Hydrotropism: Understanding the Impact of Water on Plant Movement and Adaptation
Abstract
1. Introduction
2. Literature Review
3. Hydraulic Sensing System
4. Water Physiology in Soil, Plants, and the Atmosphere
5. Effects of Water Availability on Plants
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Izzo, L.G.; Aronne, G. Root Tropisms: New Insights Leading the Growth Direction of the Hidden Half. Plants 2021, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Muthert, L.W.F.; Izzo, L.G.; van Zanten, M.; Aronne, G. Root Tropisms: Investigations on Earth and in Space to Unravel Plant Growth Direction. Front. Plant Sci. 2020, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z. Where’s the water? Hydrotropism in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 4247. [Google Scholar] [CrossRef] [PubMed]
- Falik, O.; Reides, P.; Gersani, M.; Novoplansky, A. Root navigation by self inhibition. Plant. Cell Environ. 2005, 28, 562–569. [Google Scholar] [CrossRef]
- Baluška, F.; Mancuso, S.; Volkmann, D.; Barlow, P.W. The ‘root-brain’ hypothesis of Charles and Francis Darwin: Revival after more than 125 years. Plant Signal. Behav. 2009, 4, 1121. [Google Scholar] [CrossRef]
- Marder, M. Plant intentionality and the phenomenological framework of plant intelligence. Plant Signal. Behav. 2012, 7, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Briggs, W.R. From Charles Darwin’s botanical country-house studies to modern plant biology. Plant Biol. 2009, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and Root Water Uptake. In Plant Aquaporins; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 133–153. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Ribas-Carbo, M.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef]
- Vandeleur, R.K.; Sullivan, W.; Athman, A.; Jordans, C.; Gilliham, M.; Kaiser, B.; Tyerman, S.D. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 2014, 37, 520–538. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Johanson, U.; Larsson, C.; Kjellbom, P. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta Biomembr. 2000, 1465, 324–342. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Water Deficiency (Drought). Plant Ecol. 2019, 165–202. [Google Scholar] [CrossRef]
- Miyamoto, N.; Ookawa, T.; Takahashi, H.; Hirasawa, T. Water Uptake and Hydraulic Properties of Elongating Cells in Hydrotropically Bending Roots of Pisum sativum L. Plant Cell Physiol. 2002, 43, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Carminati, A.; Zarebanadkouki, M.; Kroener, E.; Ahmed, M.A.; Holz, M. Biophysical rhizosphere processes affecting root water uptake. Ann. Bot. 2016, 118, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Carminati, A.; Schneider, C.L.; Moradi, A.B.; Zarebanadkouki, M.; Vetterlein, D.; Vogel, H.-J.; Hildebrandt, A.; Weller, U.; Schüler, L.; Oswald, S.E. How the Rhizosphere May Favor Water Availability to Roots. Vadose Zone J. 2011, 10, 988–998. [Google Scholar] [CrossRef]
- Carminati, A.; Moradi, A.B.; Vetterlein, D.; Vontobel, P.; Lehmann, E.; Weller, U.; Vogel, H.-J.; Oswald, S.E. Dynamics of soil water content in the rhizosphere. Plant Soil 2010, 332, 163–176. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; von Wirén, N. Hydropatterning—How roots test the waters. Science 2018, 362, 1358–1359. [Google Scholar] [CrossRef]
- Robbins, N.E.; Dinneny, J.R. The divining root: Moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 2015, 66, 2145–2154. [Google Scholar] [CrossRef]
- Orman-Ligeza, B.; Morris, E.C.; Parizot, B.; Lavigne, T.; Babé, A.; Ligeza, A.; Klein, S.; Sturrock, C.; Xuan, W.; Novák, O.; et al. The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water. Curr. Biol. 2018, 28, 3165–3173.e5. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Dinneny, J.R. Water transport, perception, and response in plants. J. Plant Res. 2019, 132, 311–324. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef]
- Takano, M.; Takahashil, H.; Hirasawa, T.; Suge, H. Rapid communication Hydrotropisrn in roots: Sensing of a gradient in water potential by the root cap. Planta 1995, 197, 410–413. [Google Scholar] [CrossRef]
