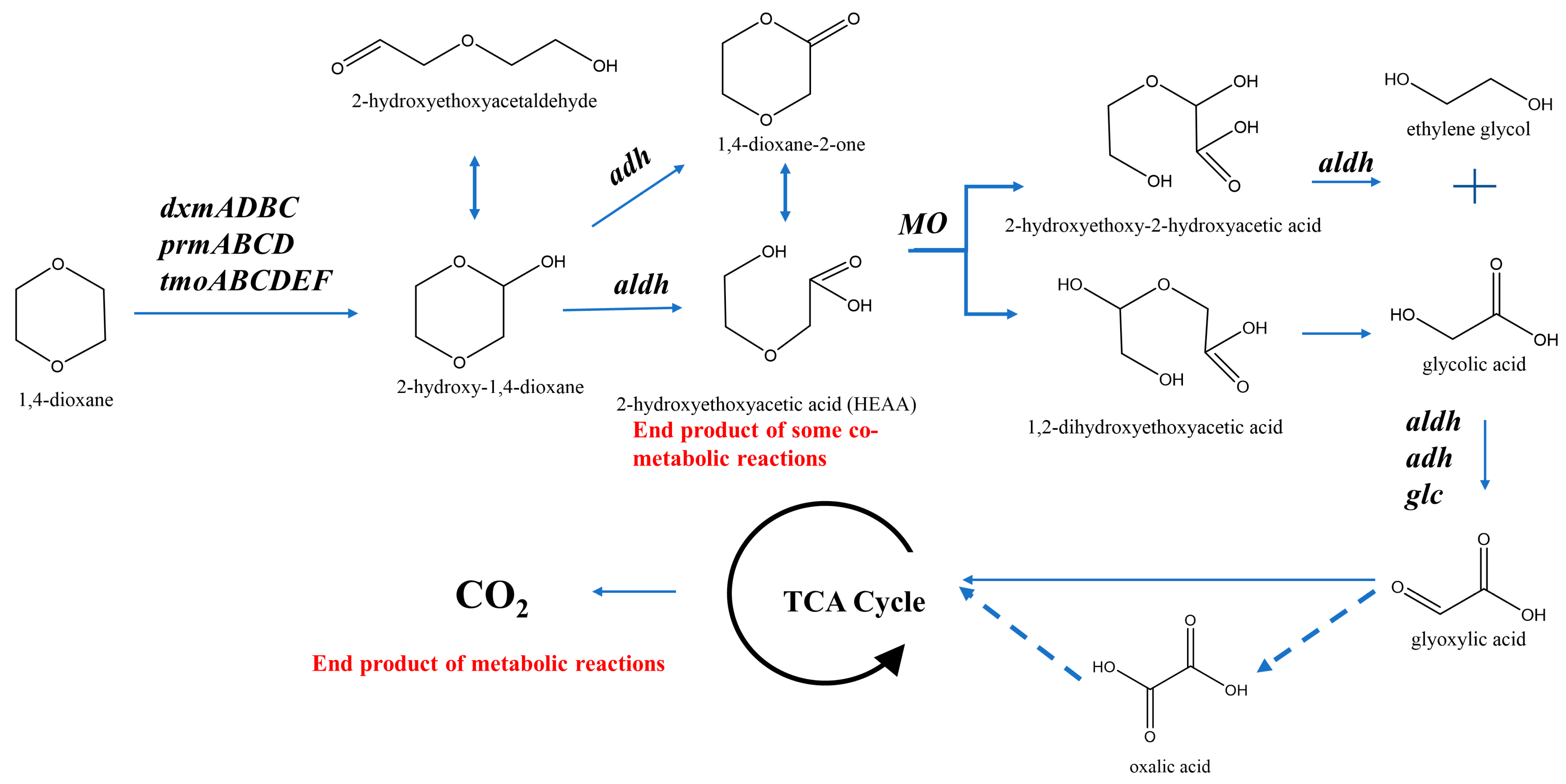
4.1.2. Functional Enzymes for 1,4-Dioxane Biodegradation
4.1.3. Effect of Co-Contaminants on 1,4-Dioxane Biodegradation
4.1.4. Anaerobic 1,4-Dioxane Biodegradation
4.2. Biotechnologies for 1,4-Dioxane Removal
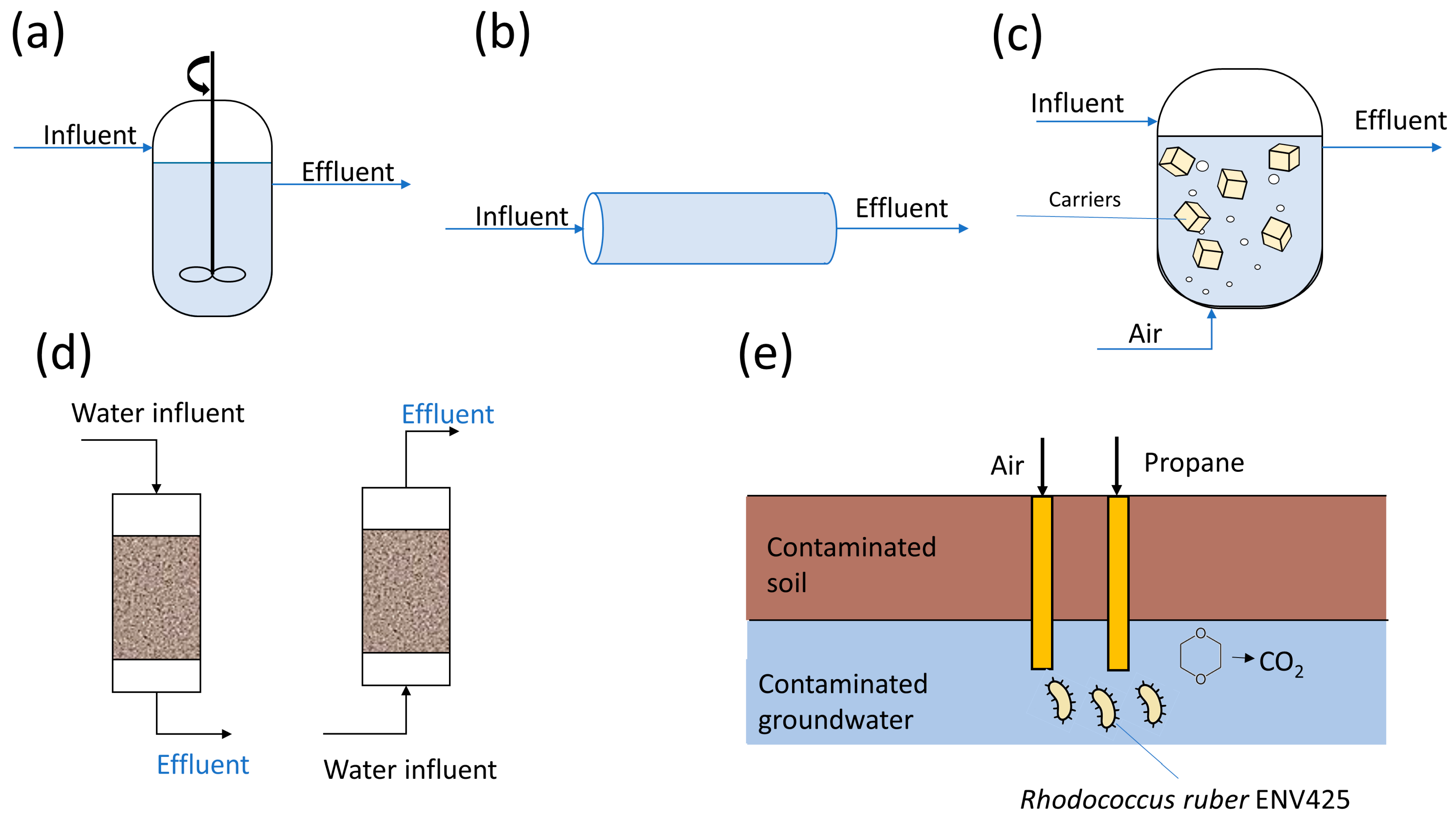
4.2.1. Suspended Growth Bioreactors for 1,4-Dioxane Removal
4.2.2. Immobilized Cell Bioreactors for 1,4-Dioxane Removal
4.2.3. Biofiltration System for 1,4-Dioxane Removal
4.2.4. Biostimulation and Natural Attenuation
5. Challenges and Opportunities of 1,4-Dioxane Removal Technologies
5.1. Metabolic 1,4-Dioxane Degradation at Environmental Relevant Conditions
5.2. Co-Existence of Chlorinated Solvents
5.3. Combined Process for 1,4-Dioxane Removal
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Occurrence and Treatment of 1,4-Dioxane in Aqueous Environments. Environ. Eng. Sci. 2003, 20, 423–432. [Google Scholar] [CrossRef]
- Mohr, T.K.G.; DiGuiseppi, W.H.; Hatton, J.W.; Anderson, J.K. Environmental Investigation and Remediation: 1,4-Dioxane and Other Solvent Stabilizers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-68578-1. [Google Scholar]
- EPA: High Production Volume List. Available online: https://comptox.epa.gov/dashboard/chemical-lists/EPAHPV (accessed on 2 March 2023).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for 1,4-Dioxane; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Anderson, R.H.; Anderson, J.K.; Bower, P.A. Co-Occurrence of 1,4-Dioxane with Trichloroethylene in Chlorinated Solvent Groundwater Plumes at US Air Force Installations: Fact or Fiction. Integr. Environ. Assess. Manag. 2012, 8, 731–737. [Google Scholar] [CrossRef]
- Doherty, A.-C.; Lee, C.-S.; Meng, Q.; Sakano, Y.; Noble, A.E.; Grant, K.A.; Esposito, A.; Gobler, C.J.; Venkatesan, A.K. Contribution of Household and Personal Care Products to 1,4-Dioxane Contamination of Drinking Water. Curr. Opin. Environ. Sci. Health 2023, 31, 100414. [Google Scholar] [CrossRef]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-Dioxane in the Aquatic Environment: From Sewage to Drinking Water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Stickney, J.A.; Sager, S.L.; Clarkson, J.R.; Smith, L.A.; Locey, B.J.; Bock, M.J.; Hartung, R.; Olp, S.F. An Updated Evaluation of the Carcinogenic Potential of 1,4-Dioxane. Regul. Toxicol. Pharmacol. 