The Chemical Weathering of Rocks and Its Carbon Sink Effect in the Naqu River Basin of the Nujiang River Source Area, Southwest China
Abstract
:1. Introduction
2. Materials and Methods
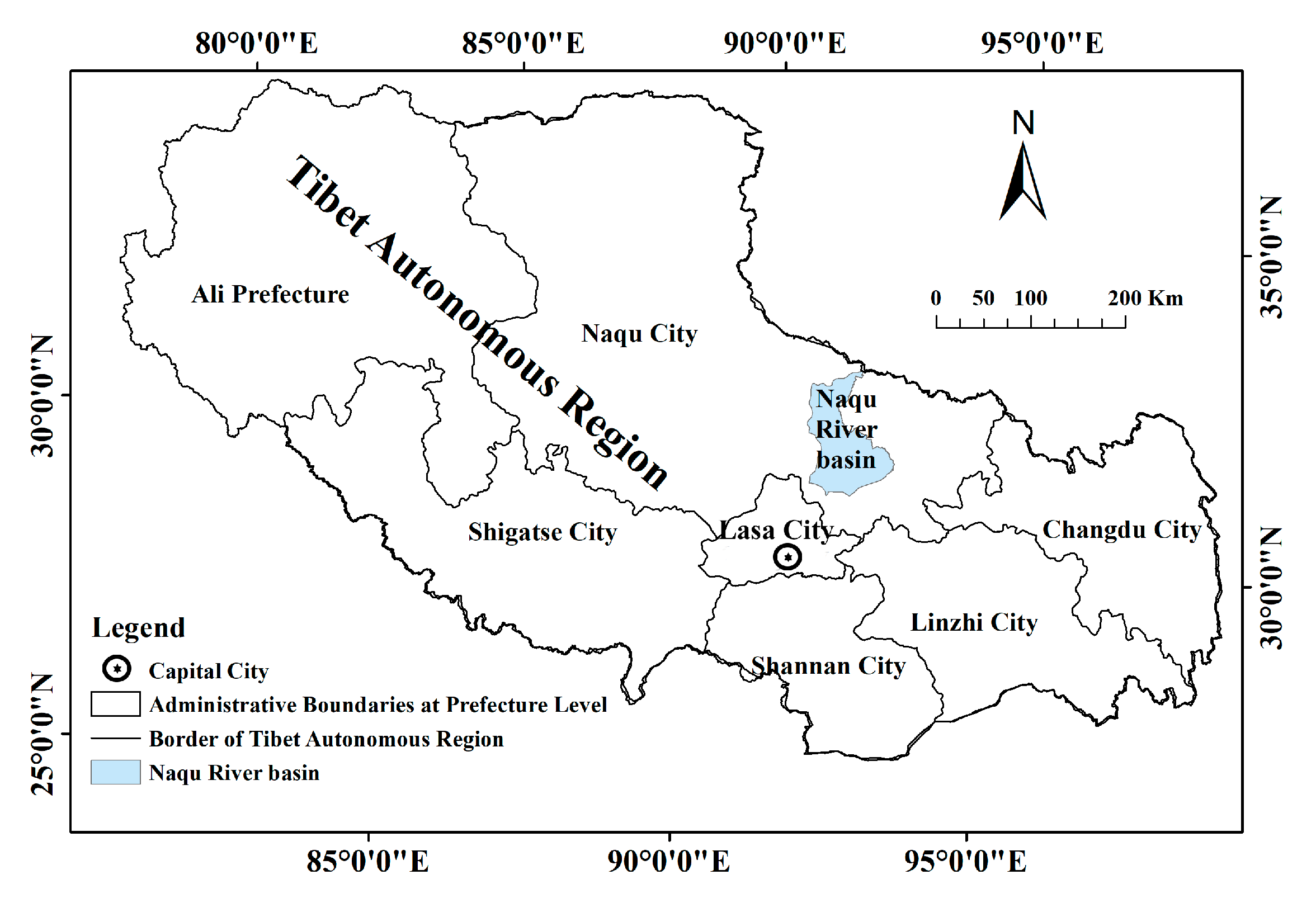
2.1. Study Area
2.2. Sample Collection and Analysis Methods
2.2.1. Sample Collection
2.2.2. Qualitative Methods for Analyzing the Sources of River Solutes
2.2.3. Quantification Methods for Analyzing the Sources of River Solutes
2.2.4. Methods for Analyzing Chemical Weathering and Carbon Sink Effects in Rocks
3. Results
3.1. Runoff
3.2. Physicochemical Parameters
4. Discussion
4.1. Provenance of Solutes in River Water
4.1.1. Qualitative Analysis of Different Sources of Riverine Solutes
4.1.2. Quantification of Different Sources of Riverine Solutes
4.2. Analysis of the Chemical Weathering Rates of Rocks and Carbon Sink Effects in Watersheds
| River | Drainage Area | [CO2]sil | [CO2]carb | Sum | Reference | |||
|---|---|---|---|---|---|---|---|---|
| 106 km2 | 108 mol/yr | 103 mol/km2/yr | 108 mol/yr | 103 mol/km2/yr | 108 mol/yr | 103 mol/km2/yr | ||
| The upstream and middle areas of Naqu River basin | 0.01 | 25.79 | 248.60 | 6.90 | 66.47 | 32.69 | 315.07 | Scenario 1 |
| 0.01 | 25.79 | 248.60 | 12.57 | 121.13 | 38.36 | 369.72 | Scenario 2 | |
| 0.01 | 25.79 | 248.60 | 9.73 | 93.80 | 35.52 | 342.40 | Scenario 3 | |
| Nujiang River | 0.11 | 120 | 110 | 650 | 590 | 890 | 810 | Wu et al. [35] |
| Tuotuo River | 0.016 | 7.3 | 45.8 | 21.4 | 135 | - | - | Li et al. [49] |
| Ganges River | 1.05 | 4710 | 448.611 | 2360 | 224.8 | - | 692 | Gaillardet et al. [50] |
5. Conclusions
- a
- The changes in terms of space of the TDS and EC values in the Naqu River watershed were the same; they decreased in concentration from upstream to downstream. The pH changed little as a function of space, but the difference was that the turbidity changed greatly. Ca-HCO3 was the main type of water chemistry in the Naqu River basin.
- b
- Based on the forward model, the contributions of different sources of river water ions were quantitatively analyzed, and they are listed in descending order: chemical weathering of carbonate rocks > chemical weathering of silicate rocks > chemical weathering of evaporite saline rocks > atmospheric precipitation input.
- c
- By analyzing the chemical weathering and carbon sink effects in the watershed, three scenarios were hypothesized based on the percentage of SO42− production from rock weathering, with carbonate chemical weathering rates being the largest. In the upstream and middle of the Naqu River basin, the chemical weathering of silicate rocks consumed more CO2 than that of carbonate rocks did.