Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Methods
2.3. DNA Isolation
2.4. PCR and Bioinformatic Processing
2.5. Statistical Analyses
3. Results
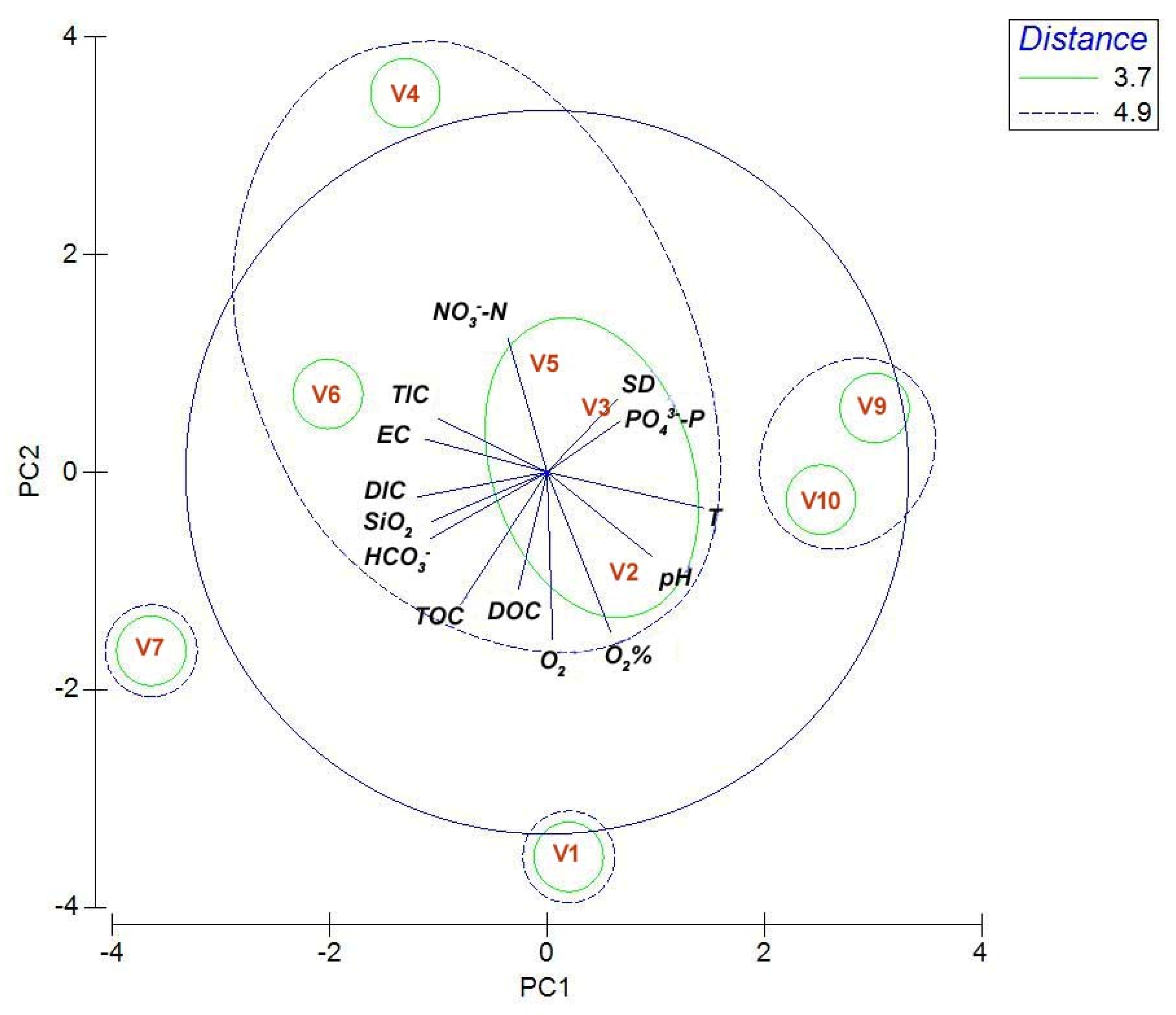
3.1. Physical and Chemical Parameters
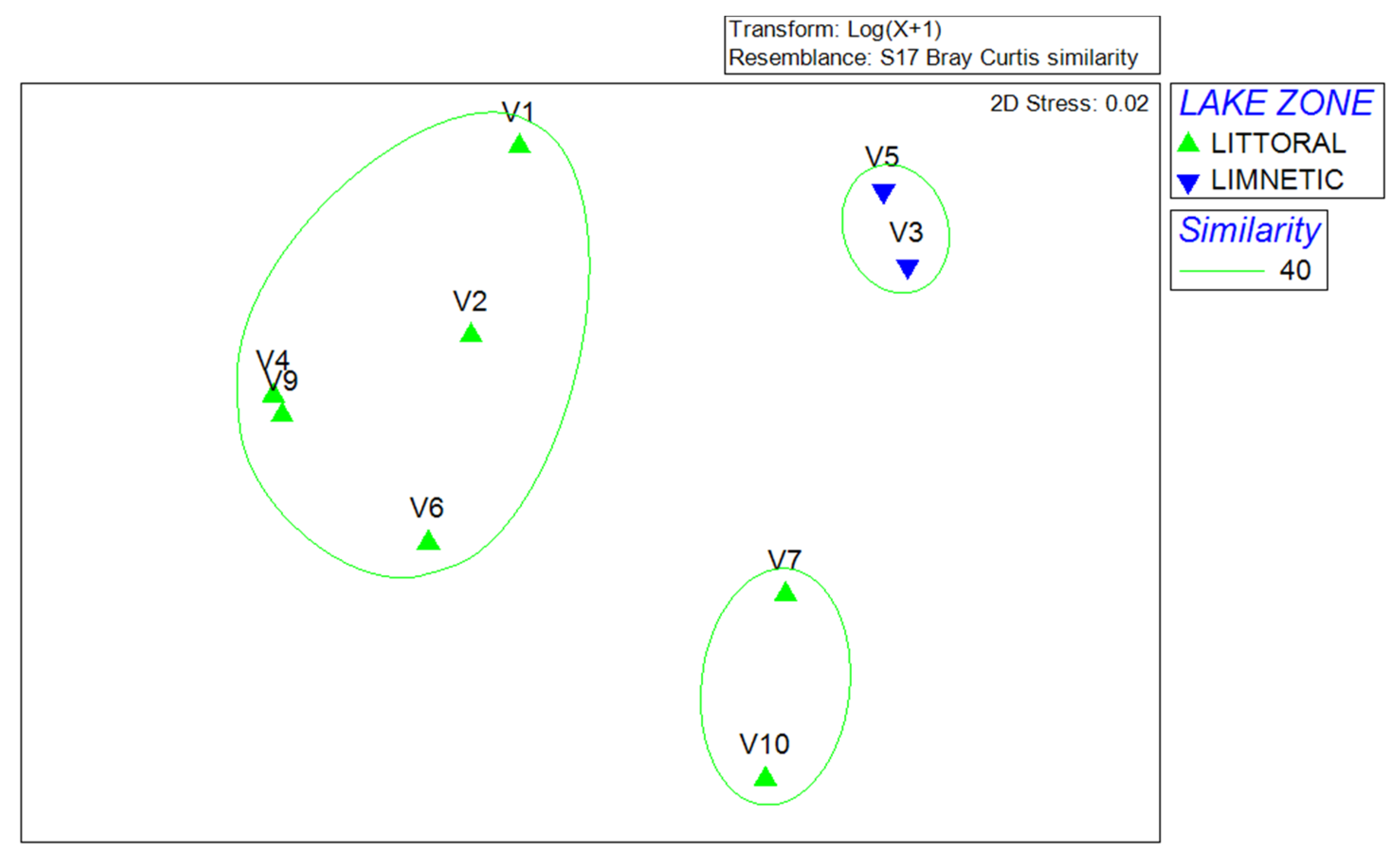
3.2. Characterization of Phytoplankton Community According to Morphological Approach
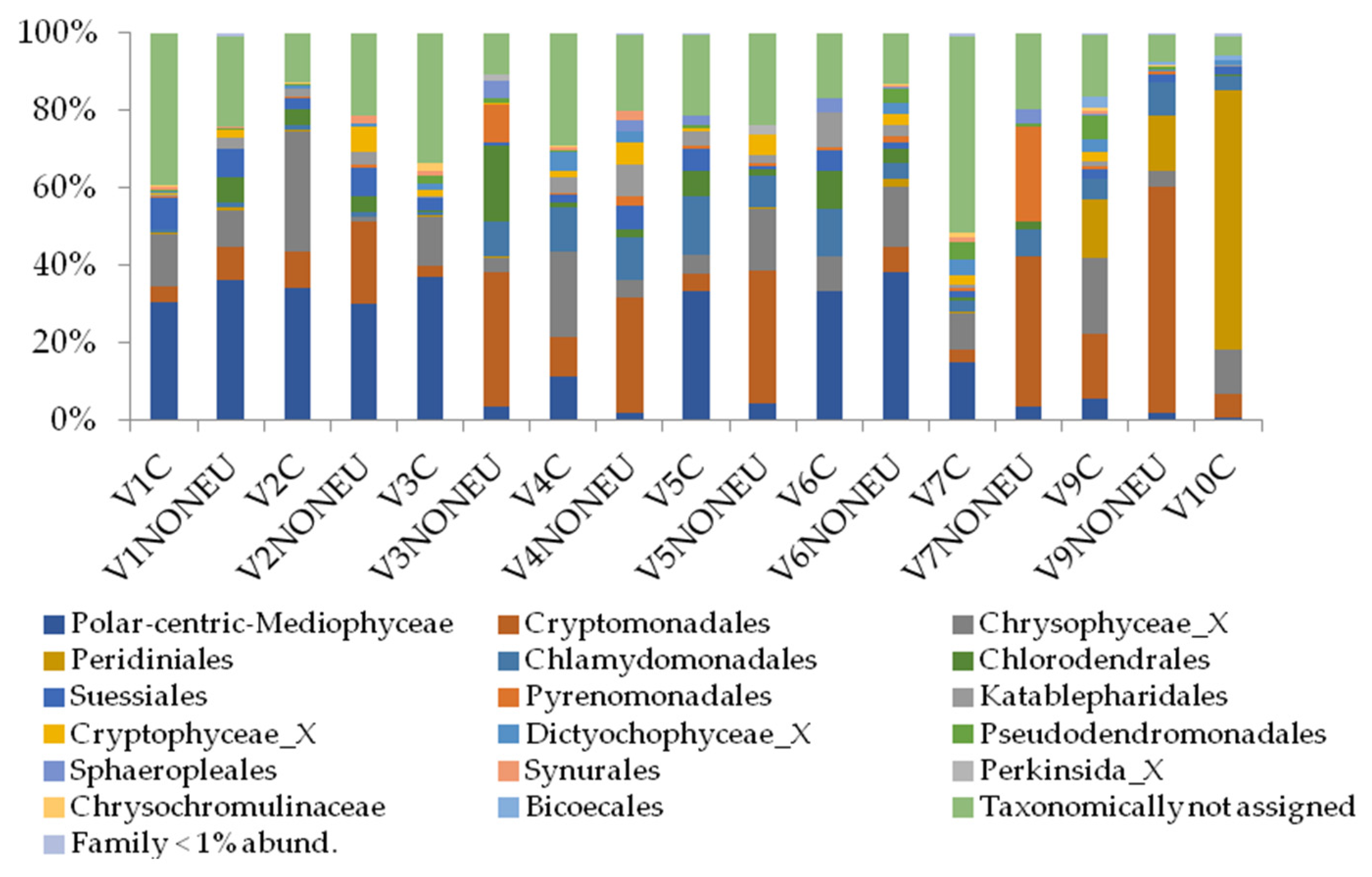
3.3. Characterization of Phytoplankton Community According to Molecular Approach
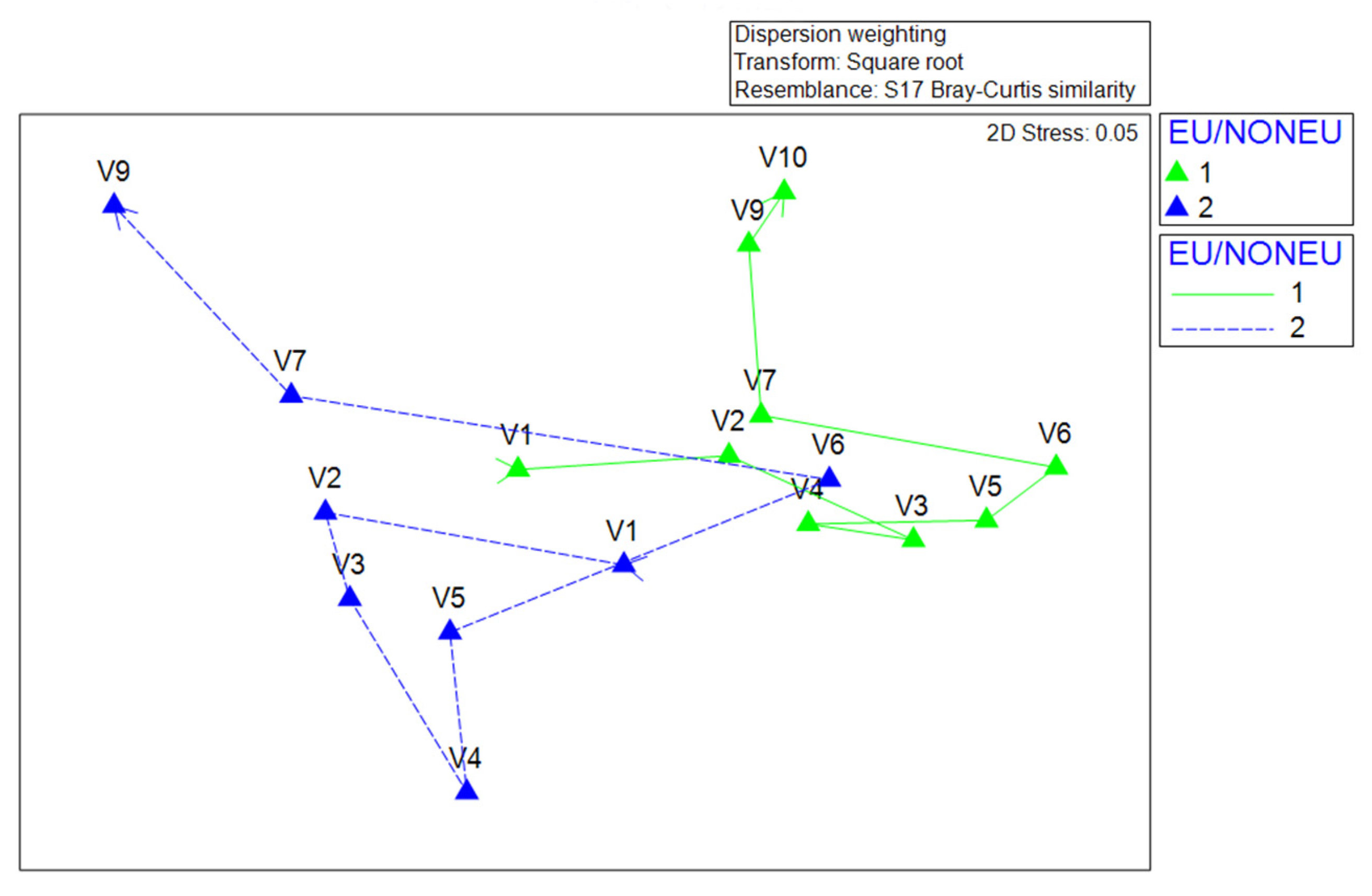
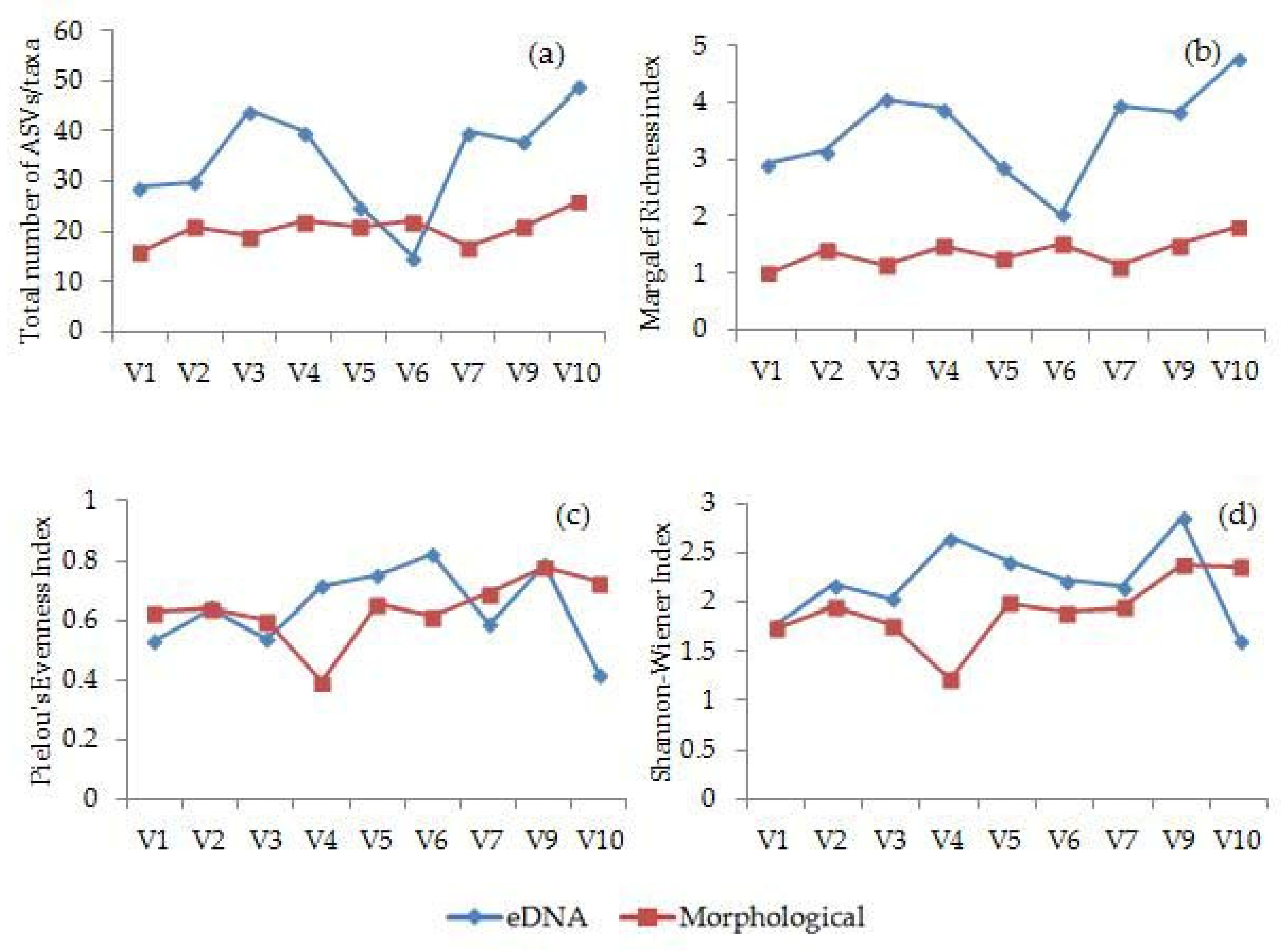
3.4. Morphological and Molecular Diversity of Phytoplankton in Lake Visovac
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mikac, I.; Fiket, Ž.; Terzić, S.; Barešić, J.; Mikac, N.; Ahel, M. Chemical Indicators of Anthropogenic Impacts in Sediments of the Pristine Karst Lakes. Chemosphere 2011, 84, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Gaedke, U. Trophic