2003, 38, 183–195. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. The Drinking Water Standards and Health Advisories; United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Kasai, T.; Saito, M.; Senoh, H.; Umeda, Y.; Aiso, S.; Ohbayashi, H.; Nishizawa, T.; Nagano, K.; Fukushima, S. Thirteen-Week Inhalation Toxicity of 1,4-Dioxane in Rats. Inhal. Toxicol. 2008, 20, 961–971. [Google Scholar] [CrossRef]
- Kasai, T.; Kano, H.; Umeda, Y.; Sasaki, T.; Ikawa, N.; Nishizawa, T.; Nagano, K.; Arito, H.; Nagashima, H.; Fukushima, S. Two-Year Inhalation Study of Carcinogenicity and Chronic Toxicity of 1,4-Dioxane in Male Rats. Inhal. Toxicol. 2009, 21, 889–897. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- An, Y.-J.; Kwak, J.; Nam, S.-H.; Jung, M.S. Development and Implementation of Surface Water Quality Standards for Protection of Human Health in Korea. Environ. Sci. Pollut. Res. 2014, 21, 77–85. [Google Scholar] [CrossRef]
- Canada, H. 1,4-Dioxane in Drinking Water—Guideline Technical Document for Public Consultation; Health Canada: Ottawa, ON, Canada, 2018.
- Yamamoto, N.; Inoue, D.; Sei, K.; Saito, Y.; Ike, M. Field Test of On-Site Treatment of 1,4-Dioxane-Contaminated Groundwater Using Pseudonocardia sp. D17. J. Water Environ. Technol. 2018, 16, 256–268. [Google Scholar] [CrossRef] [Green Version]
- New York State Department of Health. Public Water Systems and NYS Drinking Water Standards for PFAS and Other Emerging Contaminants; New York State Department of Health: Albany, NY, USA, 2020.
- Godri Pollitt, K.J.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C.; et al. 1,4-Dioxane as an Emerging Water Contaminant: State of the Science and Evaluation of Research Needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef]
- Jones, S. IRIS Toxicological Review of 1,4-Dioxane (with Inhalation Update) (Interagency Science Discussion Draft); EPA: Washington, DC, USA, 2012.
