Abstract
In membrane-based water purification technology, control of the membrane pore structure is fundamental to defining its performance. The present study investigates the effect of the preparation conditions on the final pore size distribution and on the dye removal efficiency of cellulose acetate membranes. The membranes were fabricated by means of phase inversion (using different speeds of film casting and different thicknesses of the casted solution) and introducing modifications in the preparation conditions, such as the use of a coagulation bath instead of pure water and the addition of a surfactant as a solution additive. Both isotropic and anisotropic membranes could be fabricated, and the membranes’ pore size, porosity, and water permeability were found to be greatly influenced by the fabrication conditions. The removal capacity towards different types of water contaminants was investigated, considering, as model dyes, Azure A and Methyl Orange. Azure A was removed with higher efficiency due to its better chemical affinity for cellulose acetate, and for both dyes the uptake could be fitted using a pseudo-second order model, evidencing that the rate-limiting step is chemisorption involving valency forces through the sharing or exchange of electrons between the dye and the membrane.
1. Introduction
The increasing scarcity of freshwater sources and the global demand for water are expected to grow in the upcoming decades, urging the need to develop alternative water supplies, including seawater desalination and the reuse and recycling of wastewater. Among the different procedures in use for wastewater remediation [1,2,3,4], membrane-based separation methods are playing an increasingly important role in providing adequate water resources of desirable quality for a wide spectrum of applications [5]. However, to meet the upcoming demand for adequate quality potable water and potential future regulations, membranes with enhanced permeability and selectivity must be fabricated and tested for different water treatment processes.
Phase inversion is the most commonly used process for membrane fabrication. The technique can be described as a demixing (liquid–liquid) process whereby a homogenous polymeric solution is transformed in a controlled manner from a single-phase solution to two phases (solid and liquid). At a specific stage during demixing (solvent–non-solvent interaction), the polymer-rich phase is destabilized as the local concentration of the polymer increases and the polymer solubility decreases. Therefore, the polymer becomes insoluble and precipitates [6,7].
There are many classifications for membranes, depending on several characteristics [8,9,10]. Isotropic membranes are characterized by a uniform cross-section in terms of composition, physical nature, and porous structure [11]. Anisotropic membranes, on the contrary, have heterogeneous cross-sections varying in either chemical composition or structure or both of them. They are homogeneous in chemical composition but the pore sizes and the porosity vary across the membrane thickness. Thin film composite membranes, on the other hand, are characterized by being heterogeneous in terms of both composition and porous structure. They usually consist of porous substrates coated with thin dense films of different polymers [5,12,13].
Significant efforts have been made to develop proper low-cost polymeric membranes from commercial materials. Plastic polymeric materials are popular and cost-effective, but unfortunately, their inherently low stability greatly limits their applications. Moreover, poor compatibility between the polymeric materials and the additives that could enhance their properties usually results in the formation of defects during membrane preparation, which limits the possibility of improvement. Therefore, it is highly desirable to develop polymeric membranes with enhanced intrinsic stability. In addition, many efforts have been devoted to developing bio-based materials with reduced toxicity to be employed in membrane preparations. In this context, cellulose acetate (CA) membranes present several advantages, such as the availability of the source, biocompatibility, low cost, and simple one-step synthesis method [14,15,16]. A number of reports have recently focused on the improvement of CA properties [17] using additives such as zeolites [18,19], carbon nanotubes [20,21], titanium dioxide [22], and graphene oxide (GO) [23,24].
As permeability and selectivity are controlled by the membrane structure, it is important to deeply understand the effect of the fabrication conditions on the produced membrane pore structure in order to target more efficient separation processes and improve the membrane surface physicochemical properties.
In this scenario, the current work targets the development of low-cost and biocompatible polymeric membranes with enhanced structural properties and improved water treatment characteristics. CA membranes were prepared by means of the phase inversion technique and introducing modifications to the preparation conditions, such as the use of different speeds of film casting and different thicknesses of the casted solution, the use of a coagulation bath, or the addition of salt or a surfactant to the polymer solution prior to fabrication. The effect of the different methodologies on the properties of the produced membranes was carefully investigated by analyzing the membrane cross-section morphology, surface roughness, pore size and porosity, water permeability, wettability, and removal activity towards two model dyes. A deep understanding of the interactions between the contaminants and the membranes is achieved by means of a careful analysis of the involved kinetic processes. In the frame of current research activity on the optimization of the membrane preparation conditions [25,26,27,28], the present work tackles a fundamental analysis of the effects of fine-tuning the preparation conditions on the pore structure of the final material, which eventually affects membrane separation performances and dye removal efficacy, with the added value of the biocompatibility of the polymeric substrate.
2. Materials and Methods
2.1. Materials
The following chemicals were used as received: CA (average Mn: 30,000) from Sigma-Aldrich (St. Louis, MO, USA), anhydrous dimethylformamide (DMF, max 0.005% water) from VWR Chemicals, sodium chloride (for analysis) from Carlo Erba, Pluronic F-127 from Sigma-Aldrich, AZ (7-aminophenothiazin-3-ylidene)-dimethylazanium;chloride) from Fluka, and MO (sodium;4-[[4-(dimethylamino)phenyl]diazenyl]benzenesulfonate) from Carlo Erba.
