Effects of Different Carbon and Nitrogen Ratios on Nitrogen Removal Efficiency and Microbial Communities in Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
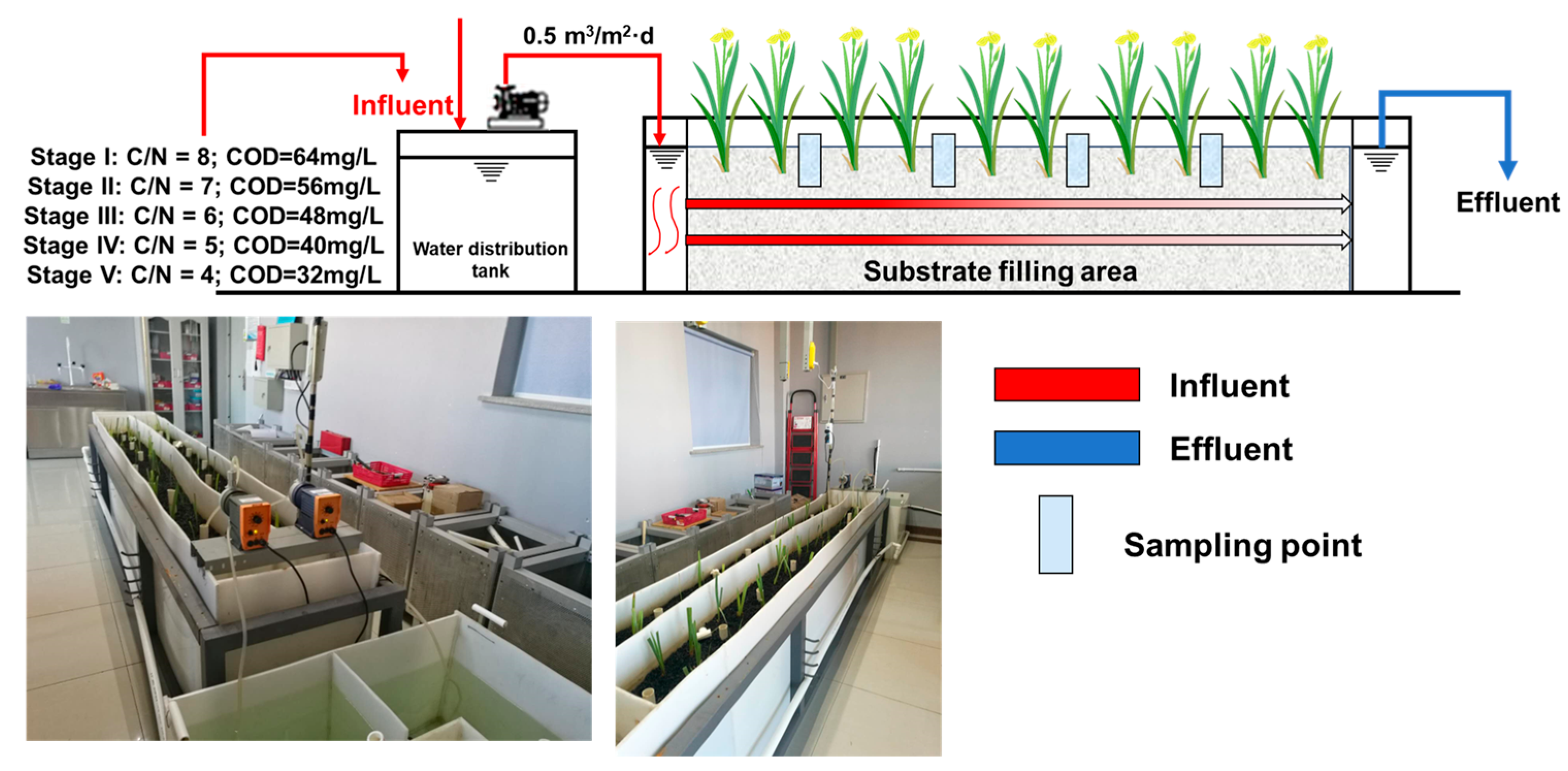
2.1. Construction of a Horizontal Submerged Constructed Wetland Simulation System
2.2. Different C/N Ratios Settings and Sampling Procedures
2.3. Statistical Analysis
3. Results and Discussion
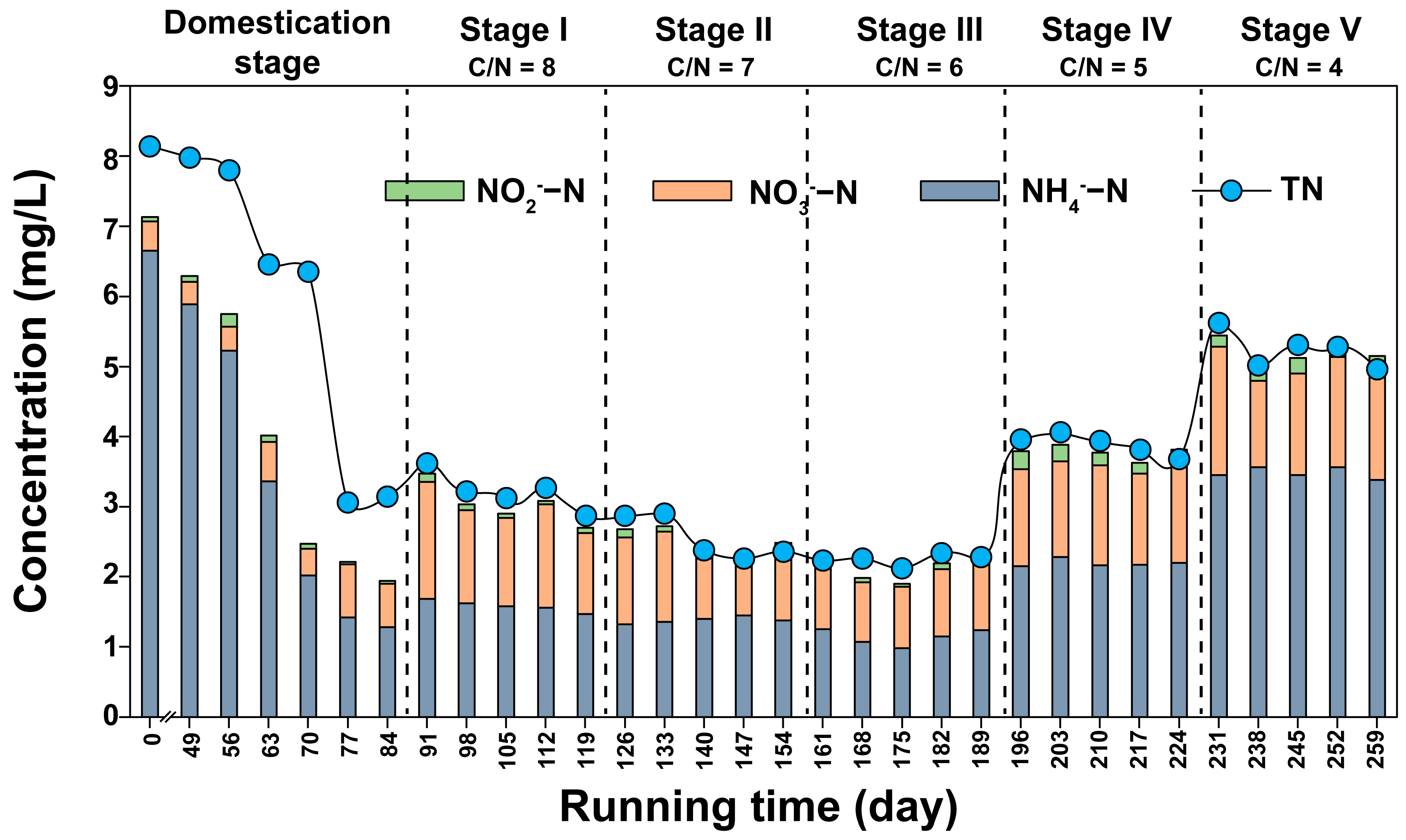
3.1. COD and Nitrogen Removal from Constructed Wetlands under Different C/N Ratios
3.2. Conversion Rates of Nitrogen in Constructed Wetlands under Different C/N Ratios
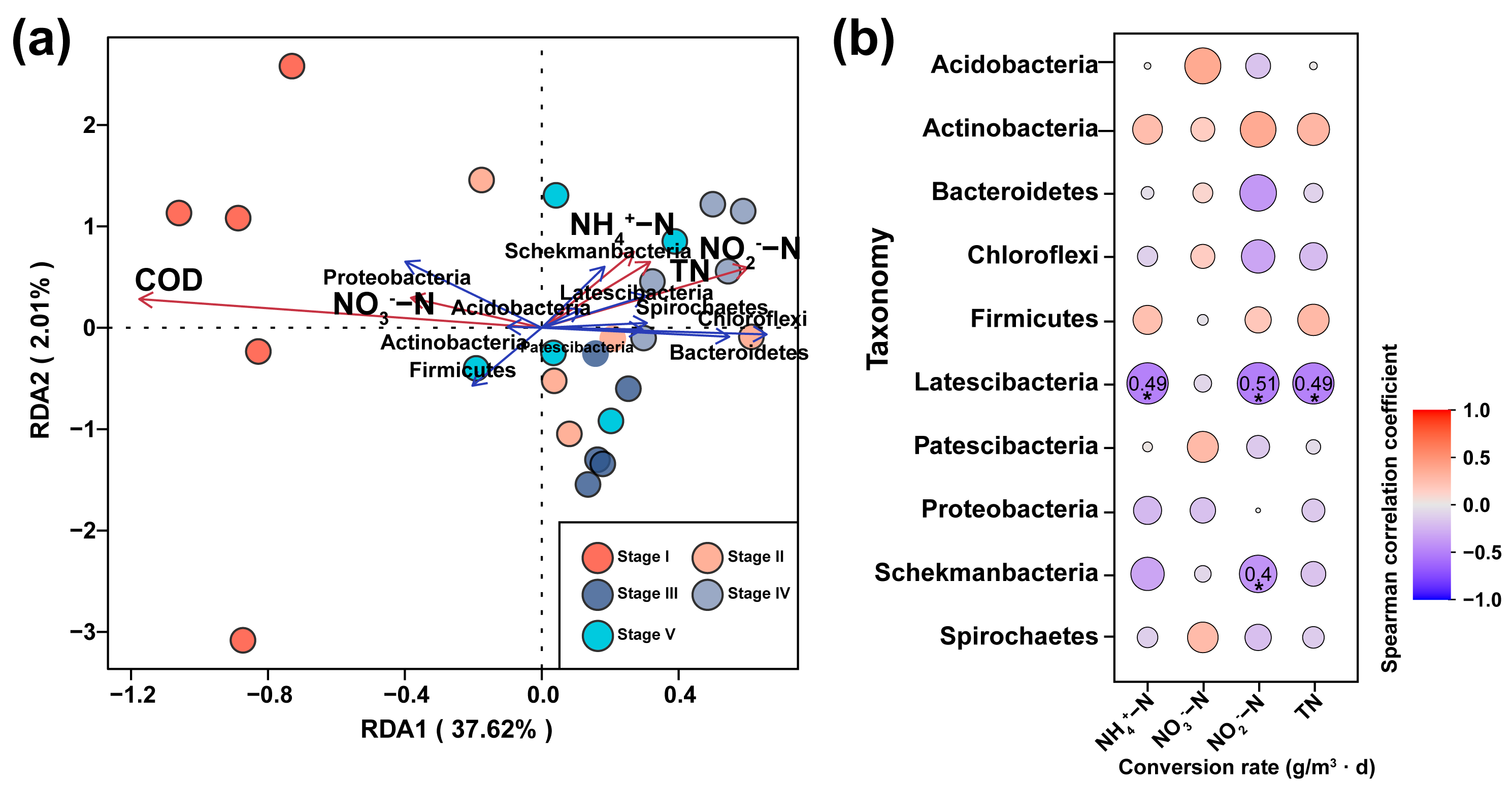
3.3. Effects on Microbial Diversity in Constructed Wetlands under Different C/N Ratios
3.4. Effects of Microorganisms on Nitrogen Transformation in Constructed Wetlands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent Research Challenges in Constructed Wetlands for Wastewater Treatment: A Review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty Years of China’s Water Pollution Control: Experiences and Challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, J.; Xiao, R.; Chai, W.; Xing, D.; Lu, H. Dissolved Organic Nitrogen in Wastewater Treatment Processes: Transformation, Biosynthesis and Ecological Impacts. Environ. Pollut. 