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 1-118-76704-7. [Google Scholar]
- Lee, I.-S.; Sim, W.-J.; Kim, C.-W.; Chang, Y.-S.; Oh, J.-E. Characteristic Occurrence Patterns of Micropollutants and Their Removal Efficiencies in Industrial Wastewater Treatment Plants. J. Environ. Monit. 2011, 13, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-C.; Choo, K.-H. Hybridization of TiO2 Photocatalysis with Coagulation and Flocculation for 1,4-Dioxane Removal in Drinking Water Treatment. Chem. Eng. J. 2013, 231, 227–235. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of Emerging Contaminants from the Environment by Adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Myers, M.A.; Johnson, N.W.; Marin, E.Z.; Pornwongthong, P.; Liu, Y.; Gedalanga, P.B.; Mahendra, S. Abiotic and Bioaugmented Granular Activated Carbon for the Treatment of 1,4-Dioxane-Contaminated Water. Environ. Pollut. 2018, 240, 916–924. [Google Scholar] [CrossRef]
- Fukuhara, T.; Iwasaki, S.; Hasegawa, T.; Ishihara, K.; Fujiwara, M.; Abe, I. Adsorption of 1,4-Dioxane from Aqueous Solutions onto Various Activated Carbons. J. Water Environ. Technol. 2011, 9, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Liu, C.; Johnson, N.W.; Zhang, L.; Mahendra, S.; Liu, Y.; Dong, Y.; Chen, M. Removal of 1,4-Dioxane by Titanium Silicalite-1: Separation Mechanisms and Bioregeneration of Sorption Sites. Chem. Eng. J. 2019, 371, 193–202. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, N.W.; Liu, C.; Chen, R.; Zhong, M.; Dong, Y.; Mahendra, S. Mechanisms of 1,4-Dioxane Biodegradation and Adsorption by Bio-Zeolite in the Presence of Chlorinated Solvents: Experimental and Molecular Dynamics Simulation Studies. Environ. Sci. Technol. 2019, 53, 14538–14547. [Google Scholar] [CrossRef]
- Saeed Alamri, M.; Hassan, H.M.A.; Alhumaimess, M.S.; Aldawsari, A.M.; Alshahrani, A.A.; Alraddadi, T.S.; Hotan Alsohaimi, I. Kinetics and Adsorption Assessment of 1,4-Dioxane from Aqueous Solution by Thiol and Sulfonic Acid Functionalized Titanosilicate. J. Mol. Liq. 2022, 362, 119786. [Google Scholar] [CrossRef]
- Woodard, S.; Mohr, T.; Nickelsen, M.G. Synthetic Media: A Promising New Treatment Technology for 1,4-Dioxane. Remediat. J. 2014, 24, 27–40. [Google Scholar] [CrossRef]
- Skala, L.P.; Yang, A.; Klemes, M.J.; Xiao, L.; Dichtel, W.R. Resorcinarene Cavitand Polymers for the Remediation of Halomethanes and 1,4-Dioxane. J. Am. Chem. Soc. 2019, 141, 13315–13319. [Google Scholar] [CrossRef] [PubMed]
- Klečka, G.M.; Gonsior, S.J. Removal of 1,4-Dioxane from Wastewater. J. Hazard. Mater. 1986, 13, 161–168. [Google Scholar] [CrossRef]
- Adams, C.D.; Scanlan, P.A.; Secrist, N.D. Oxidation and Biodegradability Enhancement of 1,4-Dioxane Using Hydrogen Peroxide and Ozone. Environ. Sci. Technol. 1994, 28, 1812–1818. [Google Scholar] [CrossRef]
- Andaluri, G.; Suri, R. Removal of 1,4-Dioxane and Volatile Organic Compounds from Groundwater Using Ozone-Based Advanced Oxidation Process. Ozone Sci. Eng. 2017, 39, 423–434. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I.; Bolton, J.R. Mechanism of the Degradation of 1,4-Dioxane in Dilute Aqueous Solution Using the UV/Hydrogen Peroxide Process. Environ. Sci. Technol. 1998, 32, 1588–1595. [Google Scholar] [CrossRef]
- Takahashi, N.; Hibino, T.; Torii, H.; Shibata, S.; Tasaka, S.; Yoneya, J.; Matsuda, M.; Ogasawara, H.; Sugimoto, K.; Fujioka, T. Evaluation of 03/UV and 03/H202 as Practical Advanced Oxidation Processes for Degradation of 1,4-Dioxane. Ozone Sci. Eng. 2013, 35, 331–337. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Oxidation Kinetics of Degradation of 1,4-Dioxane in Aqueous Solution by H2O2/Fe(II) System. J. Environ. Sci. Health 2010, 45, 395–399. [Google Scholar] [CrossRef]
- Barndõk, H.; Blanco, L.; Hermosilla, D.; Blanco, Á. Heterogeneous Photo-Fenton Processes Using Zero Valent Iron Microspheres for the Treatment of Wastewaters Contaminated with 1,4-Dioxane. Chem. Eng. J. 2016, 284, 112–121. [Google Scholar] [CrossRef]
- Zhang, Z.; Chuang, Y.-H.; Szczuka, A.; Ishida, K.P.; Roback, S.; Plumlee, M.H.; Mitch, W.A. Pilot-Scale Evaluation of Oxidant Speciation, 1,4-Dioxane Degradation and Disinfection Byproduct Formation during UV/Hydrogen Peroxide, UV/Free Chlorine and UV/Chloramines Advanced Oxidation Process Treatment for Potable Reuse. Water Res. 2019, 164, 114939. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Venkatesan, A.K.; Walker, H.W.; Gobler, C.J. Impact of Groundwater Quality and Associated Byproduct Formation during UV/Hydrogen Peroxide Treatment of 1,4-Dioxane. Water Res. 2020, 173, 115534. [Google Scholar] [CrossRef] [PubMed]
- Chitra, S.; Paramasivan, K.; Cheralathan, M.; Sinha, P.K. Degradation of 1,4-Dioxane Using Advanced Oxidation Processes. Environ. Sci. Pollut. Res. 2012, 19, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; Andersen, H.R. Comparison of UVC/S2O82- with UVC/H2O2 in Terms of Efficiency and Cost for the Removal of Micropollutants from Groundwater. Chemosphere 2015, 119, S81–S88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Patton, S.; Gleason, J.M.; Mezyk, S.P.; Ishida, K.P.; Liu, H. UV Photolysis of Chloramine and Persulfate for 1,4-Dioxane Removal in Reverse-Osmosis Permeate for Potable Water Reuse. Environ. Sci. Technol. 2018, 52, 6417–6425. [Google Scholar] [CrossRef]
- Patton, S.; Li, W.; Couch, K.D.; Mezyk, S.P.; Ishida, K.P.; Liu, H. Impact of the Ultraviolet Photolysis of Monochloramine on 1,4-Dioxane Removal: New Insights into Potable Water Reuse. Environ. Sci. Technol. Lett. 2017, 4, 26–30. [Google Scholar] [CrossRef]
