Tracking Fish Introduction in a Mountain Lake over the Last 200 Years Using Chironomids, Diatoms, and Cladoceran Remains
Abstract
1. Introduction
2. Material and Methods
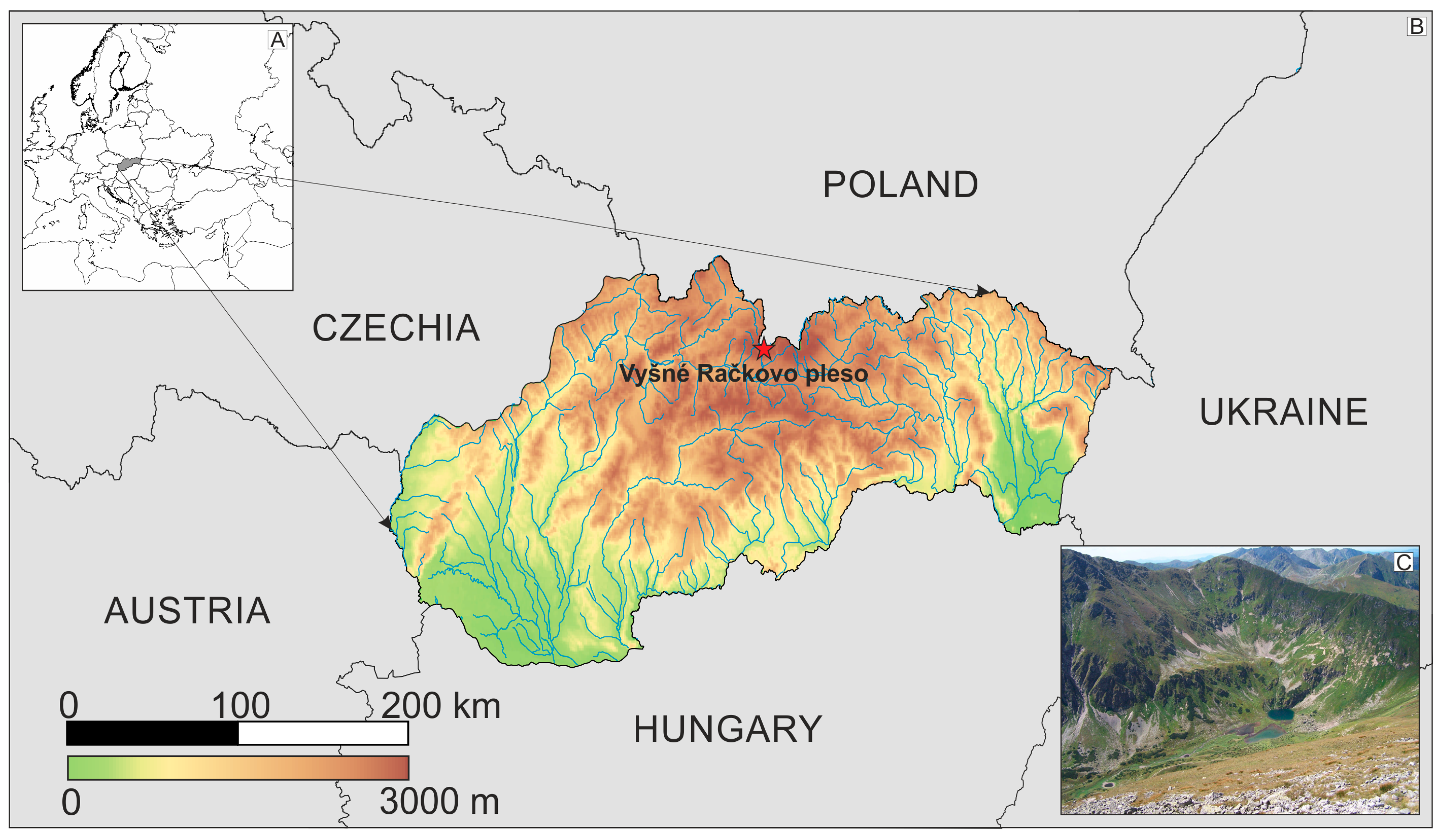
2.1. Study Lake
2.2. Sediment Sampling
2.3. Chronology
2.4. Chironomid Analysis
2.5. Cladocera Analysis
2.6. Diatom Analysis
2.7. Data Analysis
3. Results
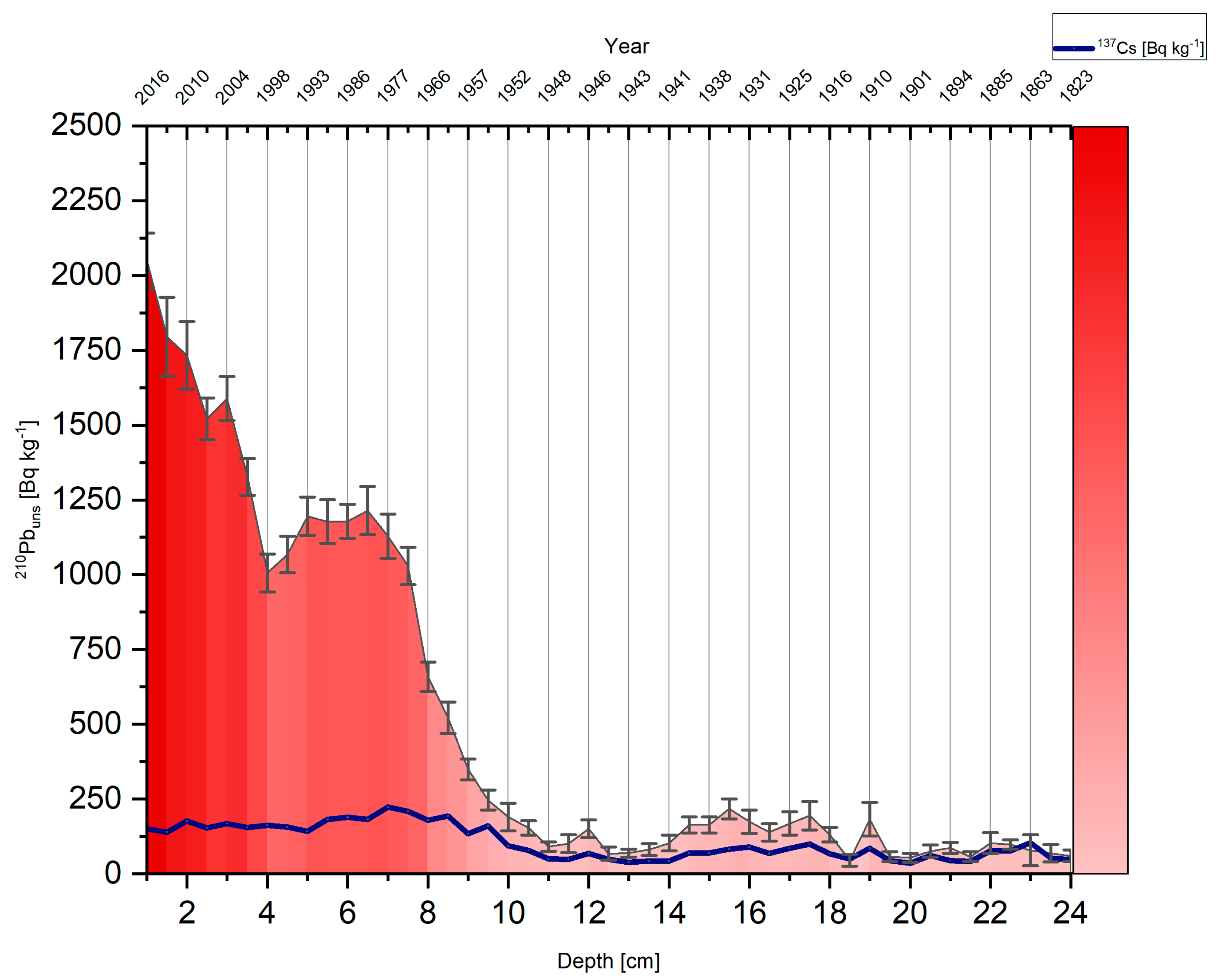
3.1. Chronology
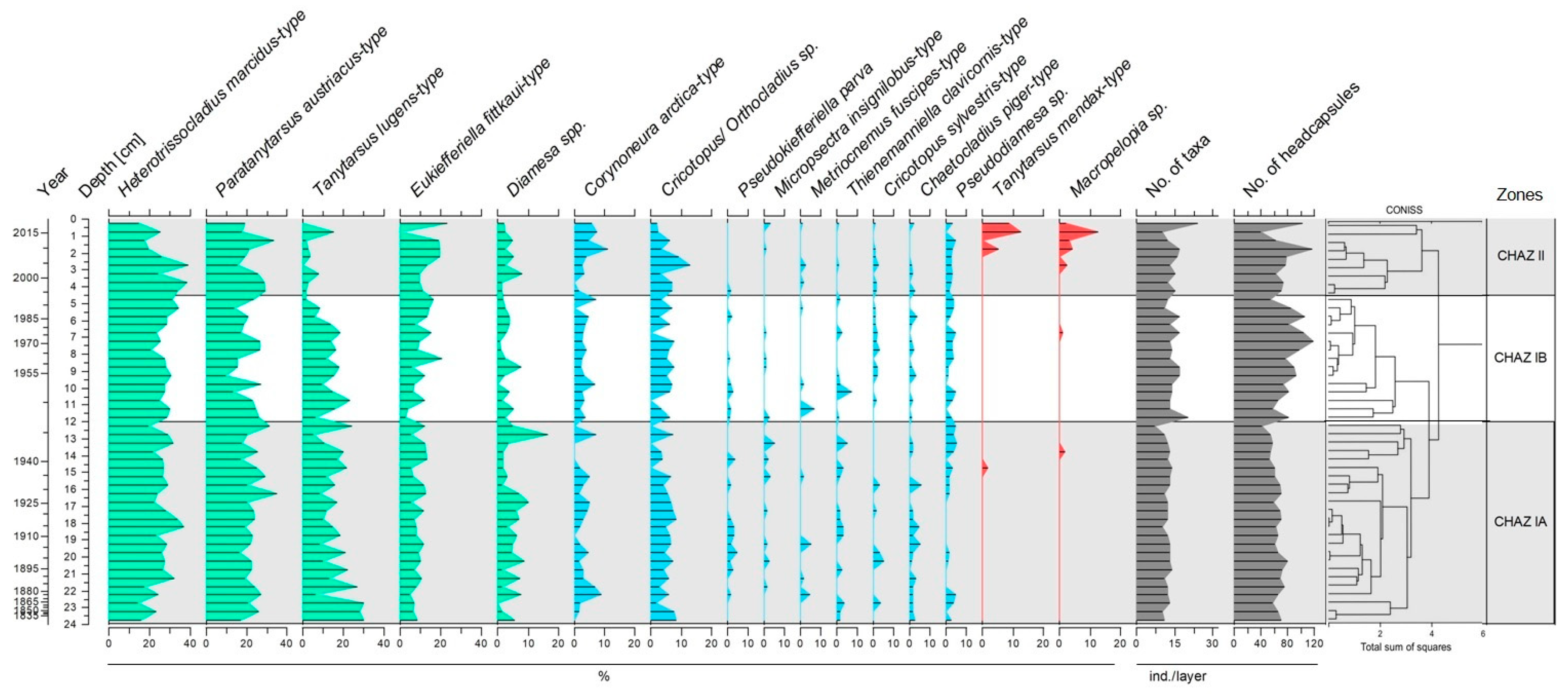
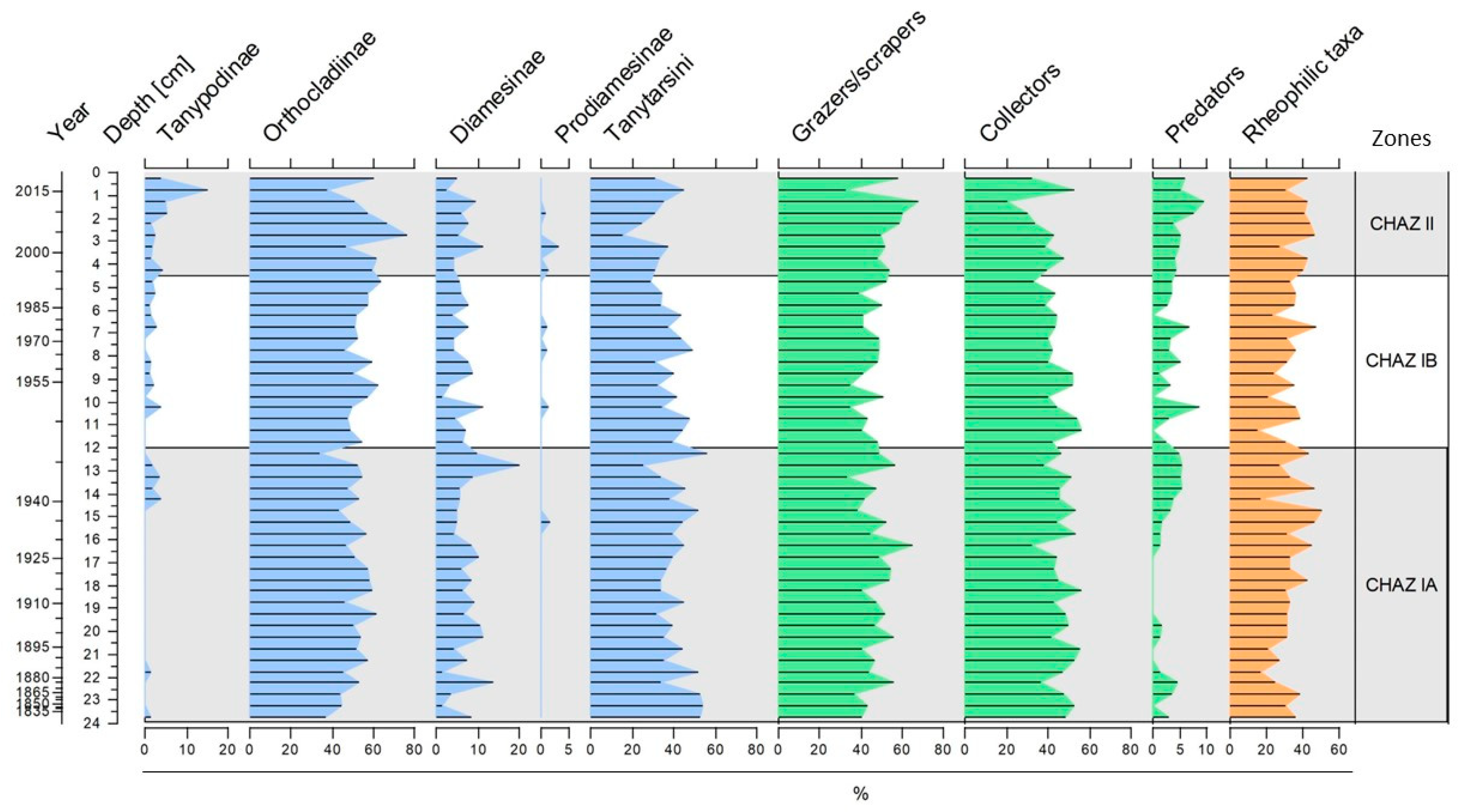
3.2. Chironomid Assemblages
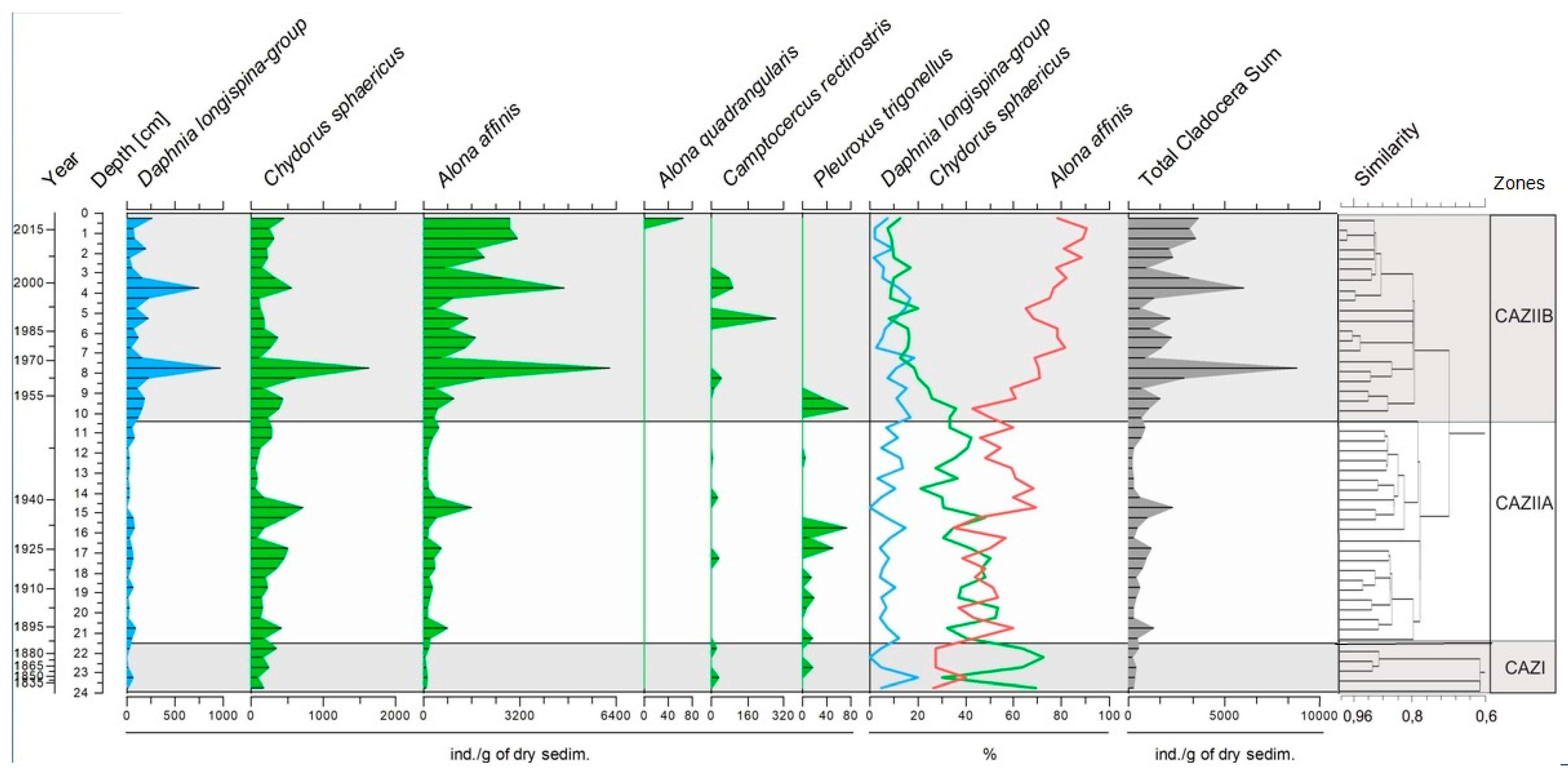
3.3. Cladocera Assemblages
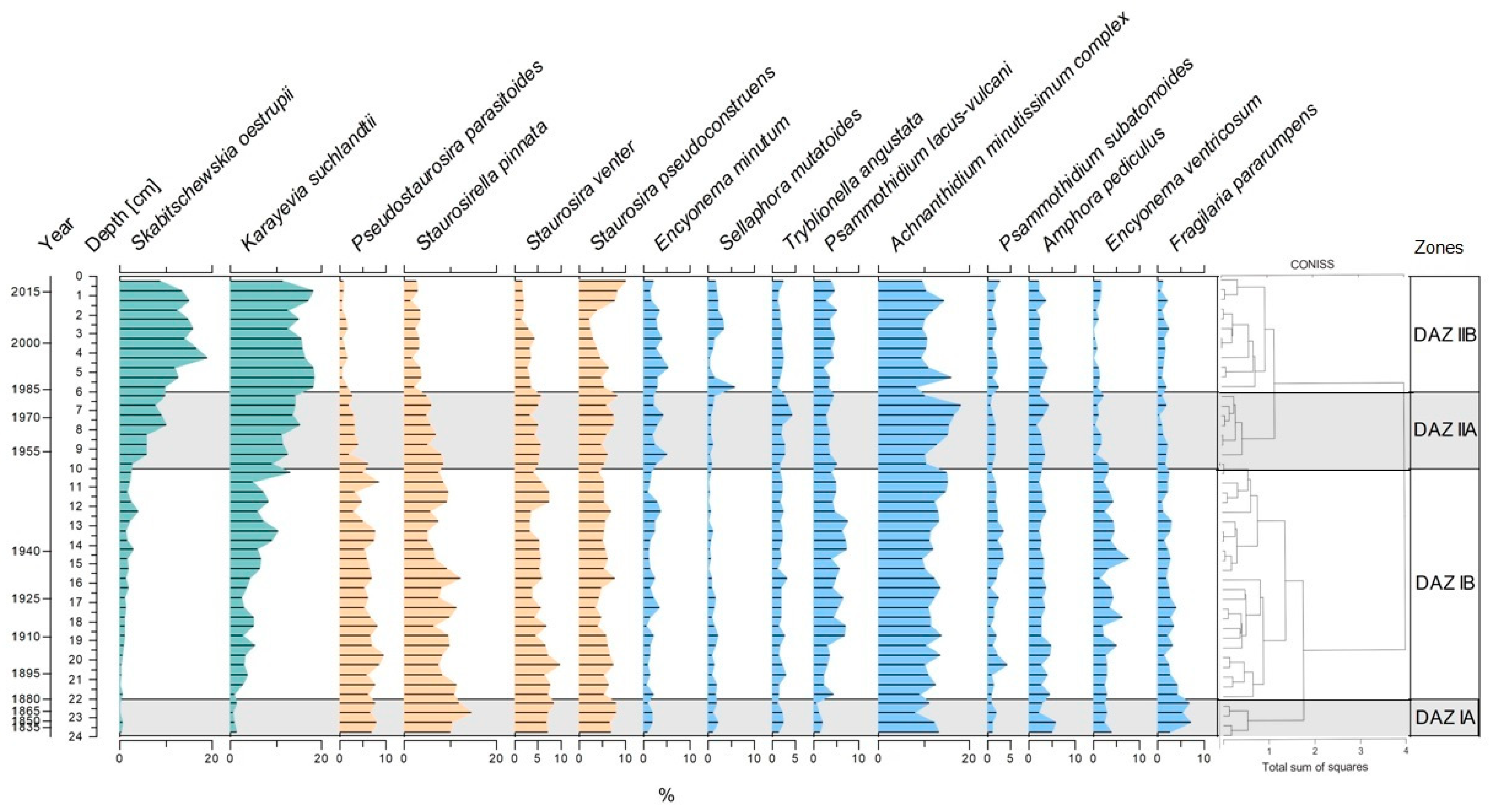
3.4. Diatom Assemblages
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Theurillat, J.P.; Guisan, A. Potential impact of climate change on vegetation in the European Alps: A review. Clim. Change 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Alverson, K.; Kull, C.; Moore, G.W.K.; Ginot, P. A dynamical perspective on high altitude paleoclimate proxy timeseries. In Global Change and Mountain Regions, 1st ed.; Huber, U.M., Bugmann, H.K.M., Reasoner, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 23, pp. 11–20. [Google Scholar]
- Nagy, L.; Grabherr, G.; Körner, C.; Thompson, D.B.A. Alpine Biodiversity in Europe: An Introduction; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; p. 16. [Google Scholar]
- Battarbee, R.W.; Patrick, S.; Kernan, M.; Psenner, R.; Thies, H.; Grimalt, J.; Rosseland, B.O.; Wathne, B.; Catalan, J.; Mosello, R.; et al. High Mountain lakes and atmospherically transported pollutants. In Global Change and Mountain Region, 1st ed.; Huber, U.M., Bugmann, H.K.M., Reasoner, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 23, pp. 113–121. [Google Scholar]
- Catalán, J.; Ninot, J.M.; Aniz, M.M. (Eds.) High Mountain Conservation in a Changing World; Springer Open: Cham, Switzerland, 2017; p. 413. [Google Scholar]
- Moser, K.A.; Baron, J.S.; Brahney, J.; Oleksy, I.A.; Saros, J.E.; Hundey, E.J.; Sadro, S.; Kopáček, J.; Sommaruga, R.; Kainz, M.J.; et al. Mountain lakes: Eyes on global environmental change. Global. Planet. Change 2019, 178, 77–95. [Google Scholar] [CrossRef]
- Schirpke, U.; Ebner, M. Exposure to global change pressures and potential impacts on ecosystem services of mountain lakes in the European Alps. J. Environ. Manag. 2022, 318, 115606. [Google Scholar] [CrossRef] [PubMed]