- Secchi, F.; Pagliarani, C.; Zwieniecki, M.A. The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ. 2017, 40, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Ihuoma, S.O.; Madramootoo, C.A. Recent advances in crop water stress detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- Gerhards, M.; Rock, G.; Schlerf, M.; Udelhoven, T. Water stress detection in potato plants using leaf temperature, emissivity, and reflectance. Int. J. Appl. Earth Obs. Geoinf. 2016, 53, 27–39. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Hill, R.S. Increases in Water Potential Gradient Reduce Xylem Conductivity in Whole Plants. Evidence from a Low-Pressure Conductivity Method. Plant Physiol. 2000, 123, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Williams, J. Root Density and Water Potential Gradients near the Plant Root. J. Exp. Bot. 1974, 25, 669–674. [Google Scholar] [CrossRef]
- Hafner, B.D.; Hesse, B.D.; Bauerle, T.L.; Grams, T.E.E. Water potential gradient, root conduit size and root xylem hydraulic conductivity determine the extent of hydraulic redistribution in temperate trees. Funct. Ecol. 2020, 34, 561–574. [Google Scholar] [CrossRef]
- Kang, Y.-I.; Park, J.-M.; Kim, S.-H.; Kang, N.-J.; Park, K.-S.; Lee, S.-Y.; Jeong, B.R. Effects of Root Zone pH and Nutrient Concentration on the Growth and Nutrient Uptake of Tomato Seedlings. J. Plant Nutr. 2011, 34, 640–652. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Soil pH extremes. In Plant Stress Physiology; CABI Publishing: Wallingford, UK, 2012; pp. 194–209. [Google Scholar]
- Antoni, R.; Dietrich, D.; Bennett, M.J.; Rodriguez, P.L. Hydrotropism: Analysis of the Root Response to a Moisture Gradient. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1398, pp. 3–9. [Google Scholar]
- So, H.B. Water potential gradients and resistances of a soil-root system measured with the root and soil psychrometer. In The Soil—Root Interface; Academic Press: Cambridge, MA, USA, 1979; pp. 99–113. [Google Scholar] [CrossRef]
- Cary, J.W.; Fisher, H.D. Plant Water Potential Gradients Measured in the Field by Freezing Point. Physiol. Plant. 1971, 24, 397–402. [Google Scholar] [CrossRef]
- Salazar-Blas, A.; Noriega-Calixto, L.; Campos, M.E.; Eapen, D.; Cruz-Vázquez, T.; Castillo-Olamendi, L.; Sepulveda-Jiménez, G.; Porta, H.; Dubrovsky, J.G.; Cassab, G.I. Robust root growth in altered hydrotropic response1 (ahr1) mutant of Arabidopsis is maintained by high rate of cell production at low water potential gradient. J. Plant Physiol. 2017, 208, 102–114. [Google Scholar] [CrossRef]
- Sperry, J.S.; Tyree, M.T. Mechanism of Water Stress-Induced Xylem Embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R.S. Plant Water Relations. Plant Physiol. Ecol. 2019, 187–263. [Google Scholar] [CrossRef]
- Miyamoto, N.; Steudle, E.; Hirasawa, T.; Lafitte, R. Hydraulic conductivity of rice roots. J. Exp. Bot. 2001, 52, 1835–1846. [Google Scholar] [CrossRef]
- Johnson, D.M.; Wortemann, R.; McCulloh, K.A.; Jordan-Meille, L.; Ward, E.; Warren, J.M.; Palmroth, S.; Domec, J.-C. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol. 2016, 36, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P.; Huiskes, R.; Søballe, K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J. Biomech. 1997, 30, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.-L.; Mathesius, U. Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2015, 43, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef]
- Vives-Peris, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Root Involvement in Plant Responses to Adverse Environmental Conditions. Agronomy 2020, 10, 942. [Google Scholar] [CrossRef]
- Dautel, R. Molecular Characterization of the Arabidopsis Thaliana Histidine Kinase 1 and Transitions from the Multistep Phosphorelay System to Ser/Thr/Tyr Phosphorylation; Eberhard Karls Universität Tübingen: Tübingen, Germany, 2016. [Google Scholar]
- Han, H.; Adamowski, M.; Qi, L.; Alotaibi, S.S.; Friml, J. PIN-mediated polar auxin transport regulations in plant tropic responses. New Phytol. 2021, 232, 510–522. [Google Scholar] [CrossRef]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Takahashi, H. Molecular mechanisms mediating root hydrotropism: What we have observed since the rediscovery of hydrotropism. J. Plant Res. 2020, 133, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, D.; Nuriel, R.; Bonza, M.C.; Costa, A.; Fromm, H. MIZ1 regulates ECA1 to generate a slow, long-distance phloem-transmitted Ca2+ signal essential for root water tracking in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 8031–8036. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.; Grimonprez, M.; Depczynski, M.; Renton, M. Tuned in: Plant roots use sound to locate water. Oecologia 2017, 184, 151–160. [Google Scholar] [CrossRef]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef]