- Youn, N.K.; Heo, J.E.; Joo, O.S.; Lee, H.; Kim, J.; Min, B.K. The Effect of Dissolved Oxygen on the 1,4-Dioxane Degradation with TiO2 and Au–TiO2 Photocatalysts. J. Hazard. Mater. 2010, 177, 216–221. [Google Scholar] [CrossRef]
- Fujioka, T.; Kodamatani, H.; Yoshikawa, T.; Inoue, D.; Ikehata, K. Assessment of 265-Nm UV-LED for Direct Photolysis and Advanced Oxidation of N-Nitrosamines and 1,4-Dioxane. Environ. Technol. Innov. 2020, 20, 101147. [Google Scholar] [CrossRef]
- Jasmann, J.R.; Borch, T.; Sale, T.C.; Blotevogel, J. Advanced Electrochemical Oxidation of 1,4-Dioxane via Dark Catalysis by Novel Titanium Dioxide (TiO2) Pellets. Environ. Sci. Technol. 2016, 50, 8817–8826. [Google Scholar] [CrossRef] [PubMed]
- Jasmann, J.R.; Gedalanga, P.B.; Borch, T.; Mahendra, S.; Blotevogel, J. Synergistic Treatment of Mixed 1,4-Dioxane and Chlorinated Solvent Contaminations by Coupling Electrochemical Oxidation with Aerobic Biodegradation. Environ. Sci. Technol. 2017, 51, 12619–12629. [Google Scholar] [CrossRef]
- Blotevogel, J.; Pijls, C.; Scheffer, B.; de Waele, J.-P.; Lee, A.; van Poecke, R.; van Belzen, N.; Staal, W. Pilot-Scale Electrochemical Treatment of a 1,4-Dioxane Source Zone. Groundw. Monit. Remediat. 2019, 39, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Even-Ezra, I.; Mizrahi, A.; Gerrity, D.; Snyder, S.; Salveson, A.; Lahav, O. Application of a Novel Plasma-Based Advanced Oxidation Process for Efficient and Cost-Effective Destruction of Refractory Organics in Tertiary Effluents and Contaminated Groundwater. Desalination Water Treat. 2009, 11, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Zhang, Q.; Wandell, R.; Bresch, S.; Wang, H.; Locke, B.R.; Tang, Y. Synergistic 1,4-Dioxane Removal by Non-Thermal Plasma Followed by Biodegradation. Chem. Eng. J. 2019, 361, 519–527. [Google Scholar] [CrossRef]
- Park, Y.-K.; Chung, K.-H.; Park, I.-S.; Kim, S.-C.; Kim, S.-J.; Jung, S.-C. Photocatalytic Degradation of 1,4-Dioxane Using Liquid Phase Plasma on Visible Light Photocatalysts. J. Hazard. Mater. 2020, 399, 123087. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baumgart, H.; Bott, C.; Ciovati, G.; Gregory, S.; Hannon, F.; McCaughan, M.; Pearce, R.; Poelker, M.; Vennekate, H.; et al. Design and Commissioning of an E-Beam Irradiation Beamline at the Upgraded Injector Test Facility at Jefferson Lab. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2022, 1039, 167093. [Google Scholar] [CrossRef]
- Pearce, R.; Li, X.; Vennekate, J.; Ciovati, G.; Bott, C. Electron Beam Treatment for the Removal of 1,4-Dioxane in Water and Wastewater. Water Sci. Technol. 2023, 87, 275–283. [Google Scholar] [CrossRef]
- Metz, D.H.; Meyer, M.; Dotson, A.; Beerendonk, E.; Dionysiou, D.D. The Effect of UV/H2O2 Treatment on Disinfection by-Product Formation Potential under Simulated Distribution System Conditions. Water Res. 2011, 45, 3969–3980. [Google Scholar] [CrossRef]
- Phungsai, P.; Kurisu, F.; Kasuga, I.; Furumai, H. Molecular Characteristics of Dissolved Organic Matter Transformed by O3 and O3/H2O2 Treatments and the Effects on Formation of Unknown Disinfection by-Products. Water Res. 2019, 159, 214–222. [Google Scholar] [CrossRef]
- Heringa, M.B.; Harmsen, D.J.H.; Beerendonk, E.F.; Reus, A.A.; Krul, C.A.M.; Metz, D.H.; IJpelaar, G.F. Formation and Removal of Genotoxic Activity during UV/H2O2–GAC Treatment of Drinking Water. Water Res. 2011, 45, 366–374. [Google Scholar] [CrossRef]
- Hofman-Caris, R.C.H.M.; Harmsen, D.J.H.; Puijker, L.; Baken, K.A.; Wols, B.A.; Beerendonk, E.F.; Keltjens, L.L.M. Influence of Process Conditions and Water Quality on the Formation of Mutagenic Byproducts in UV/H2O2 Processes. Water Res. 2015, 74, 191–202. [Google Scholar] [CrossRef]
- Li, W.; Xu, E.; Schlenk, D.; Liu, H. Cyto- and Geno-Toxicity of 1,4-Dioxane and Its Transformation Products during Ultraviolet-Driven Advanced Oxidation Processes. Environ. Sci. Water Res. Technol. 2018, 4, 1213–1218. [Google Scholar] [CrossRef]
- Sarathy, S.R.; Stefan, M.I.; Royce, A.; Mohseni, M. Pilot-Scale UV/H2O2 Advanced Oxidation Process for Surface Water Treatment and Downstream Biological Treatment: Effects on Natural Organic Matter Characteristics and DBP Formation Potential. Environ. Technol. 2011, 32, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nie, Z.; Wang, C.; Li, Y.; Xu, M.; Hofmann, R. Quenching H2O2 Residuals after UV/H2O2 Oxidation Using GAC in Drinking Water Treatment. Environ. Sci. Water Res. Technol. 2018, 4, 1662–1670. [Google Scholar] [CrossRef]
- Tang, Y.; Lee, C.-S.; Walker, H.; Gobler, C.; Apul, O.; Venkatesan, A.K.; Mao, X. Effect of Residual H2O2 on the Removal of Advanced Oxidation Byproducts by Two Types of Granular Activated Carbon. J. Environ. Chem. Eng. 2021, 9, 106838. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Advances in Bioremediation of 1,4-Dioxane-Contaminated Waters. J. Environ. Manag. 2017, 204, 765–774. [Google Scholar] [CrossRef]
- Parales, R.E.; Adamus, J.E.; White, N.; May, H.D. Degradation of 1,4-Dioxane by an Actinomycete in Pure Culture. Appl. Environ. Microbiol. 1994, 60, 4527–4530. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Jeon, J.-R.; Murugesan, K.; Kim, E.-J.; Chang, Y.-S. Biodegradation of 1,4-Dioxane and Transformation of Related Cyclic Compounds by a Newly Isolated Mycobacterium sp. PH-06. Biodegradation 2009, 20, 511–519. [Google Scholar] [CrossRef]
- Sei, K.; Miyagaki, K.; Kakinoki, T.; Fukugasako, K.; Inoue, D.; Ike, M. Isolation and Characterization of Bacterial Strains That Have High Ability to Degrade 1,4-Dioxane as a Sole Carbon and Energy Source. Biodegradation 2013, 24, 665–674. [Google Scholar] [CrossRef]