2.2. Membrane Preparation
CA membranes were prepared by the phase inversion technique described below, using 20% CA solution in DMF and an automatic film applicator (Automatic Film Applicator Compact AB3655, from TQC Sheen) with a controlled thickness of the casted solution and speed of the film spreading. In a primary experiment, three different speeds (1, 5, and 10 mm/s) and three different thicknesses (200, 500, and 1000 µm) of the casted polymer solution were used to find the optimum fabrication conditions. The final membrane thickness, after formation, was measured by scanning electron microscopy (SEM). The solution was prepared by stirring 20 wt% CA in DMF overnight using a magnetic stirrer. The homogeneous solution was carefully casted onto a plate fixed on the film applicator surface and then spread using an applicator moving with a pre-set thickness at a fixed speed. As optimum fabrication conditions (see the discussion in paragraph 3.1), the film applicator speed was fixed at 5 mm/s, and three thicknesses were set: 200, 500, and 1000 μm. After spreading, the plate with the produced casted solution was smoothly immersed into a deionized water bath. Within a few minutes, the casted film got solidified and turned from transparent to opaque, and then the produced membrane got separated from the glass plate. After complete separation, the membrane was then immersed in another fresh deionized water bath overnight. The produced membranes (named 200, 500, and 1000, as the thickness of the casted film, in μm) were stored in deionized water to keep their shape and physical and mechanical properties. Before characterization, the membranes were removed from the water and dried under vacuum at room temperature.
2.3. Preparation of Modified Membranes
For the preparation of modified membranes, the speed of the film applicator was fixed at 5 mm/s, and three samples with different thicknesses were casted: 200, 500, and 1000 μm. As a first modification, both salt coagulation baths (using a saturated NaCl bath instead of deionized water for the phase inversion) and in situ salt addition (addition of NaCl salt to the polymer solution before membrane casting) were investigated. After solidification, the membranes were transferred to a freshwater bath and washed several times to be sure that they were salt-free. The obtained modified membranes were named with the suffix –SAB, for salt coagulation bath, and with the suffix –in situ, for the in situ addition of salt. The complete removal of the salt was confirmed by elemental analysis using Energy Dispersive X-ray Analysis (EDX) in combination with SEM.
Another modification was the addition of a surfactant to the polymer solution before fabrication. In this experiment, 5 wt% of Pluronic F-127 was mixed with the polymer solution using a magnetic stirrer. The solution mixture was then fabricated as in the previous experiment. Two types of phase inversion baths were used as follows: deionized water (the produced membrane was abbreviated as 500-SURF) and a saturated salt bath (the produced membrane was abbreviated as 500-SURF-SAB).
2.4. Membrane Characterization
2.4.1. Membrane Morphology
The surface and cross-section morphologies of the prepared membranes were investigated using Scanning Electron Microscopy (SEM, LEO 1530 FEG model). To perform the membranes’ cross-section measurements, the samples were prepared using liquid nitrogen to stiffen the membrane before breaking; after that, the sample was coated with a thin gold layer (about 10 nm thickness).
2.4.2. Membrane Surface
Atomic Force Microscopy (AFM) images were collected in the air on a Multimode 8 microscope operated in PeakForce mode and equipped with a type J scanner (Bruker Nano Inc. GmbH, Berlin, Germany). Background interpolation and surface roughness parameter calculations were performed with Gwyddion 2.48 (http://gwyddion.net/, accessed on 30 March 2022).
2.4.3. Pore Size and Pore Size Distribution
Pore size and pore size distribution were measured by a Capillary Flow Porometer (Porous Materials Inc., PMI, Ithaca, NY, USA) using the wet-up/dry-up method. A fluorinated liquid (porewick) with a surface tension of 16 dynes/cm was used as a wetting liquid for the analyzed membrane samples.
2.4.4. Porosity
The measurement of the porosity was determined by applying Equation (1):
where Ww is the weight of the wet membrane, Wd is the weight of the dry membrane, ρk is the density of water (0.997 g cm−3), and ρp is the polymer density (1.305 g cm−3). The gravimetric analysis was performed using a balance where three different wetted pieces (big, medium, and small) of each membrane were weighted [29,30].
2.4.5. Pure Water Permeability (PWP)
Distilled water was pumped through the membrane (membrane area: 0.0008 m2), located in a cross-flow cell, at three different transmembrane pressures (i.e., 3/2.5/2 bar). The PWP was calculated by means of Equation (2):
where Q is the permeate volume (L), A is the membrane area (m2), t is the time (h) and p is the transmembrane pressure (bar). The permeability was measured after 20 min, when the membrane reached the steady-state condition.
2.4.6. Membrane Wettability (Contact Angle Measurement)
The water contact angles of the membrane surfaces were measured at 25 °C in the air using a contact angle meter (GBX Digidrop instrument) on the basis of the sessile drop method. All the contact angles were determined by averaging values measured on at least three different points on each sample surface and taking the standard deviation of these measures as the uncertainty.
2.4.7. Dye Removal Efficiency of the Prepared Membranes
Azure A (AZ) and Methyl Orange (MO) were chosen as representative examples of cationic and anionic dyes to study the performance of the membranes in the adsorption and rejection of differently charged species. Absorption spectra were recorded with a Perkin–Elmer Lambda 650 UV/Vis spectrophotometer. The molar extinction coefficients of AZ and MO in tri-distilled water were determined by acquiring the absorption spectra of solutions of the dyes at different concentrations (ranging from 0.1 M to 0.001 mM) and by applying the Lambert–Beer law. They were found to be 3.3 × 104 and 1.9 × 104 M−1 cm−1 at 631 and 463 nm, for AZ and MO, respectively, in agreement with literature reports [31,32,33,34].
The dye uptake efficiency, in static conditions, was calculated by measuring, spectrophotometrically, the initial (Ci = 0.01 mM) and final (Cf) concentrations of the dye solution (in molarity) prior to and after the immersion of the membrane sample (1% wt/vol) in a fixed volume of solution overnight (15 h). The uptake efficiency (%) was then calculated using the following Equation (3):
Uptake efficiency= [(Ci − Cf)/Ci] × 100
The kinetics of the dye uptake were determined by measuring the absorbance of the dye solution (0.01 mM) containing the membrane sample (in 1% wt/vol with respect to the solution volume) at 631 nm and at 463 nm for AZ and MO, respectively, as a function of time for 14 h with an Agilent Cary 100 spectrophotometer. The homogeneous and continuous contact of the membrane piece with the solution was assured by magnetic stirring.
