Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
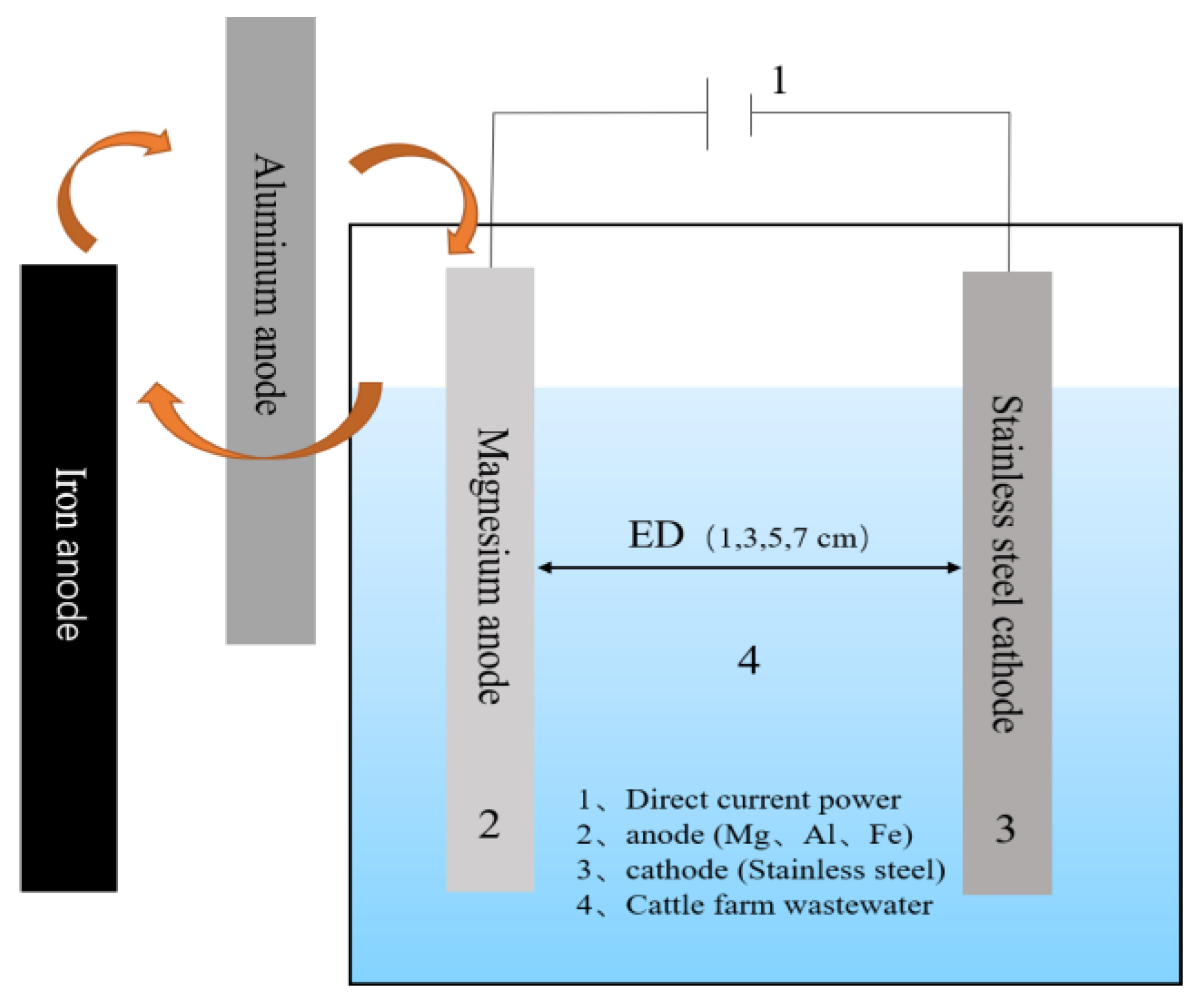
2.2. Experimental Setup
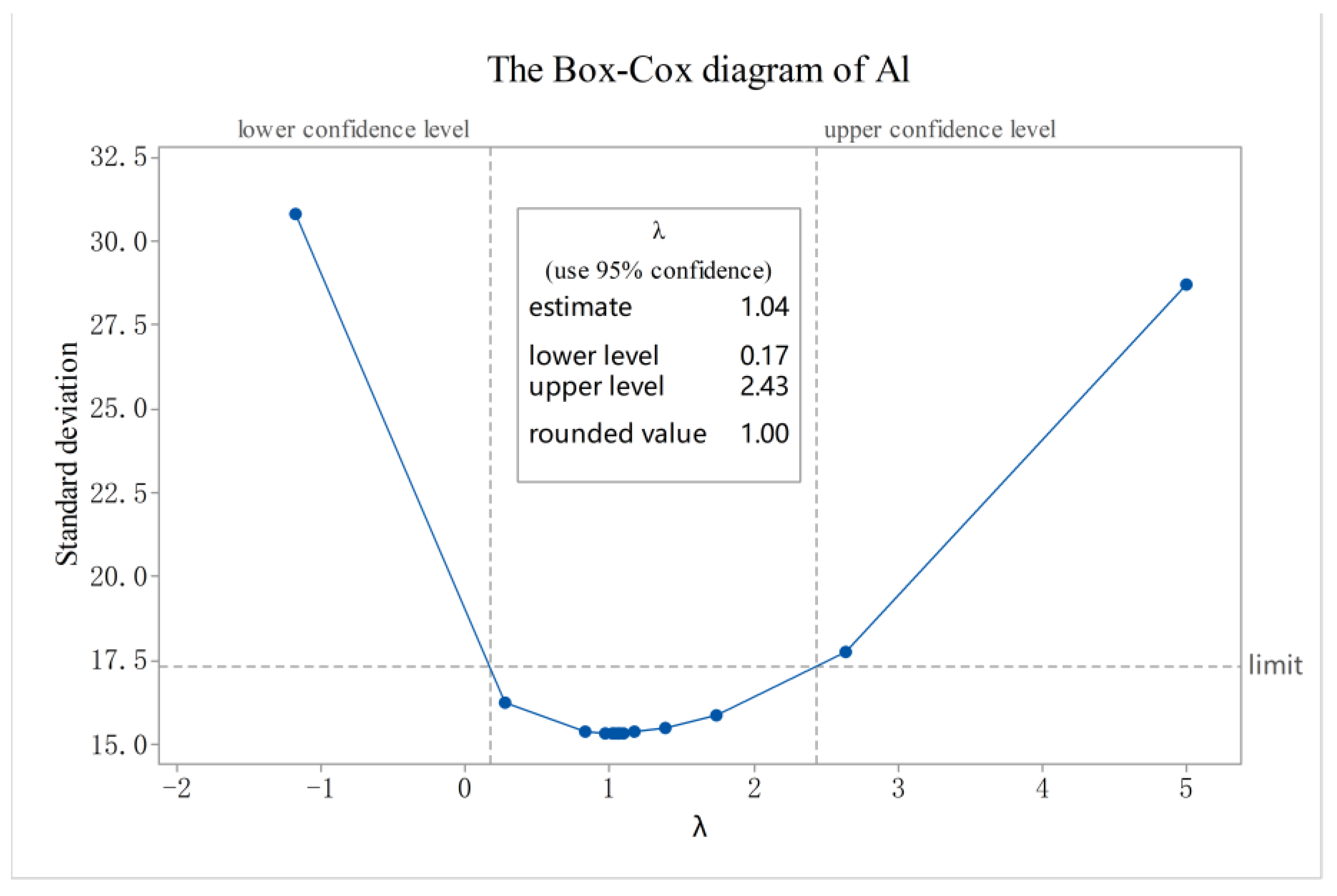
2.3. Analytical Methods
3. Results and Discussion
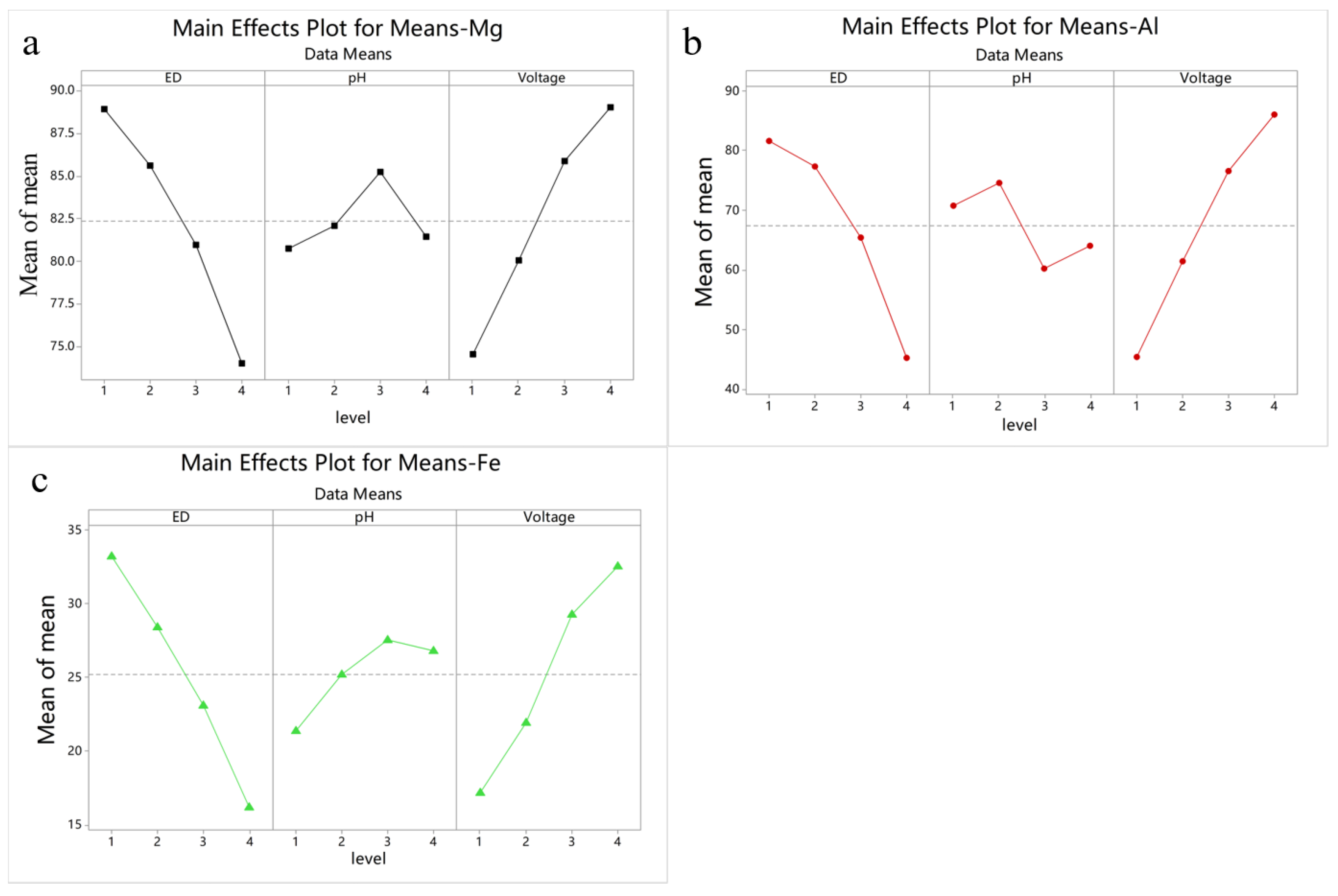
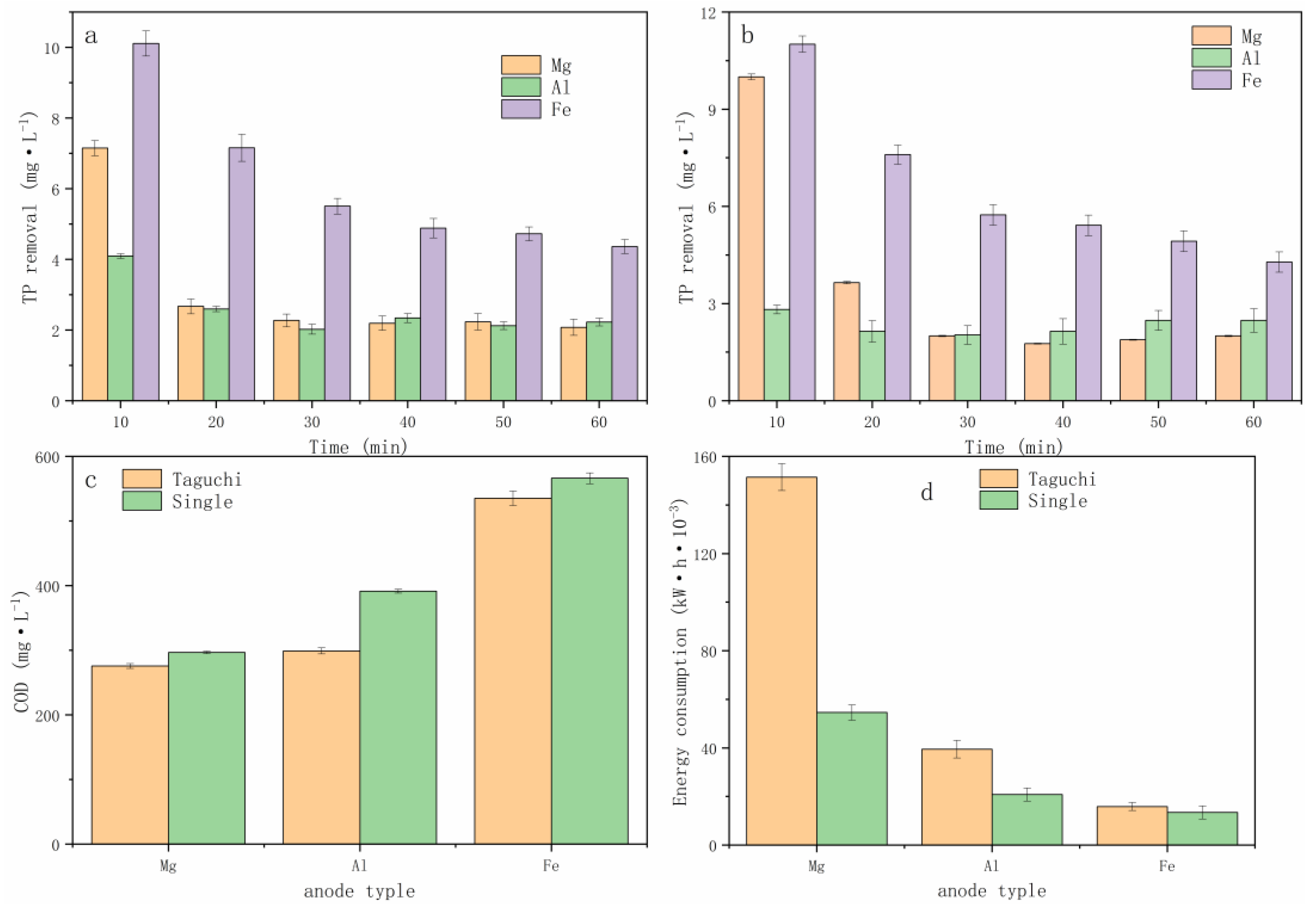
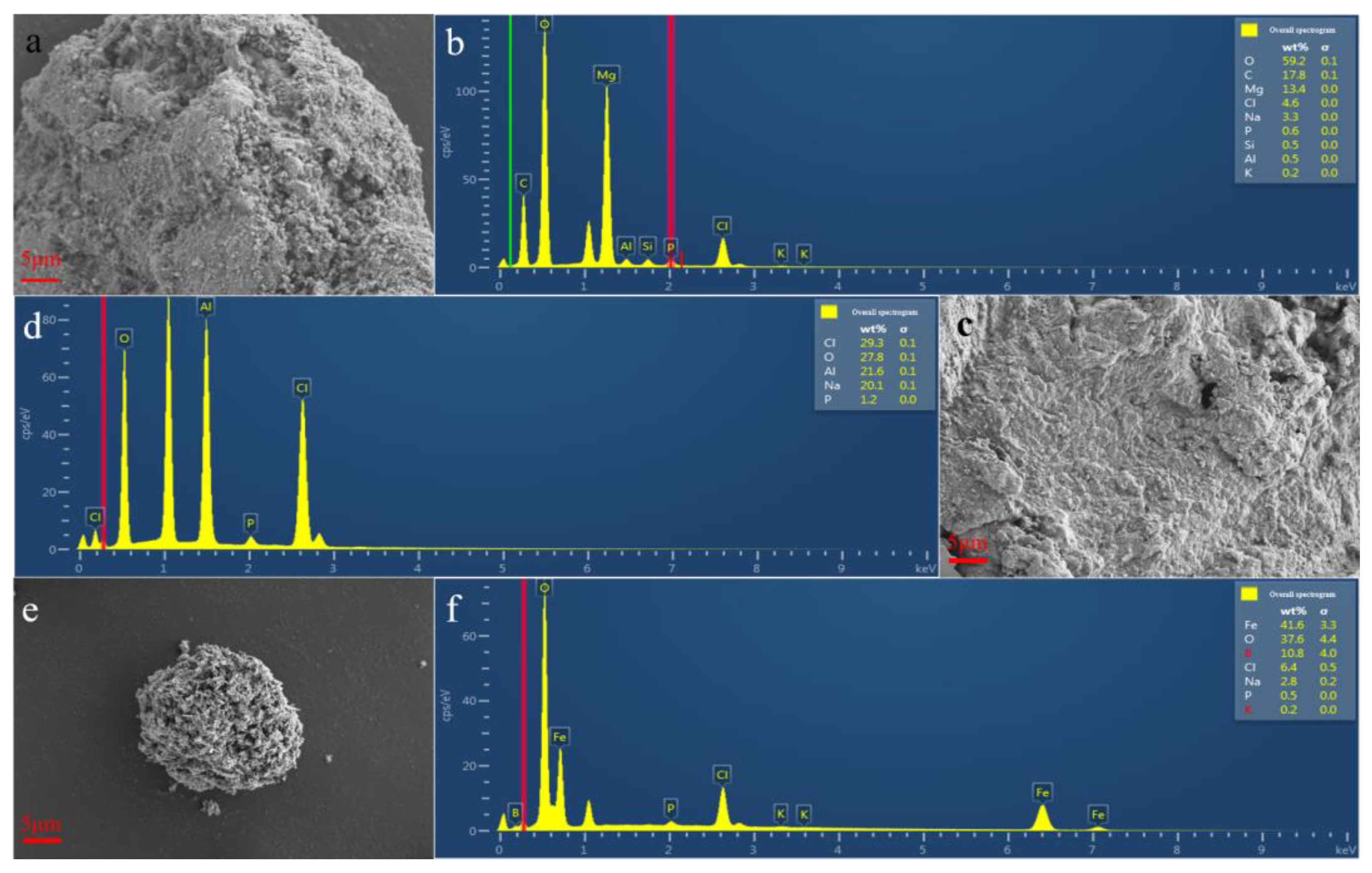
3.1. Taguchi Method Analysis
3.2. Single-Factor Method Analysis
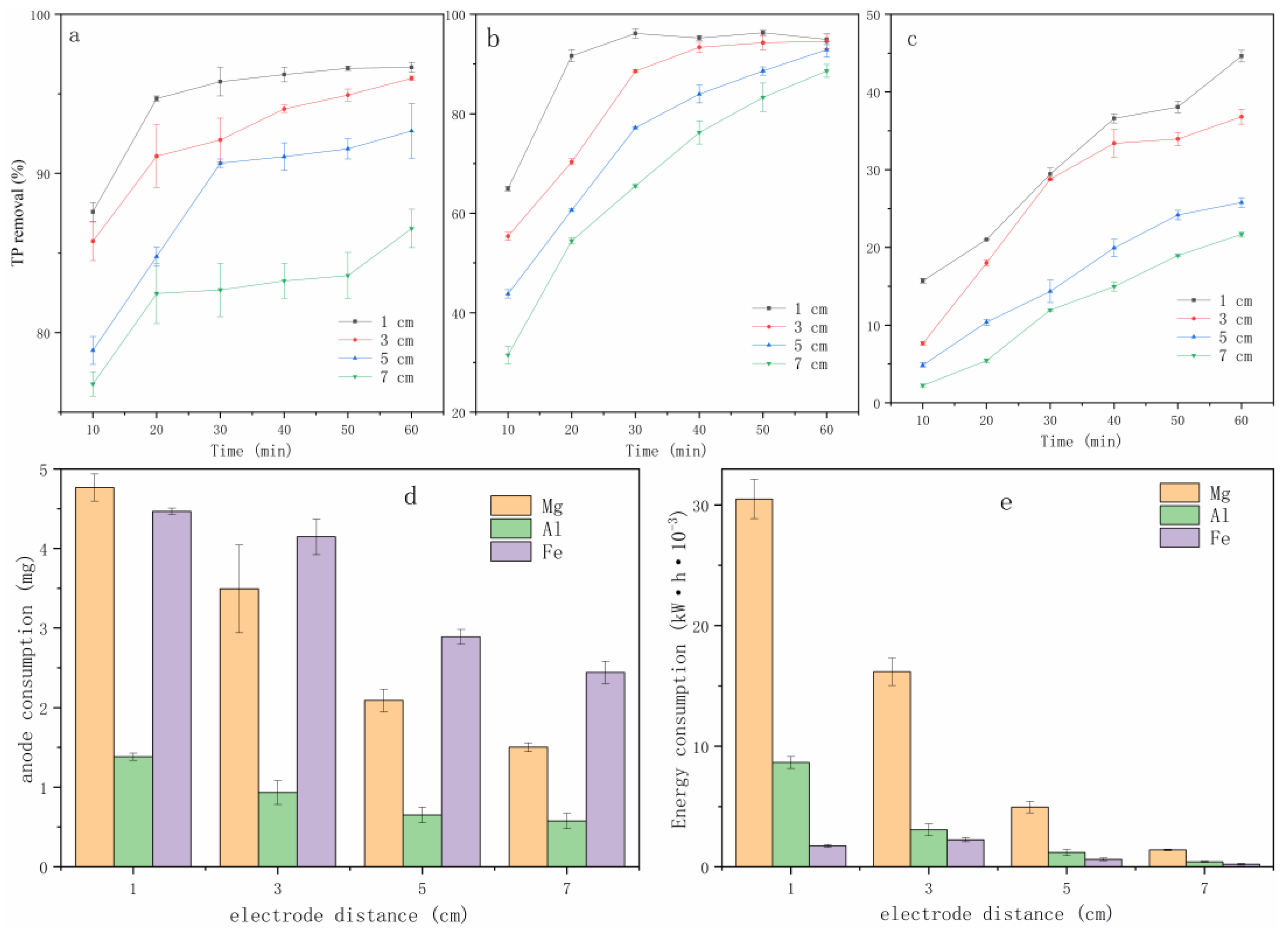
3.2.1. Effects of ED
3.2.2. Effects of Voltage
3.2.3. Effects of Initial pH
3.3. Actual Cattle Farm Wastewater Removal Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, S.L.; Wang, H.W.; Wu, T.; Yang, J.; Jiang, X.; Zhong, J.C. Vertical profiles of phosphorus fractions in the sediment in a chain of reservoirs in North China: Implications for pollution source, bioavailability, and eutrophication. Sci. Total Environ. 2019, 704, 10. [Google Scholar] [CrossRef] [PubMed]
- Pruss, A.; Kay, D.; Fewtrell, L.; Bartram, J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ. Health Perspect. 2002, 110, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.C.; Zhou, M.H.; Zhang, Q.Z.; Yin, X.Y.; Li, Y.W. Comprehensive treatment of marine aquaculture wastewater by a cost-effective flow-through electro-oxidation process. Sci. Total Environ. 2020, 722, 9. [Google Scholar] [CrossRef]
- Wei, J.; Meng, X.; Wen, X.; Song, Y. The Second National Pollution Source Census Bulletin. Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202006/W020200610353985963290 (accessed on 8 June 2020).
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2018, 231, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Meng, X.; Wen, X.; Song, Y. Adsorption and recovery of phosphate from water by amine fiber, effects of co-existing ions and column filtration. J. Environ. Sci. 2019, 87, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Z.-H.; Xiong, W.-P.; Zhou, Y.-Y.; Peng, Y.-R.; Li, X.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Barca, C.; Gerente, C.; Meyer, D.; Cliazarenc, F.; Andres, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef]