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Sun, H.; Chen, B.; Yang, M.; Zeng, Q.; Zeng, C.; Huang, J.; Luo, H.; Lin, D. Temporal variations in riverine hydrochemistry and estimation of the carbon sink produced by coupled carbonate weathering with aquatic photosynthesis on land: An example from the Xijiang River, a large subtropical karst-dominated river in China. Environ. Sci. Pollut. Res. Int. 2020, 27, 13142–13154. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Bai, X.; Luo, G.; Li, C.; Wu, L.; Chen, F.; Ran, C.; Xi, H.; Zhang, S. Climate change has enhanced the positive contribution of rock weathering to the major ions in riverine transport. Glob. Planet. Chang. 2023, 228, 104203. [Google Scholar] [CrossRef]
- Bastia, F.; Equeenuddin, S.M. Chemical weathering and associated CO2 consumption in the Mahanadi river basin, India. J. Asian Earth Sci. 2019, 174, 218–231. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpää, M.; Duo, B.; Gjessing, E.T. Water quality in the Tibetan Plateau: Metal contents of four selected rivers. Environ. Pollut. 2008, 156, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bai, X.; Zhao, C.; Tan, Q.; Luo, G.; Wang, J.; Li, Q.; Wu, L.; Chen, F.; Li, C.; et al. Global CO2 consumption by silicate rock chemical weathering: Its past and future. Earth’s Future 2021, 9, e2020EF001938. [Google Scholar] [CrossRef]
- Xi, H.; Wang, S.; Bai, X.; Tang, H.; Luo, G.; Li, H.; Wu, L.; Li, C.; Chen, H.; Ran, C.; et al. The responses of weathering carbon sink to eco-hydrological processes in global rocks. Sci. Total Environ. 2021, 788, 147706. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, G. Estimation of global karst carbon sink from 1950s to 2050s using response surface methodology. Geospat. Inf. Sci. 2023, 1–18. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W. Significance of the carbon sink produced by H2O–carbonate–CO2–aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Liu, Z.; Macpherson, G.L.; Groves, C.; Martin, J.B.; Yuan, D.; Zeng, S. Large and active CO2 uptake by coupled carbonate weathering. Earth-Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yan, N.; Wu, G.J.; Xu, T.L.; Li, F. Chemical weathering in the upstream and midstream reaches of the Yarlung Tsangpo basin, southern Tibetan Plateau. Chem. Geol. 2020, 559, 119906. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, G.; Li, F.; Chen, M.; Tran, T.V.; Liu, X.; Gao, S. Glaciation enhanced chemical weathering in a cold glacial catchment, western Nyaingêntanglha Mountains, central Tibetan Plateau. J. Hydrol. 2021, 597, 126197. [Google Scholar] [CrossRef]