The uptake capacity was measured by calculating the mass (in g) of the uptaken dye and by dividing this value for the mass (in g) of the immersed membrane. The former was derived by measuring spectrophotometrically Ci and Cf and by using Equation (4):
where V is the volume of the solution (in L) and MW is the molecular weight of the dye.
massuptaken dye = (Ci − Cf) × V × MW
The adsorption kinetics were studied by applying to the adsorption capacity versus time curves the pseudo-first, pseudo-second, and intraparticle diffusion mathematical models using the Origin Pro 8 Software.
The dyes removal efficiency was also investigated in the flow system under pressure. The effect of dissolved dyes on the water permeability was investigated by comparing the permeability of pure water and a 0.1 M dye solution under the same experimental conditions. The initial feed concentration, the filtrate concentration, and the remaining concentration in the stirred cell (the concentrate) were determined spectrophotometrically, and the remaining dye (%) was calculated as (Cflowed solution/Ci) ×100.
3. Results and Discussion
3.1. Morphological Characteristics of the Prepared Membranes
The development of CA membranes with a wide range of porous structures was achieved through modification of the fabrication conditions. The preparation was carried out using the phase inversion technique, in which a casted polymer solution is subjected to a non-solvent; the polymer starts to be thermodynamically unstable and separates into a polymer-rich phase and a polymer-poor phase, induced by the presence of the non-solvent. At this point, the system can be described as a ternary system consisting of the polymer, the solvent, and the non-solvent. This stage is followed by the replacement of the solvent with the non-solvent, which results in the solidification of the polymer and the formation of a porous membrane [35]. The thickness of the casted polymer solution and, accordingly, the amount of the polymer solution to be solidified, were found to have an influence on the rate of de-mixing and formation of the polymer-rich and polymer-poor phases. Indeed, the non-solvent needs more time to penetrate through a thick polymer phase to cause the phase inversion process, thus changing the morphological and porous structure of the final membrane.
Deionized water was used as the non-solvent coagulation medium. The speed of the film applicator and the thickness of the casted films were found to be important parameters to be controlled during the fabrication process. Accordingly, three different speeds were tested: 1, 5, and 10 mm/s, and three different membrane thicknesses were chosen: 200, 500, and 1000 µm. The upper and lower surfaces of the produced membranes are porous, as can be seen in the supplementary data, Figure S1. The fabrication conditions were found to have a significant effect also on the cross-section morphology of the membranes, as shown in the supplementary data, Figure S2a,b. Based on the observed morphological structures of the different membranes, a speed of 5 mm/s was fixed as optimum for further preparations. Actually, the membranes produced with this speed were typically anisotropic, with different pore shapes and sizes in a finger-like structure. A detailed cross-section structure can be observed in Figure 1 for a selected example.
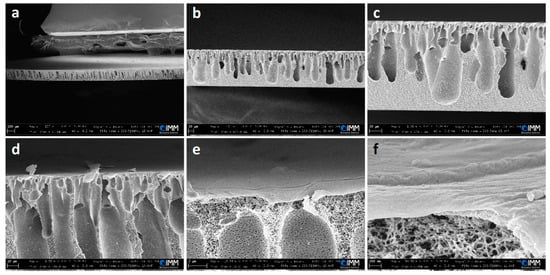
Figure 1.
SEM images with different magnifications of the cross-section of a CA membrane (fabrication conditions: film applicator speed = 5 mm/s, casted solution thickness = 200 µm). The scale bars are as follows: (a) 100 µm, (b) 20 µm, (c) 20 µm, (d) 10 µm, (e) 2 µm, and (f) 200 nm.
Figure 2 shows the morphological changes, observable from SEM cross-sections, that occurred in the membranes obtained by introducing different modifications to the preparation conditions. In the case of the use of a saturated salt solution as a coagulation bath instead of deionized water, the macrovoids or finger-like structures were replaced by a more homogeneous structure, with the presence of a porous sponge-like network closer to an isotropic structure (Figure 2c,f,i, and bottom line: magnified images). These results can be explained by considering that during the phase separation process, and due to the increase in the viscosity of the coagulation bath as a result of the presence of dissolved salt, the liquid–liquid demixing process is delayed by the slowing down of the non-solvent diffusion into the dope solution, suppressing thus the formation of channel-like macrovoids [36]. On the other hand, in the in situ addition procedure, the sodium chloride solid salt was mixed with the polymer solution before casting. Since the salt is insoluble in DMF, the salt crystals remained inside the polymer during the phase inversion process. In fact, the phase inversion process is faster than the release and dissolution of sodium chloride in water. As a result, once the salt is then completely dissolved in water, the membranes present large, irregular cavities left by the crystals (Figure 2b,e,h). The observed irregular and large pores presented low reproducibility. For these reasons, supported by pore size and porosity measurements (see below), this procedure was not taken into consideration for further modification (addition of the surfactant) in the preparation conditions.
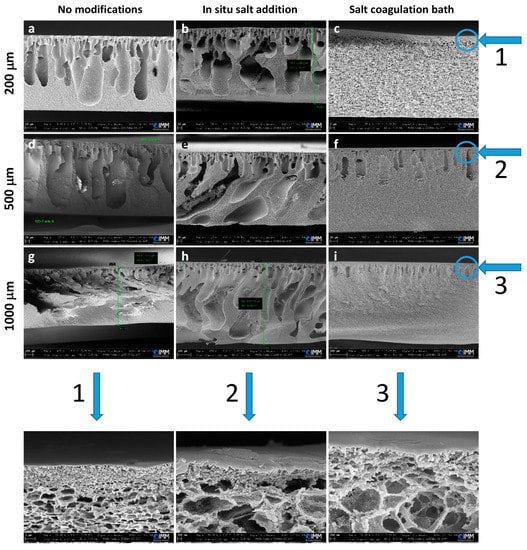
Figure 2.
