Mechanism Analysis of PFHxS Purification in Water Using Nanofiltration under the Coexistence of Sodium Alginate and Ca2+ Based on DFT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Membrane Performance Evaluation
- Precompression: the membrane was preloaded at 5 bar for 30 min with deionized water as the influent water, stabilizing the membrane effluent.
- Pure water permeability test: after conducting the above precompression process, adjust the pressure to 4 bar, test for 30 min, and record the effluent data to calculate the permeability of pure water, which is recorded as Jw1.
- Membrane fouling test: the deionized water was replaced with the above contaminant water sample and operated at 4 bar for 16 h. The water quality data were recorded in real time through the electronic balance software. The final water permeability was calculated and recorded as Jp.
- Hydraulic cleaning: after the above test, the pollutant water sample was replaced with deionized water, the inlet water pressure was adjusted to 2 bar, and the membrane was hydrologically cleaned for 2 h.
- Repeated pure water permeability test: after hydraulic cleaning, increase the inlet pressure to 4 bar, run for 30 min, and determine the permeability of pure water at this time, which is recorded as Jw2.
- Repeated membrane fouling test: the deionized water was replaced with a contaminant water sample, the inlet pressure was increased to 4 bar, and the water permeability was calculated by running the test again for another 16 h and recording the water quality in real time using the electronic balance software.
2.3. PFHxS Analysis
2.4. DFT Calculation
3. Results and Discussion
3.1. Study on the Influencing Factors of PFHxS Removal by HA-TFCiNFM
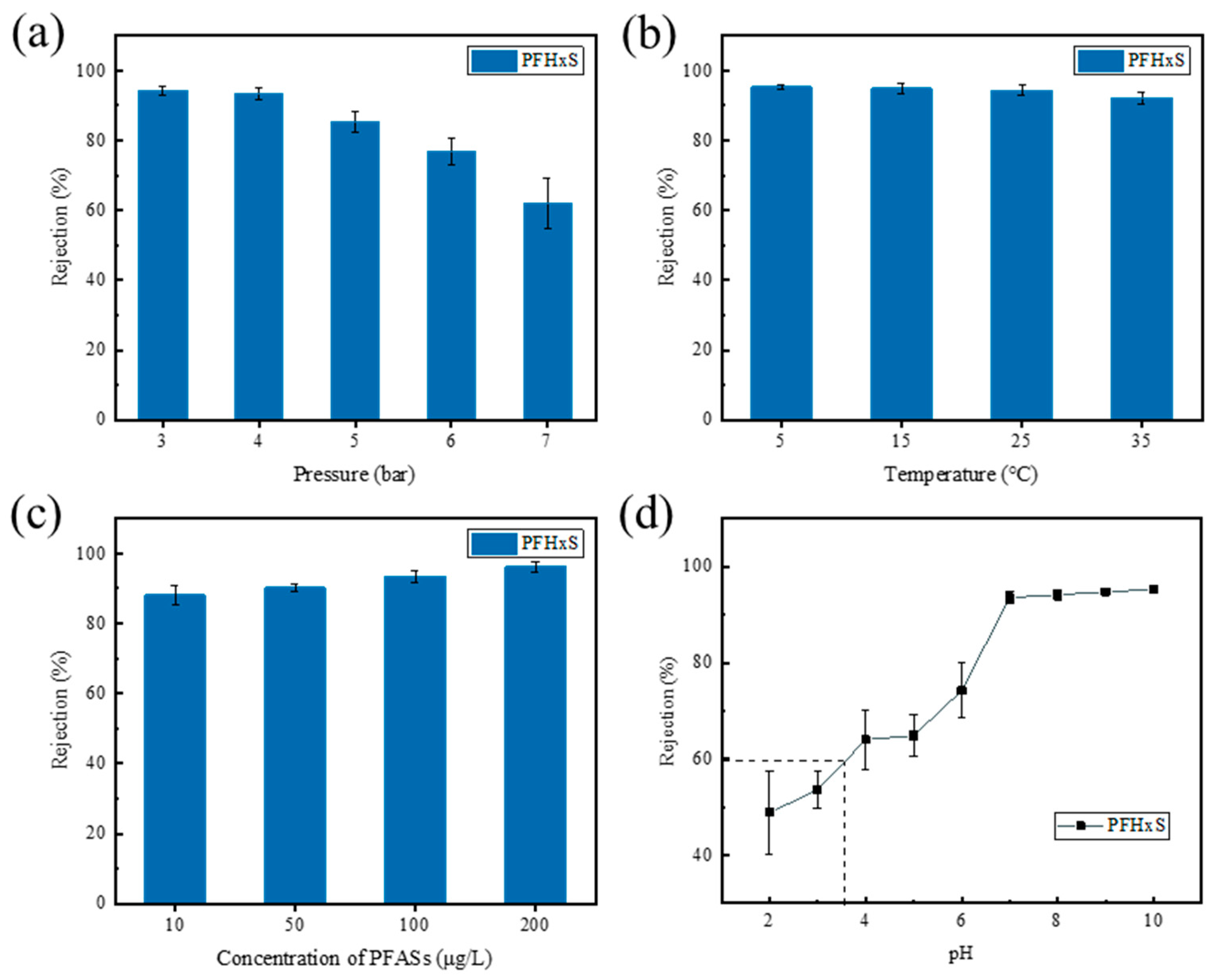
3.1.1. Effect of Operating Pressure
3.1.2. Effect of Temperature
3.1.3. Effect of PFHxS Concentration
3.1.4. Effect of pH
3.1.5. Effect of Coexisting Substances
3.2. Membrane Fouling Behavior under the Coexistence of Ca2+ and SA
3.3. HA-TFCiNFM Purification Mechanism of PFHxS
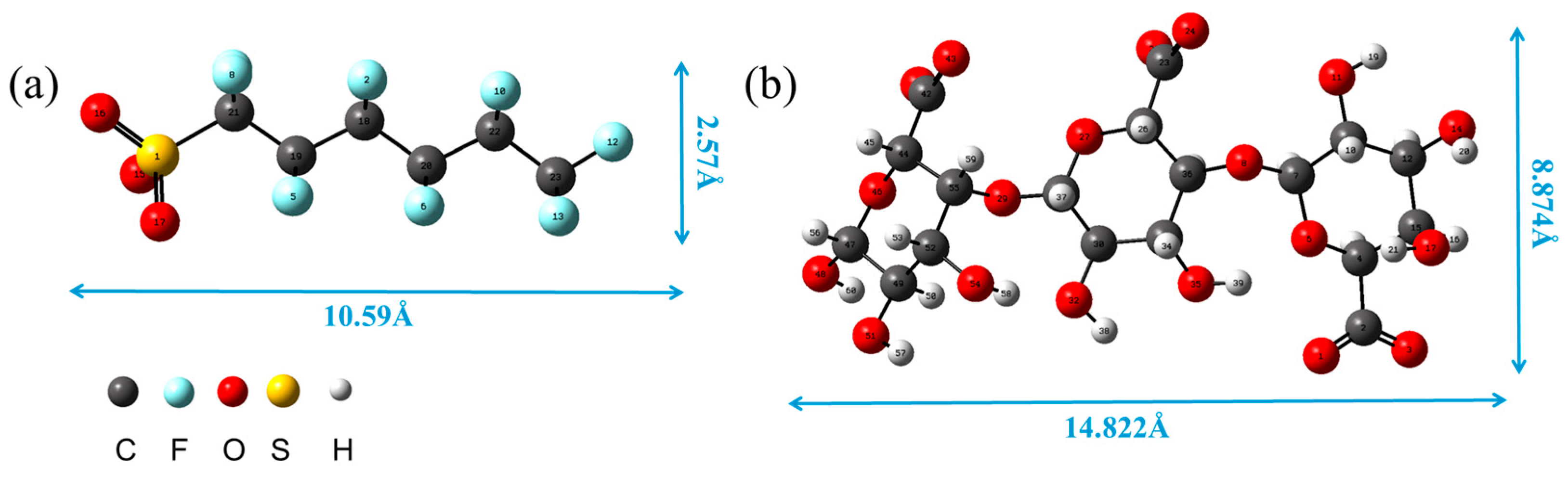
