Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Amino Acid-TiO2
2.2. Characterization of the Photocatalysts
2.3. Experimental Setup and Procedure
2.4. Experimental Design Methodology
3. Results and Discussion
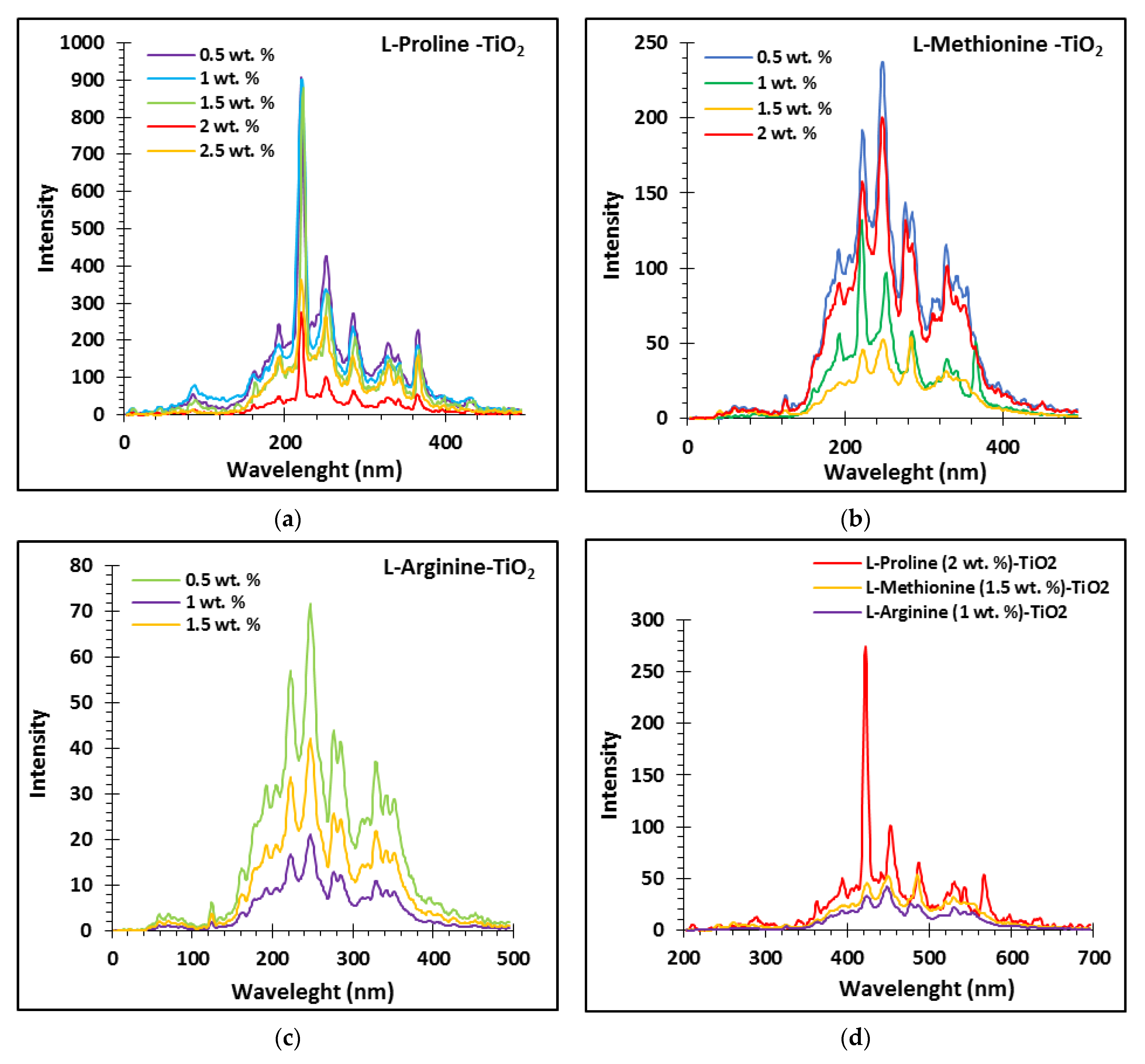
3.1. Optimization of Amino Acids Content in TiO2 Network
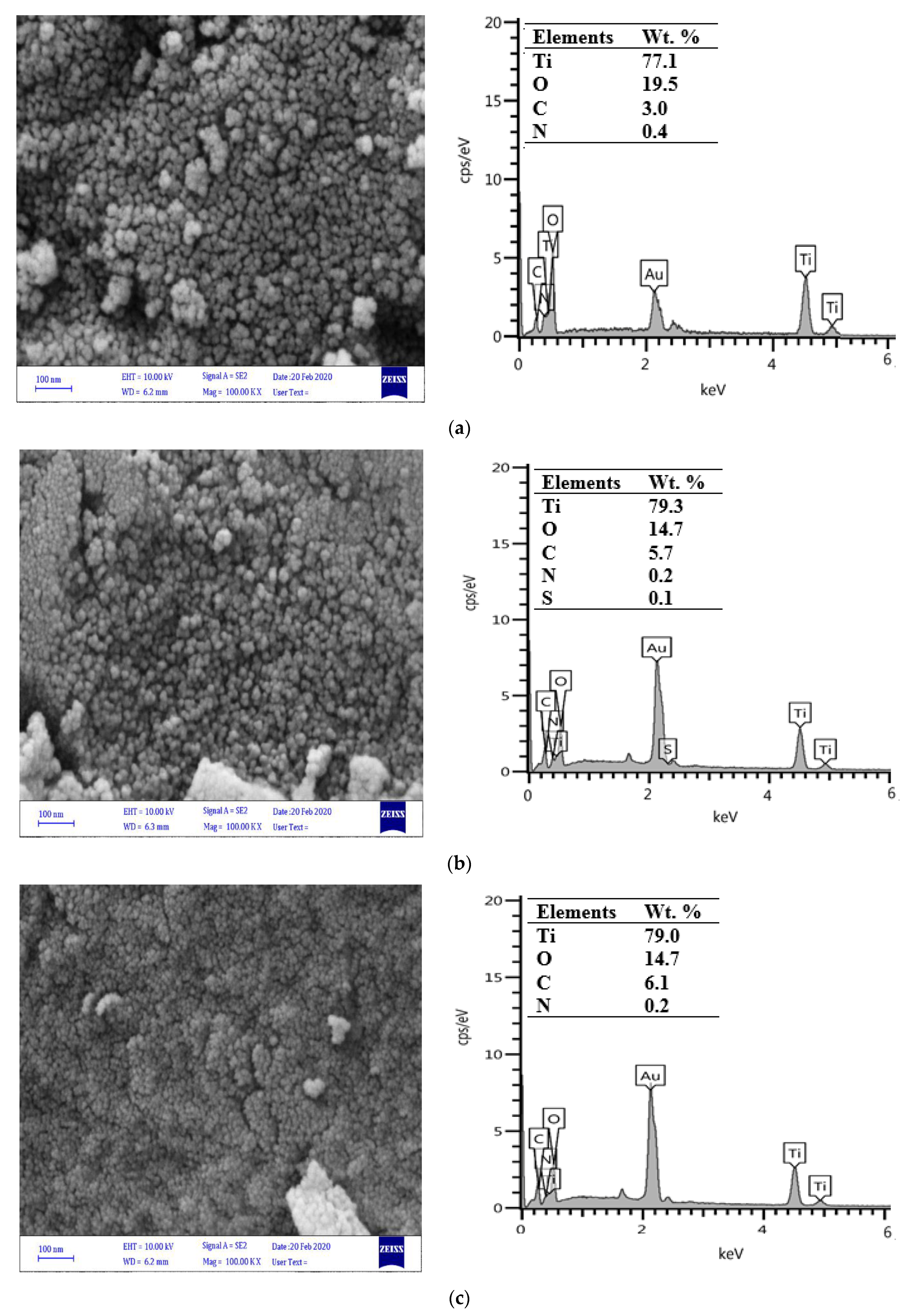
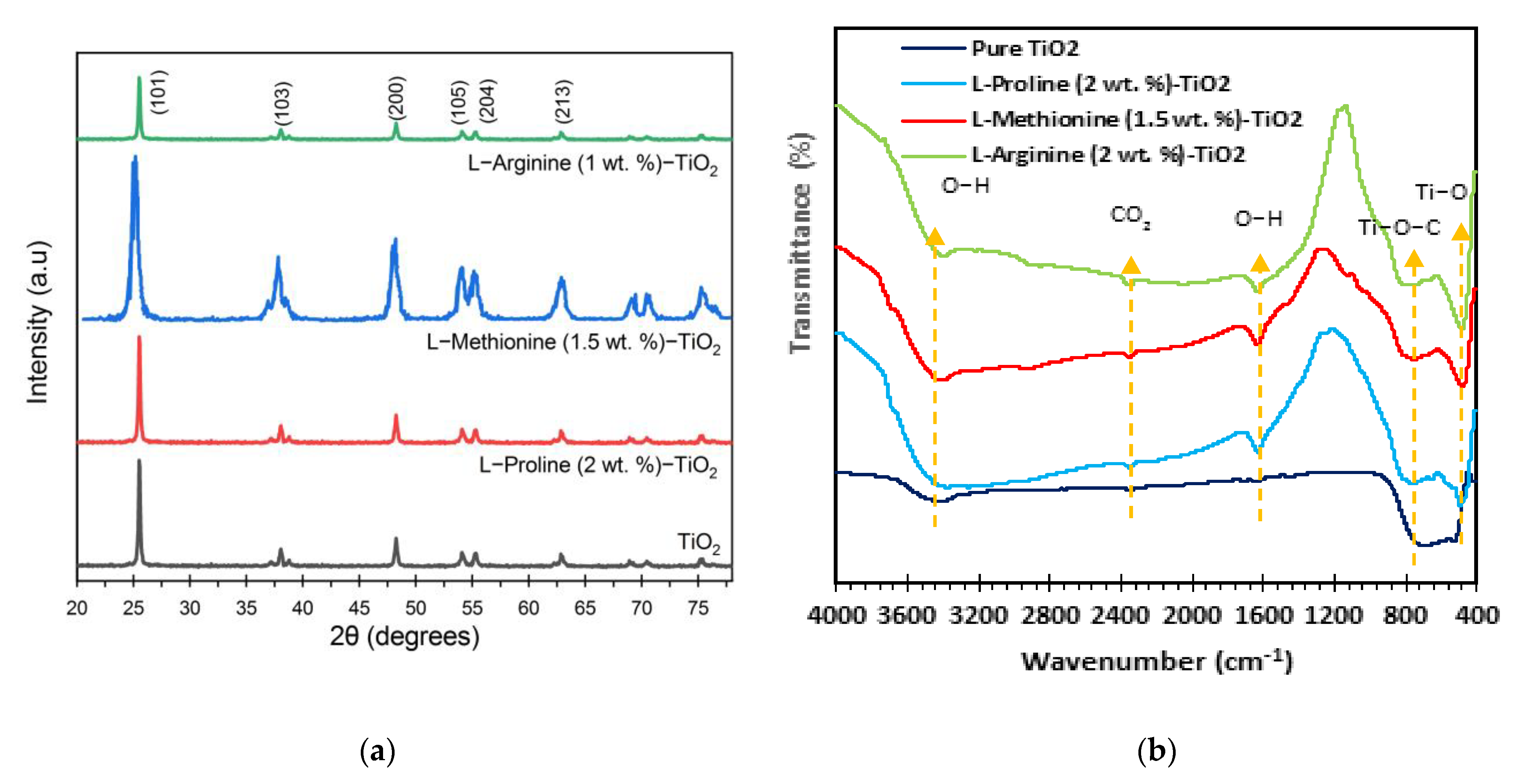
3.2. Characterization of Synthesized Catalysts
3.3. Model of Photodegradation of MNZ and CEX by L-Arginine (1 wt.%.)-TiO2
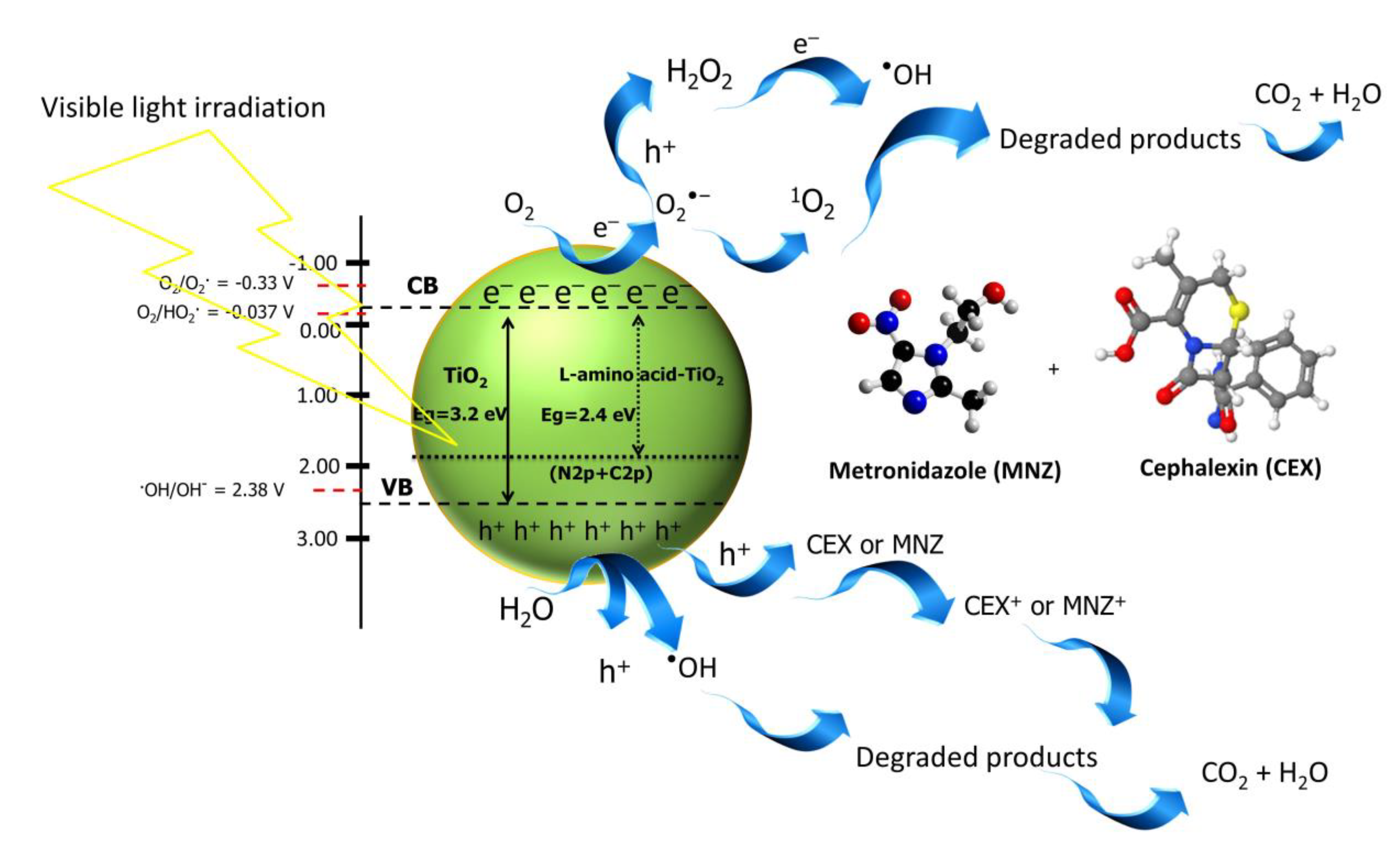
3.4. Possible Photodegradation Mechanism
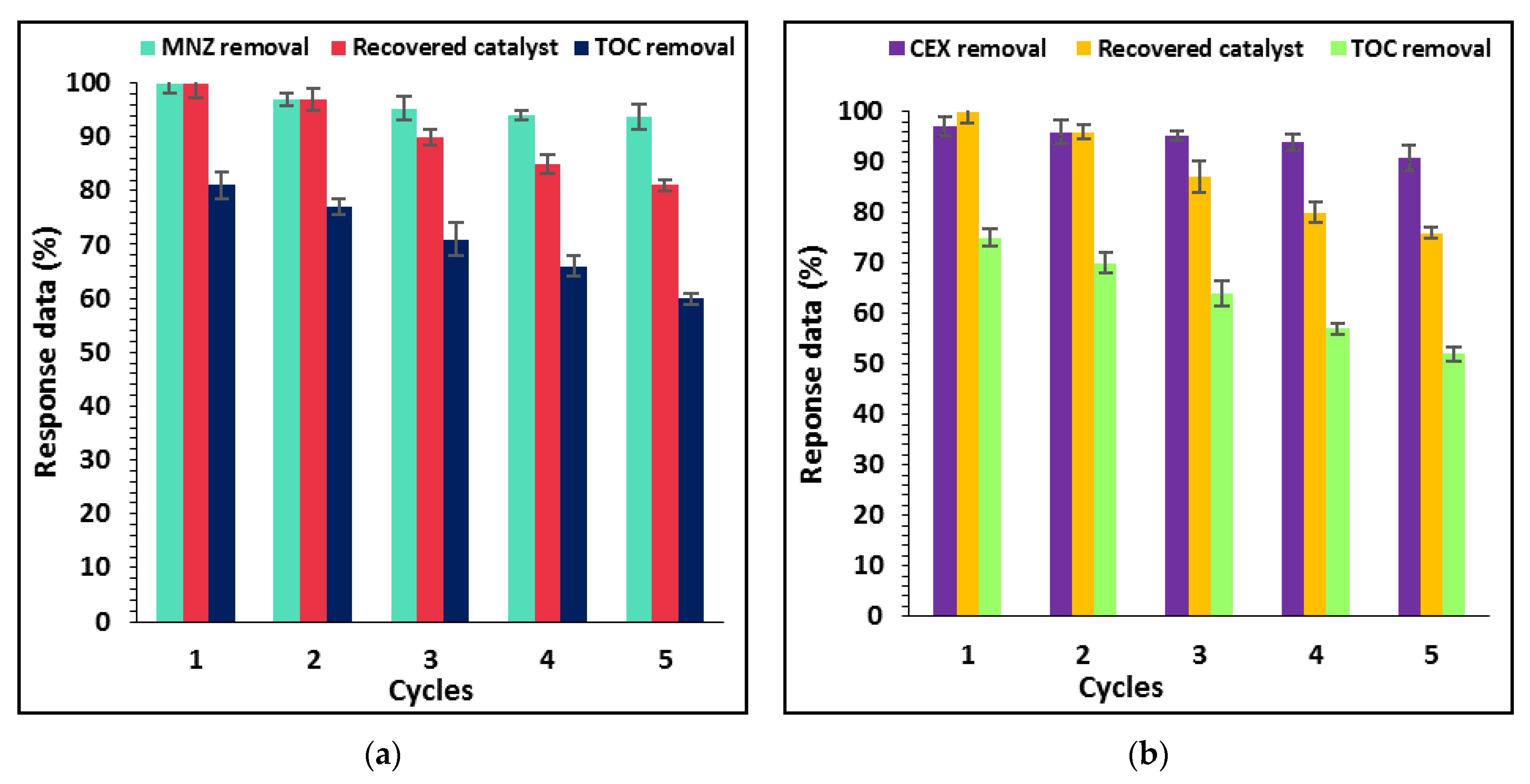
3.5. Effect of Inorganic Anions and Humic Acid on Photocatalysis Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopera, A.E.-C.; Ruiz, S.G.; Alonso, J.M.Q. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process. Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Ma, Y.-L.; Zhang, J.-T.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process. Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Ma, J.; Sun, Y. Study on the Application of Shell-Activated Carbon for the Adsorption of Dyes and Antibiotics. Water 2022, 14, 3752. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Leili, M.; Khorram, N.S.; Godini, K.; Azarian, G.; Moussavi, R.; Peykhoshian, A. Application of central composite design (CCD) for optimization of cephalexin antibiotic removal using electro-oxidation process. J. Mol. Liq. 2020, 313, 113556. [Google Scholar] [CrossRef]