- Yu, Z.L.; Wu, G.J.; Li, F.; Huang, J.; Xiao, X.; Liu, K.S. Small-catchment perspective on chemical weathering and its controlling factors in the Nam Co basin, central Tibetan Plateau. J. Hydrol. 2021, 598, 126315. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, G.; Keys, L.; Li, F.; Yan, N.; Qu, D.; Liu, X. Seasonal variation of chemical weathering and its controlling factors in two alpine catchments, Nam Co basin, central Tibetan Plateau. J. Hydrol. 2019, 576, 381–395. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Z.Q.; Li, X.D.; Li, X.D.; Zhang, L.L.; Chen, A.C. Assessing the oxidative weathering of pyrite and its role in controlling atmospheric CO2 release in the eastern Qinghai-Tibet Plateau. Chem. Geol. 2020, 543, 119605. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y. Chemical weathering of small watersheds in the upper reaches of Jinsha River on the eastern edge of the Qinghai–Tibet Plateau. J. Hydrol. 2023, 623, 129844. [Google Scholar] [CrossRef]
- Wu, W.F.; Xu, S.J.; Yang, J.D.; Yin, H.W. Silicate Weathering and CO2 Consumption Deduced from the Seven Chinese Rivers Originating in the Qinghai-Tibet Plateau. Chem. Geol. 2008, 249, 307–320. [Google Scholar] [CrossRef]
- Tao, Z.H.; Zhang, Z.Q.; Chen, D.; Li, X.D.; Liu, C.Q. Chemical Weathering in the Three Rivers (Jinsha River, Lancang River and Nujiang River) Watershed, Southwest China. Chin. J. Ecol. 2015, 34, 2297–2308. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Wang, F.Q.; Chen, X.; Ci, R.Q.J. Multivariate Statistical Analysis on Hydrochemical Characteristics of Naqu River in the Source Region of Nujiang River. J. North China Univ. Water Resour. Electr. Power Nat. Sci. Ed. 2020, 41, 63–71. (In Chinese) [Google Scholar] [CrossRef]
- Cui, B.L.; Li, X.Y. Runoff processes in the Qinghai Lake Basin, Northeast Qinghai-Tibet Plateau, China: Insights from stable isotope and hydrochemistry. Quat. Int. 2015, 380–381, 123–132. [Google Scholar] [CrossRef]
- Yan, D.; Liu, S.; Qin, T.; Weng, B.S.; Liu, J.J. Evaluation of TRMM precipitation and its application to distributed hydrological model in Naqu River Basin of the Tibetan Plateau. Hydrol. Res. 2017, 48, 822–839. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.L.; Wang, F.Q.; Yan, D.H.; Zhao, H. Characteristics of Water Isotopes and Water Source Identification During the Wet Season in Naqu River Basin, Qinghai-Tibet Plateau. Water 2019, 11, 2418. [Google Scholar] [CrossRef]
- Wang, F.Q.; Zhao, Y.; Chen, X.; Zhao, H. Hydrochemistry and Its Controlling Factors of Rivers in the Source Region of the Nujiang River on the Tibetan Plateau. Water 2019, 11, 2166. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Hou, Y.; Zhang, Z.; Zhan, J.; Gao, S.; Jin, H. Unraveling of permafrost hydrological variabilities on Central Qinghai-Tibet Plateau using stable isotopic technique. Sci. Total Environ. 2017, 605–606, 199–210. [Google Scholar] [CrossRef]
