Results of Adding Sludge Micropowder for Microbial Structure and Partial Nitrification and Denitrification in a Filamentous AGS-SBR Using High-Ammonia Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Sludge Micropowder
2.2. SBR Operation
2.3. Wastewater and Seeding Sludge
2.4. Analytical Methods
2.5. Microbial Sampling and Analysis
3. Results and Discussion
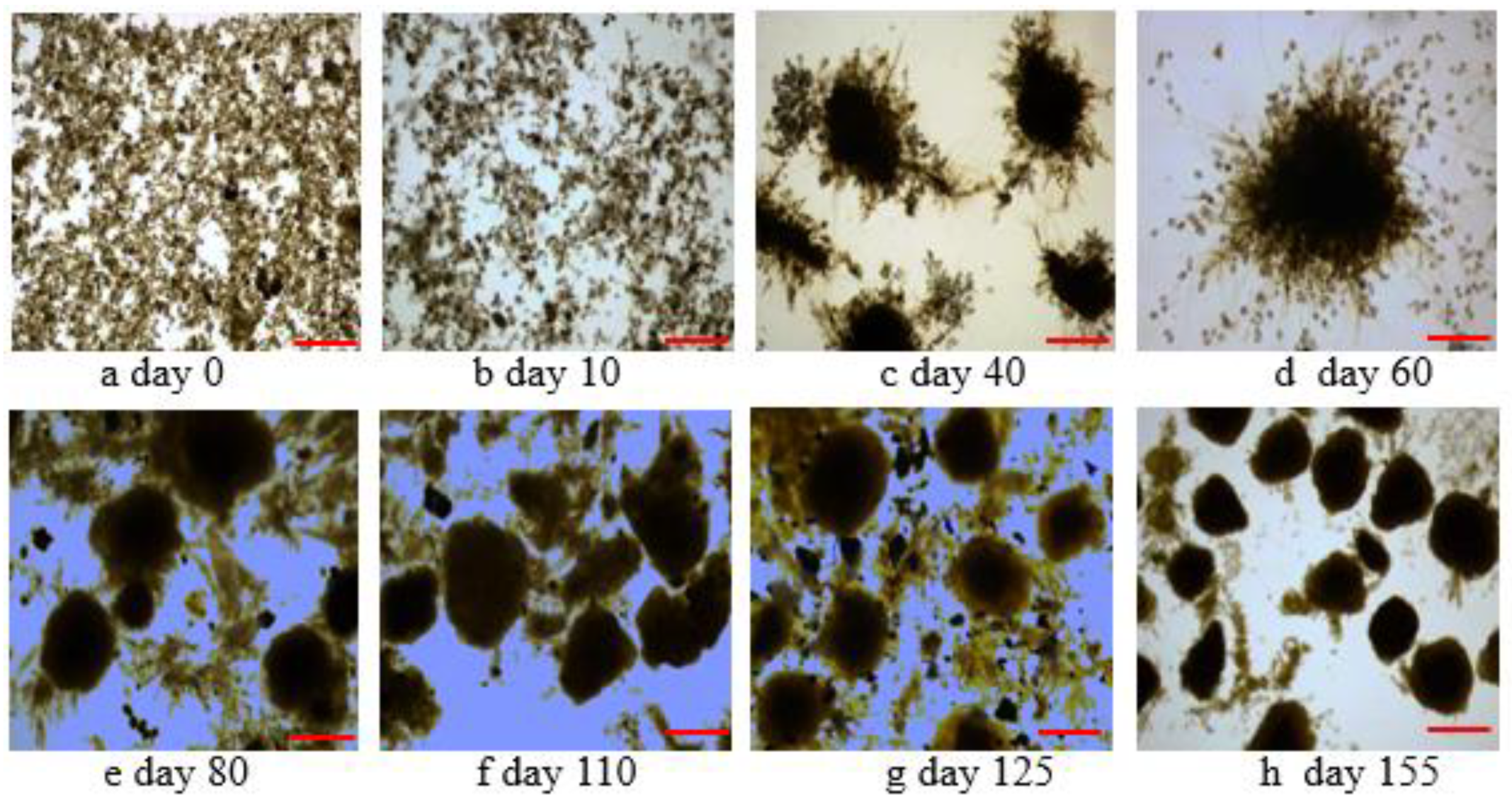
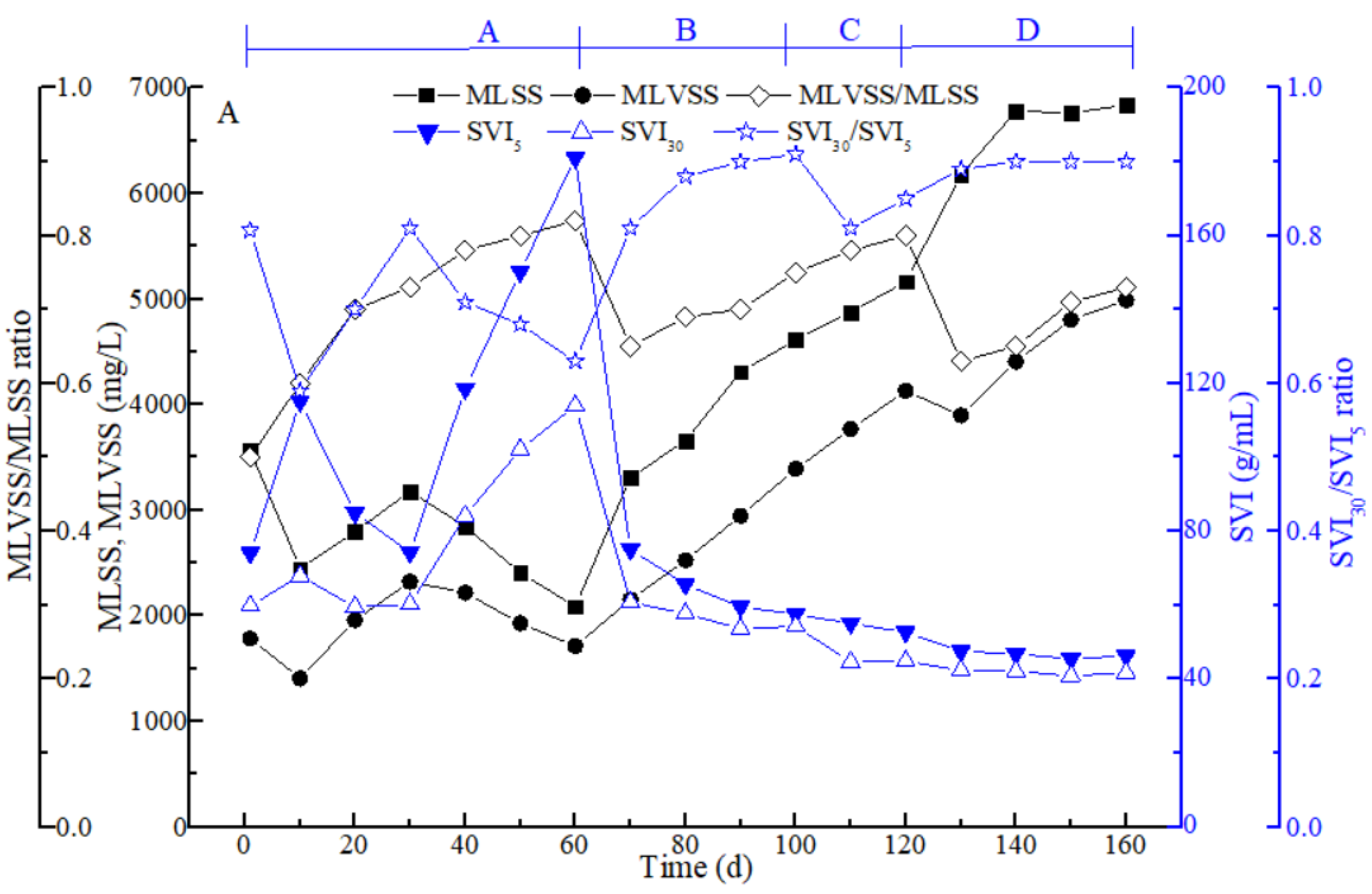
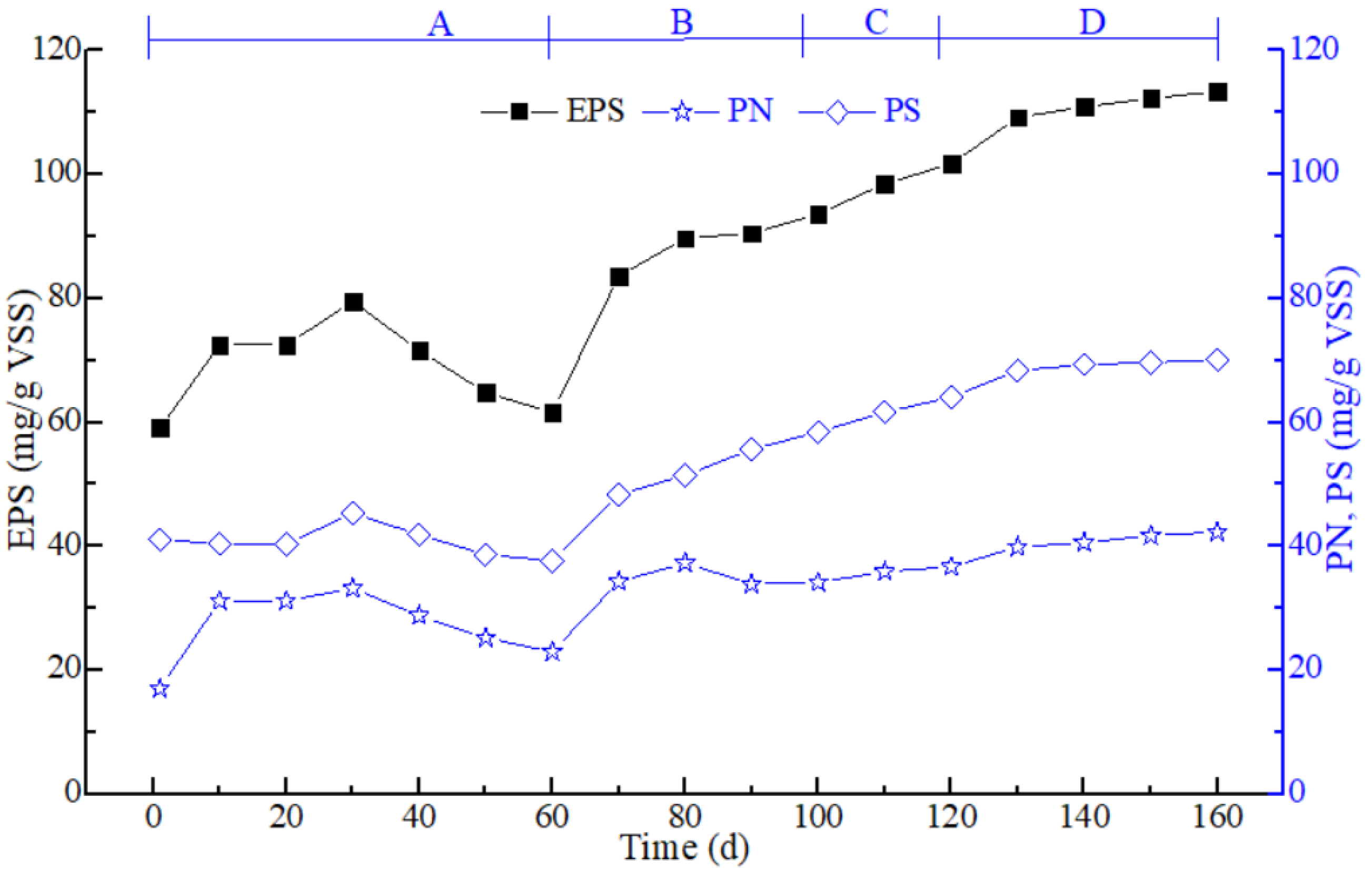
3.1. Sludge Characteristics in the Different Stages
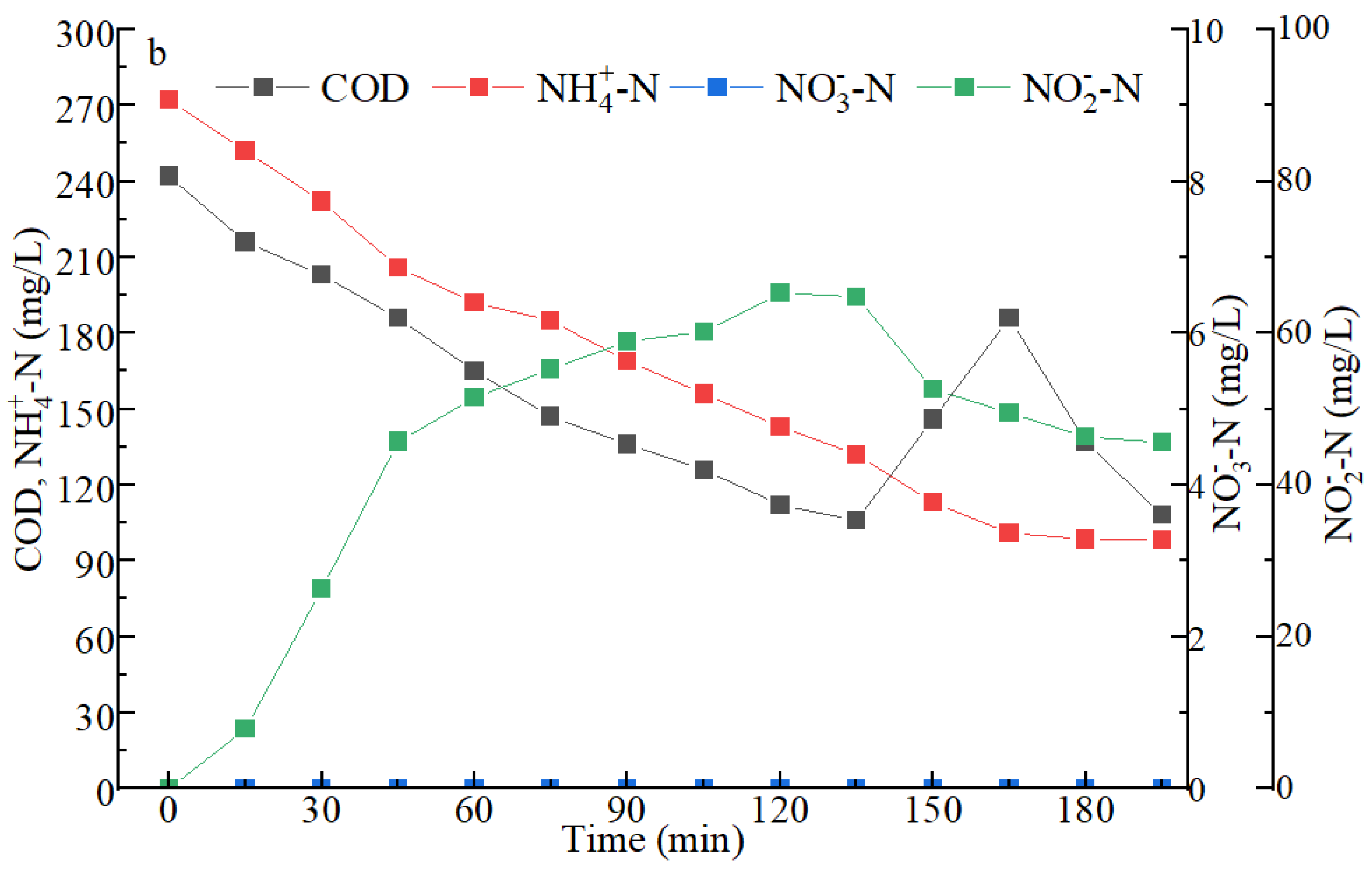
3.2. Removal Performance
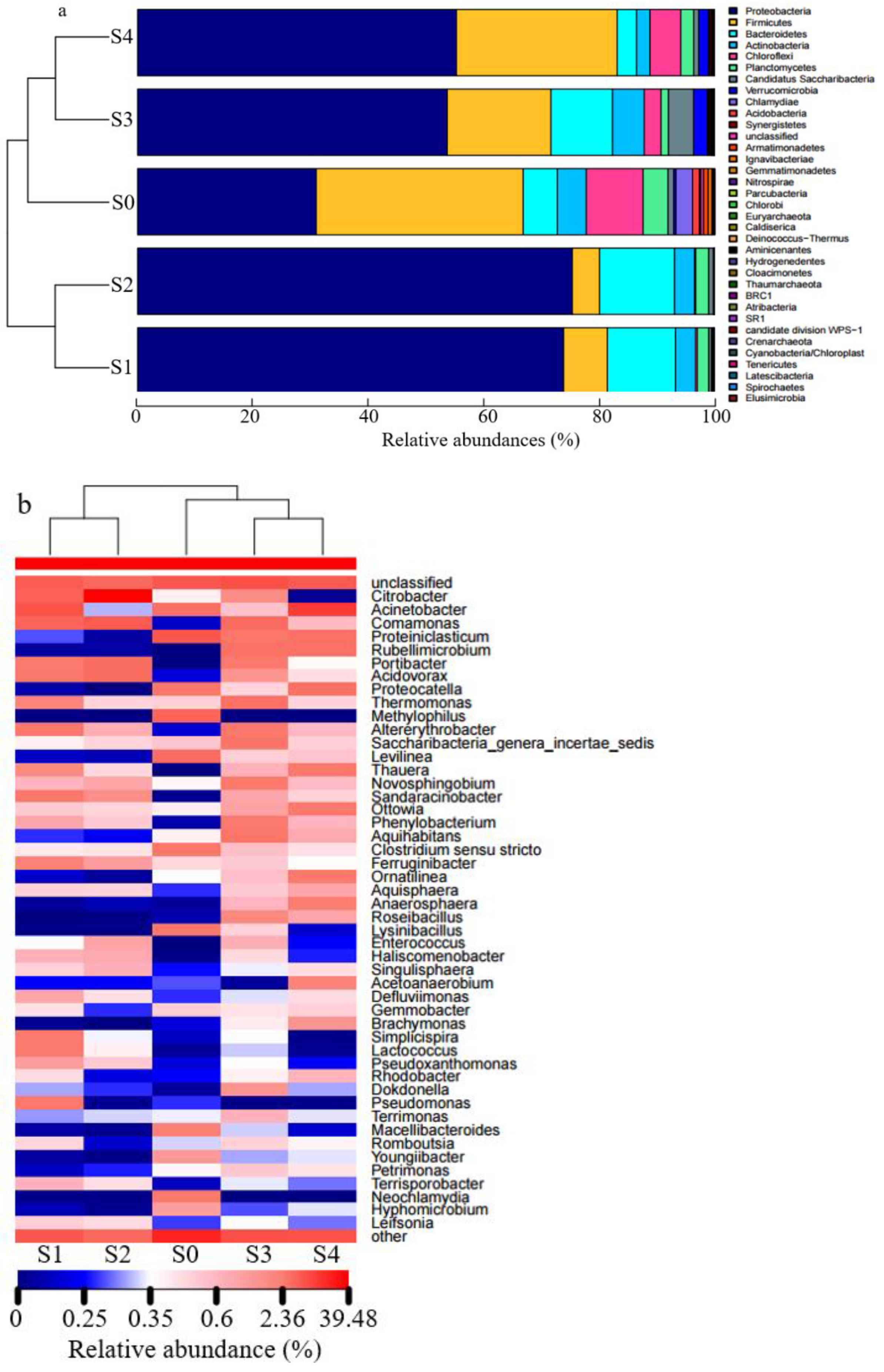
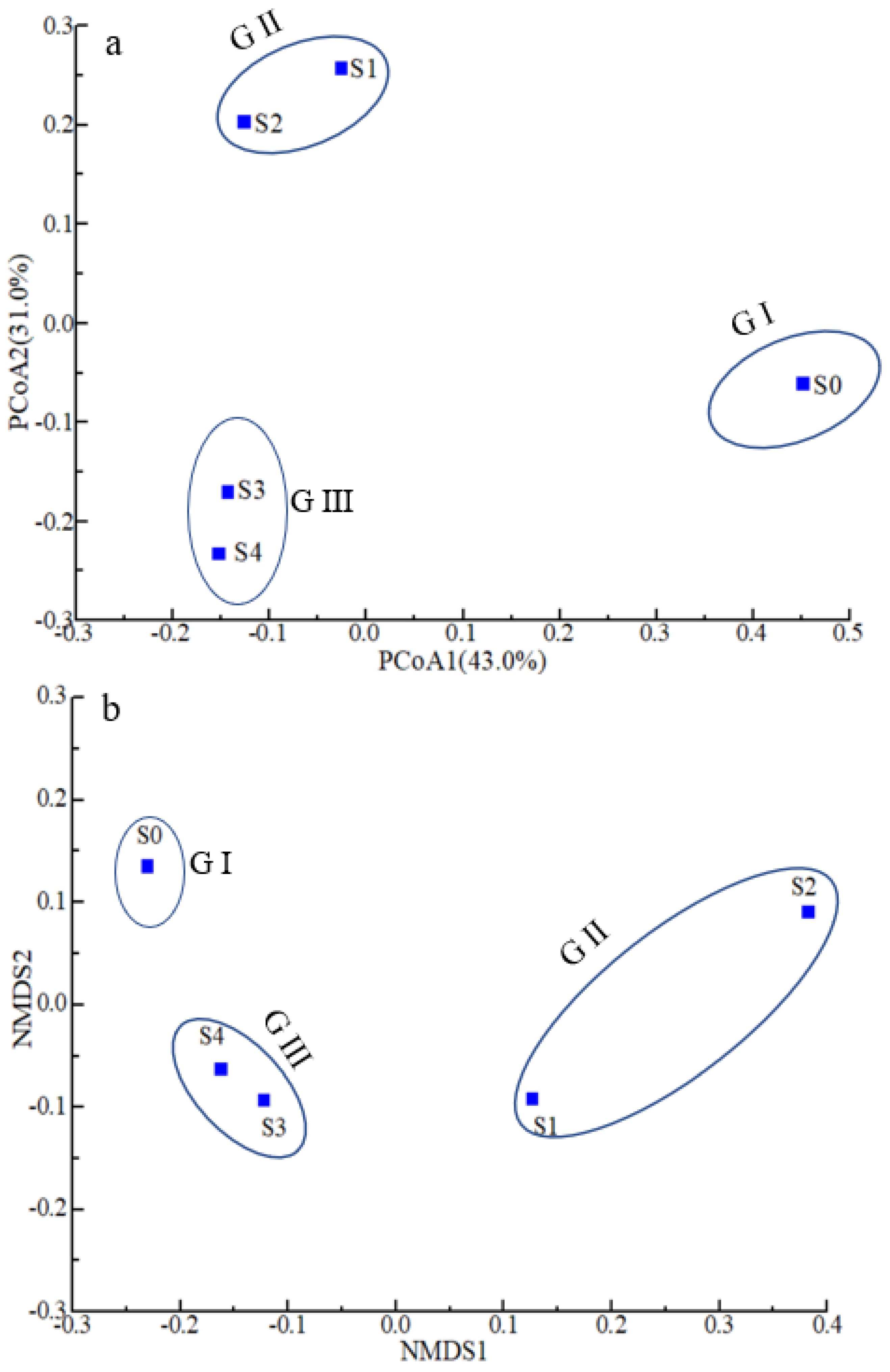
3.3. Microbial Diversity and Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgenroth, E.; Sherden, T.; Van Loosdrecht, M.C.M.; Heijnen, J.J.; Wilderer, P.A. Aerobic granule sludge in a sequencing batch reactor. Water Res. 1997, 12, 3191–3194. [Google Scholar] [CrossRef]
- Li, J.; Ding, L.B.; Cai, A.; Huang, G.X.; Horn, H. Aerobic sludge granulation in a full-scale sequencing batch reactor. BioMed Res. Int. 2014, 2014, 268789. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; De Kreuk, M.K.; De Bruin, B.; Kamminga, P.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; Meunier, C.; Henriet, O.; Mahillon, J.; Suarez-Ojeda, M.E.; Moro, G.D.; Sanctis, M.D.; Iaconi, C.D.; Weissbrodt, D.G. An integrative review of granular sludge for the biological removal of nutrients and recalcitrant organic matter from wastewater. Chem. Eng. J. 2018, 336, 489–502. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.J.; Show, K.Y.; Tay, J.H. Aerobic granular sludge: Recent advances. Biotechnol. Adv. 2008, 26, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Purba, L.D.A.; Khudzari, J.M.; Iwamoto, K.; Mohamad, S.E.; Yuzir, A.; Abdullah, N.; Shimizu, K.; Hermana, J. Discovering future research trends of aerobic granular sludge using bibliometric approach. J. Environ. Manag. 2022, 303, 114150. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.; Morgan, G.; Iorhemen, O.T. A review of the state of development of aerobic granular sludge technology over the last 20 years: Full-scale applications and resource recovery. Case Studies Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.S. Causes and control of filamentous bacteria growth in aerobic granular sludge sequencing batch reactors. Biotechnol. Adv. 2006, 24, 115–117. [Google Scholar] [CrossRef]
- He, Q.L.; Zhang, J.; Gao, S.X.; Chen, L.; Lyu, W.L.; Zhang, W.; Song, J.Y.; Hu, X.L.; Chen, R.F.; Wang, H.Y.; et al. A comprehensive comparison between non-bulking and bulking aerobic granular sludge in microbial communities. Bioresour. Technol. 2019, 294, 122151. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Xie, K.; Sellamuthu, B. Role of adding dried sludge micropowder in aerobic granular sludge reactor with extended filamentous bacteria. Bioresour. Technol. R. 2019, 5, 51–58. [Google Scholar] [CrossRef]