SEM images of the cross-section of CA membranes of different thicknesses without modification and using two different modifications: in situ salt addition and salt coagulation bath (film applicator speed = 5 mm/s). The scale bars are as follows: (a,b,d–f) 20 µm, (c) 2 µm, and (g–i) 100 µm. In the bottom line, magnifications of the images of the “salt coagulation bath” column are reported (scale bars: (1) 1 µm, (2) 200 nm, and (3) 200 nm).
The hydrophilic, water-soluble surfactant Pluronic F-127 (P127) was chosen as a structure modifier. It was mixed with the polymer solution prior to the fabrication process. As a water-soluble surfactant, P127 can be highly solubilized and form micelles within the polymer upon mixing with water during the phase inversion in the coagulation bath. This can leave uniformly porous structures, as confirmed by the SEM images of a membrane obtained with this procedure, as shown in Figure 3a–c.
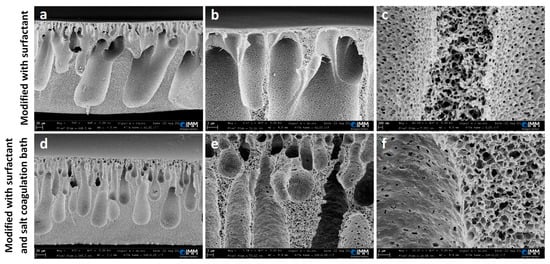
Figure 3.
SEM images at different magnifications of membranes modified with P127 surfactant (a–c) and membranes modified with P127 surfactant and salt coagulation bath (d–f). The film applicator speed was 5 mm/s and the thickness of the casted solution was 500 μm. The scale bars are, from the lowest to the highest magnification: (a,d) 20 µm, (b,e) 2 µm, (f) 1 µm, and (c) 200 nm.
The previously selected modification (use of a salt coagulation bath) was combined with the addition of P127 to the polymer solution, and the cross-section images of the produced membrane at different magnifications are shown in Figure 3d–f. It seems that the presence of the water-soluble hydrophilic surfactant can facilitate and fasten the solvent replacement during the phase inversion process, regardless and overcoming the effect of the salt coagulation bath, as confirmed by the appearance of a finger-like structure. This enhanced morphological structure is beneficial to the permeation performances of the membrane, as discussed in the following sections.
3.2. Surface Characteristics of the Prepared Membranes
To further explore the effect of the different modifications introduced in the membrane preparation on the characteristics of the membranes surface, AFM images were acquired for materials prepared with the basic procedure and by using the salt coagulation bath, the in situ addition of salt and the combination of the salt coagulation bath with the addition of P127. As shown in Figure 4 and Table 1, the roughness values of the membranes prepared using the basic procedure range from 7 to 10 nm, regardless of the thickness. No significant changes in roughness have been observed with the in situ addition of salt, whereas the manufacture of membranes with the salt coagulation bath (SAB) leads to smoother surfaces, regardless of the membrane thickness. Noteworthy, the addition of the surfactant to the basic procedure reduces the surface roughness of the produced membranes (SURF), while the addition of P127 has no significant effect on membranes obtained with SAB (SURF-SAB).
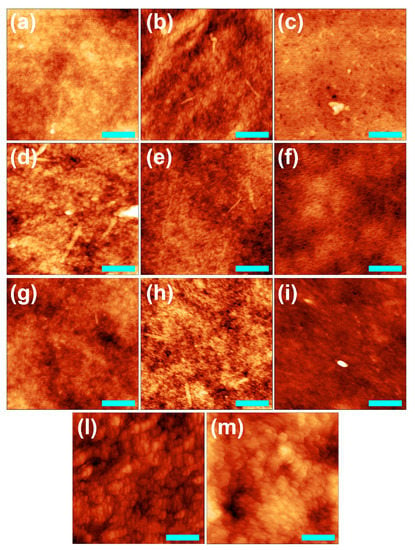
Figure 4.
AFM images of 200 (a–c), 500, (d–f,l,m) and 1000 µm (g–i) CA membranes prepared by the basic procedure (a,d,g) or in situ addition of salt (b,e,h) or SAB (c,f,i), and with the addition of P127 using the basic procedure (l) and SAB (m). The film applicator speed was 5 mm/s. Scale bar = 500 nm and false color = 70 nm.

Table 1.
Roughness (Rms) values of the membranes as a function of preparation conditions and thickness (scanning square areas of 2 µm × 2 µm).
3.3. Water Wettability: Contact Angle Measurements
The surface wettability of the membranes was monitored by recoding water contact angles at 30 s intervals over a total period of 180 s. As shown in Figure 5, all the membranes are characterized by a hydrophilic surface (contact angle < 90°), but the modifications to the preparation conditions can affect the initial wettability of the membranes. In particular, while for the lowest thickness (200 µm), modifications slightly change the surface wettability properties, SAB and in situ salt addition modifications significantly decrease the wettability of the 500 µm thickness membranes. Even for the thickest membrane, i.e., 1000 µm, in situ salt addition modification causes a decrease in wettability, but, similarly to what was observed for the 200 µm thickness membrane, the SAB does not significantly affect the wetting properties of membranes. Figure 5 also shows that the water adsorption dynamics of the membranes, i.e., the trend over time of the decrease in their contact angle values, is almost the same regardless of the conditions and thickness. Furthermore, we observed that the addition of the surfactant leads to a drastic decrease in the initial wettability of the membranes prepared with the basic procedure without altering their water adsorption dynamics, but it has a completely opposite effect on the membranes prepared by SAB.