- Inoue, D.; Tsunoda, T.; Sawada, K.; Yamamoto, N.; Saito, Y.; Sei, K.; Ike, M. 1,4-Dioxane Degradation Potential of Members of the Genera Pseudonocardia and Rhodococcus. Biodegradation 2016, 27, 277–286. [Google Scholar] [CrossRef]
- Deng, D.; Li, F.; Wu, C.; Li, M. Synchronic Biotransformation of 1,4-Dioxane and 1,1-Dichloroethylene by a Gram-Negative Propanotroph Azoarcus Sp. DD4. Environ. Sci. Technol. Lett. 2018, 5, 526–532. [Google Scholar] [CrossRef]
- He, Y.; Mathieu, J.; da Silva, M.L.B.; Li, M.; Alvarez, P.J.J. 1,4-Dioxane-Degrading Consortia Can Be Enriched from Uncontaminated Soils: Prevalence of Mycobacterium and Soluble Di-Iron Monooxygenase Genes. Microb. Biotechnol. 2018, 11, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaka, K.; Udagawa, M.; Sei, K.; Ike, M. Pilot Test of Biological Removal of 1,4-Dioxane from a Chemical Factory Wastewater by Gel Carrier Entrapping Afipia sp. Strain D1. J. Hazard. Mater. 2016, 304, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Yoshikawa, T.; Okumura, T.; Yabuki, Y.; Ike, M. Treatment of 1,4-Dioxane-Containing Water Using Carriers Immobilized with Indigenous Microorganisms in Landfill Leachate Treatment Sludge: A Laboratory-Scale Reactor Study. J. Hazard. Mater. 2021, 414, 125497. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Khan, I.A.; Inam, M.A.; Khan, R.; Wie, Y.M.; Yeom, I.T. Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture. Fermentation 2022, 8, 143. [Google Scholar] [CrossRef]
- McElroy, A.C.; Ogles, M.E.; Hyman, M.R.; Knappe, D.R.U. Pilot-Scale Biofiltration of 1,4-Dioxane at Drinking Water-Relevant Concentrations. Water Res. 2023, 231, 119652. [Google Scholar] [CrossRef]
- Dalton, H.; Stirling, D.I. Co-Metabolism. Philos. Trans. R. Soc. Lond. Biol. Sci. 1982, 297, 481–496. [Google Scholar]
- Mahendra, S.; Alvarez-Cohen, L. Kinetics of 1,4-Dioxane Biodegradation by Monooxygenase-Expressing Bacteria. Environ. Sci. Technol. 2006, 40, 5435–5442. [Google Scholar] [CrossRef]
- Vainberg, S.; McClay, K.; Masuda, H.; Root, D.; Condee, C.; Zylstra, G.J.; Steffan, R.J. Biodegradation of Ether Pollutants by Pseudonocardia sp. Strain ENV478. Appl. Environ. Microbiol. 2006, 72, 5218–5224. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Mason, O.U.; Lowe, A.; Zhang, Z.; Zhou, C.; Chen, G.; Villalonga, M.J.; Tang, Y. Investigating Promising Substrates for Promoting 1,4-Dioxane Biodegradation: Effects of Ethane and Tetrahydrofuran on Microbial Consortia. Biodegradation 2020, 31, 171–182. [Google Scholar] [CrossRef]
- Deng, D.; Pham, D.N.; Li, F.; Li, M. Discovery of an Inducible Toluene Monooxygenase That Cooxidizes 1,4-Dioxane and 1,1-Dichloroethylene in Propanotrophic Azoarcus sp. Strain DD4. Appl. Environ. Microbiol. 2020, 86, e01163-20. [Google Scholar] [CrossRef]
- Masuda, H.; McClay, K.; Steffan, R.J.; Zylstra, G.J. Characterization of Three Propane-Inducible Oxygenases in Mycobacterium sp. Strain ENV421. Lett. Appl. Microbiol. 2012, 55, 175–181. [Google Scholar] [CrossRef]
- Skinner, K.; Cuiffetti, L.; Hyman, M. Metabolism and Cometabolism of Cyclic Ethers by a Filamentous Fungus, a Graphium sp. Appl. Environ. Microbiol. 2009, 75, 5514–5522. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Shen, D.; Li, N.; Shan, D.; Shentu, J.; Zhou, Y. Biodegradation of 1,4-Dioxane by a Novel Strain and Its Biodegradation Pathway. Water Air Soil Pollut. 2014, 225, 2135. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Jin, X.-J.; Chen, J.; Ye, J.-X.; Jiang, N.-X.; Chen, J.-M. Intermediates and Substrate Interaction of 1,4-Dioxane Degradation by the Effective Metabolizer Xanthobacter flavus DT8. Int. Biodeterior. Biodegrad. 2016, 106, 133–140. [Google Scholar] [CrossRef]
- Inoue, D.; Tsunoda, T.; Yamamoto, N.; Ike, M.; Sei, K. 1,4-Dioxane Degradation Characteristics of Rhodococcus aetherivorans JCM 14343. Biodegradation 2018, 29, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Takagi, K.; Sakakibara, F.; Abe, T.; Shiiba, K. Identification and Characterization of 1,4-Dioxane-Degrading Microbe Separated from Surface Seawater by the Seawater-Charcoal Perfusion Apparatus. Biodegradation 2016, 27, 155–163. [Google Scholar] [CrossRef]
- Dai, C.; Wu, H.; Wang, X.; Zhao, K.; Lu, Z. Network and Meta-Omics Reveal the Cooperation Patterns and Mechanisms in an Efficient 1,4-Dioxane-Degrading Microbial Consortium. Chemosphere 2022, 301, 134723. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-H.; Ventura, J.-R.S.; Yeom, I.T.; Lee, Y.; Jahng, D. Structural and Kinetic Characteristics of 1,4-Dioxane-Degrading Bacterial Consortia Containing the Phylum TM7. J. Microbiol. Biotechnol. 2016, 26, 1951–1964. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wang, M.; Lee, C.-S.; Venkatesan, A.K.; Mao, X. Characterization of 1,4-Dioxane Degrading Microbial Community Enriched from Uncontaminated Soil. Appl. Microbiol. Biotechnol. 2023, 107, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Petzold, C.J.; Baidoo, E.E.; Keasling, J.D.; Alvarez-Cohen, L. Identification of the Intermediates of in Vivo Oxidation of 1,4-Dioxane by Monooxygenase-Containing Bacteria. Environ. Sci. Technol. 2007, 41, 7330–7336. [Google Scholar] [CrossRef]
- He, Y.; Mathieu, J.; Yang, Y.; Yu, P.; da Silva, M.L.B.; Alvarez, P.J.J. 1,4-Dioxane Biodegradation by Mycobacterium dioxanotrophicus PH-06 Is Associated with a Group-6 Soluble Di-Iron Monooxygenase. Environ. Sci. Technol. Lett. 2017, 4, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Nakamiya, K.; Hashimoto, S.; Ito, H.; Edmonds, J.S.; Morita, M. Degradation of 1,4-Dioxane and Cyclic Ethers by an Isolated Fungus. Appl. Environ. Microbiol. 2005, 71, 1254–1258. [Google Scholar] [CrossRef] [Green Version]