- Liu, S.; Yan, D.; Qin, T.; Weng, B.; Lu, Y.; Dong, G.; Gong, B. Precipitation phase separation schemes in the Naqu River basin, eastern Tibetan plateau. Theor. Appl. Climatol. 2018, 131, 399–411. [Google Scholar] [CrossRef]
- Deshmukh, R.Y.; Sharma, P.; Dhote, P.R. Review on hydrochemical analysis of rivers. Concern 2020, 7. [Google Scholar]
- Kai, J.; Wang, J.; Ju, J.; Huang, L.; Ma, Q.; Daut, G.; Zhu, L. Spatio-temporal variations of hydrochemistry and modern sedimentation processes in the Nam Co basin, Tibetan Plateau: Implications for carbonate precipitation. J. Great Lakes Res. 2020, 46, 961–975. [Google Scholar] [CrossRef]
- Ministry of Geology and Mineral Resources of the People’s Republic of China. Testing Methods of Underground Water Quality-Determination of Carbonate, Bicarbonate and Hydroxide-Titrimetric; China Standards Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Ministry of Geology and Mineral Resources of the People’s Republic of China. Testing Methods of Underground Water Quality-Determination of Sulfate-Turbidimetry; China Standards Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality-Determination of 32 Elements-Inductively Coupled Plasma Optical Emission Spectrometry; China Environmental Sciences Press: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Moquet, J.S.; Crave, A.; Viers, J.; Seyler, P.; Guyot, J.L. Chemical Weathering and Atmospheric/Soil CO2 Uptake in the Andean and Foreland Amazon Basins. Chem. Geol. 2011, 287, 1–26. [Google Scholar] [CrossRef]
- Xie, C.J.; Gao, Q.Z.; Tao, Z. Review and Perspectives of the Study on Chemical Weathering and Hydrochemistry in River Basin. Trop. Geogr. 2012, 32, 331–337. [Google Scholar] [CrossRef]
- Jiang, P.; Yu, G.; Zhang, Q.; Zou, Y.; Tang, Q.; Kang, Z.; Sytharith, P.; Xiao, H. Chemical weathering and CO2 consumption rates of rocks in the Bishuiyan subterranean basin of Guangxi, China. Sci. Rep. 2020, 10, 11677. [Google Scholar] [CrossRef]
- Vespasiano, G.; Apollaro, C.; De Rosa, R.; Muto, F.; Larosa, S.; Fiebig, J.; Mulch, A.; Marini, L. The Small Spring Method (SSM) for the definition of stable isotope–elevation relationships in Northern Calabria (Southern Italy). Appl. Geochem. 2015, 63, 333–346. [Google Scholar] [CrossRef]
- Wu, W.; Yang, J.; Xu, S.; Yin, H. Geochemistry of the headwaters of the Yangtze River, Tongtian He and Jinsha Jiang: Silicate weathering and CO2 consumption. Appl. Geochem. 2008, 23, 3712–3727. [Google Scholar] [CrossRef]
- Wu, W. Hydrochemistry of inland rivers in the north Tibetan Plateau: Constraints and weathering rate estimation. Sci. Total Environ. 2016, 541, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Shen, B.; Zeng, H.; Li, Y. Water Chemistry and Stable Isotopes of Different Water Types in Ta-jikistan. Environ. Process. 2018, 5, 127–137. [Google Scholar] [CrossRef]