3.3.1. Structural Optimization of PFHxS and SA Theoretical Models
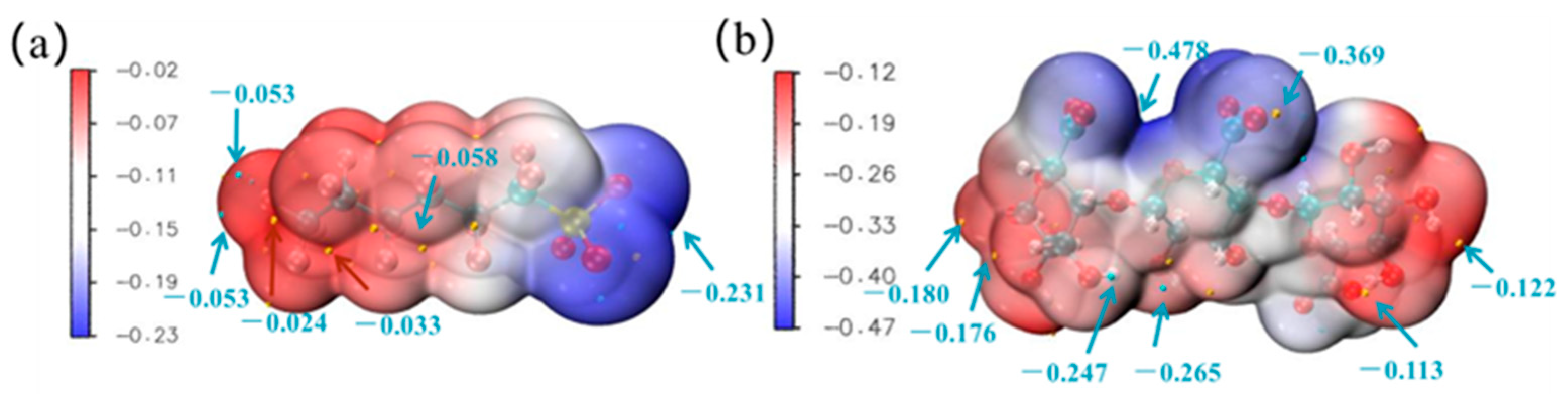
3.3.2. Active Sites of PFHxS and SA
3.3.3. Mechanism of HA-TFCiNFM Purification of PFHxS
3.4. HA-TFCiNFM Fouling Mechanism
3.4.1. Construction and Simulation Optimization of the Membrane Fouling Model
3.4.2. Analysis of Membrane–Contaminant Interactions
4. Conclusions
- It was found that the retention rate of the HA-TFCiNFM on PFHxS decreased as the operating pressure (or temperature) improved but increased with the increase in the initial pH (or concentration) of the water sample. When the coexisting substances SA and Ca2+ were present, the retention rate of the HA-TFCiNFM on PFHxS was higher compared to the single PFHxS system and increased with the concentration of SA and Ca2+. The results showed that the membrane fouling degree was greatest when SA and Ca2+ coexisted, as indicated by the water flux attenuation and anti-pollution index of the HA-TFCiNFM in the PFHxS/SA/Ca2+ water sample system.