- Akram, M.; Xu, X.; Gao, B.Y.; Yue, Q.Y.; Shang, Y.N.; Khan, R.; Inam, M.A. Adsorptive removal of phosphate by the bimetallic hydroxide nanocomposites embedded in pomegranate peel. J. Environ. Sci. 2020, 91, 189–198. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)(3)/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Koilraj, P.; Sasaki, K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: Batch and column studies. Chem. Eng. J. 2017, 317, 1059–1068. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Ding, L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. J. Clean. Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Kubar, A.A.; Huang, Q.; Sajjad, M.; Yang, C.; Lian, F.; Wang, J.; Kubar, K.A. The Recovery of Phosphate and Ammonium from Biogas Slurry as Value-Added Fertilizer by Biochar and Struvite Co-Precipitation. Sustainability 2021, 13, 3827. [Google Scholar] [CrossRef]
- Jabari, P.; Munz, G.; Oleszkiewicz, J.A. Selection of denitrifying phosphorous accumulating organisms in IFAS systems: Comparison of nitrite with nitrate as an electron acceptor. Chemosphere 2014, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Oleszkiewicz, J. Selection and enrichment of denitrifying phosphorus accumulating organisms in activated sludge. Desalination Water Treat. 2010, 22, 72–77. [Google Scholar] [CrossRef]
- Gomez, S.; Felipe Hurtado, C.; Orellana, J. Bioremediation of organic sludge from a marine recirculating aquaculture system using the polychaete Abarenicola pusilla (Quatrefages, 1866). Aquaculture 2019, 507, 377–384. [Google Scholar] [CrossRef]
- Davidson, J.; Helwig, N.; Summerfelt, S.T. Fluidized sand biofilters used to remove ammonia, biochemical oxygen demand, total coliform bacteria, and suspended solids from an intensive aquaculture effluent. Aquac. Eng. 2008, 39, 6–15. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2016, 186, 24–41. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Can, O.T.; Aygun, A.; Tek, A. Comparison of the effects of various supporting electrolytes on the treatment of a dye solution by electrocoagulation process. Colloid Interface Sci. Commun. 2019, 33, 100210. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Optimization of process parameters in electrocoagulation treating chicken industry wastewater to recover hydrogen gas with pollutant reduction. Renew. Energy 2015, 80, 101–108. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Ilhan, F.; Sapci-Zengin, Z.; Sakar, S.; Gonullu, M.T. Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. J. Hazard. Mater. 2008, 162, 120–132. [Google Scholar] [CrossRef]
- Uen, U.T.; Koparal, A.S.; Oeguetveren, U.B. Hybrid processes for the treatment of cattle-slaughterhouse wastewater using aluminum and iron electrodes. J. Hazard. Mater. 2008, 164, 580–586. [Google Scholar] [CrossRef]
- Costa, C.R.; Olivi, P. Effect of chloride concentration on the electrochemical treatment of a synthetic tannery wastewater. Electrochim. Acta 2008, 54, 2046–2052. [Google Scholar] [CrossRef]
- Wang, C.T.; Chou, W.L.; Kuo, Y.M. Removal of COD from laundry wastewater by electrocoagulation/electroflotation. J. Hazard. Mater. 2008, 164, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Yue, P.L. Separation of pollutants from restaurant wastewater by electrocoagulation. Sep. Purif. Technol. 2000, 19, 65–76. [Google Scholar] [CrossRef]
- Chen, Y.; Baygents, J.C.; Farrell, J. Evaluating electrocoagulation and chemical coagulation for removing dissolved silica from high efficiency reverse osmosis (HERO) concentrate. J. Water Process Eng. 2016, 16, 50–55. [Google Scholar] [CrossRef]
- Landels, A.; Beacham, T.A.; Evans, C.T.; Carnovale, G.; Raikova, S.; Cole, I.S.; Goddard, P.; Chuck, C.; Allen, M.J. Improving electrocoagulation floatation for harvesting microalgae. Algal Res.-Biomass Biofuels Bioprod. 2019, 39, 101446. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater. 2003, 100, 163–178. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Kobya, M.; Can, O.T.; Sozbir, M. Operating cost analysis of electrocoagulation of textile dye wastewater. Sep. Purif. Technol. 2004, 37, 117–125. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, H.A.; Kasiri, M.B. Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef]