- Jiang, L.G.; Yao, Z.J.; Wang, R.; Liu, Z.; Wang, L.; Wu, S. Hydrochemistry of the middle and upper reaches of the Yarlung Tsangpo River system: Weathering processes and CO2 consumption. Environ. Earth Sci. 2015, 74, 2369–2379. [Google Scholar] [CrossRef]
- Meybeck, M. Global occurrence of major elements in rivers. Treatise Geochem. 2003, 5, 207–223. [Google Scholar] [CrossRef]
- Wu, L.; Huh, Y.; Qin, J.; Gu, D.; Lee, S.V.D. Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim. Cosmochim. Acta 2005, 69, 5279–5294. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Dlamini, A.E.; Demlie, M. Integrated hydrogeological, hydrochemical and environmental isotope investi-gation of the area around the Kusile Power Station, Mpumalanga, South Africa. J. Afr. Earth Sci. 2020, 172, 103958. [Google Scholar] [CrossRef]
- Kobus, S.; Glińska-Lewczuk, K.; Sidoruk, M.; Skwierawski, A.; Obolewski, K.; Timofte, C.M.; Sowiński, P. Effect of hy-drological connectivity on physicochemical properties of bottom sediments of floodplain lakes-Acase study of the Lyna River, Northern Poland. Environ. Eng. Manag. J. 2016, 15, 1237–1246. [Google Scholar] [CrossRef]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, Z.Q.; Zhang, W.; Tao, Z.H.; Huang, L.; Yang, J.X.; Wu, Q.X.; Liu, C.Q. Characteristics of water chemistry and its indication of chemical weathering in Jinshajiang, Lancangjiang and Nujiang drainage basins. Environ. Earth Sci. 2016, 75, 506. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, M.; Wang, T.; Pei, Y.; Chen, B. Geochemistry of lacustrine carbonate rocks in southwestern Qaidam: Implications of silicate weathering and carbon burial triggered by the uplift of the Tibetan Plateau. Int. J. Coal Geol. 2023, 265, 104167. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, S.; Li, C.; Xiong, L.; Song, F.; Du, C.; Li, M.; Luo, Q.; Xue, Y.; Wang, S. A carbon-neutrality-capactiy index for evaluating carbon sink contributions. Environ. Sci. Ecotechnol. 2023, 15, 100237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ju, P.; Zhu, Q.; Xu, X.; Wu, N.; Gao, Y.; Feng, X.; Tian, J.; Niu, S.; Zhang, Y.; et al. Carbon and nitrogen cycling on the Qinghai–Tibetan Plateau. Nat. Rev. Earth Environ. 2022, 3, 701–716. [Google Scholar] [CrossRef]
- Chapman, H.; Bickle, M.; Thaw, S.H.; Thiam, H.N. Chemical fluxes from time series sampling of the Irrawaddy and Salween Rivers, Myanmar. Chem. Geol. 2015, 401, 15–27. [Google Scholar] [CrossRef]