- Zilly, F.E.; Halemani, N.D.; Walrafen, D.; Spitta, L.; Schreiber, A.; Jahn, R.; Lang, T. Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J. 2011, 30, 1209–1220. [Google Scholar] [CrossRef]
- Salvalaio, M.; Oliver, N.; Tiknaz, D.; Schwarze, M.; Kral, N.; Kim, S.-J.; Sena, G. Root electrotropism in Arabidopsis does not depend on auxin distribution but requires cytokinin biosynthesis. Plant Physiol. 2022, 188, 1604–1616. [Google Scholar] [CrossRef]
- Ishikawa, H.; Evans, M.L. Electrotropism of maize roots: Role of the root cap and relationship to gravitropism. Plant Physiol. 1990, 94, 913–918. [Google Scholar] [CrossRef]
- Morohashi, K.; Okamoto, M.; Yamazaki, C.; Fujii, N.; Miyazawa, Y.; Kamada, M.; Kasahara, H.; Osada, I.; Shimazu, T.; Fusejima, Y.; et al. Gravitropism interferes with hydrotropism via counteracting auxin dynamics in cucumber roots: Clinorotation and spaceflight experiments. New Phytol. 2017, 215, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Miyabayashi, S.; Sugita, T.; Kobayashi, A.; Yamazaki, C.; Miyazawa, Y.; Kamada, M.; Kasahara, H.; Osada, I.; Shimazu, T.; et al. Root-tip-mediated inhibition of hydrotropism is accompanied with the suppression of asymmetric expression of auxin-inducible genes in response to moisture gradients in cucumber roots. PLoS ONE 2018, 13, e0189827. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Cowan, I.R. Transport of Water in the Soil-Plant-Atmosphere System. J. Appl. Ecol. 1965, 2, 221. [Google Scholar] [CrossRef]
- McCarton, L.; O’Hogain, S.; Reid, A. Properties of Water. In The Worth of Water; Springer: Berlin/Heidelberg, Germany, 2021; Available online: https://link.springer.com/chapter/10.1007/978-3-030-50605-6_2 (accessed on 1 January 2023).
- Water Dynamics in Plant Production, 2nd Edition—Wilfried Ehlers, Michael Goss—Google Books. Available online: https://books.google.co.kr/books?hl=en&lr=&id=B7EtDAAAQBAJ&oi=fnd&pg=PR3&dq=During+capillary+motion+within+soil,+water+can+move+from+the+wet+parts+of+the+soil+to+the+dry+parts+of+the+soil.+The+xylem+carries+water+to+the+leaves+due+to+capillary+action+and+sur-face+tension.&ots=eY_-nlnZxB&sig=DySRyUPUnyltlXads-T_GQXO8TM&redir_esc=y#v=onepage&q&f=false (accessed on 3 December 2022).
- Water Transport in Plants: Xylem|Organismal Biology. Available online: https://organismalbio.biosci.gatech.edu/nutrition-transport-and-homeostasis/plant-transport-processes-i/ (accessed on 3 December 2022).
- Passioura, J.B. Plant-Water Relations. Encycl. Life Sci. 2010. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/9780470015902.a0001288.pub2 (accessed on 2 January 2023).
- Atangana, A. Principle of Groundwater Flow. In Fractional Operators with Constant and Variable Order with Application to Geo-hydrology; Academic Press: Cambridge, MA, USA, 2018; pp. 15–47. [Google Scholar] [CrossRef]
- Hubbert, M.K. Darcy’s law and the field equations of the flow of underground fluids. Trans. AIME 2009, 2, 23–59. [Google Scholar] [CrossRef]
- Narasimhan, T.N. Central Ideas of Buckingham (1907): A Century Later. Vadose Zone J. 2007, 6, 687–693. [Google Scholar] [CrossRef]
- Turturro, A.C.; Caputo, M.C.; Perkins, K.S.; Nimmo, J.R. Does the Darcy–Buckingham Law Apply to Flow through Unsaturated Porous Rock? Water 2020, 12, 2668. [Google Scholar] [CrossRef]
- Tyree, M. Review article. The cohesion-tension theory of sap ascent: Current controversies. J. Exp. Bot. 1997, 48, 1753–1765. [Google Scholar] [CrossRef]
- Aston, M.J.; Lawlor, D.W. The Relationship between Transpiration, Root Water Uptake, and Leaf Water Potential. J. Exp. Bot. 1979, 30, 169–181. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils—Google Books. Available online: https://www.elsevier.com/books/water-relations-of-plants-and-soils/kramer/978-0-12-425060-4 (accessed on 3 December 2022).