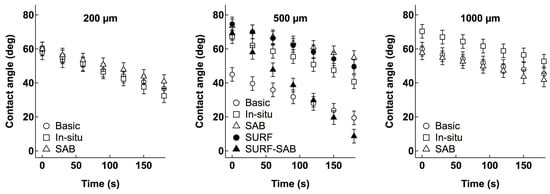
Figure 5.
Dynamic contact angles of 200, 500, and 1000 µm CA membranes fabricated by the basic procedure or by in situ addition of salt (in situ) and salt coagulation bath (SAB).
3.4. Porosity, Pore Size, and Pore Size Distribution of the Prepared Membranes
The results of the analysis of the pore size and porosity of the membranes are reported in Figure 6a. The porosity is in the range of 66 to 80%, which is in agreement with values reported for these kinds of membranes in the literature [37,38]. The membranes were formed in water, as a coagulation bath, which accelerates the demixing mechanism, according to the SEM analysis in Figure 2. When the salt was added to the bath, the phase inversion was slowed down, and the membrane resulted with a smaller pore size, as for the membrane 500-SURF-SAB (pore size of 0.02 µm). The presence of salt in the polymeric solution led to a smaller pore size in the membranes (200-SAB, 500-SAB, 1000-SAB). This result was also confirmed by a narrow pore size distribution. An example is reported in Figure 6b for the 500-SAB membrane. The clear effect of Pluronic F-127 as a pore former can be seen for the membrane 500-SURF, with a pore size of 0.06 µm, which is higher in comparison to the membrane 500 without the presence of any additive (pore size of 0.03 µm). The addition of Pluronic F-127 led also to a narrower pore size distribution, as evidenced in Figure 6b for the 500-SURF membrane. The unusually high pore size measured for the 1000 µm membrane can be tentatively ascribed to the presence of large pores that degenerated into unshaped cavities (see Figure 2g) and to the wider pore size distribution. The higher thickness of this sample could have caused a delay in the demixing of the dope solution, resulting in the formation of a membrane with a larger pore size.
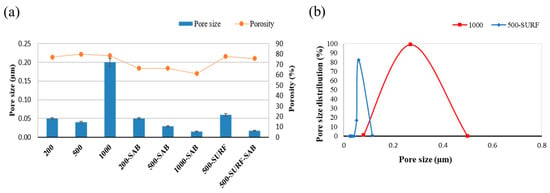
Figure 6.
(a) Porosity, pore size, and (b) pore size distribution of the prepared membranes.
3.5. Water Flux and Water Permeability of the Prepared Membranes
The water permeability of the membranes prepared using the basic procedure and with the mentioned modifications was tested by measuring the water flux through the membrane upon application of variable pressure, with a maximum of 4.0 bar. The determined values are reported in Table 2. The samples at different thicknesses (200, 500, and 1000) without additives showed a range of permeability from 47 to 539 L/m2h bar. The highest value of permeability for the 1000 µm membrane was related to the higher value of pore size placed in the microfiltration range (Figure 6). The permeability of the membranes 200-SAB and 500-SAB was not measurable until 4 bar. Confirming the results of SEM and porosity analysis, that evidenced the pore size increase for the membrane 500 SURF when using Pluronic F-127 as a pore former, the water permeability also increased from 62 L/m2h bar (for the 500 membrane) to 87 L/m2h bar (for 500-SURF). The water permeability decreased in the case of 500-SURF-SAB due to the presence of salt in the coagulation bath (23 L/m2h bar).

Table 2.
Water permeability of the prepared membranes.
The in situ salt addition produced membranes with large pores (in the μm range, as observed from the SEM analysis) due to the dissolution of the salt clusters in the phase inversion procedure. For this reason, the water flux through these membranes, at the 200 and 500 μm thickness, was too fast to be able to measure the water permeability in our experimental set-up. For the 1000 μm membrane of this series, the water permeability was measurable, with values ranging from 251 to 539 L/m2h. Overall, the permeability issue, related to the pore size and pore distribution of these particular kinds of membranes (Table 3), pointed to excluding the “in situ salt addition” procedure from the combination with the modification with the surfactant.

Table 3.
Performance in terms of water permeability, porosity, and pore size of the membranes prepared with 5 wt% of Pluronic F-127 in water (500-SURF) and in a saturated salt bath as a coagulation bath (500-SURF-SAB).
3.6. Dye Removal Efficiency of the Prepared Membranes
The prepared membranes were tested for water treatment efficacy by using two dyes, AZ and MO, chosen as models for cationic and anionic water pollutants, respectively. The analysis was aimed at exploring how the membranes interact with two dyes of different natures.
Two sets of water treatment experiments were designed, one in “static” conditions and one under pressure. In the static treatment, a sample of the membrane was immersed in the dye aqueous solution (0.01 mM) overnight, and, after removal of the membrane, the final concentration of the solution was measured spectrophotometrically (Figure S3 shows the absorption spectra of the dye solutions prior and after immersion of the membrane samples). The uptake of the dye by the membrane was calculated and expressed as uptake efficiency (%, see the Experimental Section for details). The membranes selected for this test were those considered more promising in terms of pore size/distribution and water permeability, i.e., the ones prepared: (i) in basic conditions (200, 500, and 1000 μm thicknesses), (ii) with salt coagulation bath (with 200, 500, and 1000 μm thicknesses), (iii) with the addition of the surfactant (500 μm thickness as the most promising), and (iv) with the addition of the surfactant and salt coagulation bath (500 μm thickness as the most promising). Figure 7 shows the results of this experiment. It is evident that the uptake of AZ is very high in all cases and almost quantitative for 500-SURF, while that of MO is particularly low for all kinds of membranes, except for 500-SURF-SAB, where it reaches 37%. The difference in uptake can be explained by the higher affinity of the CA structure for the cationic AZ dye.