- Aram, M.; Farhadian, M.; Nazar, A.; Tangestaninejad, S.; Eskandari, P.; Jeon, B.-H. Metronidazole and Cephalexin degradation by using of Urea/TiO2/ZnFe2O4/Clinoptiloite catalyst under visible-light irradiation and ozone injection. J. Mol. Liq. 2020, 304, 112764. [Google Scholar] [CrossRef]
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Martínez-Piernas, A.B.; Bertakis, Y.; Xekoukoulotakis, N.P.; Agüera, A.; Pérez, J.A.S. TiO2 photocatalysis under natural solar radiation for the degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions at pilot plant scale. Water Res. 2019, 166, 115037. [Google Scholar] [CrossRef]
- Chung, W.; Chun, S.; Kim, S.; Chang, S. Photocatalytic removal of tetracycline using TiO2/Ge composite optimized by response surface methodology (RSM). J. Ind. Eng. Chem. 2016, 36, 320–325. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Zamani, M.; Aminabhavi, T. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. J. Environ. Manag. 2023, 326, 116691. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Martínez, P.R.; Ortega, J.C.; Martínez, M.R.; Uribe, I.O. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar]
- Alikhani, N.; Farhadian, M.; Goshadrou, A.; Tangestaninejad, S.; Eskandari, P. Photocatalytic degradation and adsorption of herbicide 2, 4-dichlorophenoxyacetic acid from aqueous solution using TiO2/BiOBr/Bi2S3 nanostructure stabilized on the activated carbon under visible light. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100415. [Google Scholar] [CrossRef]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P. Comparison the visible photocatalytic activity and kinetic performance of amino acids (non-metal doped) TiO2 for degradation of colored wastewater effluent. Mater. Sci. Semicond. Process. 