- The active sites of PFHxS and SA were analyzed based on electrostatic potential, and the optimal configuration was determined from the perspective of energy and the product structure. The presence of Ca2+ increases the size of the complex compound by complexing two PFHxS molecules, thereby enhancing the sieving effect. The presence of SA, although unable to react with PFHxS, blocks membrane pores and increases the negative charge on the membrane surface, thus strengthening the size of the sieving and Donnan effects. When both Ca2+ and SA are present, they form a larger complex compound by complexing one PFHxS and one SA molecule, further enhancing the sieving effect. The removal mechanism of the HA-TFCiNFM on PFHxS in the coexistence system is mainly based on the sieving effect, with the Donnan effect playing a supporting role.
- During the removal of PFHxS pollutants with the HA-TFCiNFM, weak interactions, such as hydrogen bonding and van der Waals, became crucial for binding between the membranes and pollutants. The addition of Ca2+ impacted the membrane fouling process by influencing the hydrogen bonding interaction between the HA-TFCiNFM and pollutants.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, X.; Zhao, Y.; Wang, J.; Bao, J. Treatment techniques for perfluorinated compounds and their alternatives. Environ. Chem. 2018, 37, 1860–1868. [Google Scholar]
- Jian, J.-M.; Chen, D.; Han, F.-J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069. [Google Scholar] [CrossRef]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Brede, E.; Wilhelm, M.; Goeen, T.; Mueller, J.; Rauchfuss, K.; Kraft, M.; Hoelzer, J. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int. J. Hyg. Environ. Health 2010, 213, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.W.Y.; So, M.K.; Jiang, G.B.; Taniyasu, S.; Yamashita, N.; Song, M.Y.; Wu, Y.N.; Li, J.G.; Giesy, J.P.; Guruge, K.S.; et al. Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ. Sci. Technol. 2006, 40, 715–720. [Google Scholar] [CrossRef]
- Ramhoj, L.; Hass, U.; Boberg, J.; Scholze, M.; Christiansen, S.; Nielsen, F.; Axelstad, M. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol. Sci. 2018, 163, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Hong, Y.; Xie, G.; Zhang, J.; Zhang, H.; Cai, Z. Comprehensive multi-omics approaches reveal the hepatotoxic mechanism of perfluorohexanoic acid (PFHxA) in mice. Sci. Total Environ. 2021, 790, 148160. [Google Scholar] [CrossRef]
- Reemtsma, T.; Berger, U.; Arp, H.P.H.; Gallard, H.; Knepper, T.P.; Neumann, M.; Quintana, J.B.; de Voogt, P. Mind the gap: Persistent and mobile organic compounds water contaminants that slip through. Environ. Sci. Technol. 2016, 50, 10308–10315. [Google Scholar] [CrossRef] [Green Version]
- Maimaiti, A.; Deng, S.; Meng, P.; Wang, W.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chem. Eng. J. 2018, 348, 494–502. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Ostlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sukeesan, S.; Boontanon, N.; Boontanon, S.K. Improved electrical driving current of electrochemical treatment of Per- and Polyfluoroalkyl Substances (PFAS) in water using Boron-Doped Diamond anode. Environ. Technol. Innov. 2021, 23, 101655. [Google Scholar] [CrossRef]
- Kulkarni, P.R.; Richardson, S.D.; Nzeribe, B.N.; Adamson, D.T.; Kalra, S.S.; Mahendra, S.; Blotevogel, J.; Hanson, A.; Dooley, G.; Maraviov, S.; et al. Field Demonstration of a Sonolysis Reactor for Treatment of PFAS-Contaminated Groundwater. J. Environ. Eng. 2022, 148, 06022005. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Fu, J.; Wang, A.; Ding, M.; Zhang, L.; Han, L.; Gao, L. Hyaluronic acid-modified nanofiltration membrane for ultrahigh water permeance and efficient rejection of PFASs. Process Saf. Environ. Prot. 2022, 166, 214–221. [Google Scholar] [CrossRef]