- McCully, M.E. Root Xylem Embolisms and Refilling. Relation to Water Potentials of Soil, Roots, and Leaves, and Osmotic Potentials of Root Xylem Sap1. Plant Physiol. 1999, 119, 1001. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop. J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Lavrov, A. Natural Fractures in Rocks. Lost Circ. 2016, 49–80. [Google Scholar] [CrossRef]
- Andrii, R.; Vladimir, K.; Yevhen, M. Influence of Bingham fluid viscosity on energy performances of a vortex chamber pump. Energy 2021, 218, 119432. [Google Scholar] [CrossRef]
- Schopfer, P. Biomechanics of plant growth. Am. J. Bot. 2006, 93, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
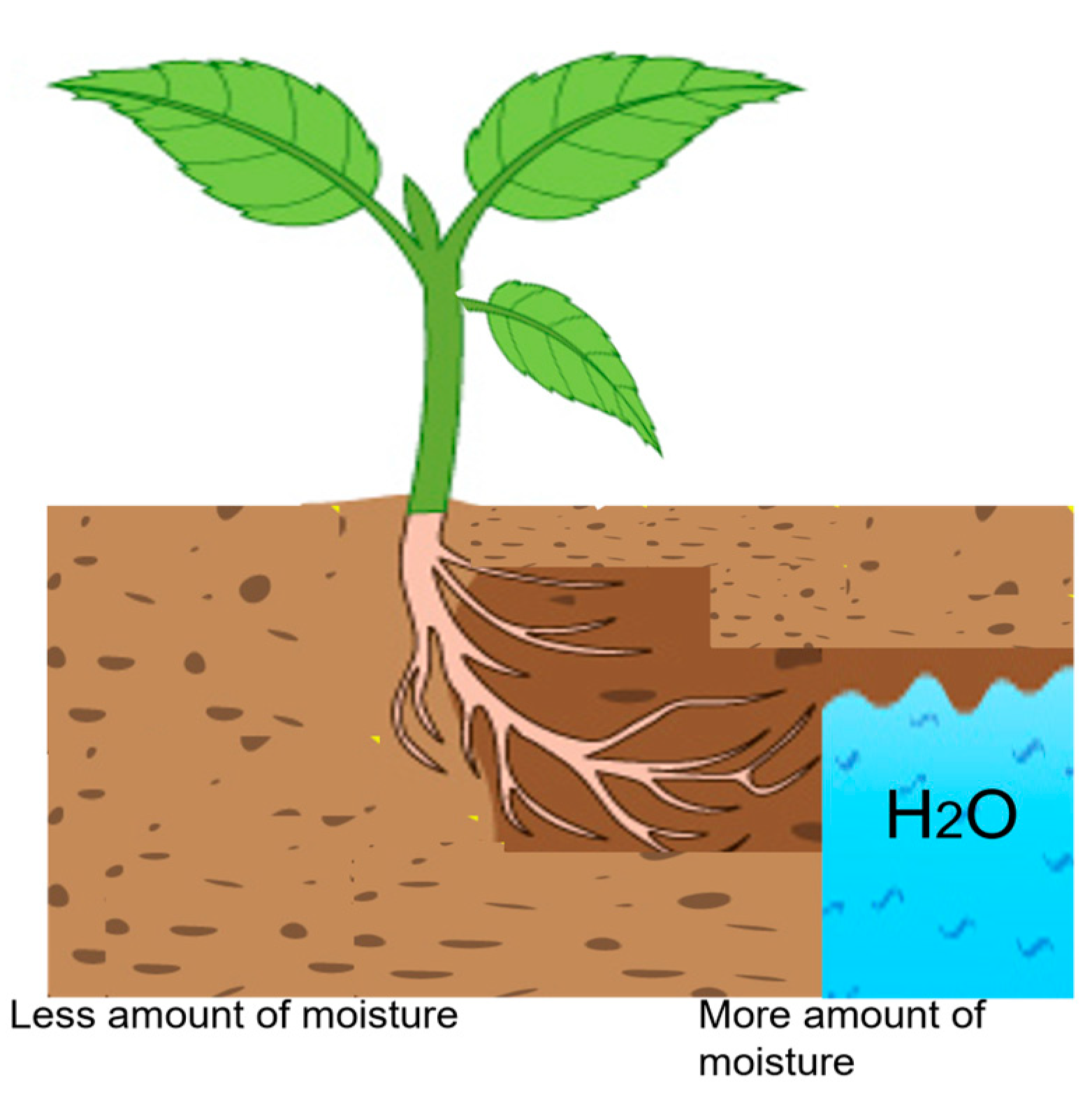

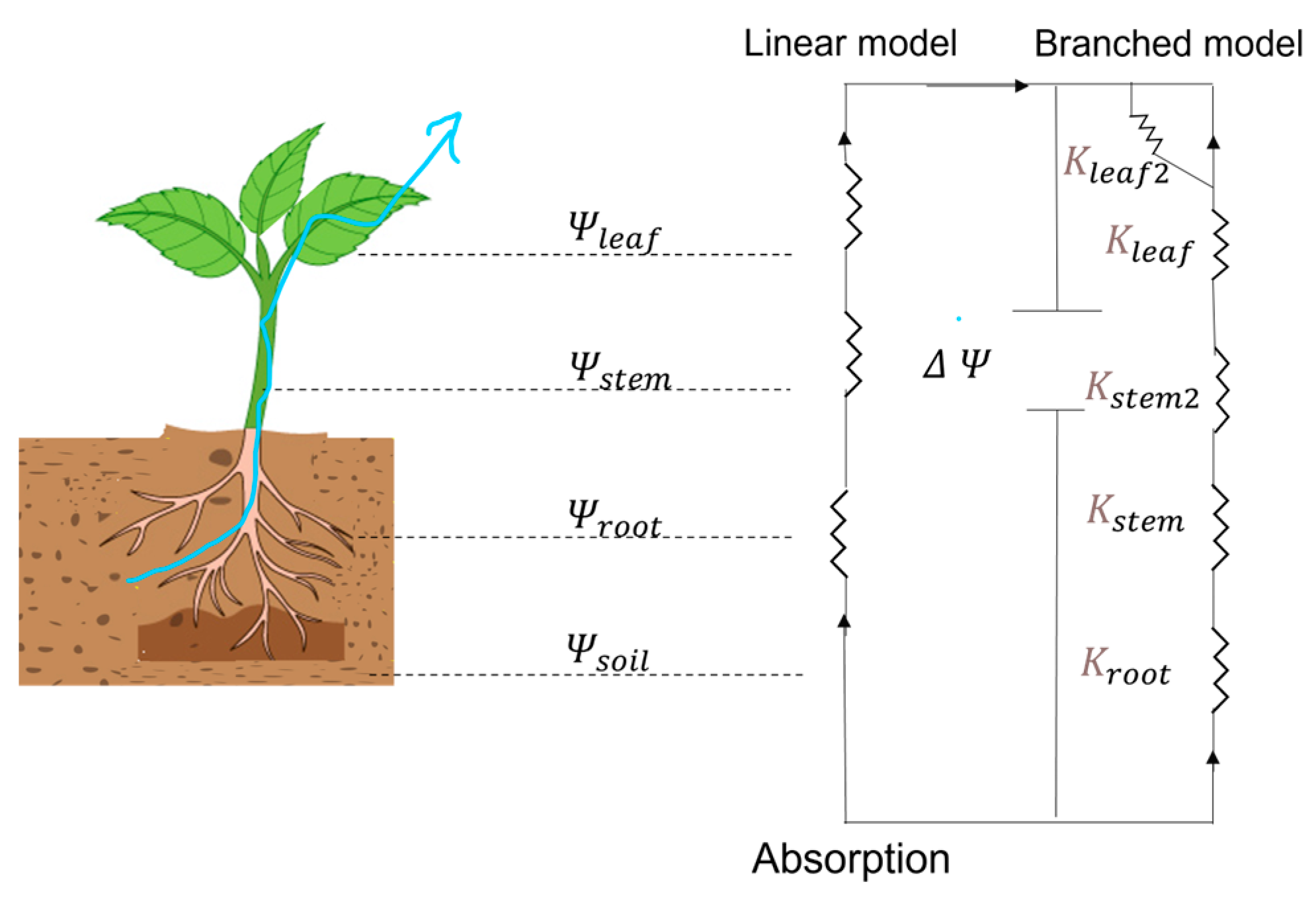
| Paper Reference | Objective | Experiment | Advantage of Research |
|---|---|---|---|
| Year | Outcome | ||
| [34] 2017 | Under low water potential gradient conditions, the ahr1 mutant is tested for root hydrotropism and growth responses. | As a result of the mutant root cells’ ability to proliferate and grow in the presence of progressively negative water potential gradients, the ahr1 phenotype is unique. | Hydrotropism can be understood with the help of this outstanding resource. |
