Genotoxicity Signatures near Brine Outflows from Desalination Plants in the Levant
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Artificial Sea Water (ASW) Control
2.3. Chemicals
2.4. LD-50 Assay
2.5. Neutral Red Test
2.6. Testing Genotoxicity by Comet Assay
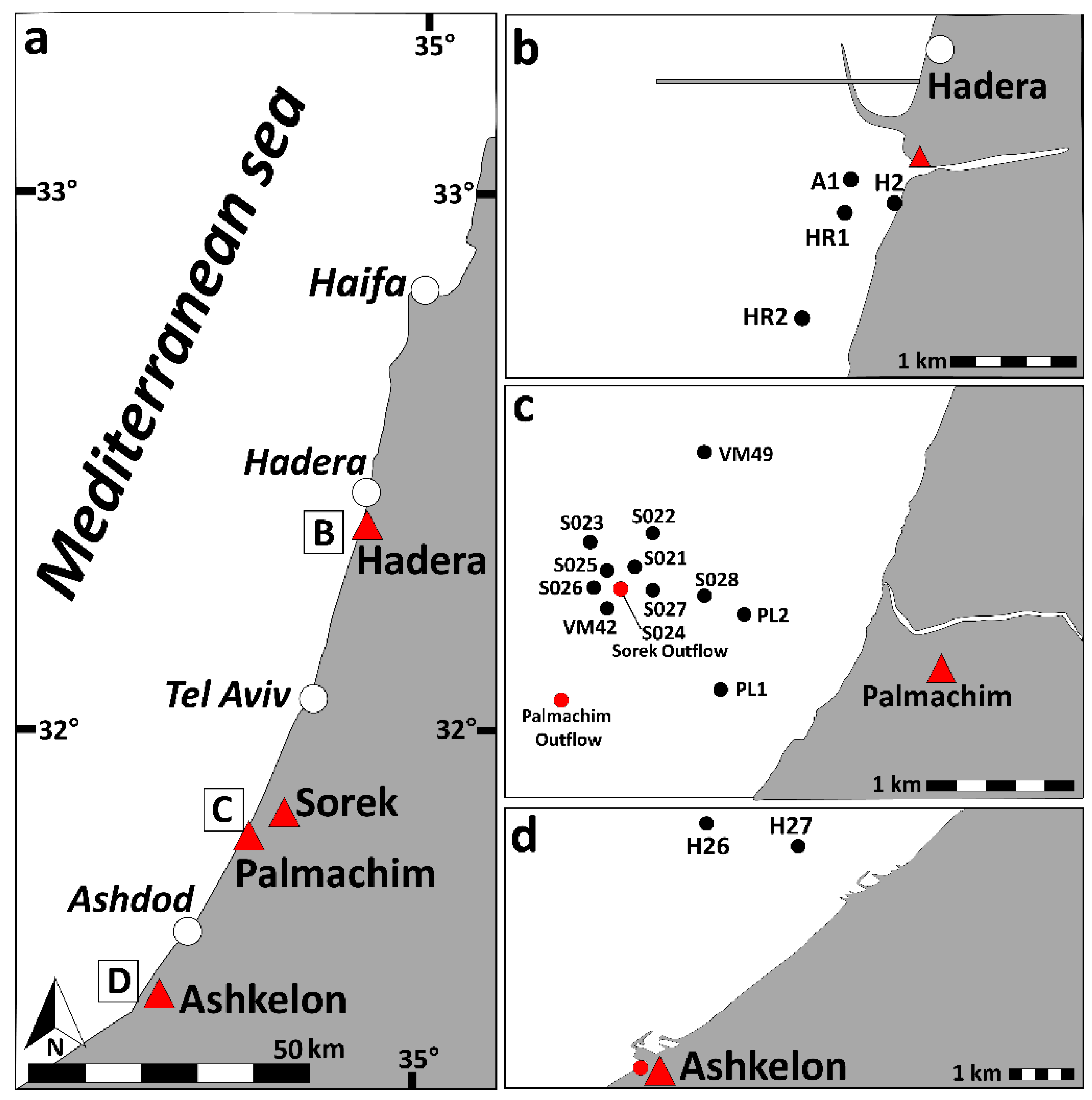
2.7. Water Sampling
3. Results
3.1. Toxic Impact of a Coagulant and an Antiscalant