- Tay, J.H.; Liu, Q.S.; Liu, Y. Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor. J. Appl. Microbiol. 2001, 91, 168–175. [Google Scholar] [CrossRef]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking and Forming; CRC Press: Boca Raton, FL, USA, 1993; Lodos Ativados. [Google Scholar]
- Martins, A.M.P.; Pagilla, K.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Filamentous bulking sludge a critical review. Water Res. 2004, 34, 793–817. [Google Scholar] [CrossRef] [PubMed]
- McSwain, B.; Irvine, R.; Wilderer, P.A. The effect of intermittent feeding on aerobic granule structure. Water Sci. Technol. 2004, 49, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbeck, N.; Borges, J.M.; Wilderer, P.A. Treatment of dairy effluents in an aerobic granular sludge sequencing batch reactor. Appl. Microbiol. Biotechnol. 2005, 66, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Han, X.S.; Jin, Y.; Yu, J.B. Rapid formation of aerobic granular sludge by bioaugmentation technology: A review. Chem. Eng. J. 2022, 437, 134971. [Google Scholar] [CrossRef]
- Kotay, S.M.; Datta, T.; Choi, J.; Geol, R. Biocontrol of biomass bulking caused by Haliscomenobacter hydrossis using a newly isolated lytic bacteriophage. Water Res. 2011, 45, 694–704. [Google Scholar] [CrossRef]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking and Other Solids Separation Problems; IWA Publishing: London, UK, 2004. [Google Scholar]