- Li, M.; Sun, F.; Shang, W.; Zhang, X.; Dong, W.; Dong, Z.; Zhao, S. Removal mechanisms of perfluorinated compounds (PFCs) by nanofiltration: Roles of membrane-contaminant interactions. Chem. Eng. J. 2021, 406, 126814. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Xu, H.; Ding, M.; Gao, L. MXene (Ti3T2CX)-reinforced thin-film polyamide nanofiltration membrane for short-chain perfluorinated compounds removal. Process Saf. Environ. Prot. 2022, 168, 275–284. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Bao, J.; Hu, X.; Zhao, X.; Shao, L.; Li, C.; Lu, M. A Review of Treatment Techniques for Short-Chain Perfluoroalkyl Substances. Appl. Sci. 2022, 12, 1941. [Google Scholar] [CrossRef]
- Tang, W.; Meng, Y.; Yang, B.; He, D.; Li, Y.; Li, B.; Shi, Z.; Zhao, C. Preparation of hollow-fiber nanofiltration membranes of high performance for effective removal of PFOA and high resistance to BSA fouling. J. Environ. Sci. 2022, 122, 14–24. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, T.; Hu, G.; Ma, J.; Song, R.; Li, J. Efficient removal of perfluorooctane sulphonate by nanofiltration: Insights into the effect and mechanism of coexisting inorganic ions and humic acid. J. Membr. Sci. 2020, 610, 118176. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, C.Y.; Li, P.; Adrian, P.; Hu, G. Perfluorooctane sulfonate removal by nanofiltration membrane—The effect and interaction of magnesium ion / humic acid. J. Membr. Sci. 2016, 503, 31–41. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Yao, C.; Chen, W.; Song, N.; Zhang, Q.; Lin, T.; Xie, Z. Understanding the membrane fouling control process at molecular level in the heated persulfate activation- membrane distillation hybrid system. Water Res. 2023, 229, 119465. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.E.; Steiner, Z.; Miao, J.; Kasher, R.; Li, Q. Studying the Role of Common Membrane Surface Functionalities on Adsorption and Cleaning of Organic Foulants Using QCM-D. Environ. Sci. Technol. 2011, 45, 6309–6315. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Cao, X.; Xue, J.; Zhang, Q.; Tian, J.; Li, X.; Qiu, X.; Pan, B.; Gu, A.Z.; et al. Effect of carboxyl and hydroxyl groups on adsorptive polysaccharide fouling: A comparative study based on PVDF and graphene oxide (GO) modified PVDF surfaces. J. Membr. Sci. 2020, 595, 117514. [Google Scholar] [CrossRef]
- Meng, S.; Wang, R.; Meng, X.; Wang, Y.; Fan, W.; Liang, D.; Zhang, M.; Liao, Y.; Tang, C. Reaction heterogeneity in the bridging effect of divalent cations on polysaccharide fouling. J. Membr. Sci. 2022, 641, 119933. [Google Scholar] [CrossRef]
- Zhu, X.-Z.; Wang, L.-F.; Zhang, F.; Lee, L.W.; Li, J.; Liu, X.-Y.; Luo, S.; Huang, M.-S.; Liu, H.-Q. Combined fouling of forward osmosis membrane by alginate and TiO2 nanoparticles and fouling mitigation mechanisms. J. Membr. Sci. 2021, 622, 119003. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zheng, M.; Ma, C.; Fu, Q.; Zhang, Y. Transparent exopolymer particles-associated membrane fouling analyses of systems containing sodium alginate, calcium, iron, alum and their combination during dead-end ultrafiltration. J. Clean. Prod. 2022, 366, 132983. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Thwala, J.M.; Mamba, B.B.; D’Haese, A.; Verliefde, A.R.D. Factors governing combined fouling by organic and colloidal foulants in cross-flow nanofiltration. J. Membr. Sci. 2015, 491, 53–62. [Google Scholar] [CrossRef]