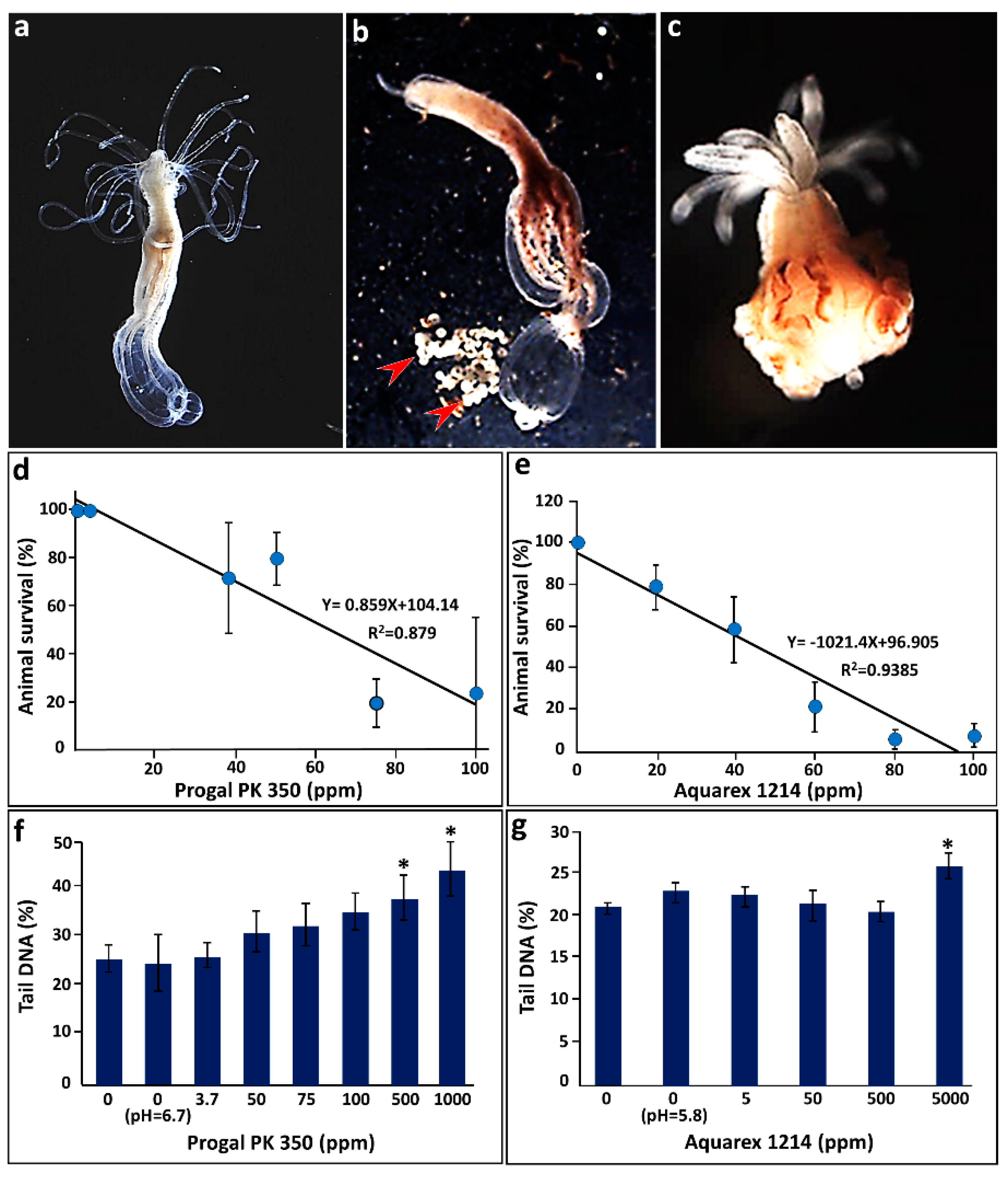
3.1.1. Setting LD50 for Progal 350 PK and Aquarex 1214
3.1.2. Cell Viability following Exposure to Progal 350 PK and Aquarex 1214
3.1.3. Genotoxicity Testing of Progal 350 PK and Aquarex 1214
3.2. Sea Water Genotoxicity
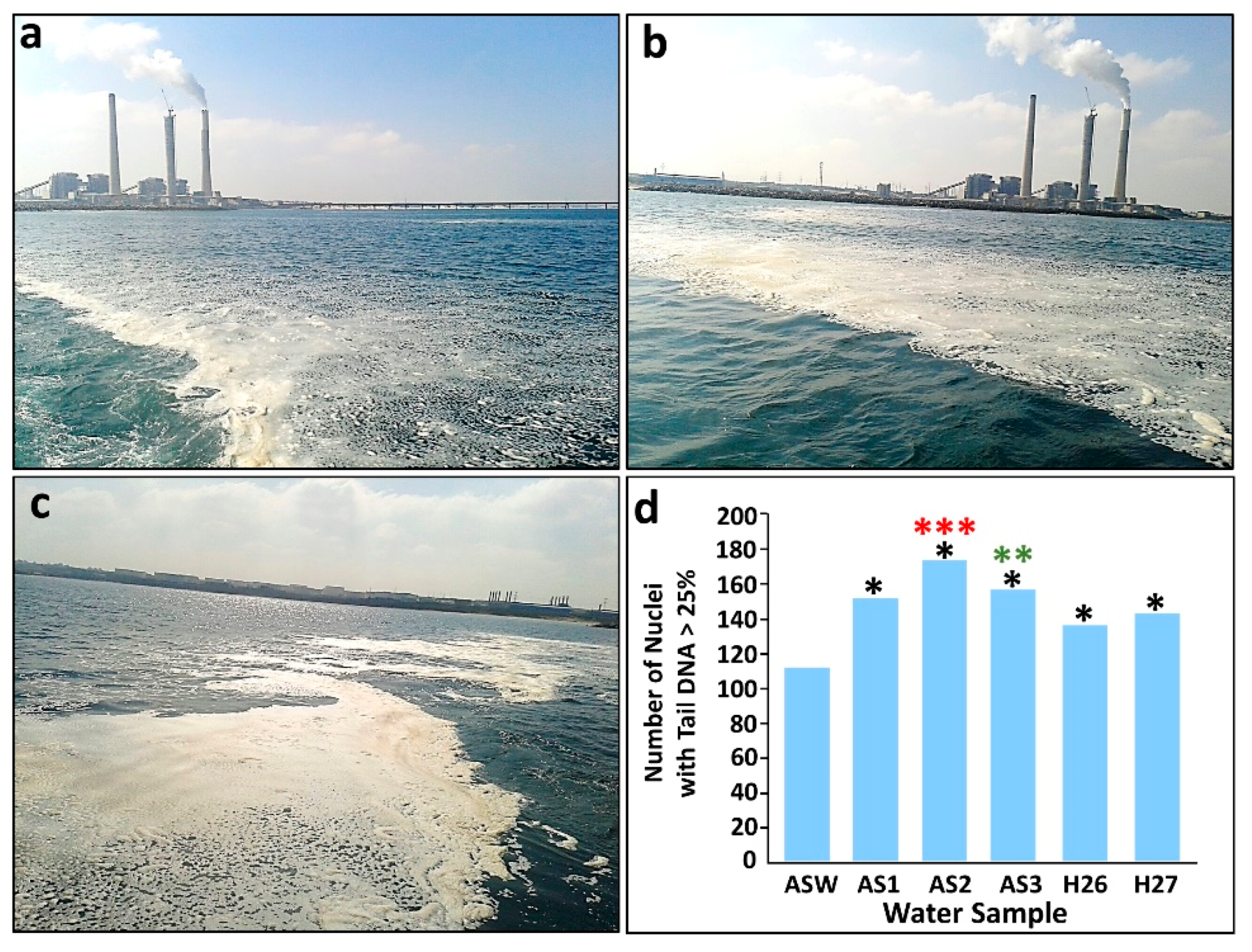
3.2.1. Ashkelon Desalination Plant