- Guo, J.H.; Peng, Y.Z.; Wang, Z.W.; Yuan, Z.G.; Yang, X.; Wang, S.Y. Control filamentous bulking by chlorine-resistant Type 021N bacteria through adding a biocide CATB. Water Res. 2012, 46, 6531–6542. [Google Scholar] [CrossRef]
- Kedves, A.; Santa, L.; Balazs, M.; Kesseru, P.; Kiss, I.; Ronavari, A.; Konya, Z. Chronic responses of aerobic granules to the presence of graphene oxide in sequencing batch reactors. J. Hazard. Mater. 2020, 389, 121905. [Google Scholar] [CrossRef]
- Kappeler, J.; Gujer, W. Verification and applications of mathematical model for aerobic bulking. Water Res. 1994, 28, 303–310. [Google Scholar] [CrossRef]
- Weissbrodt, D.G.; Lochmatter, S.; Ebrahimi, S.; Rossi, P.; Maillard, J.; Holliger, C. Bacteria selection during the formation of early-stage aerobic granules in wastewater treatment systems operated under wash-out dynamics. Front. Microbiol. 2012, 3, 1–22. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhang, L.; Peng, D.C.; Hou, Y.P.; Pei, L.Y.; Yu, L.F. Extended filaments of bulking sludge sink in the floc layer with particulate substrate. Chemosphere 2013, 93, 2715–2731. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Val, D.R.A.; Campos, J.L.; Mendez, R.; Mosquera-Corral, A. Filamentous bacteria existence in aerobic granular sludge reactors. Bioproc. Biosyst. Eng. 2015, 38, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.P.; Karahan, O.; Van Loosdrecht, M.C.M. Effect of polymeric substrate on sludge settleability. Water Res. 2011, 45, 263–273. [Google Scholar] [CrossRef]