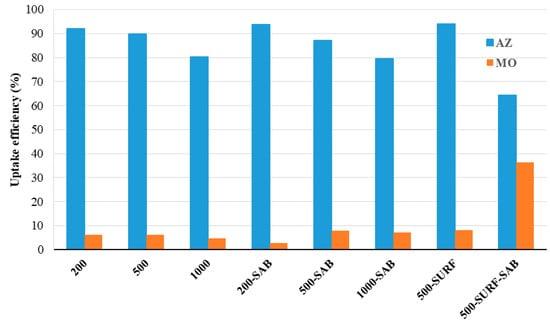
Figure 7.
AZ and MO uptake efficiency of the prepared membranes at equilibrium using a batch technique under static conditions (the dye initial concentration was 0.01 mM and the material amount was 1% wt/vol with respect to the dye solution).
The removal capacity under pressure was tested in a cross-flow cell by flowing a solution of the dye (0.01 mM) through the membrane under a pressure of 1 bar, collecting the flowed solution at different time intervals, and measuring the concentration of each collected aliquot. The membrane removal capacity could then be estimated for each time interval and expressed in terms of remaining dye in the flowed solution (as % of the initial concentration). Two membranes were selected for this application, i.e., the 500-SURF and the 500-SURF-SAB, as they turned out to be the most efficient in AZ and MO uptake, respectively, in static conditions. Figure 8 reports, for both dyes, the % of the remaining dye in the solution after flowing through the 500-SURF membrane, as a function of time. The removal of AZ is quantitative till 25 min of flow, then a slight release of dye is observed, probably due to the saturation of the membrane’s uptake capability. Conversely, the removal efficiency of MO decreases much faster with time, and after 15 min of flow, the concentration of the flowed solution is already close to the initial one. The MO removal efficiency as a function of time was compared for 500-SURF and 500-SURF-SAB (Figure 9), with the result of slightly better removal performance observed for the most modified membrane (500-SURF-SAB).
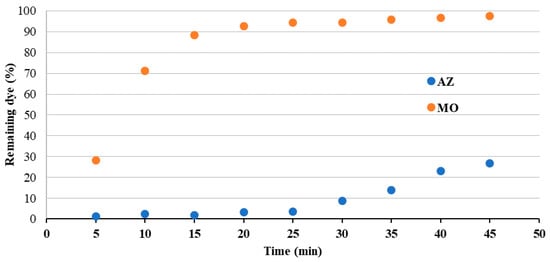
Figure 8.
AZ and MO remaining dye (%) for solutions flowed through the membrane 500-SURF under the pressure of 1 bar (the dye initial concentration was 0.01 mM and the effective membrane filtration area was 0.00115 m2).
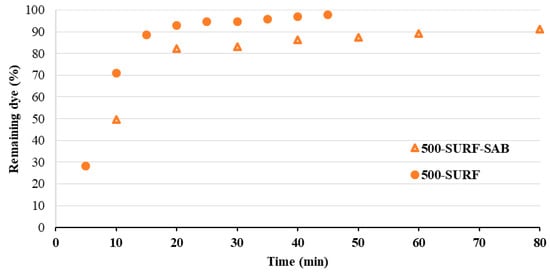
Figure 9.
MO remaining dye (%) for a solution flowed through the membranes 500-SURF and 500-SURF-SAB under the pressure of 1 bar (the dye initial concentration was 0.01 mM and the effective membrane filtration area was 0.00115 m2).
3.7. Adsorption Kinetic Study
In order to understand the adsorption mechanisms of the dyes onto the different membranes, the adsorption capacities collected during time using the AZ and MO solutions at the initial concentration of 0.01 mM (Figure 10) were fitted using the pseudo-first, the pseudo-second order, and the intraparticle diffusion models.
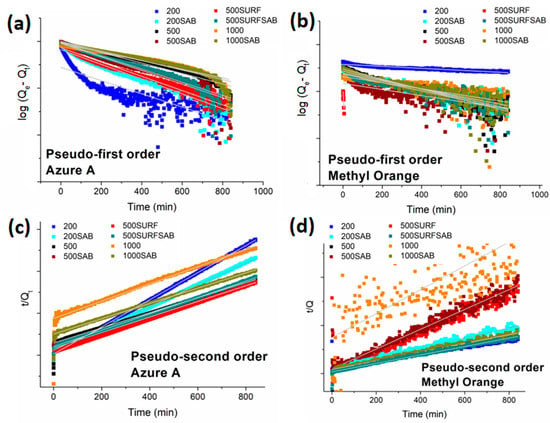
Figure 10.
Application of the pseudo-first order adsorption model onto membranes for AZ (a) and MO, (b) and application of pseudo-second order model onto membranes for AZ (c) and MO (d).
The linearized forms of the pseudo-first (Equation (5)) and the pseudo-second order (Equation (6)) equations are shown below [39]:
where and are the rate constants of adsorption, is the adsorption capacity at time t, is the equilibrium adsorption capacity.
All the kinetic parameters determined from the slopes and the intercepts of the respective plots (Figure 10) are reported in Table 4. With the exception of the membrane 1000, all the other samples displayed a better fit with the pseudo-second order model for both dyes, showing higher correlation coefficients. The applicability of the pseudo-second order model suggests that the rate-limiting step is chemisorption involving valency forces through the sharing or exchange of electrons between the dyes and membranes [40]. On the other hand, a physiosorption involving the Van der Waals forces has a significant role in the adsorption of both dyes onto the membrane 1000.

Table 4.
Kinetic parameters of the pseudo-first and pseudo-second order models for the adsorption of AZ and MO onto the membranes.
The values of Qe calculated through the pseudo-second order model are close to the experimental ones and of one order of magnitude higher for AZ than for MO. The membrane 500-SURF shows the highest kinetic constants for the adsorption of both dyes.