2022, 140, 106383. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Li, W.; Ma, Y.; Tong, Z.; Huang, W.; Chen, L.; Wu, G.; Wang, H.; Lyu, P. Synthesis of yolk-shell structure Fe3O4/P (MAA-MBAA)-PPy/Au/void/TiO2 magnetic microspheres as visible light active photocatalyst for degradation of organic pollutants. J. Alloy. Compd. 2019, 810, 151807. [Google Scholar] [CrossRef]
- Yan, X.; Xing, Z.; Cao, Y.; Hu, M.; Li, Z.; Wu, X.; Zhu, Q.; Yang, S.; Zhou, W. In-situ CNS-tridoped single crystal black TiO2 nanosheets with exposed {001} facets as efficient visible-light-driven photocatalysts. Appl. Catal. B Environ. 2017, 219, 572–579. [Google Scholar] [CrossRef]
- Xie, E.; Zheng, L.; Li, X.; Wang, Y.; Dou, J.; Ding, A.; Zhang, D. One-step synthesis of magnetic-TiO2-nanocomposites with high iron oxide-composing ratio for photocatalysis of rhodamine 6G. PLoS ONE 2019, 14, e0221221. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Zinadini, S.; Feyzi, M.; Rafiee, E.; Bahnemann, D. A novel L-Histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater. J. Hazard. Mater. 2019, 369, 384–397. [Google Scholar] [CrossRef]
- Azizi, M.; Ebadi, T.; Qaderi, F. A novel method of co-doping TiO2 with carbon and boron for enhancing photocatalytic degradation of 4-nitrophenol. Int. J. Environ. Sci. Technol. 2022, 19, 2619–2634. [Google Scholar] [CrossRef]