- Puigagut, J.; Salvado, H.; Tarrats, X.; Garcia, J. Effects of particulate and soluble substrates on microfauna populations and treatment efficiency in activated sludge systems. Water Res. 2007, 41, 3168–3176. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Wang, D.J.; Chen, T.; Ma, T.; Wang, Z.; Zhuo, W.L. Accelerating aerobic sludge granulation by adding dry sewage sludge micropowder in sequencing batch reactors. Int. J. Environ. Res. Public Heath 2015, 12, 10056–10065. [Google Scholar] [CrossRef]
- Zou, J.T.; Tao, Y.Q.; Li, J.; Wu, S.Y.; Ni, Y.J. Cultivating aerobic granular sludge in a developed continuous-flow reactor with two-zone sedimentation tank treating real and low-strength wastewater. Bioresour. Technol. 2018, 247, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation; IWA Publishing: London, UK, 2000. [Google Scholar]
- Zulkifli, M.; Hasan, H.A.; Abdullah, S.R.R.; Muhamad, M.H. A review of ammonia removal using a biofilm-based reactor and its challenges. J. Environ. Manag. 2022, 315, 115162. [Google Scholar] [CrossRef]
- De Sousa Rollemberg, S.L.; Barros, A.R.M.; Firmino, P.I.M.; Dos Santos, A.B. Aerobic granular sludge: Cultivation parameters and removal mechanisms. Bioresour. Technol. 2018, 270, 678–688. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Work Association/Water Environmental Federation: Washington, DC, USA, 2012. [Google Scholar]