- Inoue, D.; Hisada, K.; Okumura, T.; Yabuki, Y.; Yoshida, G.; Kuroda, M.; Ike, M. Carbon Sources That Enable Enrichment of 1,4-Dioxane-Degrading Bacteria in Landfill Leachate. Biodegradation 2020, 31, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Wie, Y.M.; Lee, Y.-S. Characterization of 1,4-Dioxane Biodegradation by a Microbial Community. Water 2020, 12, 3372. [Google Scholar] [CrossRef]
- Tusher, T.R.; Shimizu, T.; Inoue, C.; Chien, M.-F. Enrichment and Analysis of Stable 1,4-Dioxane-Degrading Microbial Consortia Consisting of Novel Dioxane-Degraders. Microorganisms 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Miao, Y.; Liu, Y.; Zhang, L.; Zhong, M.; Adams, J.M.; Dong, Y.; Mahendra, S. Identification of Novel 1,4-Dioxane Degraders and Related Genes from Activated Sludge by Taxonomic and Functional Gene Sequence Analysis. J. Hazard. Mater. 2021, 412, 125157. [Google Scholar] [CrossRef]
- Ramos-García, Á.A.; Walecka-Hutchison, C.; Freedman, D.L. Effect of Biostimulation and Bioaugmentation on Biodegradation of High Concentrations of 1,4-Dioxane. Biodegradation 2022, 33, 157–168. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.; Zhou, C.; Chen, H.; Chen, G.; Tang, Y. Determination of Growth Kinetics of Microorganisms Linked with 1,4-Dioxane Degradation in a Consortium Based on Two Improved Methods. Front. Environ. Sci. Eng. 2022, 16, 62. [Google Scholar] [CrossRef]
- Deng, D.; Li, F.; Li, M. A Novel Propane Monooxygenase Initiating Degradation of 1,4-Dioxane by Mycobacterium dioxanotrophicus PH-06. Environ. Sci. Technol. Lett. 2017, 5, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Gedalanga, P.B.; Pornwongthong, P.; Mora, R.; Chiang, S.-Y.D.; Baldwin, B.; Ogles, D.; Mahendra, S. Identification of Biomarker Genes To Predict Biodegradation of 1,4-Dioxane. Appl. Environ. Microbiol. 2014, 80, 3209–3218. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Y.; Mathieu, J.; Liu, Y.Y.; Van Orden, E.T.; Yang, Y.; Fiorenza, S.; Alvarez, P.J.J. The Abundance of Tetrahydrofuran/Dioxane Monooxygenase Genes (thmA/dxmA) and 1,4-Dioxane Degradation Activity Are Significantly Correlated at Various Impacted Aquifers. Environ. Sci. Technol. Lett. 2014, 1, 122–127. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Biodegradation Kinetics of 1,4-Dioxane in Chlorinated Solvent Mixtures. Environ. Sci. Technol. 2016, 50, 9599–9607. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.T.; Anderson, R.H.; Mahendra, S.; Newell, C.J. Evidence of 1,4-Dioxane Attenuation at Groundwater Sites Contaminated with Chlorinated Solvents and 1,4-Dioxane. Environ. Sci. Technol. 2015, 49, 6510–6518. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, D.; Zeng, L.; Abrams, S.; Li, M. Sequential Anaerobic and Aerobic Bioaugmentation for Commingled Groundwater Contamination of Trichloroethene and 1,4-Dioxane. Sci. Total Environ. 2021, 774, 145118. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Grostern, A.; Alvarez-Cohen, L. The Impact of Chlorinated Solvent Co-Contaminants on the Biodegradation Kinetics of 1,4-Dioxane. Chemosphere 2013, 91, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, X.; Polasko, A.; Johnson, N.W.; Miao, Y.; Yang, Z.; Mahendra, S.; Gu, B. Co-Contaminant Effects on 1,4-Dioxane Biodegradation in Packed Soil Column Flow-through Systems. Environ. Pollut. 2018, 243, 573–581. [Google Scholar] [CrossRef]
- Polasko, A.L.; Miao, Y.; Kwok, I.; Park, K.; Park, J.O.; Mahendra, S. Vinyl Chloride and 1,4-Dioxane Metabolism by Pseudonocardia Dioxanivorans CB1190. J. Hazard. Mater. Lett. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Polasko, A.L.; Zulli, A.; Gedalanga, P.B.; Pornwongthong, P.; Mahendra, S. A Mixed Microbial Community for the Biodegradation of Chlorinated Ethenes and 1,4-Dioxane. Environ. Sci. Technol. Lett. 2019, 6, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Chen, H.; Pan, S. Anaerobic Biodegradation of 1,4-Dioxane by Sludge Enriched with Iron-Reducing Microorganisms. Bioresour. Technol. 2008, 99, 2483–2487. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.Y.; Li, H. Synergistic Effects of Acclimated Bacterial Community and Zero Valent Iron for Removing 1,1,1-Trichloroethane and 1,4-Dioxane Co-Contaminants in Groundwater. J. Chem. Technol. Biotechnol. 2018, 93, 2244–2251. [Google Scholar] [CrossRef]
- Ramalingam, V.; Cupples, A.M. Anaerobic 1,4-Dioxane Biodegradation and Microbial Community Analysis in Microcosms Inoculated with Soils or Sediments and Different Electron Acceptors. Appl. Microbiol. Biotechnol. 2020, 104, 4155–4170. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendi, A.; Rajesh Banu, J.; Dhavamani, J.; Yeom, I.T. Biodegradation of 1,4-Dioxane by Rhodanobacter AYS5 and the Role of Additional Substrates. Ann. Microbiol. 2015, 65, 2201–2208. [Google Scholar] [CrossRef]
- Tawfik, A.; Al-Sayed, A.; Hassan, G.K.; Nasr, M.; El-Shafai, S.A.; Alhajeri, N.S.; Khan, M.S.; Akhtar, M.S.; Ahmad, Z.; Rojas, P.; et al. Electron Donor Addition for Stimulating the Microbial Degradation of 1,4-Dioxane by Sequential Batch Membrane Bioreactor: A Techno-Economic Approach. Chemosphere 2022, 306, 135580. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.L.B.; He, Y.; Mathieu, J.; Alvarez, P.J.J. Enhanced Long-Term Attenuation of 1,4-Dioxane in Bioaugmented Flow-through Aquifer Columns. Biodegradation 2020, 31, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Han, T.-H.; Han, J.-S.; So, M.-H.; Seo, J.-W.; Ahn, C.-M.; Min, D.H.; Yoo, Y.S.; Cha, D.K.; Kim, C.G. The Removal of 1,4-Dioxane from Polyester Manufacturing Process Wastewater Using an up-Flow Biological Aerated Filter (UBAF) Packed with Tire Chips. J. Environ. Sci. Health 2012, 47, 117–129. [Google Scholar] [CrossRef]