- Kernan, M.; Ventura, M.; Bitušík, P.; Brancelj, A.; Clarke, G.; Velle, G.; Raddum, G.G.; Stuchlík, E.; Catalan, J. Regionalisation of remote European mountain lake ecosystems according to their biota: Environmental vs. geographical patterns. Freshw. Biol. 2009, 54, 2470–2493. [Google Scholar] [CrossRef]
- Rühland, K.M.; Paterson, A.M.; Smol, J.P. Lake diatom responses to warming: Reviewing the evidence. J. Paleolimnol. 2015, 54, 1–35. [Google Scholar] [CrossRef]
- Psenner, R. Alpine waters in the interplay of global change: Complex links—simple effects? In Global Environmental Change in Alpine Region; Steininger, K.W., Weck-Hannemann, H., Eds.; Edward Eldgar: Cheltenham, UK; Northampton, MA, USA, 2002; pp. 15–40. [Google Scholar]
- Catalán, J.; Camarero, L.; Felip, M.; Pla, S.; Ventura, M.; Buchaca, T.; Bartumeus, F.; Mendoza, G.; Miró, A.; Casamayor, E.O.; et al. High Mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 64, 123–145. [Google Scholar] [CrossRef]
- Bitušík, P.; Kopáček, J.; Stuchlík, E.; Šporka, F. Biologia 61/18; Slovak Academy of Sciences: Bratislava, Slovakia, 2006; p. 221. [Google Scholar]
- Stuchlík, E.; Kopáček, J.; Fott, J.; Hořická, Z. Chemical composition of the Tatra Mountain lakes: Response to acidification. Biologia 2006, 61, 11–20. [Google Scholar] [CrossRef]
- Hořická, Z.; Stuchlík, E.; Hudec, I.; Černý, M.; Fott, J.; Kopáček, J. Acidification and the structure of crustacean zooplankton in mountain lakes: The Tatra Mountains (Slovakia and Poland). Biologia 2006, 61, 121–134. [Google Scholar] [CrossRef]
- Krno, I.; Šporka, F.; Galas, J.; Hamerlík, L.; Zaťovičová, Z.; Bitušík, P. Littoral benthic macroinvertebrates of mountain lakes in the Tatra Mountains (Slovakia, Poland). Biologia 2006, 61, 147–166. [Google Scholar] [CrossRef]
- Kopáček, J.; Hardekopf, D.; Stuchlík, E. Chemical composition of the Tatra Mountain lakes: Recovery from acidification. Biologia 2006, 61, 21–33. [Google Scholar] [CrossRef]
- Stuchlík, E.; Bitušík, P.; Hardekopf, D.W.; Hořická, Z.; Kahounová, M.; Tátosová, J.; Vondrák, D.; Dočkalová, K. Complexity in the biological recovery of Tatra Mountain lakes from acidification. Water Air Soil Poll. 2017, 228, 184. [Google Scholar] [CrossRef]