- Rabanimehr, F.; Farhadian, M.; Nazar, A.; Moghadam, M. Fabrication of Z-scheme Bi2WO6/CNT/TiO2 heterostructure with enhanced cephalexin photodegradation: Optimization and reaction mechanism. J. Mol. Liq. 2021, 339, 116728. [Google Scholar] [CrossRef]
- Zangeneh, H.; Farhadian, M.; Zinatizadeh, A.A. A reusable visible driven N and C–N doped TiO2 magnetic nanocomposites for photodegradation of direct red 16 azo dye in water and wastewater. Environ. Technol. 2020, 43, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Sun, X.; Chen, J.; Zhao, S.; Jiang, C.; Yang, L.; Cheng, L.; Cao, S. Simultaneous formation of a C/N-TiO2 hollow photocatalyst with efficient photocatalytic performance and recyclability. Chin. J. Catal. 2019, 40, 765–775. [Google Scholar] [CrossRef]
- Saha, M.S.; Li, R.; Sun, X. High loading and monodispersed Pt nanoparticles on multiwalled carbon nanotubes for high performance proton exchange membrane fuel cells. J. Power Sources 2008, 177, 314–322. [Google Scholar] [CrossRef]
- Wan, N.; Xing, Z.; Kuang, J.; Li, Z.; Yin, J.; Zhu, Q.; Zhou, W. Oxygen vacancy-mediated efficient electron-hole separation for CNS-tridoped single crystal black TiO2 (B) nanorods as visible-light-driven photocatalysts. Appl. Surf. Sci. 2018, 457, 287–294. [Google Scholar] [CrossRef]
- Arasu, M.V.; Arokiyaraj, S.; Viayaraghavan, P.; Kumar, T.S.J.; Duraipandiyan, V.; Al-Dhabi, N.A.; Kaviyarasu, K. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B Biol. 2019, 190, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, K.; Pirsaheb, M.; Gupta, V.K.; Agarwal, S.; Moradi, M.; Vasseghian, Y.; Dragoi, E.