- Li, Z.J.; Li, Z.X.; Song, L.L.; Ma, J.Z.; Song, Y. Environment significance and hydrochemical characteristics of supra-permafrost water in the source region of the Yangtze River. Sci. Total Environ. 2018, 644, 1141–1151. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]





| River | Drainage Area /km2 | Runoff /108 m3 | Runoff Depth /mm |
|---|---|---|---|
| Upstream of the main Naqu stream | 6412.6 | 0.15 | 2.29 |
| Middle of the main Naqu stream | 3962 | 0.16 | 4.01 |
| Downstream of the main Naqu stream | 4338.8 | 0.32 | 7.33 |
| Mugequ | 2103 | 0.019 | 0.92 |
| Chengqu | 1090 | 0.005 | 0.47 |
| Gongqu | 1232 | 0.012 | 0.94 |
| Luoqu | 1479 | 0.024 | 1.60 |
| Parameter | Min | Max | Mean | SD | CV/(%) |
|---|---|---|---|---|---|
| Ca2+/(mg/L) | 12.1 | 81.25 | 37.77 | 19.23 | 50.92 |
| Mg2+/(mg/L) | 1.96 | 37.83 | 13.59 | 9.76 | 71.83 |
| K+/(mg/L) | 0.39 | 5.54 | 2.60 | 1.81 | 69.45 |
| Na+/(mg/L) | 2.19 | 63.1 | 22.42 | 18.18 | 81.09 |
| Cl−/(mg/L) | 2.48 | 28.68 | 7.14 | 6.74 | 94.43 |
| HCO3−/(mg/L) | 43.63 | 321.15 | 173.24 | 87.65 | 50.60 |
| SO42−/(mg/L) | 11.72 | 188.85 | 47.21 | 44.36 | 93.95 |
| NO3−/(mg/L) | 0.58 | 3.74 | 1.58 | 0.83 | 52.71 |
| SiO2/(mg/L) | 5.04 | 11.5 | 7.42 | 1.99 | 26.82 |
| Sr/(mg/L) | 0.048 | 1.69 | 0.27 | 0.42 | 1.57 |
| TDS/(mg/L) | 53 | 586 | 220.57 | 137.84 | 62.49 |
| pH | 7.65 | 10.08 | 8.93 | 0.66 | 7.38 |
| Turbidity/NTU | 4.36 | 172 | 52.17 | 53.46 | 102.48 |
| DO/(mg/L) | 8.28 | 31.27 | 17.26 | 6.90 | 40.00 |
| ORP/(mV) | 32 | 181 | 121.14 | 35.41 | 29.23 |
| EC/(μS/cm) | 81 | 915 | 341.43 | 213.85 | 62.63 |
| Temperature/(°C) | 10.55 | 21.7 | 15.24 | 3.52 | 23.10 |
| River | Elevation | Sampling Time | Hydrochemical Type | NICB |
|---|---|---|---|---|
| (m) | (yyyy-mm-dd) | |||
| Upstream of the main Naqu stream | 4756 | 2019-08-15 | Ca-HCO3 | 0.0081 |
| Middle of the main Naqu stream | 4539 | 2019-08-15 | Mg-HCO3 | −0.0117 |
| Downstream of the main Naqu stream | 4455 | 2019-08-16 | Na-HCO3 | 0.0382 |
| Sangqu | 4628 | 2019-08-15 | Ca-HCO3 | 0.0055 |
| Basuoqu | 4710 | 2019-08-15 | Ca-HCO3 | −0.0106 |
| Mumuqu | 4625 | 2019-08-15 | Ca-HCO3 | −0.0018 |
| Upstream of Chengqu stream | 4522 | 2019-08-15 | Ca-HCO3 | 0.0036 |
| Downstream of Chengqu stream | 4499 | 2019-08-15 | Ca-HCO3 | −0.0503 |
| Zongqingqu | 4565 | 2019-08-15 | Ca-HCO3 | −0.1216 |
| Mugequ main stream | 4590 | 2019-08-14 | Na-HCO3 | 0.0058 |
| Mugequ basin tributary | 4697 | 2019-08-14 | Ca-HCO3 | −0.0314 |
| Gongqu main stream | 4574 | 2019-08-16 | Ca-HCO3 | 0.2221 |
| Gongqu basin tributary | 4489 | 2019-08-16 | Ca-HCO3 | −0.0584 |
| Luoqu main stream | 4986 | 2019-08-16 | Ca-HCO3 | −0.2119 |
| Basin | Ca2+ | Mg2+ | K+ | Na+ | SO42− | Average Contribution Rate |
|---|---|---|---|---|---|---|
| μmol/L | % | |||||
| Naqu River | 4.12 | 7.95 | 0.16 | 1.00 | 9.39 | 0.88 (0.25–2.89) |