In order to better understand the diffusion mechanism of the dyes onto the membranes, the intraparticle diffusion model was applied. This model is expressed as follows in Equation (7):
where (mmol/g min1/2) is the rate constant of intraparticle diffusion. By plotting versus t1/2, curves with different linear steps were obtained, and each one was fitted with Equation (7) (Figure 11). The obtained parameters are reported in Table 5.
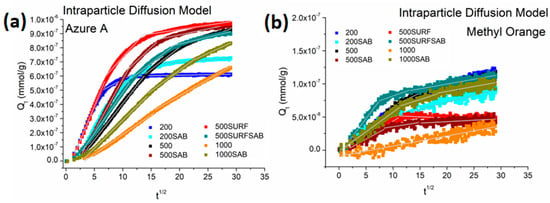
Figure 11.
Application of the intraparticle diffusion model onto membranes for AZ (a) and MO (b).

Table 5.
Kinetic parameters of the intraparticle diffusion model for the adsorption of AZ and MO onto the membranes.
With the exception of the adsorption processes of MO and AZ onto the membrane 1000, all the other adsorption processes are characterized by three diffusion steps (three different linear steps). The first linear step is associated with the external surface adsorption or diffusion in macro-pores until the exterior surface reaches saturation. A larger diffusion rate constant k corresponds to a faster diffusion process. After this first diffusion process, the adsorbate molecules entered the less accessible pores, so the diffusion resistance increased and the diffusion rate constant decreased (kI > kII and kIII).
As shown in Table 5, the diffusion processes of AZ onto the external surface of the membranes were faster than those of MO, due to the better chemical affinity of AZ for CA than that of MO. In addition, sample 500-SURF showed a faster external surface adsorption of AZ, while sample 500-SURF-SAB showed a faster external surface adsorption of MO. As concerns the adsorption process of AZ, sample 500-SURF showed the highest kI, while sample 1000 showed the lowest kI. On the other hand, sample 1000 showed the slowest external surface adsorption of both AZ and MO. Moreover, in the considered time range, the adsorption process of AZ onto this membrane is characterized by two diffusion steps, whereas that of MO is characterized by only the first diffusion step.
4. Conclusions
Anisotropic and isotropic CA porous membranes have been prepared using deionized water and a saturated salt solution as coagulation baths, respectively. The addition of a surfactant to the polymer solution prior to the phase inversion process helped to increase the pore size and thus the water permeability. Upon changing the preparation conditions, the produced CA membranes acquired a porosity ranging from 66 to 80% and an average pore size ranging from 0.017 to 0.060 µm. The porous structure of the produced membranes affected the water permeability as well as the absorption/rejection characteristics towards charged cationic and anionic molecules.
The difference in the removal efficiency of the dyes AZ and MO, almost quantitative and up to a maximum of 37% for AZ and MO, respectively, can be explained by the different mechanisms of interaction of the two dyes with the membranes. In the case of MO, the membrane pore size is the main factor governing the dye removal/rejection, so as the pore size decreases, the treatment efficiency increases, up to a maximum of 37%. For AZ, the uptake is mainly due to chemisorption, so the higher the water permeability, the higher the probability of interaction between the two oppositely charged materials.
The applicability of the pseudo-second order model for the uptake process indicates that the rate-limiting step is chemisorption involving valency forces through the sharing or exchange of electrons between the dye and the membrane. For the thickest membrane, a physiosorption involving Van der Waals forces was supposed to occur. Generally, the diffusion processes of AZ into the external surface of the membranes were faster than those of MO, due to the better chemical affinity of AZ for CA.
The results highlight the important role played by the preparation conditions in defining the physical properties and water treatment characteristics of polymeric membranes, opening the way to optimization procedures highly needed in water management technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15061061/s1. Figure S1: SEM images of the upper (a) and lower (b) surfaces of the CA membrane (fabrication conditions: film applicator speed = 5 mm/s and casted film thickness = 200 µm); Figure S2: (a) SEM images of cross-sections of CA membranes obtained with different film applicator speeds (1, 5 and 10 mm/s, the thickness was fixed at 500 µm) and (b) cross-sections of CA membranes of different thicknesses (the film applicator speed was fixed at 5 mm/s); Figure S3: Absorption spectra of the dye solutions prior (thicker curves, initial) and after (thinner curves, final) the overnight immersion of the membrane samples (0.05 g in 5 mL solution: 1% wt/vol) for (a) AZ and (b) MO.