3.2.2. Palmachim and Sorek Desalination Plants
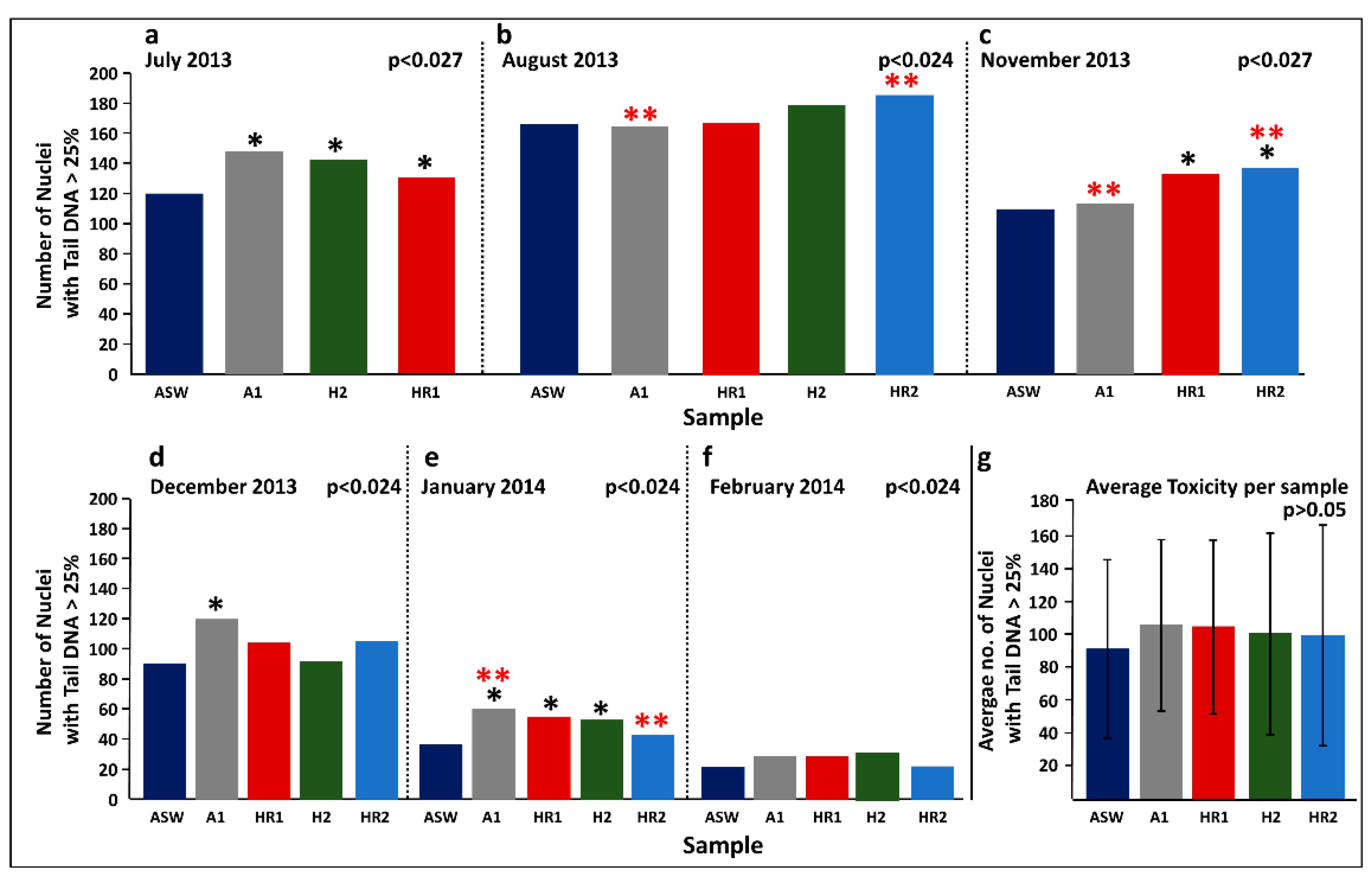
3.2.3. Hadera Desalination Plant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; Haralambous, K.-J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef]
- Kress, N. Marine Impacts of Seawater Desalination: Science, Management, and Policy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Dreizin, Y.; Tenne, A.; Hoffman, D. Integrating large scale seawater desalination plants within Israel’s water supply system. Desalination 2008, 220, 132–149. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Israeli Ministry of Environmental Protection. Available online: https://www.gov.il/he/departments/policies/desalination_facilities_moep_policy (accessed on 28 February 2023).