- Kopáček, J.; Kaňa, J.; Bičárová, S.; Brahney, J.; Navrátil, T.; Norton, S.A.; Porcal, P.; Stuchlík, E. Climate change accelerates recovery of the Tatra Mountain lakes from acidification and increases their nutrient and chlorophyll a concentration. Aquat. Sci. 2019, 81, 70. [Google Scholar] [CrossRef]
- Kopáček, J.; Kaňa, J.; Porcal, P.; Stuchlík, E. Diverse effects of accelerating climate change on chemical recovery of alpine lakes from acidic deposition in soil-rich versus scree-rich catchments. Environ. Pollut. 2021, 284, 117522. [Google Scholar] [CrossRef] [PubMed]
- Svitok, M.; Kubovčík, V.; Kopáček, J.; Bitušík, P. Temporal trends and spatial patterns of chironomid communities in alpine lakes recovering from acidification under accelerating climate change. Freshw. Biol. 2021, 66, 2223–2239. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Gozlan, R.E. Introduction of non-native freshwater fish: Is it all bad? Fish Fish. 2008, 9, 106–115. [Google Scholar] [CrossRef]
- Hulme, P.E.; Nentwig, W.; Pyšek, P. Glossary of the Main Technical Terms Used in the Handbook. In Handbook of Alien Species in Europe, Vol 3. INVADING Nature; Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2009; pp. 375–379. [Google Scholar]
- Knapp, R.A.; Matthews, K.R.; Sarnelle, O. Resistance and resilience of alpine lake fauna to fish introductions. Ecol. Monogr. 2001, 71, 401–421. [Google Scholar] [CrossRef]
- Sarnelle, O.; Knapp, R.A. Nutrient recycling by fish versus zooplankton grazing as drivers of the trophic cascade in alpine lakes. Limnol. Oceanogr. 2005, 50, 2032–2042. [Google Scholar] [CrossRef]
- Mendoza, G.; Rico, E.; Catalan, J. Predation by introduced fish constrains the thermal distribution of aquatic Coleoptera in mountain lakes. Freshw. Biol. 2012, 57, 803–814. [Google Scholar] [CrossRef]
- Tiberti, R.; Brighenti, S.; Lacobuzio, R.; Pasquini, G.; Rolla, M. Behind the impact of introduced trout in high altitude lakes: Adult, not juvenile fish are responsible of the selective predation on crustacean zooplankton. J. Limnol. 2014, 73, 593–597. [Google Scholar] [CrossRef]
- Tiberti, R.; Hardenberg, A.; Bogliani, G. Ecological impact of introduced fish in highbaltitude lakes: A case of study from the European Alps. Hydrobiologia 2014, 724, 1–19. [Google Scholar] [CrossRef]