- Khemila, B.; Merzouk, B.; Chouder, A.; Zidelkhir, R.; Leclerc, J.-P.; Lapicque, F. Removal of a textile dye using photovoltaic electrocoagulation. Sustain. Chem. Pharm. 2017, 7, 27–35. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Madani, K.; Vial, C.; Sekki, A. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation. A comparative study. Desalination 2011, 272, 246–253. [Google Scholar] [CrossRef]
- Al Aji, B.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Sep. Purif. Technol. 2011, 86, 248–254. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.-K.; Zoh, K.-D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J. Water Process Eng. 2019, 33, 101109. [Google Scholar] [CrossRef]
- Kobya, M.; Ciftci, C.; Bayramoglu, M.; Sensoy, M.T. Study on the treatment of waste metal cutting fluids using electrocoagulation. Sep. Purif. Technol. 2007, 60, 285–291. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Dedeli, A.; Sensoy, M.T. Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. J. Hazard. Mater. 2009, 173, 326–334. [Google Scholar] [CrossRef]
- Fouad, Y.O.A.; Konsowa, A.H.; Farag, H.A.; Sedahmed, G.H. Performance of an electrocoagulation cell with horizontally oriented electrodes in oil separation compared to a cell with vertical electrodes. Chem. Eng. J. 2008, 145, 436–440. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Jiang, W.; Wang, T. Experimental Study on the Treatment of Oily Wastewater by Electrocoagulation with Aluminum Electrodes at Different Heights. J. Electrochem. Soc. 2019, 166, E533–E541. [Google Scholar] [CrossRef]
- Nasrullah, M.; Zularisam, A.W.; Krishnan, S.; Sakinah, M.; Singh, L.; Fen, Y.W. High performance electrocoagulation process in treating palm oil mill effluent using high current intensity application. Chin. J. Chem. Eng. 2018, 27, 208–217. [Google Scholar] [CrossRef]
- Yang, C.-L. Electrochemical coagulation for oily water demulsification. Sep. Purif. Technol. 2006, 54, 388–395. [Google Scholar] [CrossRef]