- Qasim, M.; Badrelzaman, M.; Darwish, N.; Darwish, N.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Henthorne, L.; Boysen, B. State-of-the-art of reverse osmosis desalination pretreatment. Desalination 2015, 356, 129–139. [Google Scholar] [CrossRef]
- Prihasto, N.; Liu, Q.-F.; Kim, S.-H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Valavala, R.; Sohn, J.; Han, J.; Her, N.; Yoon, Y. Pretreatment in Reverse Osmosis Seawater Desalination: A Short Review. Environ. Eng. Res. 2011, 16, 205–212. [Google Scholar] [CrossRef]
- Ghafour, E.E. Enhancing RO system performance utilizing antiscalants. Desalination 2002, 153, 149–153. [Google Scholar] [CrossRef]
- Ketsetzi, A.; Stathoulopoulou, A.; Demadis, K.D. Being “green” in chemical water treatment technologies: Issues, challenges and developments. Desalination 2008, 223, 487–493. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Hoepner, T.; Lattemann, S. Chemical impacts from seawater desalination plants—A case study of the northern Red Sea. Desalination 2003, 152, 133–140. [Google Scholar] [CrossRef]
- Marine Coastal Pollution Report of the Israeli Ministry of Environmental Protection. Available online: https://www.gov.il/he/departments/publications/reports/pollutant_loads_sea_annual_reports (accessed on 28 February 2023).
- An Article Published by Tashtiot Magazine on 1st March 2015. Available online: https://www.tashtiot.co.il/2015/03/01/%D7%90%D7%99%D7%9B%D7%95%D7%AA-%D7%94%D7%A1%D7%91%D7%99%D7%91%D7%94-175/ (accessed on 28 February 2023).
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Benaissa, M.; Rouane-Hacene, O.; Boutiba, Z.; Habib, D.; Guibbolini-Sabatier, M.E.; Risso-De Faverney, C. Ecotoxicological effects assessment of brine discharge from desalination reverse osmosis plant in Algeria (South Western Mediterranean). Reg. Stud. Mar. Sci. 2020, 39, 101407. [Google Scholar] [CrossRef]
- Capó, X.; Tejada, S.; Ferriol, P.; Pinya, S.; Mateu-Vicens, G.; Montero-González, I.; Box, A.; Sureda, A. Hypersaline water from desalinization plants causes oxidative damage in Posidonia oceanica meadows. Sci. Total Environ. 2020, 736, 139601. [Google Scholar] [CrossRef]
- de-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Loya-Fernández, A.; Ferrero-Vicente, L.M.; Marco-Méndez, C.; Martinez-Garcia, E.; Sánchez-Lizaso, J.L. Response of amphipod assemblages to desalination brine discharge: Impact and recovery. Estuar. Coast. Shelf Sci. 2016, 172, 13–23. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Chipman, J.K. DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res. 1998, 399, 135–147. [Google Scholar] [CrossRef]
- Lee, R.; Kim, G.B.; Maruya, K.A.; Steinert, S.A.; Oshima, Y. DNA strand breaks (comet assay) and embryo development effects in grass shrimp (Palaemonetes pugio) embryos after exposure to genotoxicants. Mar. Environ. Res. 2000, 50, 553–557. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S. Disinfection by-products in desalinated and blend water: Formation and control strategy. J. Water Health 2019, 17, 1–24. [Google Scholar] [CrossRef]