Author Contributions
Conceptualization, R.E.M. and B.V.; investigation, R.E.M., F.C., D.G., F.R. and F.G.; supervision and validation, V.M., M.C., A.F. and B.V.; formal analysis, A.A.; writing—original draft preparation, all authors; writing—review and editing, R.E.M. and B.V.; project administration, B.V.; funding acquisition, R.E.M. and B.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by H2020-MSCA-IF-2017 (project number 800317 “Enhanced-MUMs”) and Italian CNR (project PHEEL). F.C. and V.M. acknowledge funding from the European Commission projects Graphene Flagship Core3, grant agreement No 881603, and CHALLENGES, grant agreement No 861857.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
E. Treossi and A. Scidà (ISOF-CNR) are acknowledged for assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Y.; Hu, J.; Zhang, X.; Yuan, D.; Duan, G.; Li, Y. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, J.; Gong, J.; Qu, J.; Niu, R. Cost-effective, scalable fabrication of self-floating xerogel foam for simultaneous photothermal water evaporation and thermoelectric power generation. Chem. Eng. J. 2023, 454, 140383. [Google Scholar] [CrossRef]
- He, P.; Bai, H.; Fan, Z.; Hao, L.; Liu, N.; Chen, B.; Niu, R.; Gong, J. Controllable synthesis of N/Co-doped carbon from metal–organic frameworks for integrated solar vapor generation and advanced oxidation processes. J. Mater. Chem. A 2022, 10, 13378–13392. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Yang, F.; Bai, W.; Zhang, X.; Li, H.; Duan, G.; Xu, Y.; Li, Y. A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Han, M.-J.; Bhattacharyya, D. Morphology and transport study of phase inversion polysulfone membranes. Chem. Eng. Commun. 1994, 128, 197–209. [Google Scholar] [CrossRef]
- Murphy, T.M.; Offord, G.T.; Paul, D.R. Fundamentals of membrane gas separation. In Membrane Operations: Innovative Separations and Transformations; Drioli, E., Giorno, L., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 63–82. [Google Scholar]
- Jose, A.; Kappen, J.; Alagar, M. Polymeric membranes: Classification, preparation, structure physiochemical, and transport mechanisms. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Duxford, UK, 2018; pp. 21–35. [Google Scholar]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges on adsorptive membranes for the removal of pollutants from wastewater. Part I: Fundamentals and classification of membranes. Case Stud. Chem. Env. Eng. 2021, 3, 100086. [Google Scholar] [CrossRef]
- Sagle, A.; Freeman, B. Fundamentals of Membranes for Water Treatment. In The Future of Desalination in Texas; Report Number 363; Texas Water Development Board: Austin, TX, USA, 2004; Volume 2, pp. 137–154. [Google Scholar]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.-N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Perera, D.H.N.; Nataraj, S.K.; Thomson, N.M.; Sepe, A.; Hüttner, S.; Steiner, U.; Qiblawey, H.; Sivaniah, E. Room-temperature development of thin film composite reverse osmosis membranes from cellulose acetate with antibacterial properties. J. Membr. Sci. 2014, 453, 212–220. [Google Scholar] [CrossRef]
- Ebrahim, S.; Morsy, A.; Kenawy, E.; Abdel-Fattah, T.; Kandil, S. Reverse osmosis membranes for water desalination based on cellulose acetate extracted from Egyptian rice straw. Desalination Water Treat. 2016, 57, 20738–20748. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- El-Dein, L.A.N.; El-Gendi, A.; Ismail, N.; Abed, K.A.; Ahmed, A.I. Evaluation of Cellulose Acetate Membrane with Carbon Nanotubes Additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- El Badawi, N.; Ramadan, A.R.; Esawi, A.M.K.; El-Morsi, M. Novel carbon nanotube-cellulose acetate nanocomposite membranes for water filtration applications. Desalination 2014, 344, 79–85. [Google Scholar] [CrossRef]
- Shaban, M.; AbdAllah, H.; Said, L.; Hamdy, H.S.; Khalek, A.A. Titanium dioxide nanotubes embedded mixed matrix PES membranes characterization and membrane performance. Chem. Eng. Res. Des. 2015, 95, 307–316. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagheri, M.; Mohammadi, T.; Bakhtiari, O. Fabrication optimization of polyethersulfone (PES)/polyvinylpyrrolidone (PVP) nanofiltration membranes using Box–Behnken response surface method. RSC Adv. 2017, 7, 24995–25008. [Google Scholar] [CrossRef]
- Cadore, Í.R.; Ambrosi, A.; Medeiros Cardozo, N.S.; Tessaro, I.C. Poly(ethylene terephthalate) phase inversion membranes: Thermodynamics and effects of a poor solvent on the membrane characteristics. Polym. Eng. Sci. 2022, 62, 1847–1858. [Google Scholar] [CrossRef]
- Russo, F.; Castro-Muñoz, R.; Galiano, F.; Figoli, A. Unprecedented preparation of porous Matrimid® 5218 membranes. J. Memb. Sci. 2019, 585, 166–174. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Drioli, E. Polymeric Membranes. In Membrane Fabrication; Hilal, N., Ismail, A.F., Wright, C., Eds.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2015; Chapter 1; pp. 3–44. [Google Scholar]
- Gilani, A.G.; Ghorbanpour, T.; Salmanpour, M. Additive effect on the dimer formation of thiazine dyes. J. Mol. Liq. 2013, 177, 273–282. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, R.; Zhu, W.J.; Sha, X.L.; Ge, J.F. Reversible Absorption and Emission Responses of Nile Blue and Azure A Derivatives in Extreme Acidic and Basic Conditions. J. Fluoresc. 2017, 27, 819–827. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef]
- Buwalda, R.T.; Engberts, J.B.F.N. Aggregation of Dicationic Surfactants with Methyl Orange in Aqueous Solution. Langmuir 2001, 17, 1054–1059. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Galiano, F. Polymeric membranes in biorefinery. In Membrane Technologies for Biorefining, 1st ed.; Figoli, A., Cassano, A., Basile, A., Eds.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 2; pp. 29–59. [Google Scholar]
- Zhang, Y.; Tong, X.; Zhang, B.; Zhang, C.; Zhang, H.; Chen, Y. Enhanced permeation and antifouling performance of polyvinyl chloride (PVC) blend Pluronic F127 ultrafiltration membrane by using salt coagulation bath (SCB). J. Membr. Sci. 2018, 548, 32–41. [Google Scholar] [CrossRef]
- Razzaghi, M.H.; Tavakolmoghadam, M.; Rekabdar, F.; Oveisi, F. Investigation of the effect of coagulation bath composition on PVDF/CA membrane by evaluating critical flux and antifouling properties in lab-scale submerged MBR. Water Environ. J. 2018, 32, 366–376. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Liang, Y.; Yao, J. Study on the control of pore sizes of membranes using chemical methods Part II. Optimization factors for preparation of membranes. Desalination 2008, 225, 123–138. [Google Scholar] [CrossRef]
- Alahabadi, A.; Moussavi, G. Preparation, characterization and atrazine adsorption potential of mesoporous carbonate-induced activated biochar (CAB) from Calligonum Comosum biomass: Parametric experiments and kinetics, equilibrium and thermodynamic modelling. J. Mol. Liq. 2017, 242, 40–52. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).