- Min, G.; Yirong, Z.; Jinglong, Z.; Liming, W.; Hongwei, Z.; Lei, S. Study on phosphorous removal by electrolytic process with iron electrode. Technol. Water Treat. 2014, 40, 39–42. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; He, X.; Wang, Z.; Zhao, C. Factors Influencing the Removal of Phosphorus and the Purity of Recycling Struvite in Wastewater by the Electrochemical Sacrificial Magnesium Anode Method. Sci. Adv. Mater. 2019, 11, 128–134. [Google Scholar] [CrossRef]
- Mores, R.; Treichel, H.; Zakrzevski, C.A.; Kunz, A.; Steffens, J.; Dallago, R.M. Remove of phosphorous and turbidity of swine wastewater using electrocoagulation under continuous flow. Sep. Purif. Technol. 2016, 171, 112–117. [Google Scholar] [CrossRef]
- Kobya, M.; Gengec, E.; Sensoy, M.T.; Demirbas, E. Treatment of textile dyeing wastewater by electrocoagulation using Fe and Al electrodes: Optimisation of operating parameters using central composite design. Color. Technol. 2014, 130, 226–235. [Google Scholar] [CrossRef]
- Kirujika, K.; Kreshaanth, S.; Gunathilake, C.; Udagedara, T.; Manipura, A. Investigation of electrochemical denitrification of prawn-farm wastewater. Sep. Sci. Technol. 2020, 8, 2862–2869. [Google Scholar] [CrossRef]
- Jia, G.; Jing, H.; Hu, B.; Ju, Z. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Tohamy, H.S.; El-Sakhawy, M.; Kamel, S. Microwave-assisted synthesis of amphoteric fluorescence carbon quantum dots and their chromium adsorption from aqueous solution. Sci. Rep. 2023, 13, 11306. [Google Scholar] [CrossRef]
- Shapiro, S.; Francia, R. An Approximate Analysis of Variance Test for Normality. J. Am. Stat. Assoc. 1972, 67, 215–216. [Google Scholar] [CrossRef]
- Brahmi, K.; Bouguerra, W.; Hamrouni, B.; Elaloui, E.; Loungou, M.; Tlili, Z. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2014, 12, 1848–1859. [Google Scholar] [CrossRef]
- Hashim, K.S.; Shaw, A.; Al Khaddar, R.; Pedrola, M.O.; Phipps, D. Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J. Environ. Manag. 2016, 189, 98–108. [Google Scholar] [CrossRef]
- Emamjomeh, M.M. Electrocoagulation as a green technology for phosphate removal from river water. Sep. Purif. Technol. 2019, 217, 85. [Google Scholar] [CrossRef]