- Mo, Y.; Xiao, K.; Shen, Y.; Huang, X. A new perspective on the effect of complexation between calcium and alginate on fouling during nanofiltration. Sep. Purif. Technol. 2011, 82, 121–127. [Google Scholar] [CrossRef]
- Yin, Z.; Wen, T.; Li, Y.; Li, A.; Long, C. Pre-ozonation for the mitigation of reverse osmosis (RO) membrane fouling by biopolymer: The roles of Ca2+ and Mg2+. Water Res. 2020, 171, 115437. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Takizawa, Y.; Nakaruk, A.; Katouda, M.; Yamanaka, A.; Ortiz-Medina, J.; Morelos-Gomez, A.; Tejima, S.; Obata, M.; Takeuchi, K.; et al. New insights in the natural organic matter fouling mechanism of polyamide and nanocomposite multiwalled carbon nanotubes-polyamide membranes. Environ. Sci. Technol. 2019, 53, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Ryu, J.; Yu, Y.; Kweon, J. Characteristics of organic fouling, reversibility by physical cleaning and concentrates in forward osmosis membrane processes for wastewater reclamation. Chemosphere 2020, 245, 125787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Deng, S.; Zhang, Q.; Fan, Q.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated sludge. Chemosphere 2010, 81, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: Measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, C.-V.; Cuhorka, J.; Mikulášek, P. Analysis of lead(II) retention from single salt and binary aqueous solutions by a polyamide nanofiltration membrane: Experimental results and modelling. J. Membr. Sci. 2013, 436, 132–144. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Manis, A.; Soldenhoff, K.; Schäfer, A.I. Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci. 2004, 242, 37–45. [Google Scholar] [CrossRef]
- Kaya, Y.; Barlas, H.; Arayici, S. Nanofiltration of Cleaning-in-Place (CIP) wastewater in a detergent plant: Effects of pH, temperature and transmembrane pressure on flux behavior. Sep. Purif. Technol. 2009, 65, 117–129. [Google Scholar] [CrossRef]
- Xu, H.; Ma, J.; Ding, M.; Xie, Z. Mechanistic insights into the removal of PFOA by 2D MXene/CNT membrane with the influence of Ca2+ and humic acid. Desalination 2022, 529, 115643. [Google Scholar] [CrossRef]
- Zeng, C.; Tanaka, S.; Suzuki, Y.; Fujii, S. Impact of feed water pH and membrane material on nanofiltration of perfluorohexanoic acid in aqueous solution. Chemosphere 2017, 183, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]




| MWCO (Da) | Zeta Potential (mV) | Water Contact Angle (°) | Pure Water Flux (L m−2 h−1 bar−1) | Rejection of Na2SO4 |
|---|---|---|---|---|
| 345.35 | 29.53 | 28.45 | 29.53 | 94.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, L.; Han, L.; Zhang, Y.; Gu, C.; Huang, J. Mechanism Analysis of PFHxS Purification in Water Using Nanofiltration under the Coexistence of Sodium Alginate and Ca2+ Based on DFT. Water 2023, 15, 792. https://doi.org/10.3390/w15040792
Liu M, Zhang L, Han L, Zhang Y, Gu C, Huang J. Mechanism Analysis of PFHxS Purification in Water Using Nanofiltration under the Coexistence of Sodium Alginate and Ca2+ Based on DFT. Water. 2023; 15(4):792. https://doi.org/10.3390/w15040792
Chicago/Turabian StyleLiu, Mingxiang, Lei Zhang, Le Han, Ying Zhang, Chengjun Gu, and Jianbo Huang. 2023. "Mechanism Analysis of PFHxS Purification in Water Using Nanofiltration under the Coexistence of Sodium Alginate and Ca2+ Based on DFT" Water 15, no. 4: 792. https://doi.org/10.3390/w15040792
APA StyleLiu, M., Zhang, L., Han, L., Zhang, Y., Gu, C., & Huang, J. (2023). Mechanism Analysis of PFHxS Purification in Water Using Nanofiltration under the Coexistence of Sodium Alginate and Ca2+ Based on DFT. Water, 15(4), 792. https://doi.org/10.3390/w15040792