- Isaka, K.; Udagawa, M.; Kimura, Y.; Sei, K.; Ike, M. Biological Wastewater Treatment of 1,4-Dioxane Using Polyethylene Glycol Gel Carriers Entrapping Afipia sp. D1. J. Biosci. Bioeng. 2016, 121, 203–208. [Google Scholar] [CrossRef]
- Isaka, K.; Udagawa, M.; Kimura, Y.; Sei, K.; Ike, M. Biological 1,4-Dioxane Wastewater Treatment by Immobilized Pseudonocardia sp. D17 on Lower 1,4-Dioxane Concentration. J. Water Environ. Technol. 2016, 14, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Isaka, K.; Masuda, T.; Omae, S.; Mishima, I.; Ike, M. Effect of Nitrogen, Phosphorus, and Sulfur on the Start-up of a Biological 1,4-Dioxane Removal Process Using Pseudonocardia sp. D17. Biochem. Eng. J. 2021, 176, 108179. [Google Scholar] [CrossRef]
- Chung, J.; Lee, G.; Chung, S.; Lee, Y.-W. Removal of 1,4-Dioxane in Water Using Specific Microbe Immobilization Cells. Water Air Soil Pollut. 2019, 230, 114. [Google Scholar] [CrossRef]
- Zenker, M.; Borden, R.; Barlaz, M. Biodegradation of 1,4Dioxane Using Trickling Filter. J. Environ. Eng. 2004, 130, 926–931. [Google Scholar] [CrossRef]
- Gibson, J.M. Peer Reviewed: Evaluating Natural Attenuation for Groundwater Cleanup. Environ. Sci. Technol. 2000, 34, 346A–353A. [Google Scholar] [CrossRef] [PubMed]
- Goff, K.L.; Hug, L.A. Environmental Potential for Microbial 1,4-Dioxane Degradation Is Sparse despite Mobile Elements Playing a Role in Trait Distribution. Appl. Environ. Microbiol. 2022, 88, e02091-21. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.T.; Mahendra, S.; Walker, K.L.; Rauch, S.R.; Sengupta, S.; Newell, C.J. A Multisite Survey To Identify the Scale of the 1,4-Dioxane Problem at Contaminated Groundwater Sites. Environ. Sci. Technol. Lett. 2014, 1, 254–258. [Google Scholar] [CrossRef]
- Adamson, D.T.; de Blanc, P.C.; Farhat, S.K.; Newell, C.J. Implications of Matrix Diffusion on 1,4-Dioxane Persistence at Contaminated Groundwater Sites. Sci. Total Environ. 2016, 562, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lippincott, D. Bioaugmentation and Propane Biosparging for In Situ Biodegradation of 1,4-Dioxane. Groundw. Monit. Remediat. 2015, 35, 81–92. [Google Scholar] [CrossRef]
- Horst, J.F.; Bell, C.H.; Lorenz, A.; Heintz, M.; Miao, Y.; Saling, J.; Favero, D.; Mahendra, S. Bioremediation of 1,4-Dioxane: Successful Demonstration of In Situ and Ex Situ Approaches. Groundw. Monit. Remediat. 2019, 39, 15–24. [Google Scholar] [CrossRef]
- Bell, C.H.; Wong, J.; Parsons, K.; Semel, W.; McDonough, J.; Gerber, K. First Full-Scale In Situ Propane Biosparging for Co-Metabolic Bioremediation of 1,4-Dioxane. Groundw. Monit. Remediat. 2022, 42, 54–66. [Google Scholar] [CrossRef]
- Simmer, R.A.; Richards, P.M.; Ewald, J.M.; Schwarz, C.; da Silva, M.L.B.; Mathieu, J.; Alvarez, P.J.J.; Schnoor, J.L. Rapid Metabolism of 1,4-Dioxane to below Health Advisory Levels by Thiamine-Amended Rhodococcus ruber Strain 219. Environ. Sci. Technol. Lett. 2021, 8, 975–980. [Google Scholar] [CrossRef]
- Barajas-Rodriguez, F.J.; Murdoch, L.C.; Falta, R.W.; Freedman, D.L. Simulation of in Situ Biodegradation of 1,4-Dioxane under Metabolic and Cometabolic Conditions. J. Contam. Hydrol. 2019, 223, 103464. [Google Scholar] [CrossRef]
- Lee, C.-S.; Asato, C.; Wang, M.; Mao, X.; Gobler, C.J.; Venkatesan, A.K. Removal of 1,4-Dioxane during on-Site Wastewater Treatment Using Nitrogen Removing Biofilters. Sci. Total Environ. 2021, 771, 144806. [Google Scholar] [CrossRef]
- Vatankhah, H.; Szczuka, A.; Mitch, W.A.; Almaraz, N.; Brannum, J.; Bellona, C. Evaluation of Enhanced Ozone–Biologically Active Filtration Treatment for the Removal of 1,4-Dioxane and Disinfection Byproduct Precursors from Wastewater Effluent. Environ. Sci. Technol. 2019, 53, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.E.; Miao, Y.; Johnson, N.W.; Ramos, P.; Mahendra, S.; Blotevogel, J. Bioelectrochemical Treatment of 1,4-Dioxane in the Presence of Chlorinated Solvents: Design, Process, and Sustainability Considerations. ACS Sustain. Chem. Eng. 2021, 9, 3172–3182. [Google Scholar] [CrossRef]
| Strain | Induced Enzyme | Co Substrate | Biodegradation Rate a | qmax (mg-Dioxane h−1 mg-Protein −1) | Ks (mg L−1) | Enrichment Source | Reference |
|---|---|---|---|---|---|---|---|
| Co-metabolic strains | |||||||
| Pseudonocardia K1 | THF MO | THF | 0.26 ± 0.013 mg hr−1 mg-protein−1 | [76] | |||
| Rhodococcus RR1 | N/A | Toluene | 0.38 ± 0.03 mg hr−1 mg-protein−1 | ||||
| Methylosinus trichosporium OB3b | sMMO | Methane | 0.38 ± 0.02 mg hr−1 mg-protein−1 | ||||
| Mycobacterium vaccae JOB5 | Propane MO | Propane | 0.40 ± 0.06 mg hr−1 mg-protein−1 | ||||
| Pseudomonas mendocina KR1 | toluene-4-MO | Toluene | 0.37 ± 0.04 mg hr−1 mg-protein−1 | ||||
| Ralstonia pickettii PKO1 | toluene-p-MO | Toluene | 0.31 ± 0.007 mg hr−1 mg-protein−1 | ||||
| Burkholderia cepacia G4 | toluene-2-MO | Toluene | 0.1 ± 0.006 mg hr−1 mg-protein−1 | ||||
| Pseudonocardia sp. strain ENV478 | N/A | THF | 0.008 mg hr−1 mg-protein−1 | [77] | |||
| Mycobacterium sp. strain ENV421 | Propane MO | Propane | N/A | [80] | |||
| Azoarcus sp. DD4 | Toluene MO | Toluene | 1.82 mg L−1 day−1 | [79] | |||
| Graphium sp. (ATCC 58400) (fungus) | MO | THF | 19 ± 10.5 mg hr−1 mg-protein−1 | [81] | |||
| Metabolic strains | |||||||