Dynamics in Aquatic Ecosystems. In Encyclopedia of Inland Waters; Elsevier: New York, NY, USA, 2009; pp. 499–504. ISBN 978-0-12-370626-3. [Google Scholar]
- European Commision Directive of the European Parliament and of the Council. 2000/60/EC. Establishing a Framework for Community Action in the Field of Water Policy (Water Framework Directive); The Publications Office of the European Union (L’Office des publications de l’Union européenne: Luxembourg, 2000. [Google Scholar]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a Functional Classification of the Freshwater Phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification, Enumeration and Use as Bioindicators, 1st ed.; Wiley-Blackwell: New York, NY, USA, 2015; ISBN 978-1-118-91716-9. [Google Scholar]
- Huo, S.; Li, X.; Xi, B.; Zhang, H.; Ma, C.; He, Z. Combining Morphological and Metabarcoding Approaches Reveals the Freshwater Eukaryotic Phytoplankton Community. Environ. Sci. Eur. 2020, 32, 37. [Google Scholar] [CrossRef]
- Hanžek, N.; Gligora Udovič, M.; Kajan, K.; Borics, G.; Várbíró, G.; Stoeck, T.; Žutinić, P.; Orlić, S.; Stanković, I. Assessing Ecological Status in Karstic Lakes through the Integration of Phytoplankton Functional Groups, Morphological Approach and Environmental DNA Metabarcoding. Ecol. Indic. 2021, 131, 108166. [Google Scholar] [CrossRef]
- Borics, G.; Abonyi, A.; Salmaso, N.; Ptacnik, R. Freshwater Phytoplankton Diversity: Models, Drivers and Implications for Ecosystem Properties. Hydrobiologia 2021, 848, 53–75. [Google Scholar] [CrossRef]
- Pawlowski, J.; Apothéloz-Perret-Gentil, L.; Mächler, E.; Altermatt, F. Environmental DNA Applications in Biomonitoring and Bioassessment of Aquatic Ecosystems; Environmental Studies. no 2010; Federal Office for the Environment: Bern, Switzerland, 2020; p. 71. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Jones, J.I.; Pont, D.; Boets, P.; Bouchez, A.; Bruce, K.; Drakare, S.; Hänfling, B.; Kahlert, M.; et al. Implementation Options for DNA-Based Identification into Ecological Status Assessment under the European Water Framework Directive. Water Res. 2018, 138, 192–205. [Google Scholar] [CrossRef]
- Kulaš, A.; Gligora Udovič, M.; Tapolczai, K.; Žutinić, P.; Orlić, S.; Levkov, Z. Diatom EDNA Metabarcoding and Morphological Methods for Bioassessment of Karstic River. Sci. Total Environ. 2022, 829, 154536. [Google Scholar] [CrossRef]
- Bonacci, O.; Andrić, I.; Roje-Bonacci, T. Hydrological Analysis of Skradinski Buk Tufa Waterfall (Krka River, Dinaric Karst, Croatia). Env. Earth Sci. 2017, 76, 669. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Croatia No. 96/19 Regulation on Water Quality Standards. Zagreb, Croatia, 2019. Available online: https://leap.unep.org/countries/hr/national-legislation/regulation-water-quality-standard (accessed on 6 March 2023).