| River | Elevation (m) | Precipitation (%) | Evaporite (%) | Silicate (%) | Carbonate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 3 | ||||
| Upstream of the main Naqu stream | 4756 | 0.25 | 7.87 | 46.64 | 27.25 | 25.01 | 66.87 | 28.11 | 47.49 |
| Middle of the main Naqu stream | 4539 | 0.50 | 5.05 | 30.65 | 17.85 | 33.14 | 61.32 | 35.72 | 48.52 |
| Downstream of the main Naqu stream | 4455 | 0.51 | 5.60 | 21.89 | 13.75 | 37.03 | 56.85 | 40.57 | 48.71 |
| Sangqu | 4628 | 0.41 | 3.37 | 28.73 | 16.05 | 26.82 | 69.40 | 44.04 | 56.72 |
| Basuoqu | 4710 | 0.49 | 2.47 | 14.31 | 8.39 | 13.09 | 83.95 | 72.11 | 78.03 |
| Mumuqu | 4625 | 0.99 | 2.23 | 12.98 | 7.61 | 14.42 | 82.36 | 71.61 | 76.99 |
| Upstream of Chengqu stream | 4522 | 0.50 | 2.72 | 16.54 | 9.63 | 37.19 | 59.58 | 45.76 | 52.67 |
| Downstream of Chengqu stream | 4499 | 0.52 | 3.42 | 21.68 | 12.55 | 32.55 | 63.51 | 45.25 | 54.38 |
| Zongqingqu | 4565 | 1.21 | 3.68 | 32.30 | 17.99 | 6.02 | 89.08 | 60.47 | 74.78 |
| Mugequ main stream | 4590 | 0.84 | 7.53 | 20.55 | 14.04 | 39.78 | 51.85 | 38.83 | 45.34 |
| Mugequ basin tributary | 4697 | 1.01 | 2.67 | 33.53 | 18.10 | 14.43 | 81.89 | 51.04 | 66.46 |
| Gongqu main stream | 4574 | 1.24 | 4.24 | 31.06 | 17.65 | 7.38 | 87.14 | 60.32 | 73.73 |
| Gongqu basin tributary | 4489 | 1.02 | 3.10 | 40.17 | 21.63 | 13.84 | 82.05 | 44.98 | 63.51 |
| Luoqu main stream | 4986 | 2.89 | 8.80 | 34.60 | 21.70 | 3.55 | 84.75 | 58.95 | 71.85 |
| EWR | SWR | CWR | Sum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 3 | |
| t/km2/yr | |||||||||
| 2.20 | 9.63 | 5.92 | 6.82 | 16.84 | 11.32 | 14.08 | 25.87 | 27.78 | 26.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Zhao, H.; Hou, X.; Zhang, H.; Wang, F.; Kang, P. The Chemical Weathering of Rocks and Its Carbon Sink Effect in the Naqu River Basin of the Nujiang River Source Area, Southwest China. Water 2023, 15, 4191. https://doi.org/10.3390/w15234191
Ren S, Zhao H, Hou X, Zhang H, Wang F, Kang P. The Chemical Weathering of Rocks and Its Carbon Sink Effect in the Naqu River Basin of the Nujiang River Source Area, Southwest China. Water. 2023; 15(23):4191. https://doi.org/10.3390/w15234191
Chicago/Turabian StyleRen, Suming, Heng Zhao, Xinli Hou, Honglu Zhang, Fuqiang Wang, and Pingping Kang. 2023. "The Chemical Weathering of Rocks and Its Carbon Sink Effect in the Naqu River Basin of the Nujiang River Source Area, Southwest China" Water 15, no. 23: 4191. https://doi.org/10.3390/w15234191
APA StyleRen, S., Zhao, H., Hou, X., Zhang, H., Wang, F., & Kang, P. (2023). The Chemical Weathering of Rocks and Its Carbon Sink Effect in the Naqu River Basin of the Nujiang River Source Area, Southwest China. Water, 15(23), 4191. https://doi.org/10.3390/w15234191