- Han, Z.Y.; Wang, L.; Duan, L.; Zhu, S.M.; Ye, Z.Y.; Yu, H.J. The electrocoagulation pretreatment of biogas digestion slurry from swine farm prior to nanofiltration concentration. Sep. Purif. Technol. 2016, 156, 817–826. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.V.; Srivastava, V.C.; Singh, S.; Bhatnagar, R.; Mall, I.D. Electrochemical treatment of alkali decrement wastewater containing terephthalic acid using iron electrodes. J. Taiwan Inst. Chem. Eng. 2013, 45, 908–913. [Google Scholar] [CrossRef]
- Attour, A.; Touati, M.; Thin, M.; Ben Amor, M.; Lapicque, F.; Leclerc, J.P. Influence of operating parameters on phosphate removal from water by electrocoagulation using aluminum electrodes. Sep. Purif. Technol. 2013, 123, 124–129. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Qian, F.; Zhang, K.J.; Liu, X.H.; Guan, W.Y.; Chen, X.D.; Song, L.R.; Fang, F.; Luo, J.Y.; Xue, Z.X.; Cao, J.S. An improved kinetic model for dephosphorization of laundry wastewater by electrocoagulation. J. Water Process Eng. 2020, 39, 8. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhang, D.D.; Guo, G.J.; Jiang, Y.; Wang, M.S.; Zhang, P.; Li, J. Dolomite application for the removal of nutrients from synthetic swine wastewater by a novel combined electrochemical process. Chem. Eng. J. 2017, 335, 665–675. [Google Scholar] [CrossRef]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Hecini, M.; Hamitouche, H. Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem. Eng. Process.-Process Intensif. 2010, 49, 1176–1182. [Google Scholar] [CrossRef]
- Bassyouni, D.G.; Hamad, H.A.; El-Ashtoukhy, E.S.Z.; Amin, N.K.; Abd El-Latif, M.M. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N. Treatment of textile dyeing factory wastewater by electrocoagulation with low sludge settling time: Optimization of operating parameters by RSM. J. Environ. Chem. Eng. 2017, 6, 635–642. [Google Scholar] [CrossRef]
- Congcong, C.; Guanglei, Q.; Chenxin, X. Influencing factors and kinetics analysis of electrocoagulation with bipolar aluminum electrodes treating high fluorine groundwater. Chin. J. Environ. Eng. 2020, 14, 1216–1223. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Ramo, J.; Lassi, U.; Roininen, J. Removal of phosphate from wastewaters for further utilization using electrocoagulation with hybrid electrodes—Techno-economic studies. J. Water Process Eng. 2014, 8, E50–E57. [Google Scholar] [CrossRef]