- Poikane, S. Water Framework Directive Intercalibration Technical Report; Part 2: Lakes; Office for Official Publications of the European Communities (OPOCE): Luxembourg, 2009. [Google Scholar]
- APHA Standard Methods for the Examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2017. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/referencespapers.aspx?referenceid=2459667 (accessed on 6 March 2023).
- Utermöhl, H. Zur Vervollkommnung Der Quantitativen Phytoplankton-Methodik. SIL Commun. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süßwasserflora von Mitteleuropa Band 2/3; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer: Jena, Germany; Stuttgart, Germany; Lübeck, Germany; Ulm, Germany, 1998. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil/ 2nd Part: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2005. [Google Scholar]
- Krammer, K. Bacillariophyceae; Spektrum Akademischer: Heidelberg, Germany, 2000. [Google Scholar]
- Kristiansen, J.; Preisig, H.R. Süßwasserflora von Mitteleuropa 1. In Chrysophyte and Haptophyte Algae: Part 2: Synurophyceae, 2nd ed.; Spektrum Akademisher Verlag: Berlin, Germany, 2007. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen Im Süßwasser—Benthos von Mitteleuropa. In Bestimmungsflora Kieselalgen Für Die Ökologische Praxis; Über 700 Der Häufigsten Arten Und Ihre Ökologie; Gantner, A.R.G., Ed.; Koeltz Scientific Books: Ruggell, Liechtenstein, 2011. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2022; Available online: https://www.algaebase.org (accessed on 3 August 2022).
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and Misuse in the Application of the Phytoplankton Functional Classification: A Critical Review with Updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- HRN EN ISO 5667-3:2018 Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples. Available online: https://repozitorij.hzn.hr/norm/HRN+EN+ISO+5667-3%3A2018 (accessed on 16 January 2019).
- HRN EN 15204:2008; Water Quality—Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique). Available online: https://repozitorij.hzn.hr/norm/HRN+EN+15204%3A2008 (accessed on 11 June 2020).
- HRN EN 16695:2015; Water Quality—Guidance on the Estimation of Phytoplankton Biovolume. Available online: https://repozitorij.hzn.hr/norm/HRN+EN+16695%3A2015 (accessed on 11 June 2020).
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.-W.; Richards, T.A. Multiple Marker Parallel Tag Environmental DNA Sequencing Reveals a Highly Complex Eukaryotic Community in Marine Anoxic Water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A Method for Studying Protistan Diversity Using Massively Parallel Sequencing of V9 Hypervariable Regions of Small-Subunit Ribosomal RNA Genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Stoeck, T.; Kochems, R.; Forster, D.; Lejzerowicz, F.; Pawlowski, J. Metabarcoding of Benthic Ciliate Communities Shows High Potential for Environmental Monitoring in Salmon Aquaculture. Ecol. Indic. 2018, 85, 153–164. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics; Babraham Institute: Cambridge, UK, 2010; Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 3 May 2019).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit RRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2012, 41, D597–D604. [Google Scholar] [CrossRef]
- QGIS A Free and Open Source Geographic Information System. Open Source. Geospatial Foundation Project. 2009. Available online: https://qgis.org/en/site/ (accessed on 21 March 2023).