| Pseudonocardia dioxanivorans CB1190 | 1,4-dioxane MO | 0.19 ± 0.007 mg hr−1 mg-protein−1 | 1.1 ± 0.0008 | 160 ± 44 | 1,4-dioxane-contaminated industrial sludge | [76] | |
| P. benzenivorans B5 | N/A | 0.01 ± 0.003 mg hr−1 mg-protein−1 | 0.1 ± 0.006 | 330 ± 82 | Contaminated soil | ||
| Mycobacterium sp. PH-06 | MO | 2.5 mg L−1 h−1 | N/A | 78 ± 10 | Contaminated sediment | [66] | |
| Acinetobacter baumannii DD1 | MO | 2.38 mg L−1 h−1 | N/A | N/A | Activated sludge | [82] | |
| Afipia sp. D1 | N/A | 0.263 mg hr−1 mg-protein−1 | 0.263 | 25.8 | Drainage of a chemical factory | [67] | |
| Mycobacterium sp. D6 | 0.139 mg hr−1 mg-protein−1 | 0.139 | 20.6 | ||||
| Mycobacterium sp. D11 | 0.052 mg hr−1 mg-protein−1 | 0.052 | 69.8 | ||||
| Pseudonocardia sp. D17 | 0.096 mg hr−1 mg-protein−1 | 0.096 | 59.7 | ||||
| Xanthobacter flavus DT8 | MO | Equivalent to CB1190 | N/A | 17.5 | Activated sludge of pharmaceutical plant | [83] | |
| Rhodococcus aetherivorans JCM 14343 | 0.0073 mg hr−1 mg-protein−1 | 0.0073 | 59.2 | N/A | [84] | ||
| Pseudonocardia carboxydivorans. RM-31 | N/A | 31.6 mg L−1 h−1 | N/A | N/A | Seawater | [85] | |
| Ancylobacter phlymorphus ZM13 | Toluene MO | N/A | N/A | N/A | [86] | ||
| Microbial community | |||||||
| Consortium CH1 | Toluene MO | 2.04 | N/A | Activated sludge | [86] | ||
| Mixed culture | N/A | 0.019 | 11.08 | Activated sludge | [73] | ||
| Consortium A | Propane MO | 0.297 ± 0.0075 (at 500 mg L−1) | N/A | Uncontaminated soil | [70] | ||
| Consortium B | Propane MO | 0.236 ± 0.0029 (at 500 mg L−1) | N/A | Uncontaminated soil | [70] | ||
| Enrichment culture-FS | N/A | 0.037 | 93.9 | Forest soil | [87] | ||
| Enrichment culture-AS | N/A | 0.078 | 181.3 | Activated sludge | [87] | ||
| Soil–enrichment | 1,4-dioxane MO | 0.044 ± 0.001 | 25 ± 1.60 | Uncontaminated soil | [88] | ||
| Reactor Type | Dimension | Water Source | Microbial | HRT | 1,4-Dioxane Loading Rate | Influent 1,4-Dioxane Concentration | Removal Efficiency | Reference |
|---|---|---|---|---|---|---|---|---|
| Packed soil flow-through column | 2.5 cm ID × 10.5 cm H (10 cm packing height) | Contaminated groundwater | Pseudonocardia dioxanivorans CB1190 | 41.9–80.8 h | 0.043–0.144 mg-1,4-dioxane d−1 | 3–10 mg L−1 | Up to 99% influent concentration of 10 mg L−1 | [105] |
| Packed sand filtration column | 5 cm ID × 120 cm H (100 cm packing height) | Contaminated groundwater | Pseudonocardia dioxanivorans CB1190 | 66–277 h of EBCT | 0.2–5 mg L−1 | 34–92% | [113] | |
| Tire chips packed up-flow biological aerated filter | 20 cm ID × 79.5 cm H (27 cm packing height) | 1,4-dioxane containing wastewater | Activated sludge | 5–7 h | 17.8–65.6 mg L−1 | 54.7–83.4% | [114] | |
| Biological activated filter | Sequential column of 2.54 cm ID × 32 cm packing height | Filtered water from water treatment plant | Co-metabolic microbial community enriched from river basin sample | 7.5–30 min of EBCT | 8.9–11.7 µg L−1 | 65–94% | [74] | |
| Continuous Stirred tank reactor (CSTR) | 15 L | Industrial wastewater | Microbial community enriched from activated sludge | 10–40 h | 200 mg L−1 | 81.6–98.6% | [73] | |
| Plug Flow reactor (PFR) | 11 L | Industrial wastewater | Microbial community enriched from activated sludge | 10–40 h | 200 mg L−1 | 96.2–99.8% | [73] | |
| Moving bed bioreactor with glycol gel carriers | 1000 mL Carrier packing ratio 15% | Synthetic industrial wastewater | Afipia sp. D1 | 16–24 h | 0.4–0.6 kg 1,4-dioxane m−3 d−1 | ~400 mg L−1 | 99% | [115] |
| Tubular carrier/polyurethane carrier | 1000 mL | Synthetic wastewater | Pseudonocardia sp. D17 | 2.4–20 h | 5–50 mg L−1 | 90–99 | [116] | |
| PEG gel beads (15% packing ratio) | 1440 mL | Synthetic wastewater | Pseudonocardia sp. D17 | 12–60 h | 20–100 mg L−1 | 70–99 | [117] | |
| PEG gel carriers | 700 mL | Synthetic wastewater | Sludge-enriched 1,4-dioxane degrading consortium | 3–6 h | 5–40 mg L−1 | 79–99.5 | [118] | |
| Immobilized gel-carrier bioreactor | 120 L | Industrial wastewater | Afipia sp. D1 | 40 h | 0.09–0.47 kg-dioxane m−3 d−1 | 2–670 mg L−1 | [71] | |
| Moving bed biofilm reactor | 1050 mL | Basal salt medium (BSM) | Microbial samples from landfill leachate treatment facility | 0.5–2 d | 5.14–20.8 mg L−1 d−1 | 10 mg L−1 | 69.4–97.9% at different conditions | [72] |
| Semicontinuous stirred tank reactor | 5 L | Industrial wastewater | Rhodanobacter AYS5 | 6 h | 263 mg L−1 | 100% in 6 days | [111] | |
| Sequential batch membrane bioreactor | 8.4 L | Synthetic wastewater | activated sludge | 7.3 h | 100–500 mg L−1 | 27.36–94.3% with different amounts of acetate addition | [112] | |
| Trickling filter packed with ceramic saddles | 1.3 cm × 1.1 m | 25% mineral medium L amended with THF and 1,4-dioxane | Co-metabolic microbial community enriched from a contaminated aquifer | 14.4 min | 0.99–1.51 mg L−1 | >93% | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Mao, X. Recent Advances in 1,4-Dioxane Removal Technologies for Water and Wastewater Treatment. Water 2023, 15, 1535. https://doi.org/10.3390/w15081535
Tang Y, Mao X. Recent Advances in 1,4-Dioxane Removal Technologies for Water and Wastewater Treatment. Water. 2023; 15(8):1535. https://doi.org/10.3390/w15081535
Chicago/Turabian StyleTang, Yuyin, and Xinwei Mao. 2023. "Recent Advances in 1,4-Dioxane Removal Technologies for Water and Wastewater Treatment" Water 15, no. 8: 1535. https://doi.org/10.3390/w15081535
APA StyleTang, Y., & Mao, X. (2023). Recent Advances in 1,4-Dioxane Removal Technologies for Water and Wastewater Treatment. Water, 15(8), 1535. https://doi.org/10.3390/w15081535