2021, 273, 116436. [Google Scholar] [CrossRef] [PubMed]
- Fouzia, H.B. Monitoring of Marine Pollution; BoD—Books on Demand: Norderstedt, Germany, 2019; ISBN 978-1-83880-811-2. [Google Scholar]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of External Carbon Source in Heterotrophic Denitrification of Domestic Sewage: A Review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, T.; Ye, J.; Zhao, J.; Yang, L.; Wu, P.; Duan, J.; Ye, G. Effects of Carbon Sources and Operation Modes on the Performances of Aerobic Denitrification Process and Its Microbial Community Shifts. J. Environ. Manag. 2019, 239, 299–305. [Google Scholar] [CrossRef]
- Stefanakis, A.I. The Role of Constructed Wetlands as Green Infrastructure for Sustainable Urban Water Management. Sustainability 2019, 11, 6981. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Chen, J.; Wang, X.; Li, D.; Hou, J.; He, X. Advanced Nitrogen and Phosphorus Removal by Combining Endogenous Denitrification and Denitrifying Dephosphatation in Constructed Wetlands. J. Environ. Manag. 2021, 294, 112967. [Google Scholar] [CrossRef]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial Coupling Mechanisms of Nitrogen Removal in Constructed Wetlands: A Review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef]
- Jia, W.; Yang, Y.; Yang, L.; Gao, Y. High-Efficient Nitrogen Removal and Its Microbiological Mechanism of a Novel Carbon Self-Sufficient Constructed Wetland. Sci. Total Environ. 2021, 775, 145901. [Google Scholar] [CrossRef]
- Kim, H.; Bae, H.-S.; Reddy, K.R.; Ogram, A. Distributions, Abundances and Activities of Microbes Associated with the Nitrogen Cycle in Riparian and Stream Sediments of a River Tributary. Water Res. 2016, 106, 51–61. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Nitrogen Removal through Collaborative Microbial Pathways in Tidal Flow Constructed Wetlands. Sci. Total Environ. 2021, 758, 143594. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Jeison, D.; Chamy, R. Development of Denitrifying and Methanogenic Activities in USB Reactors for the Treatment of Wastewater: Effect of COD/N Ratio. Process Biochem. 2006, 41, 1338–1342. [Google Scholar] [CrossRef]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and Diversity of Ammonia-Oxidizing Archaea in Water Columns and Sediments of the Ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Groffman, P.M.; Altabet, M.A.; Böhlke, J.K.; Butterbach-Bahl, K.; David, M.B.; Firestone, M.K.; Giblin, A.E.; Kana, T.M.; Nielsen, L.P.; Voytek, M.A. Methods for Measuring Denitrification: Diverse Approaches to a Difficult Problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef] [PubMed]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The Consequences of Niche and Physiological Differentiation of Archaeal and Bacterial Ammonia Oxidisers for Nitrous Oxide Emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Ruuskanen, M.O.; Colby, G.; St. Pierre, K.A.; St. Louis, V.L.; Aris-Brosou, S.; Poulain, A.J. Microbial Genomes Retrieved from High Arctic Lake Sediments Encode for Adaptation to Cold and Oligotrophic Environments. Limnol. Oceanogr. 2020, 65, S233–S247. [Google Scholar] [CrossRef]
- HJ/T91-2002; Technical Specifications for Wastewater Monitoring 2002. China Standard Press: Beijing, China, 2002.