- Zarauz, L.; Irigoien, X.; Fernandes, J.A. Changes in Plankton Size Structure and Composition, during the Generation of a Phytoplankton Bloom, in the Central Cantabrian Sea. J. Plankton Res. 2008, 31, 193–207. [Google Scholar] [CrossRef]
- Ptacnik, R.; Lepistö, L.; Willén, E.; Brettum, P.; Andersen, T.; Rekolainen, S.; Lyche Solheim, A.; Carvalho, L. Quantitative Responses of Lake Phytoplankton to Eutrophication in Northern Europe. Aquat. Ecol. 2008, 42, 227–236. [Google Scholar] [CrossRef]
- Žutinić, P.; Gligora Udovič, M.; Kralj Borojević, K.; Plenković-Moraj, A.; Padisák, J. Morpho-Functional Classifications of Phytoplankton Assemblages of Two Deep Karstic Lakes. Hydrobiologia 2014, 740, 147–166. [Google Scholar] [CrossRef]
- Clegg, M.R.; Maberly, S.C.; Jones, R.I. Behavioral Response as a Predictor of Seasonal Depth Distribution and Vertical Niche Separation in Freshwater Phytoplanktonic Flagellates. Limnol. Oceanogr. 2007, 52, 441–455. [Google Scholar] [CrossRef]
- Jamal, A.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Littoral and Limnetic Phytoplankton Distribution and Biodiversity in a Tropical Man-Made Lake, Malaysia. Adv. Stud. Biol. 2014, 6, 149–168. [Google Scholar] [CrossRef]
- Lemly, A.D.; Dimmick, J.F. Phytoplankton Communities in the Littoral Zone of Lakes: Observations on Structure and Dynamics in Oligotrophic and Eutrophic Systems. Oecologia 1982, 54, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Mulderij, G.; Van Nes, E.H.; Van Donk, E. Macrophyte–Phytoplankton Interactions: The Relative Importance of Allelopathy versus Other Factors. Ecol. Model. 2007, 204, 85–92. [Google Scholar] [CrossRef]
- Blaum, N.; Mosner, E.; Schwager, M.; Jeltsch, F. How Functional Is Functional? Ecological Groupings in Terrestrial Animal Ecology: Towards an Animal Functional Type Approach. Biodivers. Conserv. 2011, 20, 2333–2345. [Google Scholar] [CrossRef]
- Gligora Udovič, M.; Kralj Borojević, K.; Žutinić, P.; Šipoš, L.; Plenković-Moraj, A. Net-Phytoplankton Species Dominance in a Travertine Riverine Lake Visovac, NP Krka. Nat. Croat. 2011, 20, 411–424. [Google Scholar]
- Gligora Udovič, M.; Žutinić, P.; Kralj Borojević, K.; Plenković-Moraj, A. Co-Occurrence of Functional Groups in Phytoplankton Assemblages Dominated by Diatoms, Chrysophytes and Dinoflagellates. Fundam. Appl. Limnol. 2015, 187, 101–111. [Google Scholar] [CrossRef]
- Sommer, U. Growth and Survival Strategies of Plankton Diatoms. In Growth and Reproductive Strategies of Freshwater Phytoplankton; Sandgren, C.D., Ed.; Cambridge University Press: Cambridge, UK, 1988; pp. 227–260. ISBN 0-521-32722-9. [Google Scholar]
- Grigorszky, I.; Padisák, J.; Borics, G.; Schitchen, C.; Borbély, G. Deep Chlorophyll Maximum by Ceratium hirundinella (O. F. Müller) Bergh in a Shallow Oxbow in Hungary. Hydrobiologia 2003, 506, 209–212. [Google Scholar] [CrossRef]
- Olrik, K.; Cronberg, G.; Annadotter, H. Lake Phytoplankton Responses to Global Climate Changes. In Climatic Change and Global Warming of Inland Waters: Impacts and Mitigation for Ecosystems and Societies; Goldman, C.R., Kumagai, M., Robarts, R.D., Eds.; Wiley: Chichester, UK, 2012; pp. 173–199. [Google Scholar]
- Stoecker, D.K.; Johnson, M.D.; deVargas, C.; Not, F. Acquired Phototrophy in Aquatic Protists. Aquat. Microb. Ecol. 2009, 57, 279–310. [Google Scholar] [CrossRef]
- Sanders, R.W. Alternative Nutritional Strategies in Protists: Symposium Introduction and a Review of Freshwater Protists That Combine Photosynthesis and Heterotrophy1. J. Eukaryot. Microbiol. 2011, 58, 181–184. [Google Scholar] [CrossRef]
- Kamjunke, N.; Henrichs, T.; Gaedke, U. Phosphorus Gain by Bacterivory Promotes the Mixotrophic Flagellate Dinobryon Spp. during Re-Oligotrophication. J. Plankton Res. 2007, 29, 39–46. [Google Scholar] [CrossRef]
- Hansen, P.J. The Role of Photosynthesis and Food Uptake for the Growth of Marine Mixotrophic Dinoflagellates1. J. Eukaryot. Microbiol. 2011, 58, 203–214. [Google Scholar] [CrossRef]
- Wilken, S.; Huisman, J.; Naus-Wiezer, S.; Van Donk, E. Mixotrophic Organisms Become More Heterotrophic with Rising Temperature. Ecol. Lett. 2013, 16, 225–233. [Google Scholar] [CrossRef]
- Šimunović, M.; Kulaš, A.; Žutinić, P.; Goreta, G.; Gligora Udovič, M. Phytoplankton Metrics for Trophic and Ecological Status Assessment of a Natural Karstic Lake. Acta Bot. Croat. 2022, 81, 185–196. [Google Scholar] [CrossRef]