- Benaissa, M.; Rouane-Hacene, O.; Boutiba, Z.; Guibbolini-Sabatier, M.E.; Risso-De Faverney, C. Ecotoxicological impact assessment of the brine discharges from a desalination plant in the marine waters of the Algerian west coast, using a multi-biomarker approach in a limpet, Patella rustica. Environ. Sci. Pollut. Res. 2017, 24, 24521–24532. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Dusinska, M.; Collins, A.; Manjanatha, M.; Pfuhler, S.; Registre, M.; Elespuru, R. In vivo Mammalian Alkaline Comet Assay Method Adapted for Genotoxicity Assessment of Nanomaterials. Front. Toxicol. 2022, 4, 903896. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Lessing, G.; Brina, K.R.; Angeli, L.; Andriguetti, N.B.; Peruzzo, J.R.; do Nascimento, C.A.; Spilki, F.R.; Ziulkoski, A.L.; da Silva, L.B. Monitoring the Genotoxic and Cytotoxic Potential and the Presence of Pesticides and Hydrocarbons in Water of the Sinos River Basin, Southern Brazil. Arch. Environ. Contam Toxicol. 2017, 72, 321–334. [Google Scholar] [CrossRef]
- Naguib, M.; Mekkawy, I.A.; Mahmoud, U.M.; Sayed, A.E.-D.H. Genotoxic evaluation of silver nanoparticles in catfish Clarias gariepinus erythrocytes; DNA strand breakage using comet assay. Sci. Afr. 2022, 16, e01260. [Google Scholar] [CrossRef]
- Martínez-Paz, P.; Morales, M.; Martínez-Guitarte, J.L.; Morcillo, G. Genotoxic effects of environmental endocrine disruptors on the aquatic insect Chironomus riparius evaluated using the comet assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2013, 758, 41–47. [Google Scholar] [CrossRef]
- Turan, F.; Karan, S.; Ergenler, A. Effect of heavy metals on toxicogenetic damage of European eels Anguilla anguilla. Environ. Sci. Pollut. Res. 2020, 27, 38047–38055. [Google Scholar] [CrossRef]
- VID—Desalination Company Ltd. A Permit for the Discharge of Brine. Available online: https://www.gov.il/Files/Sviva/PermitFlowingToSea/doclib3/2018/07-2018.pdf (accessed on 28 February 2023).
- Fryer, J.L.; McCain, B.B.; Leong, J.C. A Cell Line Derived from Rainbow Trout (Salmo gairdneri) Hepatoma. Fish Pathol. 1981, 15, 193–200. [Google Scholar] [CrossRef]
- Kamer, I.; Rinkevich, B. In vitro application of the comet assay for aquatic genotoxicity: Considering a primary culture versus a cell line. Toxicol. Vitr. 2002, 16, 177–184. [Google Scholar] [CrossRef]
- Kamer, I.; Douek, J.; Tom, M.; Rinkevich, B. Metallothionein induction in RTH-149 cell line as an indicator for heavy metal pollution in a brackish environment: Assessment by RT-competitive PCR. Environ. Toxicol. Chem. 2003, 45, 86–91. [Google Scholar] [CrossRef]
- Rabinowitz, C.; Rinkevich, B. Epithelial cell cultures from Botryllus schlosseri palleal buds: Accomplishments and challenges. Methods Cell Sci. 2003, 25, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Avishai, N.; Rabinowitz, C.; Moiseeva, E.; Rinkevich, B. Genotoxicity of the Kishon River, Israel: The application of an in vitro cellular assay. Mutat. Res. 2002, 518, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Avishai, N.; Rabinowitz, C.; Rinkevich, B. The use of the comet assay for studying environmental genotoxicity: Comparisons between visual and image analyses. Environ. Mol. Mutagen. 2003, 42, 155–165. [Google Scholar] [CrossRef]
- Avishai, N.; Rabinowitz, C.; Rinkevich, B. A two and half year genotoxicity profile for a partly restored polluted river. Environ. Sci. Technol. 2004, 38, 3482–3487. [Google Scholar] [CrossRef]
- Rinkevich, B.; Avishai, N.; Rabinowitz, C. UV incites diverse levels of DNA breaks in different cellular compartments of a branching coral species. J. Exp. Biol. 2005, 208, 843–848. [Google Scholar]