- Liu, H.; Fang, H.H.P. Extraction of extracellular polymeric substance (EPS) of sludge. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frolund, B.; Griebe, T.; Nielsen, P.H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Sarah, P.C. The combination of external conditioning and Ca2+ addition prior to the reintroduction of effluent sludge into SBR sharply accelerates the formation of aerobic granules. J. Water Process Eng. 2020, 36, 101269. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Sarah, P.C.; Balasubramanian, S. Roles of bacterial and epistylis populations in aerobic granular SBRs treating domestic and synthetic wastewaters. Chem. Eng. J. 2018, 351, 952–958. [Google Scholar] [CrossRef]
- Zhang, L.L.; Feng, X.X.; Zhu, N.W.; Chen, J.M. Role of extracellular protein in the formation and stability of aerobic granules. Enzym. Microb. Technol. 2007, 41, 551–557. [Google Scholar] [CrossRef]
- Pan, S.; Tay, J.H.; He, Y.X.; Tay, S.T.L. The effect of hydraulic retention time on the stability of aerobically grown microbial granules. Lett. Appl. Microbiol. 2004, 38, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Peng, Y.Z.; Cao, S.B.; Li, B.K.; Wang, S.Y.; Niu, M. Mechanism and bacterial structure of partial denitrification with high nitrite accumulation. Appl. Microbiol. Biotechnol. 2016, 100, 2011–2021. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.Z.; Ji, J.T.; Shi, L.L.; Gao, R.T.; Li, X.C. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Xia, J.T.; Ye, L.; Ren, H.Q.; Zhang, X.X. Microbial community structure and function in aerobic granular sludge. Appl. Microbiol. Biotechnol. 2018, 102, 3967–3979. [Google Scholar] [CrossRef]