- Miró, A.; Ventura, M. Introduced fish in Pyrenean high mountain lakes: Impact on amphibians and other organisms, and conservation implications. Limnetica 2020, 39, 283–297. [Google Scholar] [CrossRef]
- Epanchin, P.N.; Knapp, R.A.; Lawler, S.P. Nonnative trout impact an alpine-nesting bird by altering aquatic insect subsidies. Ecology 2010, 91, 2406–2415. [Google Scholar] [CrossRef]
- Tiberti, R.; Rolla, M.; Brighenti, S.; Lacobuzio, R. Changes in the insect emergence at the water–air interface in response to fish density manipulation in high altitude lakes. Hydrobiologia 2016, 779, 93–104. [Google Scholar] [CrossRef]
- Cogalniceanu, D.; Bancila, R.; Plaiasu, R.; Samoila, C.; Hartel, T. Aquatic habitat use by amphibians with specific reference to Rana temporaria at high elevations (Retezat Mountains National Park, Romania). Ann. Limnol. 2012, 48, 355–362. [Google Scholar] [CrossRef]
- Ventura, M.; Tiberti, R.; Buchaca, T.; Buňay, D.; Sabás, I.; Miró, A. Why should we preserve fishless high mountain lakes? In High Mountain conservation in a Changing World; Catalan, J., Ninot, M.J., Aniz, M.M., Eds.; Springer: Cham, Switzerland, 2017; Volume 62, pp. 181–205. [Google Scholar]
- Zontág, M.; Kot, M. Ryby. In Tatry–Príroda, 1st ed.; Chovancová, B., Koutná, A., Eds.; Baset: Nové Město, Czech Republic, 2010; pp. 503–518. [Google Scholar]
- Bohuš, I. Tatranský Kaleidoskop, 1st ed.; Osveta: Martin, Slovakia, 1977; pp. 30–31. [Google Scholar]
- Vološčuk, I.; Bohuš, I.; Bublinec, E.; Bohušová-Hradiská, H.; Drdoš, J.; Dúbravcová, Z. Tatranský národný park. Biosférická rezervácia; Martin: Gradus, Slovakia, 1994; p. 555. [Google Scholar]
- Gliwicz, Z.M.; Rowan, M.G. Survival of Cyclops abyssorum tatricus (Copepoda, Crustacea) in alpine lakes stocked with planktivorous fish. Limnol. Oceanogr. 1984, 29, 1290–1299. [Google Scholar] [CrossRef]
- Holčík, J.; Nagy, Š. Ichtyofauna Štrbského plesa. I. Druhové zloženie. In Zborník prác o Tatranskom národnom parku, 1st ed.; Somora, J., Ed.; Obzor: Bratislava, Slovakia, 1986; Volume 27, pp. 5–24. [Google Scholar]
- Krzysztof, Ł.; Piotr, D.; Jacek, K.; Anna, Ź-S.; Konrad, W. Vertical distribution of Cottus poecilopus Heckel, 1837 in streams of Tatra National Park in Poland. In Proceedings of the International Conference on Environmental Engineering, Vilnius, Lithuania, 27 April 2017; Vilnius Gediminas Technical University Press Technika: Vilnius, Lithuania, 2017. [Google Scholar]