-N. Phenol adsorption on scoria stone as adsorbent—Application of response surface method and artificial neural networks. J. Mol. Liq. 2018, 274, 699–714. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Feyzi, M.; Zinadini, S.; Bahnemann, D. Photomineralization of recalcitrant wastewaters by a novel magnetically recyclable boron doped-TiO2-SiO2 cobalt ferrite nanocomposite as a visible-driven heterogeneous photocatalyst. J. Environ. Chem. Eng. 2018, 6, 6370–6381. [Google Scholar] [CrossRef]
- Chen, H.; Chen, K.-F.; Lai, S.-W.; Dang, Z.; Peng, Y.-P. Photoelectrochemical oxidation of azo dye and generation of hydrogen via CN co-doped TiO2 nanotube arrays. Sep. Purif. Technol. 2015, 146, 143–153. [Google Scholar] [CrossRef]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P.; Amarloo, E.; Farghelitiyan, J.; Zamani, M.R. Comparative Study on Enhanced Photocatalytic Activity of Visible Light-Active Nanostructures for Degradation of Oxytetracycline and COD Removal of Licorice Extraction Plant Wastewater. Water 2023, 15, 290. [Google Scholar] [CrossRef]
- Malini, B.; Raj, G.A.G. C,N and S-doped TiO2-characterization and photocatalytic performance for rose bengal dye degradation under day light. J. Environ. Chem. Eng. 2018, 6, 5763–5770. [Google Scholar] [CrossRef]
- Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Zangeneh, H.; Farhadian, M.; Zinatizadeh, A. N (Urea) and CN (L-Asparagine) doped TiO2-CuO nanocomposites: Fabrication, characterization and photodegradation of direct red 16. J. Environ. Chem. Eng. 2020, 8, 103639. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Zinadini, S.; Feyzi, M.; Bahnemann, D. Preparation ultrafine L-Methionine (C, N, S triple doped)-TiO2-ZnO nanoparticles and their photocatalytic performance for fouling alleviation in PES nanocomposite membrane. Compos. Part B Eng. 2019, 176, 107158. [Google Scholar] [CrossRef]
- Mousavi, S.; Mehralian, M.; Khashij, M.; Parvaneh, S. Methylene Blue removal from aqueous solutions by activated carbon prepared from N. microphyllum (AC-NM): RSM analysis, isotherms and kinetic studies. Glob. Nest J. 2017, 19, 697–705. [Google Scholar]
- Zangeneh, H.; Zinatizadeh, A.; Zinadini, S.; Nazari, S.; Sibali, L.; McKay, T. Visible light-driven photoactive L-Methionine (CNS tripledoped)-TiO2/ZnO nanocomposite aiming for highly efficient photodegradation of xenobiotic compounds in wastewater. Mater. Res. Bull. 2022, 150, 111783. [Google Scholar] [CrossRef]
- Okhovat, N.; Hashemi, M.; Golpayegani, A. Photocatalytic decomposition of Metronidazolein aqueous solutions using titanium dioxide nanoparticles. J. Mater. Environ. Sci. 2015, 6, 792–799. [Google Scholar]
- Torki, F.; Faghihian, H. Sunlight-assisted decomposition of cephalexin by novel synthesized NiS-PPY-Fe3O4 nanophotocatalyst. J. Photochem. Photobiol. A: Chem. 2017, 338, 49–59. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Nazar, A.S.; Jeon, B.-H. Adsorption and photodegradation efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/zeolite nanophotocatalysts for the removal of cyanide. Ind. Eng. Chem. Res. 2019, 58, 2099–2112. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]