- Hossain, S.; Chow, C.W.K.; Cook, D.; Sawade, E.; Hewa, G.A. Review of Nitrification Monitoring and Control Strategies in Drinking Water System. Int. J. Environ. Res. Public Health 2022, 19, 4003. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Liu, G.; Zhu, J.; Ma, X.; Piao, C.; You, S.; Wang, K. Decoding the Carbon and Nitrogen Metabolism Mechanism in Anammox System Treating Pharmaceutical Wastewater with Varying COD/N Ratios through Metagenomic Analysis. Chem. Eng. J. 2023, 457, 141316. [Google Scholar] [CrossRef]
- Ye, F.; Yan, J.; Li, T. Analysis of Municipal Sewage Pollution and Denitrification Treatment under Low Oxygen Conditions. Environ. Technol. Innov. 2021, 21, 101188. [Google Scholar] [CrossRef]
- Cherchi, C.; Onnis-Hayden, A.; El-Shawabkeh, I.; Gu, A.Z. Implication of Using Different Carbon Sources for Denitrification in Wastewater Treatments. Water Environ. Res. 2009, 81, 788–799. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhou, L. Effect of Carbon Source, C/N Ratio, Nitrate and Dissolved Oxygen Concentration on Nitrite and Ammonium Production from Denitrification Process by Pseudomonas Stutzeri D6. Bioresour. Technol. 2012, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, J.; Ma, K.; Jiang, C.; Zhu, L.; Xu, X. Tertiary Treatment of Municipal Wastewater by a Novel Flow Constructed Wetland Integrated with Biochar and Zero-Valent Iron. J. Water Process Eng. 2022, 47, 102777. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Hernández-del Amo, E.; Bañeras, L. Effects of High Nitrate Input in the Denitrification-DNRA Activities in the Sediment of a Constructed Wetland under Varying C/N Ratios. Ecol. Eng. 2021, 159, 106098. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of Heterotrophic Aerobic Denitrifying Bacteria in Nitrate Removal from Wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Negi, D.; Verma, S.; Singh, S.; Daverey, A.; Lin, J.-G. Nitrogen Removal via Anammox Process in Constructed Wetland—A Comprehensive Review. Chem. Eng. J. 2022, 437, 135434. [Google Scholar] [CrossRef]
- Gualtieri, M.; Goglio, A.; Clagnan, E.; Adani, F. The Importance of the Electron Acceptor: Comparison between Flooded and Tidal Bioelectrochemical Systems for Wastewater Treatment and Nutrients Enriched Solution Production. Bioresour. Technol. Rep. 2023, 24, 101617. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef]
- Adrados, B.; Sánchez, O.; Arias, C.A.; Becares, E.; Garrido, L.; Mas, J.; Brix, H.; Morató, J. Microbial Communities from Different Types of Natural Wastewater Treatment Systems: Vertical and Horizontal Flow Constructed Wetlands and Biofilters. Water Res. 2014, 55, 304–312. [Google Scholar] [CrossRef]
- White, B.A.; Lamed, R.; Bayer, E.A.; Flint, H.J. Biomass Utilization by Gut Microbiomes. Annu. Rev. Microbiol. 2014, 68, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Wu, D.; Ding, J.; Yuan, X.; Zhou, G.; Zhang, Y.; Kong, Q.; Zhao, C.; Du, Y.; Xu, F.; et al. Long-Term Performance and Microbial Mechanism in Intertidal Wetland Sediment Introduced Constructed Wetlands Treating Saline Wastewater. J. Clean. Prod. 2021, 310, 127409. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Niu, L.; Zhang, W.; Wang, C.; Wang, P.; Meng, F. New Insights into the Spatial Variability of Biofilm Communities and Potentially Negative Bacterial Groups in Hydraulic Concrete Structures. Water Res. 2017, 123, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Shima, S. Methane as Fuel for Anaerobic Microorganisms. Ann. N. Y. Acad. Sci. 2008, 1125, 158–170. [Google Scholar] [CrossRef]
- Yao, Z.; Ren, Y.; Li, B.; Bai, G.; Zhao, S.; Yang, L.; Chi, Y. Effects of Plant Physiological Responses under Nitrogen Stress on Pollutant Removal in Subsurface Constructed Wetlands. J. Water Process Eng. 2023, 51, 103351. [Google Scholar] [CrossRef]