- Janiga, M. Cottus poecilopus Heckel, 1836, in the river Javorinka, the Tatra mountains, Slovakia. Oecologia Mont. 2018, 27, 21–26. [Google Scholar]
- Dočkalová, K.; Holubcová, J.; Bacardit, M.; Bartrons, M.; Camarero, L.; Gallego, E.; Grimalt, J.O.; Hardekopf, D.; Hořická, Z.; Rosseland, B.O.; et al. Brown and brook trout populations in the Tatra Mountain lakes (Slovakia, Poland) and contamination by long-range transported pollutants. Biologia 2015, 70, 516–529. [Google Scholar] [CrossRef]
- Dawidowicz, P.; Gliwicz, M.Z. Food of brook charr in extreme oligotrophic conditions of an alpine lake. Environ. Biol. Fish. 1983, 8, 55–60. [Google Scholar] [CrossRef]
- Sienkiewicz, E.; Gąsiorowski, M. The effect of fish stocking on mountain lake plankton communities identified using palaeobiological analyses of bottom sediment cores. J. Paleolimnol. 2016, 55, 129–150. [Google Scholar] [CrossRef]
- Sochuliaková, L.; Sienkiewicz, E.; Hamerlík, L.; Svitok, M.; Fidlerová, D.; Bitušík, P. Reconstructing the trophic history of an alpine lake (High Tatra Mts.) using subfossil diatoms: Disentangling the effects of climate and human influence. Water Air Soil Poll. 2018, 229, 289. [Google Scholar] [CrossRef] [PubMed]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Kopáček, J.; Borovec, J.; Hejzlar, J.; Kotorová, I.; Stuchlík, E.; Veselý, J. Chemical composition of modern and pre-acidification sediments in the Tatra Mountain lakes. Biologia 2006, 61, 65–76. [Google Scholar] [CrossRef]
- Szarlowicz, K.; Reczyński, W.; Misiak, R.; Kubica, B. Radionuclides and heavy metal concentrations as complementary tools for studying the impact of industrialization on the environment. J. Radioanal. Nucl. Chem. 2013, 298, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Szarłowicz, K.; Stobiński, M.; Jedrzejek, F.; Kubica, B. Sedimentary conditions based on the vertical distribution of radionuclides in small dystrophic lakes: A case study of Toporowe Stawy Lakes (Tatra Mountains, Poland). Environ. Sci. Pollut. R. 2022, 29, 89530–89541. [Google Scholar] [CrossRef]
- Szarlowicz, K. Optimization of the radiochemical procedure of 210Po determination in small amounts of sediment samples. Int. J. Environ. Sci. Technol. 2019, 16, 5735–5740. [Google Scholar] [CrossRef]