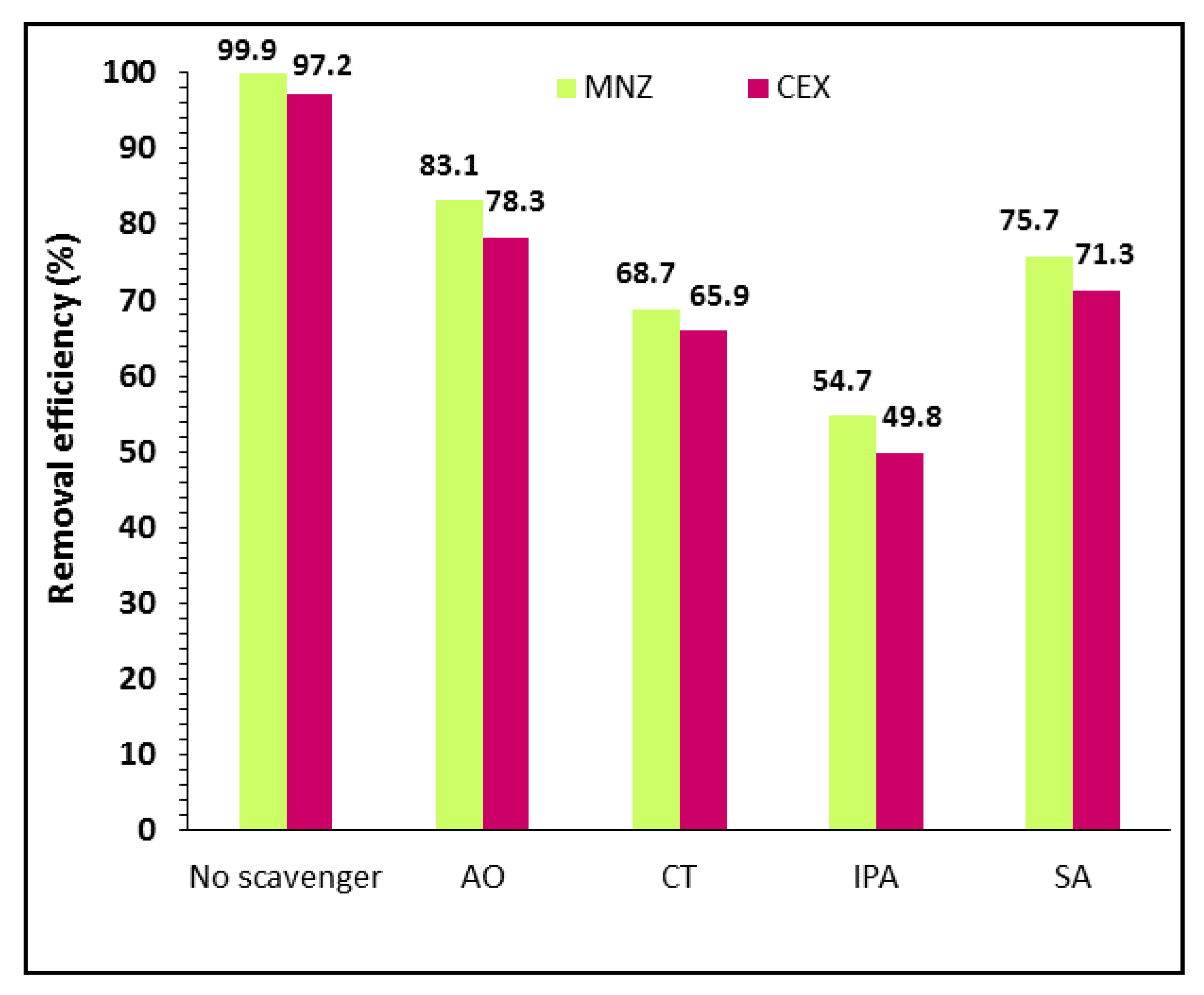

| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: [MNZ] or [CEX] (mg/L) | 50 | 75 | 100 |
| B: [Catalyst] (g/L) | 0.5 | 1 | 1.5 |
| C: pH | 4 | 7 | 10 |
| D: Irradiation time (h) | 1 | 1.5 | 2 |
| E: Type of antibiotic | MNZ | - | CEX |
| L-Amino Acid-TiO2 Photocatalysts | Weight Fraction of Amino Acid (wt.%) | Pseudo First Order Model | Pseudo Second Order Model | ||
|---|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (min−1) | R2 | ||
| MNZ | |||||
| L-Proline-TiO2 | 0.5 | 0.0096 | 0.996 | 0.0050 | 0.924 |
| 1 | 0.0141 | 0.997 | 0.0068 | 0.904 | |
| 1.5 | 0.0239 | 0.995 | 0.0155 | 0.774 | |
| 2 | 0.0321 | 0.983 | 0.0372 | 0.64 | |
| 2.5 | 0.0291 | 0.986 | 0.0251 | 0.719 | |
| L-Methionine-TiO2 | 0.5 | 0.0126 | 0.974 | 0.0056 | 0.842 |
| 1 | 0.0267 | 0.982 | 0.0193 | 0.695 | |
| 1.5 | 0.0387 | 0.991 | 0.0773 | 0.593 | |
| 2 | 0.0289 | 0.974 | 0.0264 | 0.69 | |
| L-Arginine-TiO2 | 0.5 | 0.0291 | 0.986 | 0.0254 | 0.671 |
| 1 | 0.0578 | 0.997 | 0.5823 | 0.563 | |
| 1.5 | 0.0288 | 0.971 | 0.0256 | 0.671 | |
| CEX | |||||
| L-Proline-TiO2 | 0.5 | 0.0068 | 0.986 | 0.0047 | 0.968 |
| 1 | 0.0109 | 0.984 | 0.0048 | 0.885 | |
| 1.5 | 0.0129 | 0.993 | 0.0065 | 0.917 | |
| 2 | 0.0188 | 0.991 | 0.01 | 0.862 | |
| 2.5 | 0.0158 | 0.995 | 0.0077 | 0.813 | |
| L-Methionine-TiO2 | 0.5 | 0.0131 | 0.953 | 0.0051 | 0.809 |
| 1 | 0.0169 | 0.985 | 0.0052 | 0.801 | |
| 1.5 | 0.0235 | 0.995 | 0.0141 | 0.767 | |
| 2 | 0.0182 | 0.986 | 0.0094 | 0.789 | |
| L-Arginine-TiO2 | 0.5 | 0.0144 | 0.969 | 0.0074 | 0.848 |