- Beamud, S.G.; León, J.G.; Kruk, C.; Pedrozo, F.; Diaz, M. Using Trait-Based Approaches to Study Phytoplankton Seasonal Succession in a Subtropical Reservoir in Arid Central Western Argentina. Environ. Monit. Assess. 2015, 187, 271. [Google Scholar] [CrossRef]
- Reynolds, C.S. Vegetation Processes in the Pelagic: A Model for Ecosystem Theory. Xxvii, 371 p. Oldendorf/Luhe, Germany: Ecology Institute, 1997. (Excellence in Ecology No. 9). Price DM 68.00. J. Mar. Biol. Ass. 1997, 77, 919. [Google Scholar] [CrossRef]
- Hu, R.; Han, B.; Naselli-Flores, L. Comparing Biological Classifications of Freshwater Phytoplankton: A Case Study from South China. Hydrobiologia 2013, 701, 219–233. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.S. Comparative Analyses of the V4 and V9 Regions of 18S RDNA for the Extant Eukaryotic Community Using the Illumina Platform. Sci. Rep. 2020, 10, 6519. [Google Scholar] [CrossRef]
- Rimet, F.; Vasselon, V.; A.-Keszte, B.; Bouchez, A. Do We Similarly Assess Diversity with Microscopy and High-Throughput Sequencing? Case of Microalgae in Lakes. Org. Divers. Evol. 2018, 18, 51–62. [Google Scholar] [CrossRef]
- Vasselon, V.; Domaizon, I.; Rimet, F.; Kahlert, M.; Bouchez, A. Application of High-Throughput Sequencing (HTS) Metabarcoding to Diatom Biomonitoring: Do DNA Extraction Methods Matter? Freshw. Sci. 2017, 36, 162–177. [Google Scholar] [CrossRef]
- Bruce, K.; Blackman, R.; Bourlat, S.J.; Hellström, A.M.; Bakker, J.; Bista, I.; Bohmann, K.; Bouchez, A.; Brys, R.; Clark, K.; et al. A Practical Guide to DNA-Based Methods for Biodiversity Assessment; Pensoft Publishers: Sofia, Bulgaria, 2021; ISBN 978-619-248-053-0. [Google Scholar]
- Wilmotte, A.; Dail Laughinghouse IV, H.; Capelli, C.; Rippka, R.; Salmaso, N. Taxonomic Identification of Cyanobacteria by a Polyphasic Approach. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 79–134. [Google Scholar]
- Not, F.; Zapata, M.; Pazos, Y.; Campaña, E.; Doval, M.; Rodríguez, F. Size-Fractionated Phytoplankton Diversity in the NW Iberian Coast: A Combination of Microscopic, Pigment and Molecular Analyses. Aquat. Microb. Ecol. 2007, 49, 255–265. [Google Scholar] [CrossRef]
- Xiao, X.; Sogge, H.; Lagesen, K.; Tooming-Klunderud, A.; Jakobsen, K.S.; Rohrlack, T. Use of High Throughput Sequencing and Light Microscopy Show Contrasting Results in a Study of Phytoplankton Occurrence in a Freshwater Environment. PLoS ONE 2014, 9, e106510. [Google Scholar] [CrossRef] [PubMed]
- Maritz, J.M.; Rogers, K.H.; Rock, T.M.; Liu, N.; Joseph, S.; Land, K.M.; Carlton, J.M. An 18S RRNA Workflow for Characterizing Protists in Sewage, with a Focus on Zoonotic Trichomonads. Microb. Ecol. 2017, 74, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Tragin, M.; Zingone, A.; Vaulot, D. Comparison of Coastal Phytoplankton Composition Estimated from the V4 and V9 Regions of the 18S RRNA Gene with a Focus on Photosynthetic Groups and Especially Chlorophyta. Environ. Microbiol. 2018, 20, 506–520. [Google Scholar] [CrossRef]
- Medinger, R.; Nolte, V.; Pandey, R.V.; Jost, S.; Ottenwalder, B.; Schlotterer, C.; Boenigk, J. Diversity in a Hidden World: Potential and Limitation of next-Generation Sequencing for Surveys of Molecular Diversity of Eukaryotic Microorganisms. Mol. Ecol. 2010, 19, 32–40. [Google Scholar] [CrossRef]
- Lanzén, A.; Lekang, K.; Jonassen, I.; Thompson, E.M.; Troedsson, C. High-Throughput Metabarcoding of Eukaryotic Diversity for Environmental Monitoring of Offshore Oil-Drilling Activities. Mol. Ecol. 2016, 25, 4392–4406. [Google Scholar] [CrossRef]
- Maitland, V.C.; Robinson, C.V.; Porter, T.M.; Hajibabaei, M. Freshwater Diatom Biomonitoring through Benthic Kick-Net Metabarcoding. PLoS ONE 2020, 15, e0242143. [Google Scholar] [CrossRef]
- Kahlert, M.; Bailet, B.; Chonova, T.; Karjalainen, S.M.; Schneider, S.C.; Tapolczai, K. Same Same, but Different: The Response of Diatoms to Environmental Gradients in Fennoscandian Streams and Lakes—Barcodes, Traits and Microscope Data Compared. Ecol. Indic. 2021, 130, 108088. [Google Scholar] [CrossRef]
- Ciglenečki-Jušić, I.; Ahel, M.; Mikac, N.; Omanović, D.; Vdović, N. Investigation of Natural Characteristics and Assesment of Antropogenic Influences on Water Quality of Visovac Lake; Ruđer Bošković Institute: Zagreb, Croatia, 2013; Available online: https://www.bib.irb.hr/662477 (accessed on 21 June 2020).