- Pulkka, S.; Martikainen, M.; Bhatnagar, A.; Sillanpaa, M. Electrochemical methods for the removal of anionic contaminants from water—A review. Sep. Purif. Technol. 2014, 132, 252–271. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M. Treatment of domestic wastewater phosphate by electrocoagulation using Fe and Al electrodes: A comparative study. Process Saf. Environ. Prot. 2018, 116, 34–51. [Google Scholar] [CrossRef]
- Jimenez, C.; Saez, C.; Martinez, F.; Canizares, P.; Rodrigo, M.A. Electrochemical dosing of iron and aluminum in continuous processes: A key step to explain electro-coagulation processes. Sep. Purif. Technol. 2012, 98, 102–108. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.J.; Hu, B. The effects of electrocoagulation on phosphorus removal and particle settling capability in swine manure. Sep. Purif. Technol. 2018, 200, 112–119. [Google Scholar] [CrossRef]
- Song, X.Y.; Pan, Y.Q.; Wu, Q.Y.; Cheng, Z.H.; Ma, W. Phosphate removal from aqueous solutions by adsorption using ferric sludge. Desalination 2011, 280, 384–390. [Google Scholar] [CrossRef]
- Donneys-Victoria, D.; Bermudez-Rubio, D.; Torralba-Ramirez, B.; Marriaga-Cabrales, N.; Machuca-Martinez, F. Removal of indigo carmine dye by electrocoagulation using magnesium anodes with polarity change. Environ. Sci. Pollut. Res. 2019, 26, 7164–7176. [Google Scholar] [CrossRef]
- Fulazzaky, M.A.; Salim, N.A.A.; Abdullah, N.H.; Yusoff, A.R.M.; Paul, E. Precipitation of iron-hydroxy-phosphate of added ferric iron from domestic wastewater by an alternating aerobic-anoxic process. Chem. Eng. J. 2014, 253, 291–297. [Google Scholar] [CrossRef]
- Karageorgiou, K.; Paschalis, M.; Anastassakis, G.N. Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J. Hazard. Mater. 2006, 139, 447–452. [Google Scholar] [CrossRef]



| Test Items | Value | Unit |
|---|---|---|
| Total phosphorus | 16.43~17.01 | mg·L−1 |
| pH | 8 | - |
| COD | 560.64~579.33 | mg·L−1 |
| BOD5 | 108 | mg·L−1 |