- Lehto, J.; Hou, X. Chemistry and Analysis of Radionuclides: Laboratory Techniques and Methodology; Wiley-VCH: Weinheim, Germany, 2010; p. 426. [Google Scholar]
- Brooks, S.J.; Langdon, P.G.; Heiri, O. The Identification and Use of Palaearctic Chironomidae Larvae in Palaeoecology, 1st ed.; Technical Guide No. 10.; QRA: London, UK, 2007; p. 276. [Google Scholar]
- Anderson, T.; Cranston, P.S.; Epler, J.H. (Eds.) Chironomidae of the Holarctic Region-Keys and Diagnoses—Larvae, 3rd ed.; Media-Tryck: Lund, Sweden, 2013; pp. 1–571. [Google Scholar]
- Moller Pillot, H.K.M. Chironomidae Larvae. Biology and Ecology of the aquatic Orthocladiinae, 1st ed.; KNNV Publishing: Zeist, The Netherlands, 2013; p. 314. [Google Scholar]
- Šporka, F. (Ed.) Vodné bezstavovce (makroevertebráta) Slovenska, súpis druhov a Autekologické Charakteristiky; Slovenský hydrometeorologický ústav: Bratislava, Slovakia, 2003; p. 590. [Google Scholar]
- Frey, D.G. Cladocera analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology, 1st ed.; Berglund, B.E., Ed.; John Wiley & Sons: Chichester, UK, 1986; pp. 667–692. [Google Scholar]
- Szeroczyńska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of the Lower Vistula Society: Świecie, Poland, 2007; p. 84. [Google Scholar]
- Battarbee, R.W. Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology, 1st ed.; Berglund, B.E., Ed.; John Wiley & Sons: Chichester, UK, 1986; pp. 527–570. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlang: Jena, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlang: Jena, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlang: Stuttgard, Germany; Jena, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlang: Stuttgard, Germany; Jena, Germany, 1991; p. 437. [Google Scholar]
- Lange-Bertalot, H.; Krammer, K. Achnanthes eine Monographie der Gattung; J. Cramer: Berlin, Germany; Stuttgard, Germany, 1989; p. 393. [Google Scholar]
- Coste, M.; Rosebery, J. Guide iconographique pour la mise en oeuvre de l’Indice Biologique Diatomée; Cemagref groupement de Bordeaux: Bordeaux, France, 2011; p. 236. [Google Scholar]
- Hofmann, G.; Lange-Bertalot, H.; Werum, M. Diatomeen im Süßwasser—Benthos von Mitteleuropa, 2nd ed.; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland. Available online: https://www.algaebase.org (accessed on 17 February 2023).