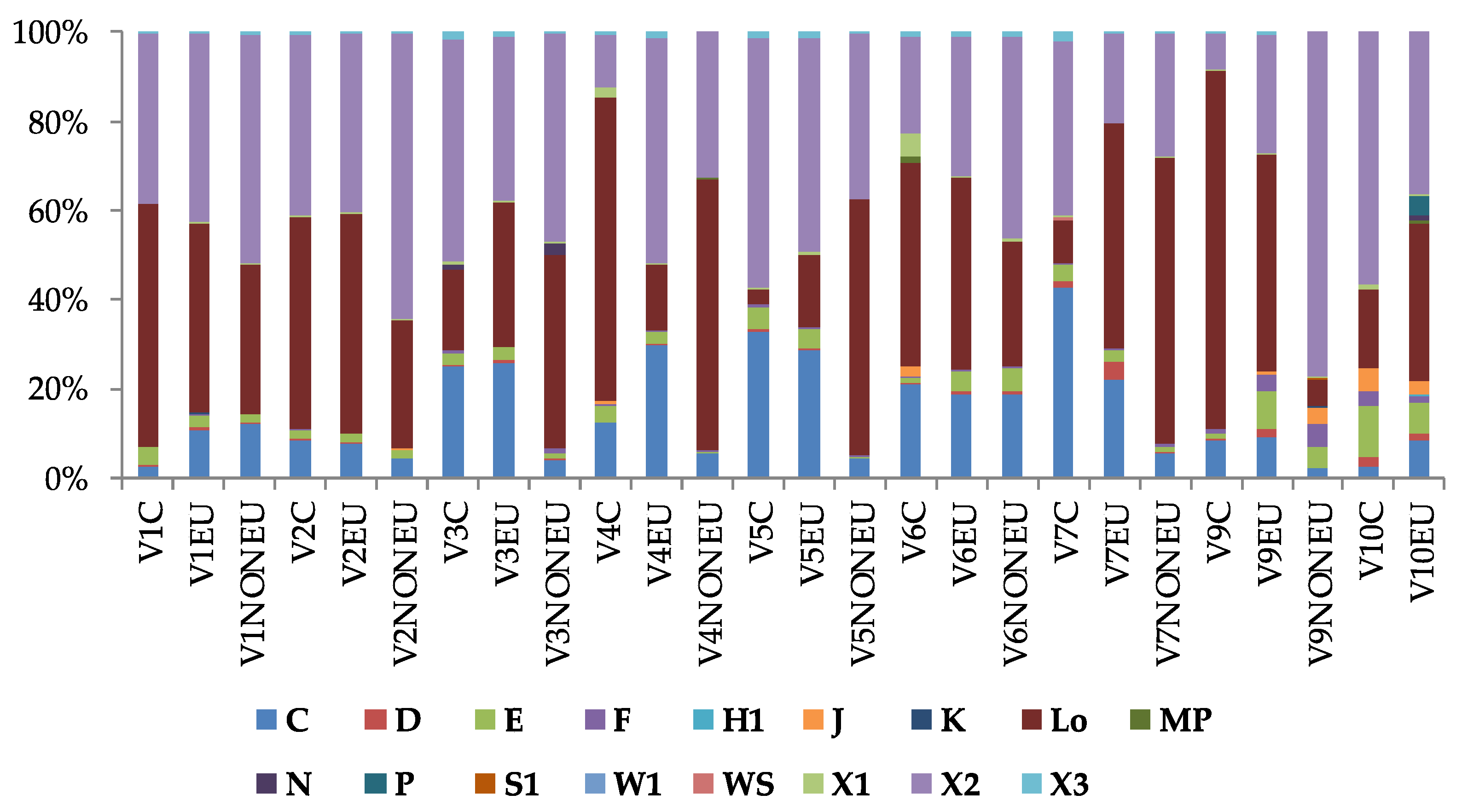


| Station | Max Depth (m) | SD * (m) | ZEU * (m) | T * (°C) | O2 * (mg L−1) | O2 (%)* | pH | EC * (µS cm−1) |
|---|---|---|---|---|---|---|---|---|
| V1 | 15.2 | 3.0 | 7.5 | 23.8 | 11.68 | 138.0 | 8.00 | 545 |
| V2 | 18.0 | 2.5 | 6.25 | 23.3 | 11.03 | 129.8 | 7.99 | 547 |
| V3 | 18.0 | 3.0 | 7.5 | 22.0 | 10.00 | 115.0 | 7.84 | 556 |
| V4 | 15.5 | 4.0 | 10.0 | 21.5 | 9.03 | 102.6 | 7.79 | 559 |
| V5 | 18.0 | 3.5 | 8.75 | 22.1 | 9.79 | 113.0 | 7.94 | 559 |
| V6 | 15.0 | 3.5 | 8.75 | 21.7 | 9.09 | 103.3 | 7.77 | 562 |
| V7 | 24.0 | 3.5 | 8.75 | 20.6 | 11.14 | 124.5 | 7.75 | 545 |
| V9 | 23.0 | 5.0 | 12.5 | 25.9 | 10.18 | 125.6 | 7.84 | 505 |
| V10 | 5.0 | 4.5 | 5.0 | 26.2 | 9.97 | 123.5 | 7.98 | 494 |
| Pairwise Tests | |||||
|---|---|---|---|---|---|
| Comparison of Samples | R Statistic | Significance Level (p) | Possible Permutations | Actual Permutations | Number >= Observed |
| C vs. EU | −0.023 | 0.548 | 24310 | 999 | 547 |
| C vs. NONEU | 0.168 | 0.042 | 24310 | 999 | 41 |
| EU vs. NONEU | 0.391 | 0.006 | 24310 | 999 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, M.; Kulaš, A.; Žutinić, P.; Gligora Udovič, M. Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches. Water 2023, 15, 1379. https://doi.org/10.3390/w15071379
Šimunović M, Kulaš A, Žutinić P, Gligora Udovič M. Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches. Water. 2023; 15(7):1379. https://doi.org/10.3390/w15071379
Chicago/Turabian StyleŠimunović, Maja, Antonija Kulaš, Petar Žutinić, and Marija Gligora Udovič. 2023. "Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches" Water 15, no. 7: 1379. https://doi.org/10.3390/w15071379
APA StyleŠimunović, M., Kulaš, A., Žutinić, P., & Gligora Udovič, M. (2023). Phytoplankton Diversity of a Natural Karst Lake Combining Morphological and Molecular Approaches. Water, 15(7), 1379. https://doi.org/10.3390/w15071379