- Glass, C.; Silverstein, J. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- Liu, B.B.; Mao, Y.J.; Bergaust, L.; Bakken, L.R.; Frostegard, A. Strains in the genus Thauera exhibit remarkably different denitrification regulatory phenotypes. Environ. Microbiol. 2013, 15, 2816–2828. [Google Scholar]
- Yang, Q.X.; Zhao, H.L.; Du, B.B. Bacteria and bacteriophage communities in bulking and non-bulking activated sludge in full-scale municipal wastewater treatment systems. Biochem. Eng. J. 2017, 119, 101–111. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, X.D.; Zhang, Q.; Littleton, H. Rapid aerobic granulation in an SBR treating piggery wastewater by seeding sludge from a municipal WWTP. J. Environ. Sci. 2017, 51, 332–341. [Google Scholar] [CrossRef] [PubMed]

| SBR Process | Running Stages | |||
|---|---|---|---|---|
| Stage A (Day 0–60) | Stage B (Day 61–100) | Stage C (Day 101–120) | Stage D, (Day 121–160) | |
| Influent | 5 min | |||
| Aeration | 180 min 135 min | |||
| Stirring (60 rad/min) | 0 min | 60 min | ||
| Settling (min) | 5 min | |||
| Effluent (min) | 5 min | |||
| Idling (min) | 45 min 30 min | |||
| Sample | Effective Reads | Mean Length (bp) | OTU | Shannon | Simpson | ACE | Chao1 | Coverage |
|---|---|---|---|---|---|---|---|---|
| S0 | 47,293 | 415.14 | 1109 | 4.6 | 0.03 | 1291.45 | 1247.65 | 0.99 |