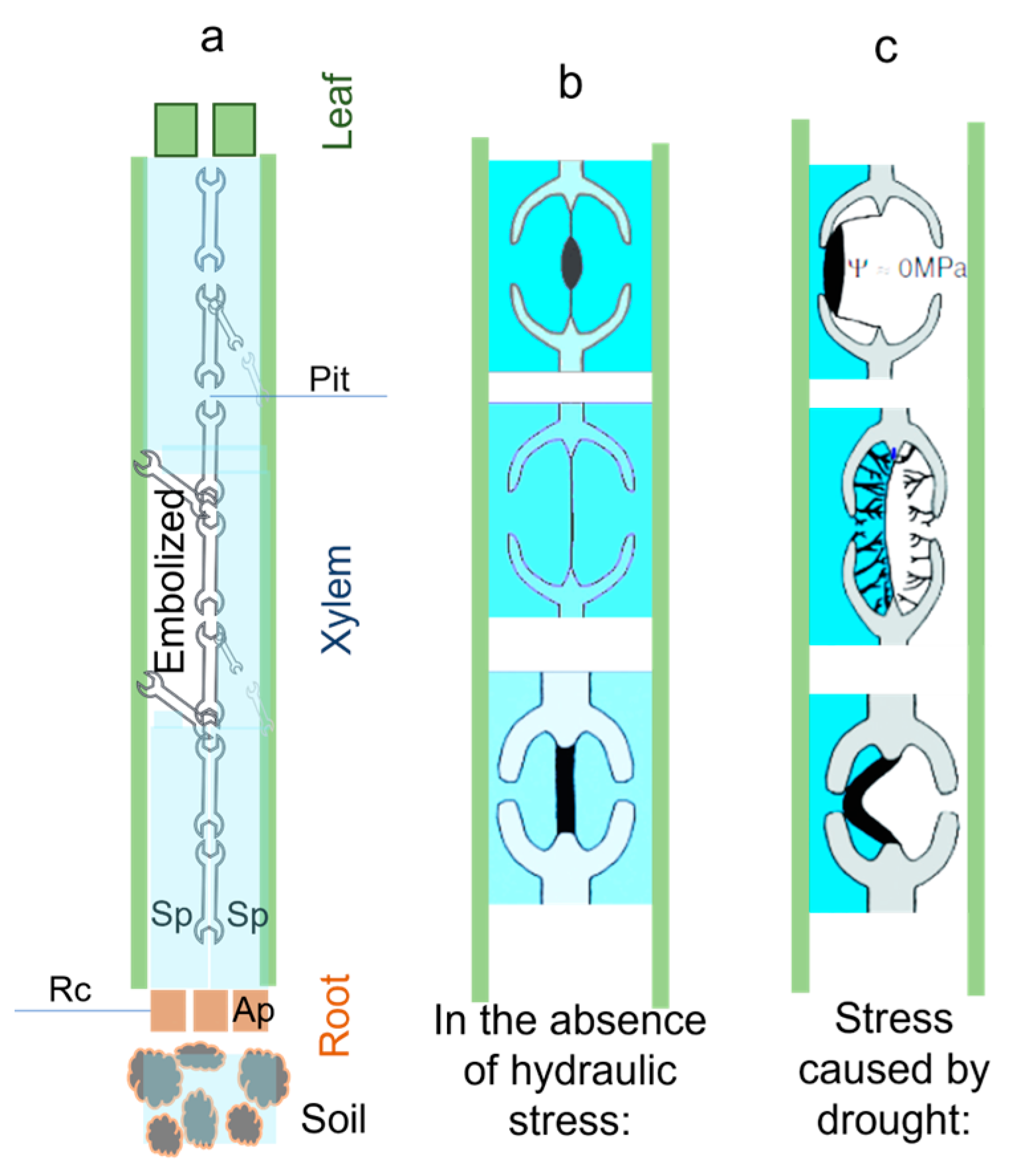
| [35] 1988 | Xylem embolisms caused by water stress are believed to be caused by the influx of air into functional vessels from embolized adjacent vessels (for example, due to physical damage) | Research suggests that rainforest species are more vulnerable to embolism due to differences in inter vessel pit membrane permeability. A species’ habitat influences its pit membrane pore size, making it adaptive. | Xylem embolism can be caused by an air-filled tracheid or vessel. Embolism can be caused by water stress, winter freezing, and dieback. Hydraulic conductivity of the xylem was reduced by 80% by the end of the winter in northern Vermont, even during a wet growing season |
| [36] 2013 | Study examines how plants communicate water availability information to remote organs. Research on long-distance signaling using hydraulic cues and potential sensors | Hydraulic signals are generated by changes in osmotic potential, water tension, or turgor. Water’s cohesion and tension