| Conductivity | 922 | μS·cm−1 |
| Run | Independent Variable | TP Removal (%) | Anode Consumption (mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ED | pH | Voltage | Mg | Al | Fe | Mg | Al | Fe | |
| 1 | 1 | 5 | 3 | 87.60 | 80.06 | 40.23 | 3.79 | 2.31 | 5.08 |
| 2 | 1 | 7 | 5 | 95.17 | 75.50 | 40.08 | 8.01 | 4.39 | 8.01 |
| 3 | 1 | 9 | 7 | 74.85 | 82.91 | 37.18 | 8.21 | 7.12 | 10.11 |
| 4 | 1 | 11 | 9 | 86.26 | 81.07 | 22.58 | 13.64 | 7.48 | 12.96 |
| 5 | 3 | 5 | 5 | 85.54 | 94.56 | 17.58 | 3.50 | 1.52 | 4.48 |
| 6 | 3 | 7 | 3 | 95.14 | 64.60 | 33.80 | 1.61 | 0.78 | 2.12 |
| 7 | 3 | 9 | 9 | 82.92 | 83.33 | 21.38 | 5.11 | 3.75 | 3.80 |
| 8 | 3 | 11 | 7 | 63.84 | 82.33 | 19.02 | 2.90 | 2.64 | 5.78 |
| 9 | 5 | 5 | 7 | 95.32 | 76.35 | 22.62 | 4.21 | 1.92 | 5.53 |
| 10 | 5 | 7 | 9 | 78.86 | 91.08 | 31.33 | 5.16 | 2.40 | 6.00 |
| 11 | 5 | 9 | 3 | 79.61 | 10.66 | 12.30 | 1.50 | 0.60 | 1.62 |
| 12 | 5 | 11 | 5 | 80.82 | 83.42 | 18.36 | 2.36 | 1.21 | 3.05 |
| 13 | 7 | 5 | 9 | 77.75 | 67.52 | 18.07 | 3.32 | 1.79 | 4.32 |
| 14 | 7 | 7 | 7 | 72.80 | 26.23 | 17.61 | 2.73 | 1.33 | 3.13 |
| 15 | 7 | 9 | 5 | 75.77 | 40.09 | 15.35 | 1.87 | 0.87 | 2.02 |
| 16 | 7 | 11 | 3 | 79.49 | 61.67 | 36.28 | 0.73 | 0.42 | 1.01 |
| Source | p-Value | ||
|---|---|---|---|
| Mg | Al | Fe | |
| ED | 0.045 | 0.229 | 0.048 |
| pH | 0.901 | 0.993 | 0.838 |
| voltage | 0.048 | 0.016 | 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, J.; Xie, N. Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes. Water 2023, 15, 4134. https://doi.org/10.3390/w15234134
Chen P, Li J, Xie N. Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes. Water. 2023; 15(23):4134. https://doi.org/10.3390/w15234134
Chicago/Turabian StyleChen, Peng, Junfeng Li, and Ningning Xie. 2023. "Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes" Water 15, no. 23: 4134. https://doi.org/10.3390/w15234134
APA StyleChen, P., Li, J., & Xie, N. (2023). Study on Influencing Parameters of Total Phosphorus Degradation in Cattle Farm Wastewater by Electrocoagulation Using Magnesium, Aluminum, and Iron Electrodes. Water, 15(23), 4134. https://doi.org/10.3390/w15234134