- Gao, F.; Liu, G.; She, Z.; Ji, J.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Effects of Salinity on Pollutant Removal and Bacterial Community in a Partially Saturated Vertical Flow Constructed Wetland. Bioresour. Technol. 2021, 329, 124890. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tao, R.; Tam, N.F.; Huang, W.; Zhang, L.; Man, Y.; Xu, X.; Dai, Y.; Yang, Y. Differences in Bacterial N, P, and COD Removal in Pilot-Scale Constructed Wetlands with Varying Flow Types. Bioresour. Technol. 2020, 318, 124061. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Wen, X.; Zhang, G.; Zhang, C.; Cui, B.; Liu, X. Microbial Resistance and Resilience in Response to Environmental Changes under the Higher Intensity of Human Activities than Global Average Level. Glob. Chang. Biol. 2020, 26, 2377–2389. [Google Scholar] [CrossRef]
- Ratzke, C.; Barrere, J.; Gore, J. Strength of Species Interactions Determines Biodiversity and Stability in Microbial Communities. Nat. Ecol. Evol. 2020, 4, 376–383. [Google Scholar] [CrossRef]
- Zeng, L.; Dai, Y.; Zhang, X.; Man, Y.; Tai, Y.; Yang, Y.; Tao, R. Keystone Species and Niche Differentiation Promote Microbial N, P, and COD Removal in Pilot Scale Constructed Wetlands Treating Domestic Sewage. Environ. Sci. Technol. 2021, 55, 12652–12663. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Tian, L.; Wen, H. Optimal Influent COD/N Ratio for Obtaining Low GHG Emissions and High Pollutant Removal Efficiency in Constructed Wetlands. J. Clean. Prod. 2020, 267, 122003. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Pang, W.; Tang, J.; Guo, Z.; Hu, Z. Biochar Immobilized Bacteria Enhances Nitrogen Removal Capability of Tidal Flow Constructed Wetlands. Sci. Total Environ. 2022, 836, 155728. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Tang, K.W.; Tong, C.; Lai, D.Y.F.; Zhang, L.; Lin, X.; Yang, H.; Tan, L.; Zhang, Y.; Hong, Y.; et al. Conversion of Coastal Wetland to Aquaculture Ponds Decreased N2O Emission: Evidence from a Multi-Year Field Study. Water Res. 2022, 227, 119326. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Espenberg, M.; Pärn, J.; Lehtovirta-Morley, L.; Anslan, S.; Kasak, K.; Kõljalg, U.; Liira, J.; Maddison, M.; Moora, M.; et al. Structure and Function of the Soil Microbiome Underlying N2O Emissions from Global Wetlands. Nat. Commun. 2022, 13, 1430. [Google Scholar] [CrossRef]
- Sun, D.; Liu, M.; Hou, L.; Zhao, M.; Tang, X.; Zhao, Q.; Li, J.; Han, P. Community Structure and Abundance of Comammox Nitrospira in Chongming Eastern Intertidal Sediments. J. Soils Sediments 2021, 21, 3213–3224. [Google Scholar] [CrossRef]
- Yu, B.; Liu, C.; Wang, S.; Wang, W.; Zhao, S.; Zhu, G. Applying Constructed Wetland-Microbial Electrochemical System to Enhance NH4+ Removal at Low Temperature. Sci. Total Environ. 2020, 724, 138017. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, P.; Zhang, J.; Xie, Z.; Ji, J.; Ghulam, A. Substrates and Pathway of Electricity Generation in a Nitrification-Based Microbial Fuel Cell. Bioresour. Technol. 2014, 161, 208–214. [Google Scholar] [CrossRef]






| C6H12O6 | NH4Cl | KH2PO4 | Mg2+ | Ca2+ | Fe2+ | Zn2+ | Cu2+ | Mn2+ | Mo2+ | Co2+ | B5+ | |
| Concentration (mol/L) | 0.41 | 0.57 | 0.03 | 8.43 | 1.83 | 0.03 | 0.02 | 0.03 | 0.03 | 0.01 | 0.03 | 0.26 |
| COD | DO | Ammonia Nitrogen | Total Phosphorus | |
| Concentration | ≤20 mg/L | ≥6 mg/L | ≤0.15 mg/L | ≤0.02 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Li, J.; Chang, S. Effects of Different Carbon and Nitrogen Ratios on Nitrogen Removal Efficiency and Microbial Communities in Constructed Wetlands. Water 2023, 15, 4272. https://doi.org/10.3390/w15244272
Bai X, Li J, Chang S. Effects of Different Carbon and Nitrogen Ratios on Nitrogen Removal Efficiency and Microbial Communities in Constructed Wetlands. Water. 2023; 15(24):4272. https://doi.org/10.3390/w15244272
Chicago/Turabian StyleBai, Xueyuan, Jianwei Li, and Sheng Chang. 2023. "Effects of Different Carbon and Nitrogen Ratios on Nitrogen Removal Efficiency and Microbial Communities in Constructed Wetlands" Water 15, no. 24: 4272. https://doi.org/10.3390/w15244272