- Juggins, S. C2: Software for Ecological and Palaeoecological Data Analysis and Visualisation (User Guide Version 1.7.7); Newcastle University: Newcastle upon Tyne, UK, 2007; p. 680. [Google Scholar]
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012; p. 496. [Google Scholar]
- Szarlowicz, K.; Kubica, B. 137Cs and 210Pb radionuclides in open and closed water ecosystems. J. Radioanal. Nucl. Chem. 2014, 299, 1321–1328. [Google Scholar] [CrossRef]
- Henderson, M.A.; Northcote, T.G. Visual prey detection and foraging in sympatric cutthroat trout (Salmo clarki clarki) and Dolly Varden (Salvelinus malma). Can. J. Fish. Aquat. Sci. 1985, 42, 785–790. [Google Scholar] [CrossRef]
- Neverman, D.; Wurtsbaugh, W.A. Visual feeding by juvenile Bear Lake sculpin. Trans. Am. Fish. Soc. 1992, 121, 395–398. [Google Scholar] [CrossRef]
- Holmen, J.; Olsen, E.N.; Vøllestad, L.A. Interspecific competition between stream-dwelling brown trout and Alpine bullhead. J. Fish Biol. 2003, 62, 1312–1325. [Google Scholar] [CrossRef]
- Hesthagen, T.; Heggenes, J. Competitive habitat displacement of brown trout by Siberian sculpin: The role of size and density. J. Fish Biol. 2003, 62, 222–236. [Google Scholar] [CrossRef]
- Christoffersen, K.; Riemann, B.; Klysner, A.; Søndergaard, M. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnol. Oceanogr. 1993, 38, 561–573. [Google Scholar] [CrossRef]
- Jeppesen, E.; Madsen, E.A.; Jensen, J.P.; Anderson, N. Reconstructing the past density of planktivorous fish and trophic structure from sedimentary zooplankton fossils: A surface sediment calibration data set from shallow lakes. Freshw. Biol. 1996, 36, 115–127. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Meerhoff, M.; Lacerot, G.; Clemente, J.M.; Scasso, F.; Kruk, C.; Goyenola, G.; García-Alonso, J.; Amsinck, S.L.; et al. High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: Evidence from lakes, fish exclosures and surface sediments. Hydrobiologia 2011, 667, 133–147. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Lafferty, K.D.; Knudsen, R.; Primicerio, R.; Kristoffersen, R.; Klemetsen, A.; Kuris, A.M. New parasites and predators follow the introduction of two fish species to a subarctic lake: Implications for food-web structure and functioning. Oecologia 2013, 171, 993–1002. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jacobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N.; Branco, C.W.C. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Jeppesen, E.; Lauridsen, T.L.; Mitchell, S.F.; Christoffersen, K.; Burns, C.W. Trophic structure in the pelagial of 25 shallow New Zealand lakes: Changes along nutrient and fish gradients. J. Plank. Res. 2000, 22, 951–968. [Google Scholar] [CrossRef]
- Vranovský, M. Zooplanktón jazier v Západných Tatrách a antropogénne vplyvy. In Zborník prác o Tatranskom Národnom Parku; Osveta: Martin, Slovakia, 1992; Volume 32, pp. 105–128. [Google Scholar]
- Sacherová, V.; Kršková, R.; Stuchlík, E.; Hořická, Z.; Hudec, I.; Fott, J. Long-term change of the littoral Cladocera in alpine lakes in the Tatra Mountains through a major acidification event. Biologia 2006, 61, 109–119. [Google Scholar] [CrossRef]
- Western, J.R.H. Studies of the diet, feeding mechanism and alimentary tract in two closely related teleosts, the freshwater Cottus gobio L. and the marine Parenophrys bubalis Euphrasen. Acta. Zool. 1969, 50, 185–205. [Google Scholar] [CrossRef]
- Hesthagen, T.; Austigard, A.; Holmedal, K. Diurnal and seasonal resource partitioning in young brown trout (Salmo trutta), Arctic charr (Salvelinus alpinus) and Alpine bullhead (Cottus poecilopus) in a subalpine lake in southeastern Norway. Boreal Environ. Res. 2011, 16, 149–157. [Google Scholar]
- Kneslová, P.; Dargocká, J.; Stuchlík, E. Zooplankton of eight High Tatra Mountain lakes in different stage of acidification. Štúdie TANAP 1997, 2, 123–134. [Google Scholar]
- Radzikowski, J. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. J. Plankton Res. 2013, 35, 707–723. [Google Scholar] [CrossRef]
- Louhi, P.; Mäki-Petäys, A.; Huusko, A.; Muotka, T. Resource use by juvenile brown trout and Alpine bullhead: Influence of interspecific versus intraspecific competition. Ecol. Freshw. Fish 2014, 23, 234–243. [Google Scholar] [CrossRef]
- Ruetz, C.R., III; Hurford, A.L.; Vondracek, B. Interspecific interactions between brown trout and slimy sculpin in stream enclosures. Tran. Am. Fish. Soc. 2003, 132, 611–618. [Google Scholar] [CrossRef]
- Dahl, J. Effects of a benthivorous and a drift-feeding fish on a benthic stream assemblage. Oecologia 1998, 116, 426–432. [Google Scholar] [CrossRef]
- Bitušík, P.; Svitok, M.; Kološta, P.; Hubková, M. Classification of the Tatra Mountains lakes (Slovakia) using chironomids (Diptera, Chironomidae). Biologia 2006, 61, 191–202. [Google Scholar] [CrossRef]
- Hamerlík, L.; Bitušík, P. Aplikovaná limnológia. Časť 1. Paleolimnologický prústup, 1st ed.; Občianske združenie Príroda: Banská Bystrica, Slovensko, 2016; p. 68. [Google Scholar]
- Goyke, A.P.; Hershey, A.E. Effects of fish predation on larval chironomid (Diptera: Chironomidae) communities in an arctic ecosystem. Hydrobiologia 1992, 240, 203–211. [Google Scholar] [CrossRef]
- Raposeiro, P.M.; Rubio, M.J.; González, A.; Hernández, A.; Sánchez-López, G.; Vázquez-Loureiro, D.; Rull, V.; Bao, R.; Costa, A.C.; Gonçalves, V.; et al. Impact of the historical introduction of exotic fishes on the chironomid community of Lake Azul (Azores Islands). Palaeogeogr. Palaeocl. 2017, 466, 77–88. [Google Scholar] [CrossRef]
- Krno, I. Macrozoobenthos of the Tatra lakes littoral (the High Tatras) and its affection by acidification. Biologia 1991, 46, 495–506. [Google Scholar]
- Dyk, V. Trout in the Poprad lake. In Sborník prác o Tatranskom národnom parku; Osveta: Bratislava, Slovakia, 1960; Volume 4, pp. 219–246. [Google Scholar]
- Hudec, I. Fauna Slovenska III. Anomopoda, Ctenopoda, Haplopoda, Onychopoda (Crustacea: Branchiopoda); Vydavateľstvo SAV: Bratislava, Slovakia, 2010; p. 496. [Google Scholar]
- Karst-Riddoch, T.L.; Pisaric, M.F.J.; Smol, J.P. Diatom responses to 20th century climate-related environmental changes in high-elevation mountain lakes of the northern Canadian Cordillera. J. Paleolimnol. 2005, 33, 265–282. [Google Scholar] [CrossRef]