properties spread local changes quickly throughout the plant. In plants, hydraulic signals spread more slowly than in rigid pipes because of cellular resistances. | It is still unclear how sensors relay signals after perception. The solution to this conundrum lies in screening for plant mutants affected in hydraulic signaling. |
| [37] 2019 | In the soil, roots are branched and follow tortuous paths. Root segments can be considered cylinders to which water flows down a gradient of pressure in soil water despite their complex geometry. | The water status of plants is determined by hydrostatic and osmotic pressures. Plants and soil are driven by hydrostatic pressure gradients. Over microscopic distances they are driven by gradients in water potential. | Water potential is also found in surface water, cells, and xylem vessels. At atmospheric pressure, pure water’s water potential is zero, so it is always negative in plants. Adding solutes or imposing suction lowers the water potential in plants. |
| [38] 2001 | Rice cannot respond to higher transpiration demands when growing in a hydrostatic or osmotic environment. It is concluded that this may account for rice’s water shortage in the shoot even in flooded fields. | Two varieties of rice (cv. Azucena and cv. IR64) were grown for 31–40 days at 27 °C daytime and 22 °C nighttime. Transient and steady-state water flow conditions were used to measure root Lpr. | The exodermis and sclerenchyma, as well as the endodermis, have apoplastic barriers. Screening for genotypes with weaker apoplastic barriers or different chemical composition may be worthwhile. |
| [39] 2016 | Researchers compared vulnerability to loss of hydraulic function, leaf and xylem water potentials, and hydraulic safety margins (compared to water potentials causing a 50% loss of hydraulic conductivity) among four angiosperms and four coniferous tree species. | Measuring one type does not accurately reflect an overall hydraulic strategy. | There is strong support for the HVSH, especially in distal organs. Leaves and roots were more vulnerable to hydraulic dysfunction than branches or trunks. |
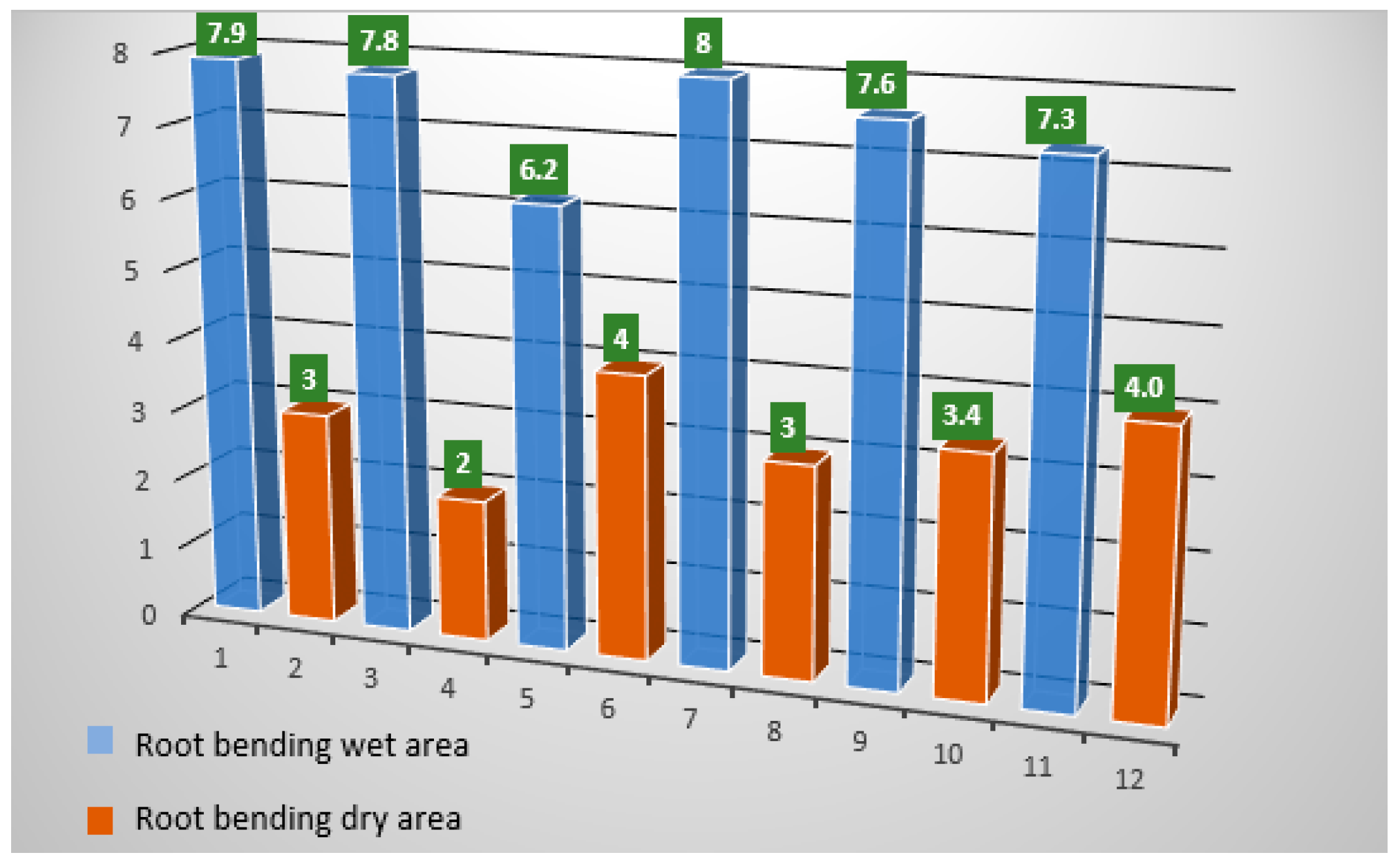
| Time (h) of Hydrosimulation | Gravitational Condition during Hydrosimulation | Moisture Condition | Side of Roots | Root Bending | |