| 1 | 0.0161 | 0.972 | 0.0260 | 0.691 | |
| 1.5 | 0.0288 | 0.991 | 0.0091 | 0.866 | |
| Source | Mean Square | df | f-Value | p-Value |
|---|---|---|---|---|
| Model | 1512.32 | 12 | 69.69 | <0.0001 |
| A: [MNZ] or [CEX] | 7361.64 | 1 | 339.26 | <0.0001 |
| B2: [Catalyst] | 1408.75 | 1 | 64.92 | <0.0001 |
| C: pH | 2384.69 | 1 | 109.90 | <0.0001 |
| D: Irradiation time | 835.21 | 1 | 38.49 | <0.0001 |
| E: Type of antibiotic | 803.74 | 1 | 37.04 | <0.0001 |
| AC | 90.79 | 1 | 4.18 | 0.0464 |
| BC | 158.87 | 1 | 7.32 | 0.0095 |
| CE | 337.33 | 1 | 15.55 | 0.0003 |
| A2 | 244.52 | 1 | 11.27 | 0.0016 |
| B2 | 743.78 | 1 | 34.28 | <0.0001 |
| C2 | 451.14 | 1 | 20.79 | <0.0001 |
| D2 | 88.42 | 1 | 4.07 | 0.0493 |
| Residual | 21.70 | 47 | - | - |
| R2 = 0.95 | R2adj = 0.93 | Lack of fit = 0.25 | C.V. = 7.14 | Adeq Precision = 34.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangeneh, H.; Mousavi, S.A.; Eskandari, P.; Amarloo, E.; Farghelitiyan, J.; Mohammadi, S. Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics. Water 2023, 15, 535. https://doi.org/10.3390/w15030535
Zangeneh H, Mousavi SA, Eskandari P, Amarloo E, Farghelitiyan J, Mohammadi S. Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics. Water. 2023; 15(3):535. https://doi.org/10.3390/w15030535
Chicago/Turabian StyleZangeneh, Hadis, Seyyed Alireza Mousavi, Parisa Eskandari, Ehsan Amarloo, Javad Farghelitiyan, and Sahar Mohammadi. 2023. "Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics" Water 15, no. 3: 535. https://doi.org/10.3390/w15030535