- Uziel, O.; Beery, E.; Dronichev, V.; Samocha, K.; Gryaznov, S.; Weiss, L.; Slavin, S.; Kushnir, M.; Nordenberg, Y.; Rabinowitz, C.; et al. Telomere shortening sensitizes cancer cells to selected cytotoxic agents: In vitro and in vivo studies and putative mechanisms. PLoS ONE 2010, 5, e9132. [Google Scholar] [CrossRef]
- Narum, S.R. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conserv. Genet. 2006, 7, 783–787. [Google Scholar] [CrossRef]
- Klein, S.; Frazier, V.; Readdean, T.; Lucas, E.; Diaz-Jimenez, E.P.; Sogin, M.; Ruff, E.S.; Echeverri, K. Common Environmental Pollutants Negatively Affect Development and Regeneration in the Sea Anemone Nematostella vectensis Holobiont. Front. Ecol. Evol. 2021, 9, 786037. [Google Scholar] [CrossRef]
- Kültz, D.; Chakravarty, D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Lykkebo Petersen, K.; Paytan, A.; Rahav, E.; Levy, O.; Silverman, J.; Barzel, O.; Potts, D.; Bar-Zeev, E. Impact of brine and antiscalants on reef-building corals in the Gulf of Aqaba—Potential effects from desalination plants. Water Res. 2018, 144, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.A.; Gafni, A.; Adler, O.; Levy, O.; Edo Bar-Zeev, E. Antiscalants used in the desalination industry impact the physiology of the coral Montipora capricornis. Water Res. 2022, in press. [Google Scholar] [CrossRef]
- Vaal, M.; van der Wal, J.T.; Hoekstra, J.; Hermens, J. Variation in the sensitivity of aquatic species in relation to the classification of environmental pollutants. Chemosphere 1997, 35, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, D.; Lahive, E.; Robinson, A.; Short, S.; Kille, P. Species Sensitivity to Toxic Substances: Evolution, Ecology and Applications. Front. Environ. Sci. 2020, 8, 588380. [Google Scholar] [CrossRef]
- Svanfeldt, K.; Lundqvist, L.; Rabinowitz, C.; Sköld, H.N.; Rinkevich, B. Repair of UV-induced DNA damage in shallow water colonial marine species. J. Exp. Mar. Biol. Ecol. 2014, 452, 40–46. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Ferraris, D.J.; Norenburg, J.L.; Burg, M.B. The Saltiness of the Sea Breaks DNA in Marine Invertebrates: Possible Implications for Animal Evolution. Cell Cycle 2006, 5, 1320–1323. [Google Scholar] [CrossRef]
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. Enhanced salinities, as a proxy of seawater desalination discharges, impact coastal microbial communities of the eastern Mediterranean Sea. Environ. Microbiol. 2015, 17, 4105–4120. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosner, A.; Grossmark, Y.; Gertner, Y.; Rabinowitz, C.; Reem, E.; Rinkevich, B. Genotoxicity Signatures near Brine Outflows from Desalination Plants in the Levant. Water 2023, 15, 1079. https://doi.org/10.3390/w15061079
Rosner A, Grossmark Y, Gertner Y, Rabinowitz C, Reem E, Rinkevich B. Genotoxicity Signatures near Brine Outflows from Desalination Plants in the Levant. Water. 2023; 15(6):1079. https://doi.org/10.3390/w15061079
Chicago/Turabian StyleRosner, Amalia, Yaara Grossmark, Yaron Gertner, Claudette Rabinowitz, Eitan Reem, and Baruch Rinkevich. 2023. "Genotoxicity Signatures near Brine Outflows from Desalination Plants in the Levant" Water 15, no. 6: 1079. https://doi.org/10.3390/w15061079
APA StyleRosner, A., Grossmark, Y., Gertner, Y., Rabinowitz, C., Reem, E., & Rinkevich, B. (2023). Genotoxicity Signatures near Brine Outflows from Desalination Plants in the Levant. Water, 15(6), 1079. https://doi.org/10.3390/w15061079