|---|---|---|---|---|---|
| 0 | - | - | Wet | 7.9 | |
| Dry | 3 | ||||
| 4 | 1G | K2CO3 | Wet | 7.8 | |
| Dry | 2 | ||||
| H2O | Wet | 6.2 | |||
| Dry | 4 | ||||
| 3D | K2CO3 | Wet | 8 | ||
| Dry | 3 | ||||
| H2O | Wet | 7.6 | |||
| Dry | 3.4 | ||||
| Wet | 7.3 | ||||
| Dry | 4.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, M.U.; Paul, A.; S, M.; Chehri, A. Hydrotropism: Understanding the Impact of Water on Plant Movement and Adaptation. Water 2023, 15, 567. https://doi.org/10.3390/w15030567
Gul MU, Paul A, S M, Chehri A. Hydrotropism: Understanding the Impact of Water on Plant Movement and Adaptation. Water. 2023; 15(3):567. https://doi.org/10.3390/w15030567
Chicago/Turabian StyleGul, Malik Urfa, Anand Paul, Manimurugan S, and Abdellah Chehri. 2023. "Hydrotropism: Understanding the Impact of Water on Plant Movement and Adaptation" Water 15, no. 3: 567. https://doi.org/10.3390/w15030567
APA StyleGul, M. U., Paul, A., S, M., & Chehri, A. (2023). Hydrotropism: Understanding the Impact of Water on Plant Movement and Adaptation. Water, 15(3), 567. https://doi.org/10.3390/w15030567