| S1 | 42,545 | 422.77 | 780 | 4.35 | 0.03 | 1267.47 | 1104.56 | 0.99 |
| S2 | 47,825 | 425.5 | 784 | 3.3 | 0.17 | 1101.28 | 1063.43 | 0.99 |
| S3 | 55,914 | 417.43 | 1088 | 4.96 | 0.02 | 1292.89 | 1292.90 | 0.99 |
| S4 | 64,649 | 417.53 | 1014 | 4.3 | 0.06 | 1236.15 | 1198.51 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, D.; He, W.; He, Q.; Chu, W.; Li, S.; Li, J. Results of Adding Sludge Micropowder for Microbial Structure and Partial Nitrification and Denitrification in a Filamentous AGS-SBR Using High-Ammonia Wastewater. Water 2023, 15, 508. https://doi.org/10.3390/w15030508
Liu J, Xu D, He W, He Q, Chu W, Li S, Li J. Results of Adding Sludge Micropowder for Microbial Structure and Partial Nitrification and Denitrification in a Filamentous AGS-SBR Using High-Ammonia Wastewater. Water. 2023; 15(3):508. https://doi.org/10.3390/w15030508
Chicago/Turabian StyleLiu, Jun, Dong Xu, Weiqiang He, Qiulai He, Wenhai Chu, Songbo Li, and Jun Li. 2023. "Results of Adding Sludge Micropowder for Microbial Structure and Partial Nitrification and Denitrification in a Filamentous AGS-SBR Using High-Ammonia Wastewater" Water 15, no. 3: 508. https://doi.org/10.3390/w15030508
APA StyleLiu, J., Xu, D., He, W., He, Q., Chu, W., Li, S., & Li, J. (2023). Results of Adding Sludge Micropowder for Microbial Structure and Partial Nitrification and Denitrification in a Filamentous AGS-SBR Using High-Ammonia Wastewater. Water, 15(3), 508. https://doi.org/10.3390/w15030508