- Lotter, A.F.; Bigler, C. Do diatoms in the Swiss Alps reflect the length of ice-cover? Aquat. Sci. 2000, 62, 125–141. [Google Scholar] [CrossRef]
- Küfner, W. Response of Diatoms to Climate Change in Mountain Lakes in the Northern Calcareous Alps with Indications for the Future Development of the Lake Biota. Master’s Thesis, TUM School of Life Sciences, Freising, Germany, 2021. [Google Scholar]
- Vološčuk, I. (Ed.) The National Parks and Biosphere Reserves in Carpathians; The Last Nature Paradises; SLZA: Poprad, Slovakia, 1999; p. 244. [Google Scholar]
- Stone, J.R.; Saros, J.E.; Spanbauer, T.L. The influence of Fetch on the Holocene Thermal Structure of Hidden Lake, Glacier National Park. Front. Earth Sci. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Cartier, R.; Brisset, E.; Paillés, C.; Guiter, F.; Sylvestre, F.; Ruaudel, F.; Anthony, E.J.; Miramont, C. 5000 years of lacustrine ecosystem changes from Lake Petit (Southern Alps, 2200 m a. s. l.): Regime shift and resilience of algal communities. Sage J. 2015, 25, 1231–1245. [Google Scholar] [CrossRef]
- Woelders, L.; Lenaerts, J.T.M.; Hagemans, K.; Akkerman, K.; van Hoof, T.; Hoek, W.Z. Recent climate warming drives ecological change in a remote high-Arctic Lake. Sci. Rep. 2018, 8, 6858. [Google Scholar] [CrossRef] [PubMed]
- Rimet, F.; Bouchez, A. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Managt. Aquat. Ecosyst. 2012, 406, 12. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Telford, R.J.; Birks, H.J.B.; Saros, J.E.; Hazewinkel, R.R.O.; Perren, B.B.; Saulnier-Talbot, E.; Wolfe, A.P. Quantifying recent ecological changes in remote lakes of North America and Greenland using sediment diatom assemblages. PLoS ONE 2010, 5, e10026. [Google Scholar] [CrossRef] [PubMed]
- Holtgrieve, G.W.; Schindler, D.E.; Hobbs, W.O.; Leavitt, P.R.; Ward, E.J.; Bunting, L.; Chen, G.; Finney, B.P.; Gregory-Eaves, I.; Holmgren, S.; et al. A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science 2011, 334, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, C.; Rühland, K.M.; Smol, J.P.; Jones, V.J.; Conde-Porcuna, J.M. Long-term ecological changes in Mediterranean mountain lakes linked to recent climate change and Saharan dust deposition revealed by diatom analyses. Sci. Total Environ. 2020, 727, 138519. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, C.; Conde-Porcuna, J.M.; Ramos-Rodríguez, E.; Moreno, E.; Rühland, K.M.; Jeziorski, A.; Smol, J.P.; García-Alix, A.; Heiri, O.; Corral-Arredondo, E.; et al. Paleolimnological Indicators of Global Change. In The Landscape of the Sierra Nevada: A Unique Laboratory of Global Processes in Spain; Zamora, R., Oliva, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 279–291. [Google Scholar]
- Eggermont, H.; Heiri, O. The chironomid-temperature relationship: Expression in nature and palaeoenvironmental implications. Biol. Rev. 2011, 87, 430–456. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.I.; Dokulil, M.T.; Marszelewski, W.; Schmid, M.; Bouffard, D.; Merchant, C.J. Warming of Central European lakes and their response to the 1980s climate regime shift. Clim. Change 2017, 142, 505–520. [Google Scholar] [CrossRef]
- Reid, P.C.; Hari, R.E.; Beaugrand, G.; Livingstone, D.M.; Marty, C.; Straile, D.; Barichivich, J.; Goberville, E.; Adrian, R.; Aono, Y.; et al. Global impacts of the 1980s regime shift. Glob. Change Biol. 2016, 22, 682–703. [Google Scholar] [CrossRef] [PubMed]
- Agustí-Panareda, A.; Thompson, R. Reconstructing air temperature at eleven remote alpine and arctic lakes in Europe from 1781 to 1997 AD. J. Paleolimnol. 2002, 28, 7–23. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Warwick, W.F. Chironomids, lake development and climate: A commentary. J. Paleolimnol. 1989, 2, 15–17. [Google Scholar] [CrossRef]
- Hamerlík, L.; Bitušík, P. The distribution of littoral chironomids along an altitudinal gradient in High Tatra Mountain lakes: Could they be used as indicators of climate change? Ann. Limnol.-Int. J. Lim. 2009, 45, 145–156. [Google Scholar] [CrossRef]

| Variable/Site Name | Unit | Value |
|---|---|---|
| Coordinates | 49°11′59″ N | |
| 19°48′06″ E | ||
| Altitude | m | 1697 |
| Max. depth | m | 12.3 |
| Area | ha | 0.74 |
| Littoral habitat description (R:S:O) | % | 75:15:10 |
| Catchment characteristics | ||
| Maximum altitude | m | 2137 |
| Catchment area | ha | 56 |
| Rock | % | 20 |
| Moraine | % | 20 |
| Meadow, dwarf pines | % | 60 |
| Forest | % | 0 |
| Water chemistry | ||
| pH | 7.49 | |
| Alkalinity | µeq L−1 | 219.15 |
| Conductivity | µS cm L−1 | 29.11 |
| ANC | µmol L−1 | 254 |
| TON | µmol L−1 | 7.7 |
| TP | µmol L−1 | 0.094 |
| TOC | µmol L−1 | 100 |
| Cl¯ | µmol L−1 | 4.9 |
| µmol L−1 | 32 | |
| µmol L−1 | 13 | |
| µmol L−1 | 0.7 | |
| Na+ | µmol L−1 | 21 |
| K+ | µmol L−1 | 2.5 |
| Ca2+ | µmol L−1 | 81 |
| Mg2+ | µmol L−1 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodníková, V.; Hamerlík, L.; Wojewódka-Przybył, M.; Sochuliaková, L.; Szarlowicz, K.; Buczkó, K.; Chamutiová, T.; Sedlačková Přidalová, M.; Bitušík, P. Tracking Fish Introduction in a Mountain Lake over the Last 200 Years Using Chironomids, Diatoms, and Cladoceran Remains. Water 2023, 15, 1372. https://doi.org/10.3390/w15071372
Slobodníková V, Hamerlík L, Wojewódka-Przybył M, Sochuliaková L, Szarlowicz K, Buczkó K, Chamutiová T, Sedlačková Přidalová M, Bitušík P. Tracking Fish Introduction in a Mountain Lake over the Last 200 Years Using Chironomids, Diatoms, and Cladoceran Remains. Water. 2023; 15(7):1372. https://doi.org/10.3390/w15071372
Chicago/Turabian StyleSlobodníková, Veronika, Ladislav Hamerlík, Marta Wojewódka-Przybył, Lucia Sochuliaková, Katarzyna Szarlowicz, Krisztina Buczkó, Tímea Chamutiová, Marcela Sedlačková Přidalová, and Peter Bitušík. 2023. "Tracking Fish Introduction in a Mountain Lake over the Last 200 Years Using Chironomids, Diatoms, and Cladoceran Remains" Water 15, no. 7: 1372. https://doi.org/10.3390/w15071372
APA StyleSlobodníková, V., Hamerlík, L., Wojewódka-Przybył, M., Sochuliaková, L., Szarlowicz, K., Buczkó, K., Chamutiová, T., Sedlačková Přidalová, M., & Bitušík, P. (2023). Tracking Fish Introduction in a Mountain Lake over the Last 200 Years Using Chironomids, Diatoms, and Cladoceran Remains. Water, 15(7), 1372. https://doi.org/10.3390/w15071372










